Abstract
Sodium‐glucose cotransporter 2 (SGLT2) inhibitors are a unique class of oral anti‐hyperglycaemic medications that act to reduce glucose reabsorption in the renal proximal tubules, thereby enhancing urinary glucose excretion. Large randomized placebo‐controlled trials in people with diabetes at high cardiovascular risk have demonstrated that SGLT2 inhibitors reduce heart failure hospitalization within months of commencing therapy. These findings are of considerable interest, as diabetes is associated with an increased risk of both heart failure with reduced ejection fraction and heart failure with preserved ejection fraction. In addition, left ventricular (LV) hypertrophy and impaired diastolic function is thought to be more prevalent in people with diabetes. Although many hypotheses have been proposed, the underlying mechanisms through which SGLT2 inhibitors reduce the risk of heart failure in people with diabetes are not fully understood. Given the rapid reduction in heart failure hospitalization, it is conceivable that the benefits of SGLT2 inhibitors are due to favourable haemodynamic and metabolic effects on LV function. Several clinical studies have been conducted to investigate the effect of SGLT2 inhibitors on LV structure and function and have found that LV mass index and diastolic function improve following SGLT2 inhibitor therapy in people with type 2 diabetes. If these findings are confirmed in future studies utilizing novel cardiac imaging modalities and large randomized controlled trials, then this will bring new hope for the prevention and management of heart failure with preserved ejection fraction, for which no current treatments have been shown to reduce mortality. At the present time, SGLT2 inhibitors are indicated for the treatment of type 2 diabetes; however, the results of ongoing trials in participants with heart failure but without diabetes are eagerly awaited. The purpose of this review is to summarize current knowledge regarding the effects of SGLT2 inhibitors on LV function, particularly the findings from clinical studies, proposed biological mechanisms, and future directions.
Keywords: Heart failure, Diabetes mellitus, Echocardiography, SGLT2 inhibitor, Prevention, Treatment
Introduction
Sodium‐glucose cotransporter 2 (SGLT2) inhibitors are a unique class of oral anti‐hyperglycaemic medications that act primarily to reduce glucose reabsorption in the renal proximal tubules, thereby enhancing urinary glucose excretion.1 In contrast to many other anti‐hyperglycaemic medications, SGLT2 inhibitors reduce plasma glucose levels via an insulin‐independent mechanism.1 Furthermore, the use of SGLT2 inhibitors can result in modest reductions to body weight and systolic and diastolic blood pressures.1
Large randomized placebo‐controlled trials on the SGLT2 inhibitors, empagliflozin (EMPA‐REG OUTCOME),2 canagliflozin (CANVAS Program),3 and dapagliflozin (DECLARE‐TIMI 58),4 have demonstrated somewhat unexpected, but favourable cardiovascular outcomes in people with type 2 diabetes mellitus. In particular, significant reductions in heart failure (HF) hospitalization were seen in all three trials within months of initiating a SGLT2 inhibitor.2, 3, 4 At baseline, only 10–15% of trial participants had a history of investigator‐reported HF, which was not further phenotyped by echocardiography.2, 3, 4 Sub‐analyses have demonstrated that the reduction in HF hospitalization is significant regardless of HF or cardiovascular disease history at baseline.4, 5 However, whilst composite cardiovascular outcomes were significantly reduced, individual outcomes more directly related to atherosclerosis, such as myocardial infarction and ischaemic stroke, were not significantly different compared with placebo.2, 3, 4
Diabetes is associated with an increased risk of both HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF).6, 7 Current guidelines define HFpEF as a constitution of symptoms and signs of HF, a left ventricular (LV) ejection fraction of 50% or greater (although between 40% and 49% is often classified as HFpEF in clinical trials), elevated levels of natriuretic peptides, and evidence of structural heart disease or diastolic dysfunction.8 Key echocardiography findings associated with HFpEF are structural alterations such an increased left atrial volume index or LV mass index, functional alterations such as an increased mitral peak E‐wave velocity to early mitral or septal annular tissue Doppler velocity ratio (E/e′) or reduced e′, and indirect measures such as increased tricuspid regurgitation velocity.8
Subclinical LV diastolic dysfunction, an independent predictor of adverse outcomes and a major causative factor to the development of HFpEF, is highly prevalent in people with diabetes.7, 9 Therefore, it is intuitive to speculate that a large proportion of participants in the landmark SGLT2 inhibitor trials might have had occult LV diastolic dysfunction or even undiagnosed HF. Given the rapid reductions in HF hospitalization but not atherosclerosis‐related events observed with SGLT2 inhibitors, it is conceivable that the benefits of this class of medications are due to early favourable haemodynamic effects on LV function rather than via modulation of atherothrombotic mechanisms.10
The complex interplay between HF and diabetes has consequently been of increased interest amongst the scientific community in recent times. The expectation that SGLT2 inhibitors may prevent the onset of HFpEF in people with diabetes is now high. Several clinical studies have been conducted to investigate the effect of SGLT2 inhibitors on LV structure and function. However, although many hypotheses have been put forward, the biological mechanisms through which SGLT2 inhibitors reduce HF hospitalization remain uncertain. The purpose of this review article is to summarize current knowledge regarding the effects of SGLT2 inhibitors on LV function, particularly the findings from clinical studies, proposed biological mechanisms and future directions.
