Abstract
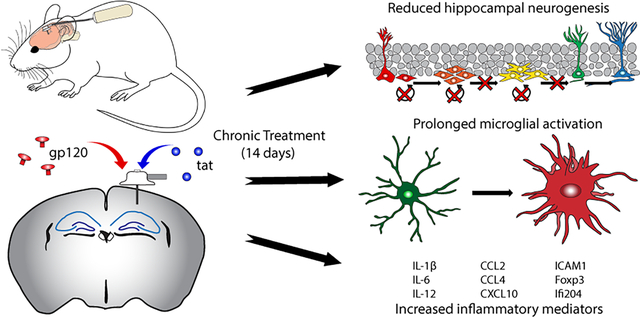
HIV-1 infection causes chronic neuroinflammation resulting in cognitive decline associated with diminution of survival of neural stem cells (NSC). In part, this is attributable to production of toxic viral proteins (gp120 and tat) by infected cells in the brain that can activate microglia. Here, we evaluated a novel model for HIV-1 neuropathogenesis by direct administration of viral proteins into the hippocampus. Chronic administration of either HIV-1 gp120 or tat over 14 days significantly decreased NSC proliferation, survival and neuroblast formation (by 32–37%) within the hippocampal subgranular zone as detected by doublecortin/BrdU or Ki67-positive cells. Intrahippocampal administration of gp120 or tat induced microglial activation within the hippocampus as determined by increases in microglial number and increases in the volume of the microglia (2.5–3-fold, evaluated by double IBA-1/CD68 staining). We further assessed inflammatory responses within the hippocampus by RNAseq and Ingenuity Pathway Analysis. There was a significant mRNA upregulation of numerous inflammatory mediators including Il1b, Icam1, Il12a, Ccl2, and Ccl4. These data suggest that chronic administration induces a prolonged inflammatory state within the hippocampus that negatively affects NSC survival potentially leading to cognitive dysfunction.
Keywords: HIV-1, gp120, tat, neural stem cell, microglia, neuroinflammation
Graphical Abstract
Introduction
The development of combined antiretroviral therapy (cART) has drastically extended the lifespan of patients living with HIV-1 infection. Unfortunately, cART can only suppress the virus and allows for persistent activation of the immune system especially within the central nervous system (CNS). Chronic neuroinflammation due to viral infection has been shown to induce neuronal death ultimately leading to cognitive dysfunction commonly referred to as HIV-1 associated neurocognitive disorder (HAND)(Spudich and Gonzalez-Scarano 2012).
Neural stem cells (NSCs) are undifferentiated precursors which can generate neurons, astrocytes, and oligodendrocytes. The two major NSC niches within the CNS are the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus within the hippocampus. Neurogenesis, or the generation of new neurons from NSCs, is continuous throughout adulthood and neurons generated within the hippocampus integrate into existing neural networks which is imperative for functions such as learning and memory(Sahay and Hen 2007). Recent studies of human tissue have shown conflicting results of detectable levels of hippocampal neurogenesis post adolescence(Boldrini et al. 2018; Sorrells et al. 2018). Numerous recent reviews have assessed the current literature concerning adult hippocampal neurogenesis across numerous animal species in order reconcile the reports of limited neurogenesis in humans. Even though the debate as to whether human hippocampal neurogenesis exists in the adult brain remains unresolved, comparative analysis suggests that neurogenesis rates fall to low levels for much of adult life in all species(Snyder 2019).
Hippocampal NSCs are targets of HIV-1 infection through direct interaction with HIV-1 neurotoxic proteins (envelope glycoprotein gp120, transactivator of transcription [tat]) or indirectly due to an increased inflammatory milieu which is detrimental to proper NSC function(Hauser and Knapp 2014). Animal models of HIV-1 infection show negative regulation of hippocampal NSC proliferation and neurogenesis which could contribute to the progression of HAND(Putatunda et al. 2018). Most of these animal models utilize transgenic creation of HIV-1 neurotoxic proteins which can be used to study the long-term effects of viral proteins in the CNS(Avraham et al. 2014; Putatunda et al. 2018). A drawback of these models is how long neurogenic deficits manifest in vivo. Here, we sought to generate a mouse model which allows for chronic administration of HIV-1 neurotoxic proteins while rapidly producing similar effects (decreased neurogenesis, increased inflammatory milieu, microglial activation) seen in transgenic animal models.
