Abstract
Surface plasmons are collective oscillations of free electrons at metallic surfaces. These oscillations can give rise to the intense colors of solutions of plasmon resonance nanoparticles and/or very intense scattering. While the use of plasmonic particle absorption based bioaffinity sensing is now widespread throughout biological research, the use of their scattering properties is relatively ill explored. We refer to the use, utility and control of surface plasmons as plasmonics. In this review and forward-looking article, we discuss the current opinions and uses of plasmonics, as well as speculate on areas of future research. These include the use of plasmon scatter for long-range immunosensing and macromolecular conformation studies, as well as the ability to Stokes shift plasmon scatter, a plasmonics phenomenon recently referred to as metal-enhanced fluorescence.
Introduction
The localized plasmon resonance of noble metal nanoparticles has led to the development of many sensors with unique properties in the past decade [1,2]. These nanoparticles exhibit strong UV-Vis absorption bands that are not present in the bulk metal [1], and which can lead to the brilliant colors observed for nanoparticles in solution, a function of both their absorption and scattering properties [1,2]. It is this fascination of the optical properties of gold nanoparticles which led to their early colorimetric use in cathedral windows. While the first and limited demonstrations of plasmonics occurred hundreds of years ago, their colorimetric uses are now entrenched in modern biotechnology, particularly for bioaffinity sensing and, even more recently, in cellular imaging [1–6].
The majority of sensors utilizing nanoparticle surface plasmon resonance have been solution-based [1], where the sensitivity of the sensors is typically determined by the sensitivity of the surface plasmons themselves to inter-particle coupling. When many particles, all supporting a surface plasmon resonance, are in close proximity, they are able to interact electromagnetically, primarily through a dipole-dipole coupling mechanism [1,2]. This mechanism, which can occur up to two and half times the diameter of the particles [7], broadens and red shifts the plasmon resonance bands, where smaller clusters of particles have similar plasmon resonance properties as compared to that of a larger single particle [1]. This has primarily lead to two main solution-sensing formats using the nanoparticles, namely absorption/colorimetrically based [1,2], and those which look for changes in plasmon light scattering properties [1,2]. Although biosensing using plasmonic scatter is inherently a much more powerful technique, it is considerably less published [3–6,8,9•,10••]. We note a recently published method of wavelength-ratiometric plasmonic light-scattering for glucose sensing [9•]. Intuitively, these properties can be considered as a function of the nanoparticle’s crosssection, Cext, which comprises both absorption, Cabc, and scattering, Csca, components, where:
| (1) |
In addition to these two properties of nanoparticles, several other properties are known, but have been relatively ill explored for biosensing. These include the spatial distribution of scatter and its subsequent polarization dependence, and the ability for noble metal nanostructures to dipole-couple over very large distances, effectively breaking the fluorescence resonance energy transfer (FRET) limit imposed by current organic fluorophores. It is these cutting-edge areas of nanoparticle plasmonics that this article considers; the absorption (colorimetric) properties of nanoparticles for sensing are covered in other reviews [11–14], as are the plasmon scattering properties from surfaces [15••].