Methodology
We performed an electronic search of PubMed, EMBASE, CENTRAL, Google Scholar, and ClinicalTrials.gov in March 2019 for studies published between January 2000 and February 2019. Abstracts and conference proceedings were included, as no restrictions were placed on publication status. The search strategy included a mix of subject headings and free text terms such as SGLT2, sodium glucose cotransporter 2, empagliflozin, canagliflozin, dapagliflozin, luseogliflozin, tofogliflozin, sotagliflozin, ertugliflozin, cardiac, cardiac failure, heart failure, diastolic, systolic, left ventricular, diabetes, and diabetes mellitus. The literature was then screened for studies investigating the effect of SGLT2 inhibitors on LV structure and function.
The effect of sodium‐glucose cotransporter 2 inhibitors on left ventricular function
Pre‐clinical research
Studies involving obese mice with diabetes and LV diastolic dysfunction have demonstrated that administration of empagliflozin can improve LV diastolic function.11, 12, 13 However, measures of LV systolic function remained unchanged after treatment.12 Furthermore, in non‐obese mice with diabetes, LV hypertrophy, and diastolic and systolic dysfunction, administration of dapagliflozin resulted in a decrease in LV hypertrophy and an improvement in both LV diastolic and systolic function.14
In mice without diabetes but with HFpEF, empagliflozin has been shown to reduce LV mass and improve diastolic function.15, 16 Interestingly, in one study on mice without diabetes but with HFrEF, empagliflozin treatment did not affect LV diastolic parameters but was found to ameliorate the decline in LV systolic function.17 In contrast to this, a study conducted on explanted human hearts from people with end‐stage HFrHF demonstrated that empagliflozin significantly reduced LV diastolic stiffness and improved diastolic function independent of diabetes history but did not improve systolic force.18 In the same study, the beneficial effects on LV diastolic function, but not systolic function, were confirmed in mice with and without diabetes.18
The beneficial effects of SGLT2 inhibitors on systolic function have been inconsistent based on these pre‐clinical studies. It should be noted that these studies did not report data regarding speckle‐tracking echocardiography, which may have demonstrated changes in strain parameters. However, the results of these studies do suggest that SGLT2 inhibitors may have a potential role for the management of LV dysfunction, which may theoretically underlie the observed reductions in HF hospitalization with these medications.
Clinical research
There are relatively few clinical studies on human participants that have assessed the effect of SGLT2 inhibitors on LV structure and function. The results of such studies are summarized in Table 1.
Table 1.
Review of previous studies on SGLT2 inhibitors and LV function
| Author | SGLT2 inhibitor | Cohort | Imaging modality | Imaging findings |
|---|---|---|---|---|
| Verma S., et al. | Empagliflozin | 10 people with T2DM and CVD | TTE before and 3 months after | • Improved LV diastolic function according to early lateral e′ |
| • Reduced LV mass index | ||||
| • No difference in LV volumes and LV EF | ||||
| Matsutani D., et al. | Canagliflozin | 37 people with T2DM and ≥2 CVD risk factors or CVD | TTE before and 3 months after | • Improved LV diastolic function according to the E/e′ ratio |
| • Reduced LV mass index | ||||
| • No difference in LV diameters, LV EF, and left atrial diameter | ||||
| Soga F., et al. | Dapagliflozin | 53 people with T2DM and stable HFrEF or HFpEF | TTE before and 6 months after | • Improved LV diastolic function according to the E/e′ ratio |
| • Reduced LV mass index and left atrial volume index | ||||
| • No difference in LV volumes | ||||
| • Improved LV EF | ||||
| Sakai T., et al.a | Empagliflozin | 59 people with T2DM and HFpEF | TTE before and 3 months after | • Improved LV diastolic function according to the E/A and E/e′ ratios |
| Luseogliflozin | 63 people with T2DM and HFpEF | |||
| Tofogliflozin | 62 people with T2DM and HFpEF | |||
| Verma S., et al.a | Empagliflozin vs. placebo | 97 people with T2DM and CVD (49 drug and 48 placebo) | Cardiac MRI before and 6 months after | • Improved LV mass index |
| • No difference in LV EF and LV end‐systolic volume | ||||
| Cohen N., et al. | Empagliflozin vs. placebo | 25 people with T2DM (17 drug and 8 placebo) | Cardiac MRI before and 6 months after | • Reduced LV end‐diastolic volume |
| • No difference in LV mass, LV EF, atrial volumes, and markers of cardiac fibrosis |
A, mitral peak A‐wave velocity; CVD, cardiovascular disease; E, mitral peak E‐wave velocity; e′, early annular tissue Doppler velocity; EF, ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular; MRI, magnetic resonance imaging; SGLT2, sodium‐glucose cotransporter 2; T2DM, type 2 diabetes mellitus; TTE, transthoracic echocardiography.
Preliminary data obtained from abstract or conference presentation.