Methods
Animals
C57BL/6 mice used for this study were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Male mice 12 weeks of age at the beginning of the study were single-housed on a 12:12 light/dark cycle (7:00am – 7:00pm) at 22.1 ± 1°C with ad libitum access to food and water. Animals were randomly assigned to treatment group with equal numbers of animals per group at the start of the study.
HIV-1 neurotoxic proteins
Commercially available recombinant biologically active HIV-1 neurotoxic proteins were purchased from ImmunoDiagnostics (Woburn, MA, USA). Full length recombinant HIV-1 envelope protein gp120 was cloned from the dual-tropic HIV-1 MN strain. Full length recombinant tat (101 amino acids) was produced from the R5 tropic HIV-1 BaL strain in an E. coli expression system. Both gp120 and tat were stored at −80°C until used for intrahippocampal injection.
Intrahippocampal infusion of HIV-1 neurotoxic proteins
Surgery for implantation of cannulae and osmotic pumps for HIV-1 neurotoxic protein delivery was performed as previously described(Hill et al. 2018a; Hill et al. 2018b). Stereotaxic coordinates for cannula placement were calculated from Bregma (in mm) as follows: −2.0 anterio-posterior, +1.5 medial-lateral, −1.5 dorso-ventral. Osmotic mini-pumps used were from Alzet Durect (Cupertino, CA, USA; model 1002) as were cannulae (Alzet, brain infusion kit 3). Pumps were filled with either vehicle (sterile artificial cerebrospinal fluid (ACSF), Tocris Bioscience, Bristol, UK) or gp120 or tat diluted in ACSF at appropriate doses. Infusion doses were set to 36 ng/day (gp120) or 18 ng/day (tat) continuously for 14 days. Doses were based on previous pilot work performed by the laboratory.
BrdU
Animals were injected with 100 mg/kg 5-bromo-2’-deoxyuridine (BrdU; Sigma-Aldrich, St. Louis, MO, USA) i.p. twice per day for days 1 and 2 of the experimental schedule and once per day for days 3 and 4 for a total of six injections.
Histology
Animal tissue was harvested and processed 14 days after pump/cannula implantation as described previously(Hill et al. 2018b).
Immunofluorescence staining
All samples were stained as previously described by our laboratory(Hill et al. 2018a; Hill et al. 2018b). Antibodies used were anti-Ki67 (1:500, Abcam, Cambridge, UK; Cat# ab15580 Lot# GR264777 RRID:AB_443209), anti-DCX (1:1000, Millipore, Burlington, MA, USA; Cat# AB2253 Lot# 2828588 RRID:AB_1586992), anti-BrdU (1:200, Abcam, Cat# ab6326 Lot# GR267766–1 RRID:AB_305426), anti-IBA-1 (1:500, Wako Chemicals, Richmond, VA, USA; Cat# 019–19741 Lot#WDK2121 RRID:AB_839504), and anti-CD68 (1:1000, Bio-Rad Laboratories, Hercules, CA, USA; Cat# MLA1957 Lot#1708 RRID:AB_322219). Secondary antibodies used were Alexa-488® goat anti-guinea pig (Thermo Fisher Scientific, Waltham, MA, USA; Cat# A-11073 Lot#1841755 RRID:AB_2534117), Alexa-594® donkey anti-rat (Thermo Fisher Scientific; Cat# A-21209 Lot#45081A RRID:AB_2535795), Alexa-488® donkey anti-rabbit (Thermo Fisher Scientific; Cat# A-21206 Lot#1608521 RRID:AB_2535792). All secondary antibodies were used at a dilution of 1:400. Imaging for in vivo quantification was performed using a CoolSNAP EZ CCD camera (Photometrics, Tucson, AZ, USA) coupled to a Nikon i80 Eclipse (Nikon Instruments Inc., Melville, NY, USA). Confocal images were taken on a Nikon A1R confocal microscope (Nikon).
In vivo NSC quantification
Quantification of positive immunofluorescent cells in the SGZ was performed as previously described(Hill et al. 2018a; Hill et al. 2018b).
Microglial activation analysis
Microglial analysis was performed as previously described by our laboratory (Hill et al. 2018b). Total animals analyzed per group are as follows; vehicle n=6, gp120 n=6, tat n=5.