Scattering-based properties of plasmonic nanoparticles
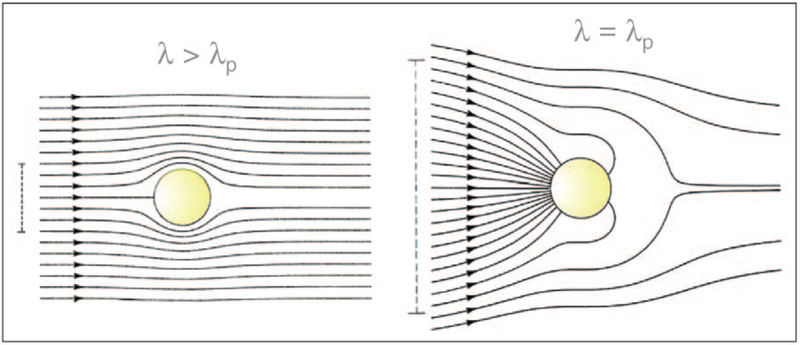
When a metallic nanoparticle is exposed to an electromagnetic wave, the electrons in the metal (plasmons) oscillate at the same frequency as the incident wave. Subsequently, the oscillating electrons radiate electro-magnetic radiation with the same frequency as the oscillating electrons. It is this re-radiation of light at the same incident wavelength that is often referred to as plasmon scatter [16,17]. If the colloid is illuminated with light (λ) which matches the principle plasmon absorption maxima (λp), the nanoparticles can then both absorb and scatter light outside of their physical cross-sections. For wavelengths longer than λp, light is only influenced over the physical constraints of its cross section (Figure 1). For a 30 nm diameter Ag particle, at 380 nm, CScat = 8 × 10−12 cm2, cf. Equation 1. Subsequently, this generally leads to the high sensitivity detection of both gold and silver particles at concentrations as low as 10−16M [16]. Yguerabide has reported the scattering powers of 30 nm noble metal particles in a medium refractive index of 1.33 [16]. In normalized terms, silver and gold have been shown to be efficient plasmon scatterers, Ag (1000), > Au (112.7), > Cu (46.8) and Al (46.6). In comparison, a Rayleigh scattering polystyrene particle of 30 nm has a relative Cscat of 0.47 [16]. As compared to fluorophores, a 60 nm gold nanoparticle can have the same scattering intensity as 3 × 105 fluorescing fluorescein molecules [16].
Figure 1.
Poynting Vector, or energy flow lines around a subwavelength metallic colloid Illuminated at the plasmon wavelength, λp, (right), and at a wavelength longer than the plasmon wavelength (left). The vertical lines on the left indicate the diameter of the cross sections for absorption. Adapted from [2] with permission. Copyright 2004, Wiley-VCH.
The scattering of light by very small subwavelength sized particles is well described by Rayleigh theory [16,17]. For incident light horizontally polarized and observed in the same plane, the intensity of light scattered, Iscatt, in the direction Θ by a homogeneous spherical particle with radius a, that is much smaller than the wavelength, λ, of the incident beam, is given by the Rayleigh expression [16,17],
| (2) |
where I0 is the incident intensity of monochromatic light, nmed is the refractive index surrounding the particle, m is the refractive index of the bulk particle material and r is the distance between the particle and where the scattered light is detected. From equation (2) the scattered light in the horizontal plane is 100% polarized. Interestingly the scattered light intensity is highest at the observation angles θ = 0° and 180°, is zero at θ = 90° and 270°, and is proportional to Cos2θ at all other angles. This spatial or angular distribution of plasmon scatter is characteristic of an electric dipole emitter [16,17]. For light vertically polarized and perpendicular to the scattering pane, the intensity of scatter is given by the well-known form of the Rayleigh expression:
| (3) |
Here, there is no angular-dependence of scatter. The change in polarization of light due to scattering has also been known for many years, as first described by Rayleigh [10••,16,17]. For the case where the incident light is polarized perpendicular to the scattering plane, then the extent of polarization, P, at any angle θ is given by the expression:
| (4) |
where IPERP and IPAR are the scattered intensities in the perpendicular and parallel planes, respectively. P can be positive or negative and |P| ≤ 1 [16,17]. For plane polarized light, the plasmon scattered light by a homogenously sized and dilute solution approaches 1.
Plasmon scatter for bioaffinity sensing
Compared with absorption-based (colorimetric) measurements, there have been relatively few reports of plasmon light scattering for sensing [3–6,8,9•,10••]. Of particular note are the recent reports of wavelength-ratiometric plasmon light scattering [9•]. Similar to absorbance-based measurements, plasmon supporting colloids also red-shift their scattering spectra as a function of coupling to other close-proximity colloids. By simply taking the ratio of the scattered intensities at two different wavelengths, one can measure any biospecies that either causes the aggregation or dissociation of the nanostructures. This has recently been shown for both a model colloidal system using biotin and streptavidin [18•], as well for glucose sensing using 20 nm gold colloids [9•]. In these measurements, the ratio of the scattered light intensities are independent of nanoparticle concentration, as well as fluctuations in the scattering light source, notable features for sensing.