Studies that have utilized transthoracic echocardiography have demonstrated that empagliflozin,19, 20 canagliflozin,21 dapagliflozin,22 luseogliflozin,20 and tofogliflozin20 may reduce LV mass index and diastolic function after only 3 to 6 months duration of therapy in people with diabetes. The first study showing improved LV diastolic function based on changes in early lateral annular tissue Doppler velocity (e′) was hypothesis generating, having been conducted with empagliflozin in only 10 participants with diabetes and cardiovascular disease.19 Echocardiography parameters that were used by subsequent studies to assess LV diastolic function include the ratio of mitral peak E‐wave velocity to early mitral or septal annular tissue Doppler velocity ratio (E/e′ ratio), mitral peak E‐wave velocity to A‐wave velocity ratio (E/A ratio) and left atrial size. Notably, improvements in diastolic function, based on the E/A and E/e′ ratios, have also been demonstrated in a study that enrolled primarily people with diabetes who had HFpEF.20
Whilst the majority of echocardiography studies did not find any difference in LV systolic function or LV diameters and volumes, one study found an improvement in LV ejection fraction after initiating dapagliflozin in people with diabetes who had stable HFrEF or HFpEF.22 Although considered as a statistically significant change, the difference in LV ejection fraction from baseline and 6 months duration of therapy was only 1.3%.22 However, it should be noted that these echocardiography studies were not randomized controlled trials, were performed in relatively small cohorts of participants with short periods of follow‐up, and are subject to the limitations that are inherent to transthoracic echocardiography.
More recently, preliminary results from EMPA‐HEART, a randomized placebo‐controlled study on SGLT2 inhibitors that utilized cardiac magnetic resonance imaging, were presented at the American Heart Association Annual Scientific Sessions 2018.23, 24 This study demonstrated that empagliflozin may reduce LV mass index in people with diabetes and cardiovascular disease, particularly in those with LV mass index >60 g/m2 at baseline.23, 24 In contrast, a separate but smaller placebo‐controlled study that utilized cardiac magnetic resonance imaging demonstrated that empagliflozin reduces LV end‐diastolic volume, likely due to a reduction in plasma volume, but did not affect LV mass.25 Both studies did not find any statistically significant change in LV ejection fraction, which may be a reflection of the small sample sizes. However, taken together with the results of previous echocardiography studies, the overall findings suggest that SGLT2 inhibitors may have the potential to reverse LV hypertrophy and improve diastolic function. Similar imaging studies have not yet, to the best of our knowledge, been reported in people without diabetes.26
Ongoing clinical research
Further larger studies utilizing novel cardiac imaging modalities to more precisely quantify cardiac structure and function, particularly indicators of early LV dysfunction, would be of great benefit. There are currently ongoing clinical studies that are also utilizing cardiac magnetic resonance imaging to assess the effects of SGLT2 inhibitors on cardiac structure and function, as shown in Table 2.26, 27, 28 In addition, studies utilizing speckle‐tracking echocardiography for the measurement of global longitudinal strain and three‐dimensional echocardiography for more accurate quantification of LV structure in people with diabetes and normal LV ejection fraction are underway.29 Although these studies also involve a relatively small cohort of participants, they will be able to provide useful insights and hypotheses for further larger studies to be conducted in this area.
Table 2.
Ongoing studies on SGLT2 inhibitors and LV function
| Study name | SGLT2 inhibitor | Anticipated cohort | Imaging modality | Imaging outcome | Estimated end date |
|---|---|---|---|---|---|
| Research Into the Effect of SGLT2 Inhibition on Left Ventricular Remodeling in Patients With Heart Failure and Diabetes Mellitus (REFORM) (NCT02397421) | Dapagliflozin vs. placebo | 56 people with T2DM and HFrEF | Cardiac MRI before and 12 months after |
Primary: Change in LV end‐systolic and end‐diastolic volumes Secondary: Change in LV mass and EF, RV volumes and EF, atrial size, and LV remodelling index |
August 2017 (not reported) |
| Does Dapagliflozin Regress Left Ventricular Hypertrophy In Patients With Type 2 Diabetes? (DAPA‐LVH) (NCT02956811) | Dapagliflozin vs. placebo | 64 people with T2DM and LV hypertrophy | Cardiac MRI before and 12 months after |
Primary: Change in LV mass Secondary: Change in LV diastolic function and global longitudinal strain |
March 2019 |
| Effects of Empagliflozin on Left Ventricular Diastolic Function Compared to Usual Care in Type 2 Diabetics (EmDia) (NCT02932436) | Empagliflozin vs. placebo | 158 people with T2DM and LV diastolic dysfunction (E/e′ ratio ≥ 8) | TTE before and 3 months after |
Primary: Change in E/e′ ratio Secondary: Change in LV EF and end‐diastolic volume |
June 2019 |
| EMPA‐HEART triala | Empagliflozin vs. sitagliptin | 75 people with T2DM and subclinical LV dysfunction | TTE before and at 1 month and 6 months after |
Primary: Change in global longitudinal strain Secondary: Change in EF, left atrial volume, and E/e′ by 3‐D TTE |
July 2019 |
| Are the “Cardiac Benefits” of Empagliflozin Independent of Its Hypoglycemic Activity? (ATRU‐4) (EMPA‐TROPISM) (NCT03485222) | Empagliflozin vs. placebo | 80 people with T2DM and HFrEF | Cardiac MRI before and 6 months after |
Primary: Change in LV end‐systolic and end‐diastolic volumes Secondary: Change in LV EF |
December 2020 |
| ERtugliflozin triAl in DIabetes With Preserved or Reduced ejeCtion FrAcTion mEchanistic Evaluation in Heart Failure (ERADICATE‐HF) (NCT03416270) | Ertugliflozin vs. placebo | 36 people with T2DM and HF | TTE before and at 1 week and 3 months after |
Primary: N/A Secondary: Change in systolic and diastolic function |
March 2021 |
3‐D, three‐dimensional; E, mitral peak E‐wave velocity; e′, early annular tissue Doppler velocity; EF, ejection fraction; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LV, left ventricular; MRI, magnetic resonance imaging; SGLT2, sodium‐glucose cotransporter 2; T2DM, type 2 diabetes mellitus.