RNAseq
C57BL/6 mice were chronically treated with either vehicle, gp120, or tat via osmotic mini-pump for 14 days as outlined above. Animal numbers used for RNAseq analysis were as follows: vehicle n=4, gp120 n=6, tat n=5. At the end of the treatment schedule, animals were perfused with saline and brains were harvested. RNA was prepared using the tissue from the hippocampal region and gene expression was analyzed by RNAseq using the mouse inflammation and immunity transcriptome array (RMM005Z) from Qiagen (Hilden, Germany). Data from primary analysis from Qiagen was uploaded onto GeneGlobe Data Analysis Center (Qiagen). Data was normalized for Total Unique Molecular Index Count for each sample. This method was used to normalize the Unique Molecular Index count of every gene by the Total Unique Molecular Index count in each sample. Heatmaps were generated using this data to visualize changes in the proinflammatory profile. Specific genes were selected and bar graphs were plotted using the normalized Total Unique Molecular Index Count for each gene.
Statistics
For all analyses, data was processed randomly and quantification was performed by experimenters who were blinded to experimental groups. All values are expressed as mean ± SEM. Analyses were performed using GraphPad Prism 7.0 software (San Diego, CA, USA; RRID:SCR_002798). The significance of the differences between groups was evaluated by Student’s T-test and a p value <0.05 was considered statistically significant. For linear correlation, Spearman correlation test was performed. Results are expressed by the Spearman correlation coefficient (ρ) and p-value.
Results
Direct intrahippocampal infusion of HIV-1 neurotoxic proteins gp120 or tat reduces hippocampal neurogenesis in vivo.
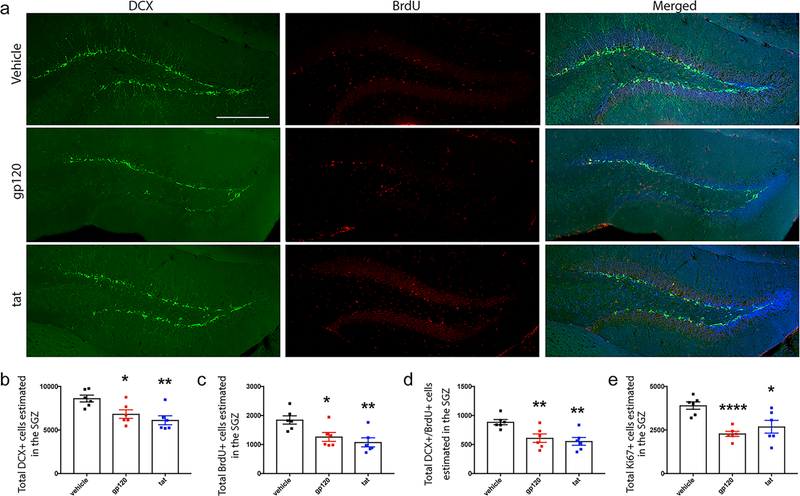
Animals were implanted with osmotic mini-pumps containing either gp120 (36 ng/day) or tat (18 ng/day) connected to cannula which were placed to infuse directly into the left hippocampus. BrdU was administered during the first four days of treatment schedule as outlined in the Methods section. Hippocampal NSC proliferation was assessed by positive staining for Ki67 while survival of NSCs was assessed by positive staining for BrdU and neuroblast/immature neuron formation was determined by staining for doublecortin (DCX) within the SGZ of the dentate gyrus. Representative images can be seen in Figure 1a. Our results demonstrate that the number of DCX+ cells were significantly reduced in gp120 (6827 ±492, p=0.0173) or tat (6121 ±517, p=0.0032) treated animals as compared to vehicle treatment (8619 ±392, Fig 1b). This was also found for BrdU+ cells (vehicle, 1847 ±143; gp120, 1262 ±153, p=0.0189; tat, 1073 ±155, p=0.0043, Fig 1c), and DCX+/BrdU+ neuroblasts (vehicle, 886 ±45; gp120, 609 ±73, p=0.0189; tat, 554 ±67, p=0.0021, Fig 1d). Ki67+ cells were also found to be significantly decreased due to gp120 or tat treatment (vehicle, 3902 ±212; gp120, 2280 ±149, p<0.0001; tat, 2687 ±366, p=0.0166, Fig 1e). Thus, chronic administration of gp120 or tat displayed significant inhibitory effects on hippocampal NSC proliferation, survival and neuroblast formation.
Fig 1. Direct intrahippocampal infusion of HIV-1 neurotoxic proteins gp120 or tat reduces hippocampal neurogenesis in vivo.