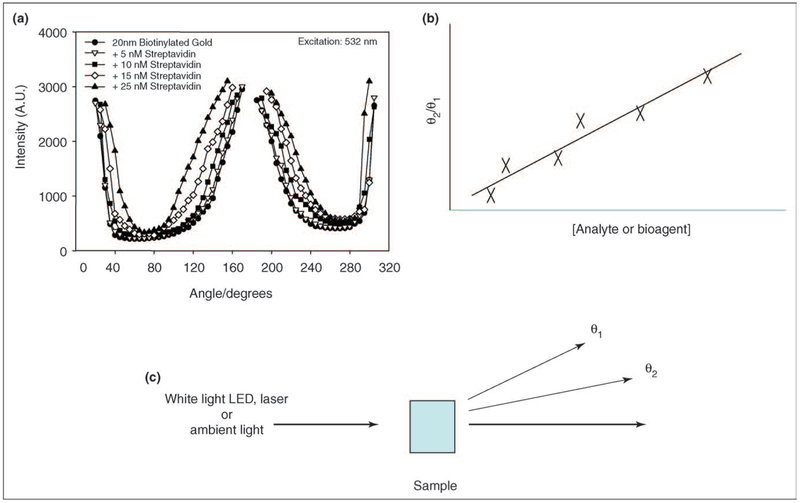
Other recent advances using plasmon scatter include utilizing the angular dependence of plasmon scatter for sensing [10••]. As described above, horizontally polarized illumination of colloids demonstrates a Cos2θ dependence of plasmon scatter in the same plane for particles that scatter in the Rayleigh limit. For 532 nm laser light, particles < 40 nm can still be considered to be in the Rayleigh limit [16]. Geddes et al. have recently shown that 20 nm particles, which initially scatter in the Rayleigh limit, change their angular dependence of scatter upon a bioaffinity induced aggregation (Figure 2) [10••].
Figure 2.
(a) Angular dependent scattering from 20 nm BSA-biotin colloids crosslinked by streptavidin after a 45 min incubation. (b) Simple angular-ratiometric analyte sensing. (c) The simplicity of angular-ratiometric plasmon scatter for bioaffinity sensing. Adapted from [10••] with permission. Copyright 2005, The American Chemical Society.
Subsequently, the extent of forward scatter (180° with respect to the excitation) significantly increases as the particles no longer purely scatter in a Cos2θ dependence, but indeed scatter in the Mie limit. By taking the ratio of scattered intensities at two arbitrary wavelengths, one can readily follow a bioaffinity reaction. Interestingly, the dynamic sensing range is extended by both choosing to measure one scattered intensity at 90°, while also choosing particles that are initially pure Rayleigh scatterers. Additionally, the measurements are independent of total nanoparticle concentration, which is not the case for other techniques such as dynamic light scattering. These findings suggest a very simple approach for field deployable biosensors; using either LEDs or ambient room light, the observer simply takes the ratio of the scattered intensity at two angles to determine the concentration of the biospecies of interest (Figure 2).
Finally, one other new approach that employs plasmon scatter for sensing is based on the angular dependence of polarization, and how this also changes upon the aggregation of solution-based nanoparticles. 20 nm gold nanoparticles coated with biotinylated-BSA readily scatter light in a Rayleigh manner, where the polarization of the colloids is known to be dependent on both the size of the colloids and/or the degree of coupling (proximity) of smaller sized colloids [16]. Upon aggregation of the nanoparticles, then the solution polarization rapidly decreases due to near-field plasmon coupling. Interestingly, by choosing particles that initially scatter incident light in a Rayleigh manner, Geddes et al. have also been able to show that the spatial distribution of polarized scatter also changes upon particle aggregation as the particles now scatter in an increased forward direction (i.e. in the Mie limit) [19••]. With an initial solution optical density of ≈1, significant depolarization occurs at angles greater than 140°, less than 220° and maximum around 180° from the incident excitation. Subsequently, this approach allows the determination of solution protein or analyte concentrations using polarized scatter, the dynamic sensing range determined by the angle of observation [19••].