There is another trial with a similar name.
Proposed mechanisms for improved left ventricular function with sodium‐glucose cotransporter 2 inhibitors
Heart failure is a complex clinical syndrome whereby the heart is unable to deliver sufficient oxygen to peripheral organs and can arise as a result of LV systolic and/or diastolic dysfunction. In people with diabetes, LV diastolic dysfunction is prevalent and may be the earliest pathological alteration resulting in an increased risk of HF.7, 9 Furthermore, LV hypertrophy is a strong determinant of cardiovascular outcomes and mortality and may be present in up to 70% of people with type 2 diabetes due to aberrant myocardial remodelling.30 The link between diabetes and the development of HF or ‘diabetic cardiomyopathy’ is thought to be multifactorial and has been previously reviewed in detail.6
In studies of biopsies from healthy, ischaemic, and hypertrophic human hearts, SGLT1 but not SGLT2 receptors were identified in cardiac tissue.31 In addition, although the SGLT2 inhibitor canagliflozin is a low‐potency inhibitor of SGLT1, similar improvements in LV mass index and diastolic function have been demonstrated with more selective SGLT2 inhibitors such as empagliflozin and dapagliflozin (Table 1), suggesting that the benefits of SGLT2 inhibitors on the heart are unlikely to be mediated via SGLT1. Indeed, studies that have systemically examined tissue expression of SGLT receptors in humans have found that SGLT2 is highly specific to renal tissue.32 Therefore, the potential effect of SGLT2 inhibitors on LV structure and function is hypothesized to be multifaceted and mediated predominantly by systemic haemodynamic and metabolic effects, as summarized in Figure 1.10 The beneficial effect of SGLT2 inhibitors on renal function and the potential to reduce renal‐related outcomes has been previously reviewed in detail and is beyond the scope of this review article.33
Figure 1.
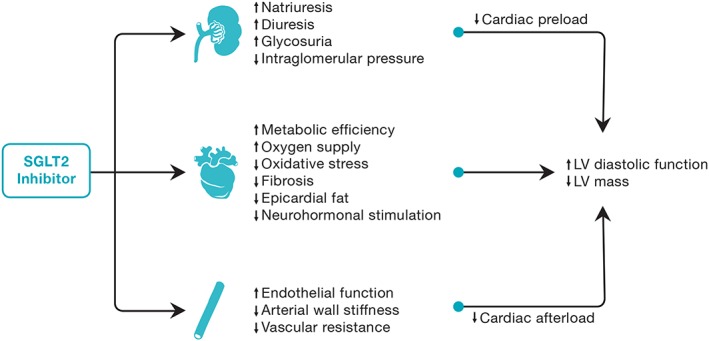
Potential mechanisms for improved left ventricular diastolic function and reduced left ventricular mass with sodium‐glucose cotransporter 2 inhibitors. LV, left ventricular; SGLT2, sodium‐glucose cotransporter 2.
Haemodynamic effects
Activation of compensatory mechanisms in HF such as the renin–angiotensin–aldosterone system, sympathetic nervous system, and other neuro‐hormonal pathways leads to fluid and sodium retention.10 However, SGLT2 inhibitors can potently reduce circulating intravascular volume through osmotic diuresis and natriuresis.10 This is reflected by an increase in haematocrit, which has also been found to be a key determinant of HF outcomes according to a recent exploratory analysis of the EMPA‐REG OUTCOME trial.34 It is hypothesized that the sustained reduction in intravascular volume and blood pressure may lead to a reduction in cardiac preload and afterload, respectively, thereby alleviating cardiac workload and improving LV function.10 Such haemodynamic changes in intravascular volume and blood pressures are seen without an increase in heart rate, suggesting that SGLT2 inhibitors may reduce reflex sympathetic nervous system activation or may have influence over other neuro‐hormonal pathways affecting the heart.35, 36
Myocardial energy supply
It is has also been hypothesized that SGLT2 inhibitors may increase myocardial energy supply and metabolic efficiency through a number of mechanisms, thereby improving myocardial performance. Firstly, SGLT2 inhibitors have been associated with increased circulating levels of β‐hydroxybutyrate, a ketone body, likely due to glucagon‐mediated ketogenesis.37 Ketones are freely taken up by myocardial cells and, compared with fatty acids, may potentially be a more efficient source of adenosine triphosphate for the failing heart.37 Secondly, SGLT2 inhibitors have been associated with an increase in erythropoietin, which in itself may have cardio‐protective effects, and an increase in haemoglobin, which may result in enhanced oxygen delivery to the myocardium.38, 39 The underlying mechanism for the increase in erythropoietin is thought to be due to favourable renal haemodynamic effects such as a reduced intra‐glomerular pressure rather than haemoconcentration from diuresis.38 Thirdly, an emerging hypothesis is that SGLT2 inhibitors can directly inhibit the myocardial sodium‐hydrogen (Na+/H+) exchanger, which leads to increased mitochondrial calcium levels, improved mitochondrial function, reduced oxidative stress, and potentially reduced arryhthmias.40 However, whether or not these metabolic changes translate into clinically meaningful effects remains unclear.