Osmotic mini-pumps (Alzet) were implanted sub-dermally into C57BL/6 mice for a continuous dispersion time of 14 days. Pumps contained either vehicle, recombinant gp120 (36 ng/day), or recombinant tat (18 ng/day) and each pump was connected to a stainless-steel cannula which was implanted into the left hippocampus. (a) Representative images depicting the dentate gyrus from 30 μm brain sections of 14-week-old C57BL/6 mice immunostained with anti-BrdU (red) and anti-DCX antibodies (green). Scale bar = 250 μm. Quantitative analysis of DCX (b), BrdU (c), DCX/BrdU (d), and Ki67 (e) total estimated positive cells within the left hippocampus per mouse is shown. Bars represent mean ±SEM, n=6 mice per group. *, p<0.05; **, p<0.01; ****, p<0.0001 as compared to C57BL/6 vehicle group by students T-test.
Microglial activation is induced by chronic intrahippocampal infusion of HIV-1 neurotoxic proteins gp120 or tat.
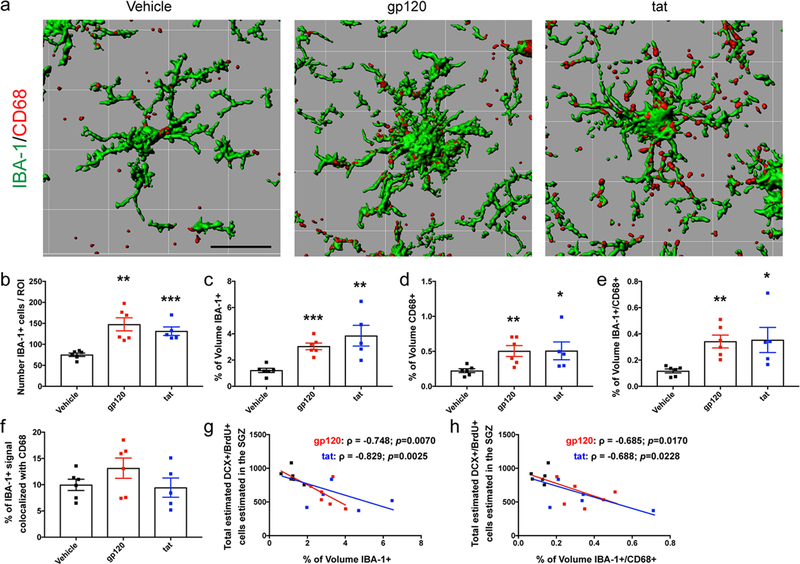
We next sought to determine if administration of neurotoxic proteins altered microglial activation state. Representative 3D-rendered images of IBA-1 and CD68 stained microglia are shown in Fig 2a. Our data indicate that there is a significant increase in the total number of IBA-1 positive microglia per ROI within the hippocampus of gp120 (147.7 ±15.5, p=0.0011) or tat (131.2 ±10.2, p=0.0004) treated animals as compared to vehicle (75.1 ±4.0, Fig 2b). Data also showed significant increases in the percent volume of IBA-1+ (vehicle, 1.212 ±0.16; gp120, 3.042 ±0.26 p=0.0001; tat, 3.852 ±0.79 p=0.0058, Fig 2c), percent volume of CD68+ (vehicle, 0.223 ±0.028; gp120, 0.504 ±0.078 p=0.0069; tat, 0.507 ±0.128 p=0.042, Fig 2d), and percent volume of IBA-1/CD68+ (vehicle, 0.117 ±0.015; gp120, 0.342 ±0.049 p=0.0013; tat, 0.353 ±0.096 p=0.0254, Fig 2e) of gp120 or tat treated animals as compared to vehicle treated animals. Interestingly, we did not see differences in the percent of IBA-1+ signal that was colocalized with CD68 (vehicle, 9.965 ±1.07; gp120, 13.15 ±1.92; tat, 9.445 ±1.83, Fig 2f). We further correlated the intensity of microglial activation with the formation of neuroblasts within the same animal. Data show a strong correlation of increased percent volume of IBA-1 with decreased DCX+/BrdU+ cells (gp120 ρ= −0.748, p=0.0070; tat ρ= −0.829, p=0.0025, Fig 2g) as well as increased percent volume of co-labeled IBA-1/CD68 with decreased DCX+/BrdU+ cells (gp120 ρ= −0.685, p=0.0170; tat ρ= −0.688, p=0.0228, Fig 2h). Together, these results suggest that administration of HIV-1 neurotoxic proteins increases microglial number and expression of IBA-1 and CD68 within the hippocampus which correlate with dysfunction of neuroblast formation.