Plasmon scatter for imaging
The high scattering cross sections for nanoparticles coupled with their relatively small sizes and available surface chemistries has made them ideal choices for cellular-based imaging applications [3–6]. In addition, although quantum dots are known to be suitably bright for imaging, the toxicity of the semiconductor material undermines their continued use in in vitro and in vivo applications. Subsequently, antibody labeled metallic colloids are slowly finding widespread use in imaging applications, and their continued use, we predict, will become more widespread. Of particular utility are the noble metal-nanoshells [20–22], which display much narrower plasmon resonance bands than the bulk colloids, suggesting the possibility for multicolor imaging based on plasmon scatter. Finally, the widely used epi-geometry for fluorescence-based imaging is unlikely to be the optimum for plasmon scatter based imaging. For larger nanoparticles, particles illuminated by shorter wavelengths or for clusters of particles, such as would be observed for high surface densities of cell surface receptors, a 180° geometry is likely to be the most appropriate for high sensitivity detection, as described recently [10••].
Stokes-shifted plasmon scatter: metal-enhanced fluorescence
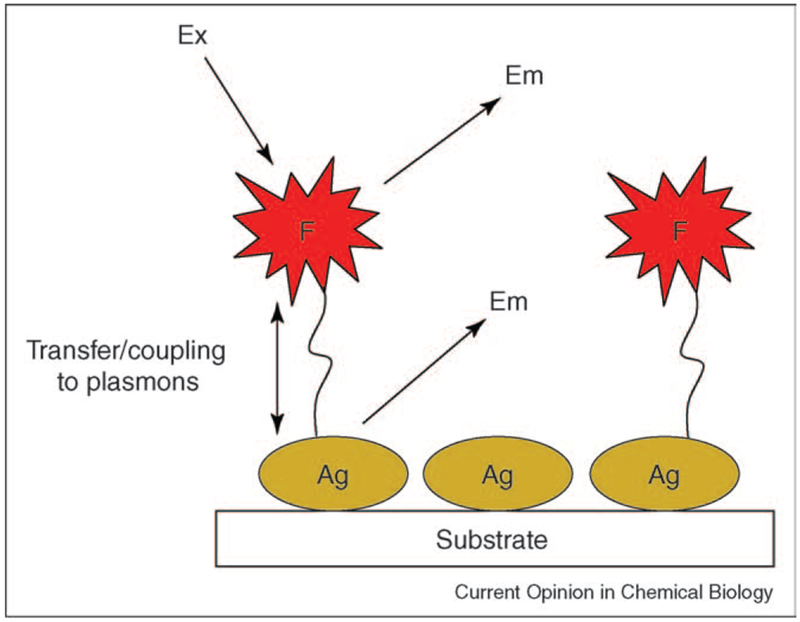
One particular disadvantage of plasmon scatter from nanoparticles is the lack on any shift in the scattered wavelength(s) compared with the excitation, as is the case with organic fluorophores. Fluorophores typically display a ‘Stokes-shifted’ emission that is particularly attractive for discriminating against unwanted excitation. Although plasmonic structures have not been shown to intrinsically scatter light at different wavelengths than their excitation at λp, one successful approach has been to utilize the near fields created by plasmon resonances with fluorophores [23,24,25•,26–29,30•]. Excited fluorophores can undergo interactions with metals, creating plasmons, which then radiate into free space [15••] (Figure 3). Remarkably, the plasmons radiate with the almost identical photophysical characteristics of the fluorophores, except for lifetime, which is inherently very short, reflecting the plasmon lifetime [15••]. The interactions of fluorophores with metallic structures has been named both radiative decay engineering [25•] and metal-enhanced fluorescence [23,30•], and promises to change both the way we think and use current fluorescent technology. In this regard, fluorescence detection is the basis of many measurements in biological research. The principles and applications of fluorescence have undergone extensive development since its introduction in biochemistry in the early 1950s. Since that time, fluorescence has developed into a mature technology with most of the fundamental principles of fluorescence being developed. Most advances in fluorescence today are therefore accomplished within the framework of specific applications, and significant advances to the principles of fluorescence are uncommon. Subsequently, the use of near-field plasmon coupled fluorescence is likely to expand both our uses and the fundamental principles of fluorescence.