Other effects
The approximately 0.5–1% reduction in mean glycated haemoglobin with SGLT2 inhibitors is only modest, which lends support to the notion that the large reductions in HF hospitalization are due to mechanisms distinct from glycaemic control.2, 3, 4, 34 This is further supported by previous studies demonstrating that intensive glycaemic control may reduce microvascular but not macrovascular events in people with longstanding type 2 diabetes.41 Indeed, SGLT2 inhibitors have been shown to affect multiple other common modifiable risk factors and co‐morbidities associated with cardiovascular disease, such as blood pressure, body weight, renal function, uric acid, and plasma lipids.2, 3, 4
A number of other biological effects have also been demonstrated with SGLT2 inhibitors, which may ultimately contribute to improved LV structure and function.34 These include favourable changes to vascular endothelial function, arterial stiffness, vascular resistance, and myocardial fibrosis.35, 42 In addition, reductions in visceral adiposity and epicardial fat may reduce insulin resistance and the production of pro‐inflammatory adipokines, such as leptin, from adipocytes.43, 44 However, these mechanisms, although intriguing, are considered insufficient to account for the large reductions in HF hospitalization observed with SGLT2 inhibitors as compared with the aforementioned hypotheses regarding diuresis, natriuresis, and myocardial energy supply. Furthermore, despite improvements in vascular function and blood pressure, SGLT2 inhibitors have not been shown to significantly reduce the risk of myocardial infarction or stroke in landmark trials.2, 3, 4
Future directions for sodium‐glucose cotransporter 2 inhibitors in heart failure
A paradigm shift in the management of diabetes has become evident with recent international guidelines now strongly recommending SGLT2 inhibitors as the preferred second‐line anti‐hyperglycaemic therapy after metformin for cardiovascular risk reduction in people with type 2 diabetes and established cardiovascular disease.45 According to the European Society of Cardiology 2016 guidelines, empagliflozin should be considered in people with type 2 diabetes and cardiovascular disease, in order to prevent or delay the onset of HF or prolong life (class IIA, level B).8 An expert consensus document in 2019 recommended that canagliflozin and dapagliflozin should also be considered for people with type 2 diabetes and established cardiovascular disease or at high cardiovascular risk, in order to prevent or delay the onset of HF and hospitalizations for HF.46 However, no specific guideline recommendations for SGLT2 inhibitor use in people with established HF could be made.46 Current recommendations for SGLT2 inhibitor use are listed in Table 3.45, 46
Table 3.
Current recommendations for sodium‐glucose cotransporter 2 inhibitor use
| Type 2 diabetes and45, 46: |
|
1. Insufficient glycaemic control despite first‐line therapya
2. At high cardiovascular risk 3. Cardiovascular disease 4. Cardiovascular disease and coexisting heart failure or at high risk of heart failure 5. Chronic kidney diseaseb |
First‐line therapy, metformin and lifestyle modifications.
Assuming adequate estimated glomerular filtration rate.
However, it must be noted that SGLT2 inhibitors are not free of undesirable effects relating to marked glycosuria, such as an increased risk of genital infections and volume depletion, and rarely, diabetic ketoacidosis, and very rarely, Fournier's gangrene.2, 3, 4 In addition, unanswered questions remain. Whether or not SGLT2 inhibitors can reduce cardiovascular risk in people with diabetes who are at a lower risk of cardiovascular disease, such as those without other cardiovascular risk factors, is a key research question.
Ongoing heart failure trials
At the present time, SGLT2 inhibitors are indicated for the treatment of type 2 diabetes; however, the results of ongoing randomized placebo‐controlled SGLT2 inhibitor trials in participants with HF but without diabetes are eagerly awaited. If a therapeutic role is proven for SGLT2 inhibitors against HF decompensation even in people without diabetes, then SGLT2 inhibitors could be deemed a group of medications able to reduce cardiac preload and afterload, characterized also by a marked reduction in blood glucose. The haemodynamic effects would then become the main property of these medications, thereby outweighing their glucose‐lowering effect in the scope of their therapeutic applications.