Fig 2. Microglial activation is induced by chronic intrahippocampal infusion of HIV-1 neurotoxic proteins gp120 or tat.
C57BL/6 mice had osmotic mini-pumps (Alzet) implanted sub-dermally for a continuous dispersion time of 14 days. Pumps contained either vehicle, recombinant gp120 (36 ng/day), or recombinant tat (18 ng/day). Each pump was connected to a stainless-steel cannula which was implanted into the left hippocampus. (a) Surface rendered images of IBA-1 positive microglia (green) co-labeled with CD68 (red) are shown. Scale bar = 20 μm. 3D surface renderings for representative images were generated using Imaris software. Confocal 3D z-stack images were taken on a Nikon A1R confocal microscope at 0.5 μm steps over a range of 24–26 μm per section using a 20x objective. Using NIS-Elements software, images were processed and a fixed threshold was set to include all positive immunofluorescent signal in the region of interest (ROI). ROIs were set to a 600 μm × 600 μm area which included the dentate gyrus, hilus, and molecular layer. Comprehensive 3D representations within the NIS-Elements software was used to determine the total number of IBA-1+ cells within each ROI (b) as well as the percentage of volume within the ROI that was either IBA-1+ (c), CD68+ (d), or both IBA-1+/CD68+ (e). To determine changes in microglial (IBA-1+) expression of CD68, the total volume of IBA-1+/CD68+ signal was divided by the total IBA-1+ volume giving changes in the percent of IBA-1+ signal colocalized with CD68 (f). Data from three separate z-stacks per animal was averaged together to obtain a single biological replicate for analysis. Further correlation analysis was performed to assess any relationship between increased percent of volume that was IBA-1+ (g) or IBA-1+/CD68+ (h) with neuroblast (DCX+/BrdU+) formation in each animal. Bars represent mean ±SEM. *, p<0.05; **, p<0.01; ***, p<0.001 as compared to vehicle-treated animals by Student’s T-test. For correlation, Spearman’s correlation test was used, ρ: coefficient of Spearman; p: value of significance.
Direct administration of gp120 or tat into the hippocampus induces an inflammatory immune response in vivo.
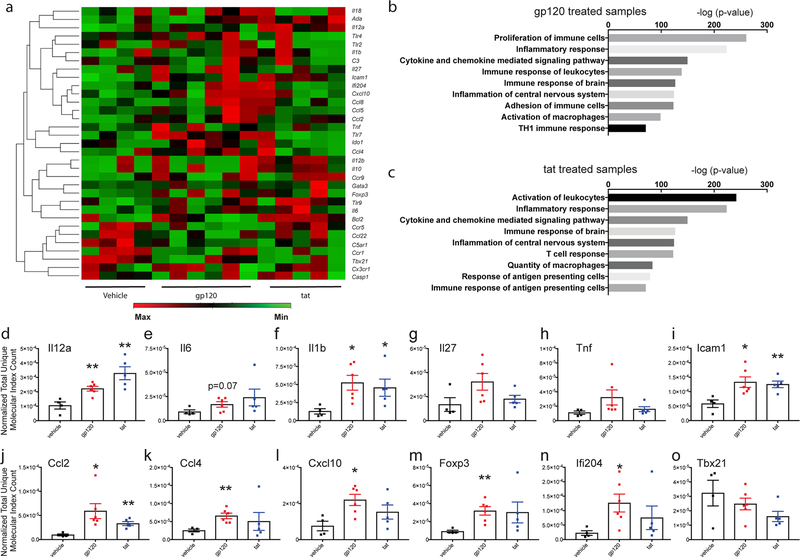
We further assessed inflammatory responses within the hippocampus after 14 days of continuous administration of gp120 or tat. At the end of the treatment schedule, hippocampal samples were analyzed for gene expression via the QIAseq mouse inflammation and immunity transcriptome array (RMM005Z, Qiagen). Heatmaps were generated (Figure 3a) from the resulting analysis. Ingenuity pathway analysis was used to assess enriched molecular pathways due to gp120 or tat treatment (Fig 3b and 3c respectively). Select targets were then chosen and graphed individually to show differences between vehicle and gp120 or tat treated animals (Fig 3d–o). Results from IPA analysis of both gp120 and tat treated animals showed significant enrichment of numerous inflammatory pathways including cytokine and chemokine mediated signaling pathways, immune responses of the brain, and inflammation of the CNS. Individual assessment showed significant increases in Il12a, Il1b, Ccl2, Ccl4, and Cxcl10 after 14 days of treatment with neurotoxic proteins. There was also significant upregulation of the adhesion molecule Icam1, the transcriptional regulator Foxp3, and interferon activable protein 204 (Ifi204). These results suggest that chronic administration of gp120 or tat induces a constant inflammatory milieu as evidenced by significant increases in a number of cytokines, chemokines, and immune modulatory mediators within the hippocampus.