Figure 3.
Schematic for radiating plasmon model. The fluorophore emission is partially plasmon coupled.
The relationship between plasmon scatter and metal-enhanced fluorescence (MEF) manifests itself in the ability of plasmon-coupled fluorescence to radiate (Figure 3) [15••] (i.e. the scattering component of the metallic particles extinction, cf. Equation 1). The model for MEF assumes that the fluorescence wavelengths are longer than the interband transitions of the metal. MEF is thought to occur when the absorption and/or emission bands of the fluorophore overlap with the scattering wavelength of the metal structure and is referred to as the radiating plasmon model [15••]. As briefly mentioned, Ag nanostructures offer greater relative scattering as compared with other noble metals, Ag being 10-fold brighter than Au 30 nm particles. This property also manifests itself in the MEF phenomenon, with Ag nanostructures being preferred in applications of MEF, effectively scattering coupled fluorescence with high efficiency [31]. From Equations 1–4, one might expect there to also be angular-dependent emission from the plasmon coupled fluorescence; however, in all the principles and applications of metal-enhanced fluorescence thus-far described [23,24,25•,26–29,30•,31], this has not been observed.
Plasmon resonance particles
In the above section we described the coupling of fluorescence and plasmons [23,24,25•,26–29,30•,31]. In this regard, one emerging area of research is in the development of plasmon resonance particles (PRPs), which take advantage of this effect. Two classes of PRPs can be found today and are at the forefront of leading edge plasmonic scattering technology. These include nanostructures that contain immobilized fluorophores both outside of, and within, a metal colloid or nanoshell (Figures 4, 5 respectively).
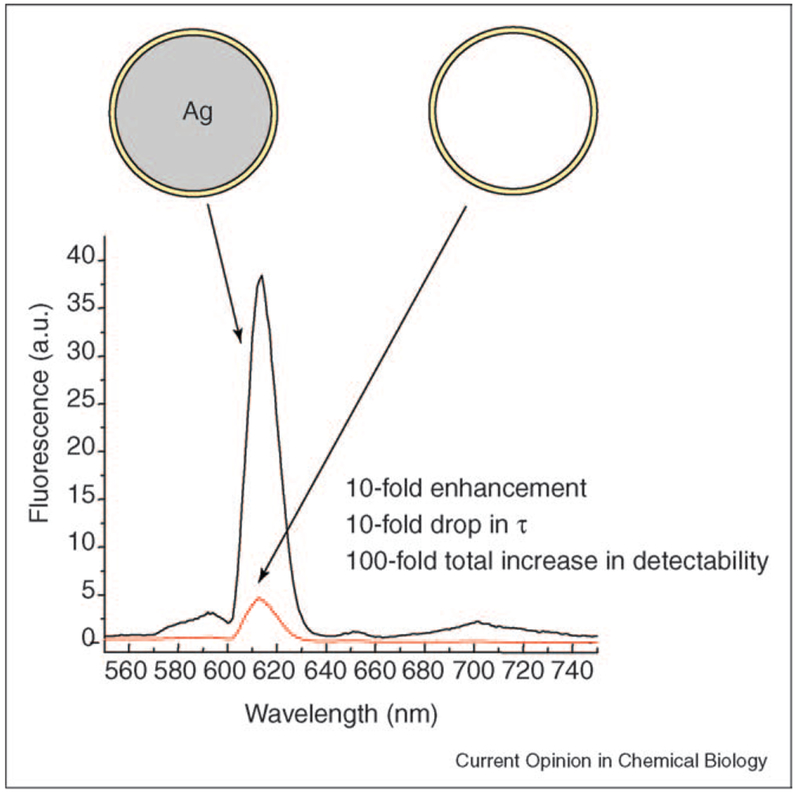
Figure 4.
Lanthanide-coated silver colloids, PRPs.