Notably, the EMPEROR‐Preserved trial (NCT03057951) is currently aiming to recruit 6000 participants with chronic HFpEF, whilst the EMPEROR‐Reduced trial (NCT03057977) is aiming to recruit 2850 participants with chronic HFrEF. Both of these trials are being conducted using empagliflozin, with a follow‐up of 38 months and a composite primary outcome of time to cardiovascular death or hospitalization for HF. In regard to dapagliflozin, the DELIVER trial (NCT03619213) is currently aiming to recruit 4700 participants with HFpEF, whilst the DAPA‐HF trial (NCT03036124) is aiming to recruit 4744 participants with HFrEF. The follow‐up for these trials is 33 months and 3 years, respectively, and the primary outcome for both trials is a composite of cardiovascular death or hospitalization for HF or urgent HF visit. It should be noted that these large cardiovascular outcome trials were explicitly designed for people with HF (i.e. diabetes was not an inclusion criteria).
In addition, the SOLOIST‐WHF trial (NCT03521934) is an ongoing placebo‐controlled cardiovascular outcome trial investigating the use of sotagliflozin in people with diabetes and known HFrEF who are admitted to the hospital with worsening HF. The trial aims to recruit 4000 participants with a composite primary outcome of time to cardiovascular death or hospitalization for HF.
Ongoing mechanistic studies
Although there have been many hypotheses regarding the biological effects of SGLT2 inhibitors on LV function, the precise mechanism remains uncertain. However, it is entirely plausible that numerous haemodynamic and metabolic mechanisms may play a role in its cardio‐protection, as per Figure 1. Further mechanistic studies are therefore required to better determine the cardiovascular actions of SGLT2 inhibitors in people with and without diabetes and in people with and without existing HF. In particular, the potential for a beneficial effect of SGLT2 inhibitors on LV mass and/or diastolic function is currently being investigated in ongoing clinical trials, as per Table 2. If these studies are able to confirm the beneficial effects, then this will potentially open new avenues for the prevention and management of HFpEF, for which no effective treatments have thus far been shown to convincingly reduce long‐term morbidity or mortality.8
Other unanswered questions
Over the last decade, the results of various cardiovascular outcome trials have transformed the landscape of cardiovascular medicine. Angiotensin–neprilysin inhibitors (PARADIGM‐HF) have been shown to significantly reduce HF hospitalization and cardiovascular mortality compared with enalapril in people with HFrEF.47 If ongoing studies investigating angiotensin–neprilysin inhibitors as a treatment for HFpEF, such as the PARAGON‐HF (NCT01920711), are found to be positive, this will raise questions as to whether or not SGLT2 inhibitors have a role as add‐on therapy in HFpEF. In addition, trials on the glucagon‐like peptide 1 analogues, liraglutide (LEADER), semaglutide (SUSTAIN‐6), and albiglutide (Harmony Outcomes), have demonstrated that these anti‐hyperglycaemic medications can significantly reduce cardiovascular events, but not HF hospitalization, in people with diabetes.48, 49, 50 The EMPA‐REG OUTCOME trial was the first SGLT2 inhibitor trial using empagliflozin to demonstrate all‐cause and cardiovascular mortality reduction in people with diabetes and established cardiovascular disease.2 However, whether or not SGLT2 inhibitors have an additive effect in reducing cardiovascular risk, HF hospitalization and/or mortality when used in combination with angiotensin–neprilysin inhibitors or glucagon‐like peptide 1 analogues is yet to be elucidated.
Conclusions
The impressive results of the landmark SGLT2 inhibitor trials has led to this class of anti‐hyperglycaemic medications becoming a powerful adjunctive therapy for the management of people with diabetes and HF. Although many theories currently exist, the biological effects of SGLT2 inhibitors on cardiac structure and function remain uncertain and require further elucidation through detailed mechanistic studies. Investigations thus far in animals and humans with diabetes have demonstrated that SGLT2 inhibitors can potentially improve LV mass index and diastolic function. Larger scale studies are anticipated, as there may be scope for the future use of SGLT2 inhibitors as a novel therapeutic strategy in the prevention and management of HFpEF in people with and without diabetes.
Conflict of interest
None declared.
Acknowledgements
Nick S. R. Lan is supported by a WA Health Research Fellowship. Girish Dwivedi holds the Wesfarmers Chair in Cardiology at University of Western Australia. We also acknowledge the medical illustrations department of Fiona Stanley Hospital for assisting in the design of Figure 1 .
Lan, N. S. R. , Fegan, P. G. , Yeap, B. B. , and Dwivedi, G. (2019) The effects of sodium‐glucose cotransporter 2 inhibitors on left ventricular function: current evidence and future directions. ESC Heart Failure, 6, 927–935. 10.1002/ehf2.12505.