Fig 3. Direct administration of gp120 or tat into the hippocampus induces an inflammatory immune response in vivo.
Osmotic pumps (Alzet) were implanted sub-dermally into C57BL/6 mice for a continuous dispersion time of 14 days. Pumps contained either vehicle, recombinant gp120 (36 ng/day), or recombinant tat (18 ng/day). Each pump was connected to a stainless-steel cannula which was implanted into the left hippocampus. After harvest, the left hippocampus was removed and a portion was processed for RNA. The expression of inflammation-related genes in the hippocampus was analyzed using the Qiagen mouse Inflammation and Immunity Transcriptome array. (a) Heat map analysis of inflammatory array data showing differentially expressed genes between all samples. Graphs for significantly enriched molecular functions identified by Ingenuity Pathway Analysis software for animals treated with either gp120 or tat are shown in (b) or (c) respectively. Results are displayed at -log of p-value (Fisher’s exact test) for each pathway as assessed by group. Normalized Total Unique Molecular Index Count for each gene was compared between vehicle and HIV-1 neurotoxic protein (gp120 or tat) treated animals. Quantitative analysis of select targets are shown in d – o. Bars represent mean ±SEM and individual points are data from each animal. p<0.05 was considered significant, represented as *p<0.05; ** p <0.01. Analysis was by Student’s T-test.
Discussion
During chronic HIV-1 infection, infected and activated resident mononuclear phagocytes (macrophages, microglia) release immune factors and neurotoxic proteins which are involved in CNS inflammation and alter hippocampal neurogenesis(Kaul et al. 2001; Peng et al. 2011). Of these, the HIV-1 associated neurotoxic proteins gp120 and tat are among the most studied. Both gp120 and tat have been shown to decrease NSC proliferation and decrease neurogenesis rates in the hippocampus(Avraham et al. 2014; Fan et al. 2016). An unfortunate aspect of animal models which utilize these proteins is that administration of gp120 or tat are under control of transgenic mechanisms and biological and cognitive deficits take time to manifest. In the current study, we wanted to generate a mouse model which would induce both neurogenesis deficits and inflammatory responses due to chronic administration of gp120 or tat in a shorter amount of time, yet still in a chronic manner. Further, we wanted to assess whether HIV-1 proteins can induce inflammatory responses on their own.
Our data show that chronic administration of HIV-1 gp120 or tat over 14 days decreases NSC proliferation, survival and neuroblast formation within the hippocampal SGZ. This is not surprising as many studies have shown detrimental effects of both gp120 and tat on hippocampal neurogenesis, yet no study to our knowledge has utilized direct intrahippocampal chronic administration of recombinant protein by osmotic minipump. An upside of using this style of administration is an ability to have a continuous infusion of recombinant protein over a number of days without separate, individual anesthesia events for cannula administration of viral proteins. This may alleviate any unwanted effects from either the type of anesthesia used or anxiety produced from handling each animal numerous times. It also better mimics a slow generation of neurotoxic viral proteins as would be expected in an infected HIV-1 individual on cART therapy without the length of time needed from transgenic generation of viral proteins. This model allows evaluation of neuroprotective strategies and effects of comorbidities (like alcohol and drug abuse) on neurogenesis in HIV-1 infection.