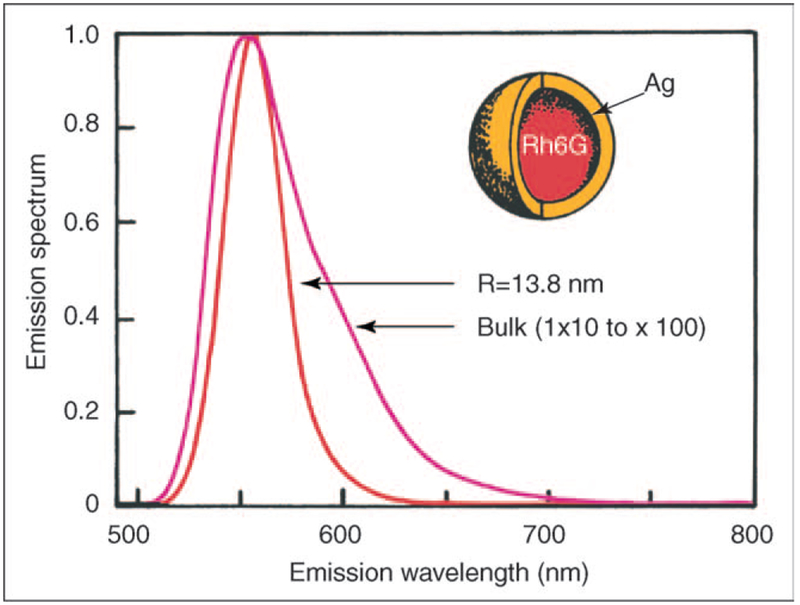
Figure 5.
Theoretical calculations for rhodamine 6G in a silver shell, PRP. Adapted from [32,33] with permission. Copyright 2002, The American Institute of Physics and The Royal Society of Chemistry.
For fluorophores immobilized outside of a metal colloid, remarkable changes in both total intensity and lifetime have been observed (Figure 4). In Figure 4, a lanthanide chelate is immobilized within a sol-gel coating < 10 nm thick, the silver colloid was 30 nm in diameter. For this particle, the lanthanide luminescence increased over 10fold, while the lifetime was reduced 10-fold, as compared with an identical nanobubble that contained no silver. Hence, the particles offer a ≈ 100-fold increase in total detectability. Of particular interest is the fact that the lifetime of the lanthanide chelate is still significantly long enough to alleviate (off-gate) unwanted biological auto-fluorescence, yet offers a 100-fold increase in detectability.
Another class of particles that also promise to offer unique properties are those where fluorophores are contained within silver nanoshells (Figure 5). Although no published experimental reports of these particles have been made, several theoretical predictions have [32,33]. The difficulty of making perfect 3D particles containing the fluorophores will challenge current nanotechnology workers. Current predictions for a 13.8 nm Ag particle incorporating a rhodamine 6G molecule include [32,33]:
A predicted 10-100 fold increase in fluorescence intensity, a combination of both an increased crosssection and the MEF effect. Interestingly, the fluorophores cross section is expected to be far greater than its own physical cross section.
Narrowed emission spectra, similar to that of quantum dots.
A reduced lifetime, decreased 10–1000 fold.
In addition, our extensive experience with MEF [23,24,25•,26–29,30•,31] suggests several other properties are likely to be observed, such as:
The use of high concentrations of fluorophores and the release of self-quenching [34] within the particle.
Enhanced fluorophore photostability of the fluorophores in the nanoshell.
The choice of a whole range of fluorophores/quantum dots/lanthanides whose spectral characteristics overlap well with the plasmon resonance of the nanoshells.