References
- 1. Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res 2015; 12: 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 3. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 4. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 5. Fitchett D, Butler J, van de Borne P, Zinman B, Lachin JM, Wanner C, Woerle HJ, Hantel S, George JT, Johansen OE, Inzucchi SE. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA‐REG OUTCOME(R) trial. Eur Heart J 2018; 39: 363–370. [DOI] [PubMed] [Google Scholar]
- 6. McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol 2014; 2: 843–851. [DOI] [PubMed] [Google Scholar]
- 7. Bouthoorn S, Valstar GB, Gohar A, den Ruijter HM, Reitsma HB, Hoes AW, Rutten FH. The prevalence of left ventricular diastolic dysfunction and heart failure with preserved ejection fraction in men and women with type 2 diabetes: a systematic review and meta‐analysis. Diab Vasc Dis Res 2018; 15: 477–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 9. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre‐clinical diastolic dysfunction a population‐based study. J Am Coll Cardiol 2010; 55: 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 inhibition and cardiovascular events: why did EMPA‐REG outcomes surprise and what were the likely mechanisms? Diabetologia 2016; 59: 1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Habibi J, Aroor AR, Sowers JR, Jia G, Hayden MR, Garro M, Barron B, Mayoux E, Rector RS, Whaley‐Connell A, DeMarco VG. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc Diabetol 2017; 16: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hammoudi N, Jeong D, Singh R, Farhat A, Komajda M, Mayoux E, Hajjar R, Lebeche D. Empagliflozin improves left ventricular diastolic dysfunction in a genetic model of type 2 diabetes. Cardiovasc Drugs Ther 2017; 31: 233–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeMarco VG, Aroor AR, Nistala R, Garro M, Mayoux E, Whaley‐Connell A, Sowers JR. Sodium glucose transporter type 2 (SGLT2) inhibitor, empagliflozin, improves diastolic function in female diabetic db/db mice. Diabetes 2015; 64: A552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joubert M, Jagu B, Montaigne D, Marechal X, Tesse A, Ayer A, Dollet L, Le May C, Toumaniantz G, Manrique A, Charpentier F, Staels B, Magre J, Cariou B, Prieur X. The sodium‐glucose cotransporter 2 inhibitor dapagliflozin prevents cardiomyopathy in a diabetic lipodystrophic mouse model. Diabetes 2017; 66: 1030–1040. [DOI] [PubMed] [Google Scholar]
- 15. Desjardins J, Zhang Y, Thai K, Kabir G, Gilbert R, Connelly K. Empagliflozin reduces LV mass and improves diastolic function in an experimental model of heart failure with preserved EF. Can J Cardiol; 33: S137–S138. [Google Scholar]
- 16. Connelly KA, Zhang Y, Visram A, Advani A, Batchu SN, D'esjardins J, Thai K, Gilbert RE. Empagliflozin improves diastolic function in a nondiabetic rodent model of heart failure with preserved ejection fraction. JACC: Basic to Translational Science 2019; 4: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bryne NJ, Parajuli N, Levasseur JL, Boisvenue J, Beker D, Masson G, Fedak PWM, Verma S, Dyck JRB. Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload‐induced heart failure. JACC: Basic to Translational Science 2017; 2: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pabel S, Wagner S, Bollenberg H, Bengel P, Kovacs A, Schach C, Tirilomis P, Mustroph J, Renner A, Gummert J, Fischer T, Van Linthout S, Tschope C, Streckfuss‐Bomeke K, Hasenfuss G, Maier LS, Hamdani N, Sossalla S. Empagliflozin directly improves diastolic function in human heart failure. Eur J Heart Fail 2018; 20: 1690–1700. [DOI] [PubMed] [Google Scholar]
- 19. Verma S, Garg A, Yan AT, Gupta AK, Al‐Omran M, Sabongui A, Teoh H, Mazer CD, Connelly KA. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA‐REG OUTCOME trial? Diabetes Care 2016; 39: e212–e213. [DOI] [PubMed] [Google Scholar]
- 20. Sakai T, Miura S. Abstract 17041: effect of sodium‐glucose cotransporter 2 inhibitor on vascular endothelial function and diastolic function in patients with heart failure with preserved ejection fraction (HFpEF). Circulation 2018; 136: A17041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol 2018; 17: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, Matsumoto K, Shite J, Takaoka H, Doi T, Hirata KI. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol 2018; 17: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verma S, Mazer CD, Yan AT, Mason T, Slabiak A, Bello OO, Opingari E, Fitchett DH, Goldenberg RM, Goodman SG, Farkouh ME, Partridge AC, Bonneau C, Bakbak E, Dhingra N, Williams AH, Lambotharan SG, Gupta N, Chowdhury B, Dai DW, Juni P, Teoh H, Quan A, Liuni A, Leiter LA, Bhatt DL, Zinman B, Connelly KA. EMPA‐HEART cardiolink‐6 trial: a randomized trial evaluating the effect of empagliflozin on left ventricular structure, function and biomarkers in people with type 2 diabetes (T2D) and coronary heart disease. Circulation 2018; 138: e762. [Google Scholar]
- 24. American College of Cardiology . EMPA‐ HEART Cardiolink‐6 ‐ EMPA‐HEART [Internet]. American College of Cardiology; 2018. https://www.acc.org/latest‐in‐cardiology/clinical‐trials/2018/11/10/01/55/empa‐heart‐cardiolink‐6 (1 March 2019).