Chronic HIV-1 infection and subsequent progression to HAND is well correlated with increased numbers of microglia and production of immunoactive and inflammatory substances such as viral proteins and inflammatory cytokines and chemokines(Glass et al. 1995; Keblesh et al. 2008; Persidsky et al. 1997). In this study, we found that chronic direct intrahippocampal administration of gp120 or tat induces microglial activation within the hippocampus as determined by increases in microglial number and increases in the volume percentage of the microglial marker IBA-1 and CD68, a protein commonly used as a marker for phagocytic microglia(Hoeijmakers et al. 2017). Interestingly, we did not see an increase in CD68 colocalized within IBA-1 positive signal as we would expect if CD68 was upregulated within microglia. It is possible that the phagocytic activity of microglia activated by HIV-1 neurotoxic proteins is not as robust as microglia activated by a different inflammatory mediator such as lipopolysaccharide (LPS). This may be the case since a non-human primate model of simian-human immunodeficiency virus (SHIV) showed no differences of CD68+ macrophages/microglia between infected and non-infected animals(Hsu et al. 2018). Our results also do not dispute increases in CD68 seen from administration of gp120 directly into the CNS since our results also show increases in the total volume percentage of CD68 within the hippocampus(Bachis et al. 2010).
As stated above, persistent production of inflammatory cytokines, chemokines and viral proteins is a hallmark of chronic HIV-1 infection(Persidsky et al. 1997). Previous studies have highlighted TNFα, ICAM-1, IL-1β, IL-6, CXCL10 and CCL2 as inflammatory mediators that are upregulated due to HIV-1 infection or gp120/tat treatment(Persidsky et al. 1997; Pu et al. 2003). Therefore, we further determined immune responses within the hippocampus of chronic direct intrahippocampal administration of gp120 or tat via osmotic minipump by RNAseq and IPA analysis. Our results showed significant mRNA upregulation of numerous inflammatory mediators including Il1b, Icam1, Il12a, Ccl2, Ccl4, Cxcl10, Foxp3, and Ili204. These data suggest that 14 days of chronic administration induces a prolonged inflammatory state within the hippocampus that could potentially induce further NSC and cognitive dysfunction. As expected, data also showed differences in gene expression between gp120 and tat treatment for a number of targets. We believe this is due to cellular target and downstream signaling cascade differences between gp120 and tat which has been reported previously. Investigation into molecular pathways further confirmed that inflammatory and immune responses were significantly upregulated by direct administration of gp120 or tat into the hippocampus and that these effects were still prominent 14 days after the start of treatment. It was imperative to determine that inflammatory effects from this style of administration were continuous, and not simply acute, to show development of an animal model that better mimics the inflammatory state seen from human tissue after HIV-1 infection.
It should be noted that we are not suggesting that all of the effects seen on NSC proliferation and differentiation are due specifically to viral protein interactions. It is entirely possible that there is a combination of effects, both directly on NSCs of the SGZ and indirectly through activation of resident microglia and subsequent release of pro-inflammatory molecules. There is also the possibility of interaction with other cell types within the CNS, specifically astrocytes, which we have overlooked in this study. Astrocytes have been shown to have structural changes and are implicated as playing a role in hippocampal-associated behavioral impairments due to HIV-1 viral protein administration(Fitting et al. 2013; Harricharan et al. 2015).
Taken together, these data suggest that chronic direct intrahippocampal infusion of HIV-1 neurotoxic proteins (gp120, tat) via osmotic minipump is a viable animal model to replicate inflammatory responses and NSC impairment. Utilization of this model allows for a more rapid onset of inflammatory mediators and NSC deficits as compared to transgenic animal models while minimizing handling and invasive techniques on the animals.
Acknowledgments
This study was supported in part by NIH grants T32DA007237–28 (JH), P30 DA013429, R37AA015913, MH115786 and U01AA023552 (YP).
Footnotes
Conflict of interest
Authors declare no conflict of interests.
References
- Avraham HK et al. (2014) The cannabinoid CB(2) receptor agonist AM1241 enhances neurogenesis in GFAP/Gp120 transgenic mice displaying deficits in neurogenesis British journal of pharmacology 171:468–479 doi: 10.1111/bph.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Cruz MI, Mocchetti I (2010) M-tropic HIV envelope protein gp120 exhibits a different neuropathological profile than T-tropic gp120 in rat striatum The European journal of neuroscience 32:570–578 doi: 10.1111/j.1460-9568.2010.07325.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M et al. (2018) Human Hippocampal Neurogenesis Persists throughout Aging Cell stem cell 22:589–599.e585 doi: 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Gao X, Chen J, Liu Y, He JJ (2016) HIV Tat Impairs Neurogenesis through Functioning As a Notch Ligand and Activation of Notch Signaling Pathway The Journal of neuroscience : the official journal of the Society for Neuroscience 36:11362–11373 doi: 10.1523/jneurosci.1208-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S et al. (2013) Synaptic dysfunction in the hippocampus accompanies learning and memory deficits in human immunodeficiency virus type-1 Tat transgenic mice Biological psychiatry 73:443–453 doi: 10.1016/j.biopsych.2012.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC (1995) Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia Annals of neurology 38:755–762 doi: 10.1002/ana.410380510 [DOI] [PubMed] [Google Scholar]
- Harricharan R, Thaver V, Russell VA, Daniels WM (2015) Tat-induced histopathological alterations mediate hippocampus-associated behavioural impairments in rats Behavioral and brain functions : BBF 11:3 doi: 10.1186/s12993-014-0047-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Knapp PE (2014) Interactions of HIV and drugs of abuse: the importance of glia, neural progenitors, and host genetic factors International review of neurobiology 118:231–313 doi: 10.1016/b978-0-12-801284-0.00009-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JD, Zuluaga-Ramirez V, Gajghate S, Winfield M, Persidsky Y (2018a) Activation of GPR55 increases neural stem cell proliferation and promotes early adult hippocampal neurogenesis British journal of pharmacology 175:3407–3421 doi: 10.1111/bph.14387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JD, Zuluaga-Ramirez V, Gajghate S, Winfield M, Sriram U, Rom S, Persidsky Y (2018b) Activation of GPR55 induces neuroprotection of hippocampal neurogenesis and immune responses of neural stem cells following chronic, systemic inflammation Brain, behavior, and immunity doi: 10.1016/j.bbi.2018.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers L, Ruigrok SR, Amelianchik A, Ivan D, van Dam AM, Lucassen PJ, Korosi A (2017) Early-life stress lastingly alters the neuroinflammatory response to amyloid pathology in an Alzheimer’s disease mouse model Brain, behavior, and immunity 63:160–175 doi: 10.1016/j.bbi.2016.12.023 [DOI] [PubMed] [Google Scholar]
- Hsu DC et al. (2018) Central Nervous System Inflammation and Infection during Early, Nonaccelerated Simian-Human Immunodeficiency Virus Infection in Rhesus Macaques Journal of virology 92 doi: 10.1128/jvi.00222-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA (2001) Pathways to neuronal injury and apoptosis in HIV-associated dementia Nature 410:988–994 doi: 10.1038/35073667 [DOI] [PubMed] [Google Scholar]
- Keblesh JP, Reiner BC, Liu J, Xiong H (2008) Pathogenesis of Human Immunodeficiency Virus Type-1 (HIV-1)-Associated Dementia: Role of Voltage-Gated Potassium Channels Retrovirology : research and treatment 2:1–10 [PMC free article] [PubMed] [Google Scholar]
- Peng H, Sun L, Jia B, Lan X, Zhu B, Wu Y, Zheng J (2011) HIV-1-infected and immune-activated macrophages induce astrocytic differentiation of human cortical neural progenitor cells via the STAT3 pathway PloS one 6:e19439 doi: 10.1371/journal.pone.0019439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman HE (1997) An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis Journal of neurovirology 3:401–416 [DOI] [PubMed] [Google Scholar]
- Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, Toborek M (2003) HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain Molecular and cellular neurosciences 24:224–237 [DOI] [PubMed] [Google Scholar]
- Putatunda R, Zhang Y, Li F, Yang XF, Barbe MF, Hu W (2018) Adult neurogenic deficits in HIV-1 Tg26 transgenic mice Journal of neuroinflammation 15:287 doi: 10.1186/s12974-018-1322-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R (2007) Adult hippocampal neurogenesis in depression Nature neuroscience 10:1110–1115 doi: 10.1038/nn1969 [DOI] [PubMed] [Google Scholar]
- Snyder JS (2019) Recalibrating the Relevance of Adult Neurogenesis Trends in neurosciences doi: 10.1016/j.tins.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Sorrells SF et al. (2018) Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults Nature 555:377–381 doi: 10.1038/nature25975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich S, Gonzalez-Scarano F (2012) HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment Cold Spring Harbor perspectives in medicine 2:a007120 doi: 10.1101/cshperspect.a007120 [DOI] [PMC free article] [PubMed] [Google Scholar]