- 25. Cohen ND, Gutman SJ, Briganti EM, Taylor AJ. The effects of empagliflozin treatment on cardiac function and structure in patients with type 2 diabetes—a cardiac MR study. Intern Med J 2019. 10.1111/imj.14260 [DOI] [PubMed] [Google Scholar]
- 26. Santos‐Gallego CG, Garcia‐Ropero A, Mancini D, Pinney SP, Contreras JP, Fergus I, Abascal V, Moreno P, Atallah‐Lajam F, Tamler R, Lala A, Sanz J, Fuster V, Badimon JJ. Rationale and design of the EMPA‐TROPISM trial (ATRU‐4): are the “cardiac benefits” of empagliflozin independent of its hypoglycemic activity? Cardiovasc Drugs Ther 2019; 33: 87–95. [DOI] [PubMed] [Google Scholar]
- 27. Brown AJM, Lang C, McCrimmon R, Struthers A. Does dapagliflozin regress left ventricular hypertrophy in patients with type 2 diabetes? A prospective, double‐blind, randomised, placebo‐controlled study. BMC Cardiovasc Disord 2017; 17: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh JS, Fathi A, Vickneson K, Mordi I, Mohan M, Houston JG, Pearson ER, Struthers AD, Lang CC. Research into the effect Of SGLT2 inhibition on left ventricular remodelling in patients with heart failure and diabetes mellitus (REFORM) trial rationale and design. Cardiovasc Diabetol 2016; 15: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Natali A, Nesti L, Fabiani I, Calogero E, Di Bello V. Impact of empagliflozin on subclinical left ventricular dysfunctions and on the mechanisms involved in myocardial disease progression in type 2 diabetes: rationale and design of the EMPA‐HEART trial. Cardiovasc Diabetol 2017; 16: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dawson A, Morris AD, Struthers AD. The epidemiology of left ventricular hypertrophy in type 2 diabetes mellitus. Diabetologia 2005; 48: 1971–1979. [DOI] [PubMed] [Google Scholar]
- 31. Di Franco A, Cantini G, Tani A, Coppini R, Zecchi‐Orlandini S, Raimondi L, Luconi M, Mannucci E. Sodium‐dependent glucose transporters (SGLT) in human ischemic heart: a new potential pharmacological target. Int J Cardiol 2017; 243: 86–90. [DOI] [PubMed] [Google Scholar]
- 32. Chen J, Williams S, Ho S, Loraine H, Hagan D, Whaley JM, Feder JN. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther 2010; 1: 57–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 inhibitors and the diabetic kidney. Diabetes Care 2016; 39: S165–S171. [DOI] [PubMed] [Google Scholar]
- 34. Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, Hantel S, Bluhmki E, Lachin JM. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care 2018; 41: 356–363. [DOI] [PubMed] [Google Scholar]
- 35. Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 2015; 17: 1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sano M. A new class of drugs for heart failure: SGLT2 inhibitors reduce sympathetic overactivity. J Cardiol 2018; 71: 471–476. [DOI] [PubMed] [Google Scholar]
- 37. Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium‐glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 2016; 65: 1190–1195. [DOI] [PubMed] [Google Scholar]
- 38. Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium‐glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res 2016; 8: 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van der Meer P, Lipsic E. Erythropoietin: repair of the failing heart. J Am Coll Cardiol 2006; 48: 185–186. [DOI] [PubMed] [Google Scholar]
- 40. Packer M, Anker SD, Butler J, Filippatos G, Zannad F. Effects of sodium‐glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA Cardiol 2017; 2: 1025–1029. [DOI] [PubMed] [Google Scholar]
- 41. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 42. Lee TM, Chang NC, Lin SZ. Dapagliflozin, a selective SGLT2 inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radic Biol Med 2017; 104: 298–310. [DOI] [PubMed] [Google Scholar]
- 43. Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, Ikeda Y, Kitazawa H, Takahashi M, Sato M, Okabe M. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol 2018; 17: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Packer M. Do sodium‐glucose co‐transporter‐2 inhibitors prevent heart failure with a preserved ejection fraction by counterbalancing the effects of leptin? A novel hypothesis. Diabetes Obes Metab 2018; 20: 1361–1366. [DOI] [PubMed] [Google Scholar]
- 45. Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018; 41: 2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seferovic PM, Ponikowski P, Anker SD, Bauersachs J, Chioncel O, Cleland JGF, de Boer RA, Drexel H, Ben Gal T, Hill L, Jaarsma T, Jankowska EA, Anker MS, Lainscak M, Lewis BS, McDonagh T, Metra M, Milicic D, Mullens W, Piepoli MF, Rosano G, Ruschitzka F, Volterrani M, Voors AA, Filippatos G, Coats AJS. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of The Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019. 10.1002/ejhf.1531. [DOI] [PubMed] [Google Scholar]
- 47. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 48. Marso SP, Daniels GH, Brown‐Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375: 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsboll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: 1834–1844. [DOI] [PubMed] [Google Scholar]
- 50. Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double‐blind, randomised placebo‐controlled trial. Lancet 2018; 392: 1519–1529. [DOI] [PubMed] [Google Scholar]