Future applications of plasmon scatter
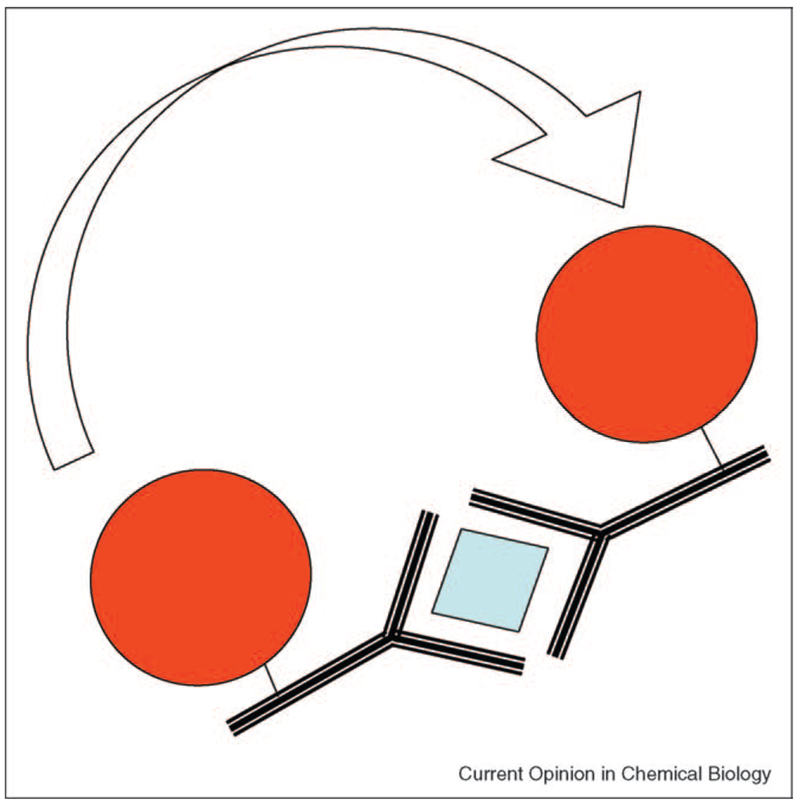
Perhaps one of the most profound future applications of plasmon scatter, is likely to be in the measurement of distances in the range 10–300 nm for biological systems. Today, optical distance measurements less than 10 nm are undertaken using FRET between a fluorescent donor and an acceptor. Distances ranging from macroscopic to about λ/2, typically about 300 nm, can be measured using confocal, multiphoton and/or laser scanning methods [35,36]. While atomic force microscopy and scanning tunneling microscopy can measure these distances, these approaches are not readily compatible with biological species, such as live cells. However, it may be possible to use the long-range plasmon coupling between particles to measure such distances, where the coupling occurs to a minimum value at about 2.5 times the diameter of the colloids [7]. Subsequently, we predict that long-range FRET utilizing plasmonics will be possible, based on the changes in the scattering, absorption and polarization properties of suitably sized colloids (Figure 6). Interestingly, the coupling distance (transfer distance in FRET) will be dependent on the wavelength of light and the initial choice of colloid size. This approach may be of significant importance for studying macromolecular dynamics and particularly in immunoassays, which typically have dimensions far too large for classical FRET [37]. While the theory for distance measurements using plasmonics has yet to be realized, the lack of a discrete physical dipole may prove simpler than fluorophore dipole-dipole coupling.
Figure 6.
Notion of long range plasmon coupling for immunosensing. The coupling distances are far larger than traditional FRET distances for organic fluorophores. Distance measurements could be made using either changes in plasmon absorption, intensity of scatter, angular dependence of scatter or even polarization based scatter.
Conclusions
In this timely article, we have both reviewed and speculated on the present and possible future uses of plasmon-based light scattering. Given the high scattering power of plasmon-based structures, combined with both their simple preparation and readily available surface chemistries, we are likely to see rapid and continued development in this area of important research.
Acknowledgements
This work was supported by the NIH (National Institutes of Health, USA), GM070929, EB00682 and RR008119. Partial salary support to CDG and JRL from UMBI is also acknowledged.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Stuart DA, Haes AJ, Yonzon CR, Hicks EM, Van Duyne RP: Biological applications of localized surface plasmonic phenomenae. IEE Proc Nanobiotechnol 2005, 152:13–22. [DOI] [PubMed] [Google Scholar]
- 2.Hutter E, Fendler JH: Exploitation of localized surface plasmon resonance. Adv Mater 2004, 16:1685–1706. [Google Scholar]
- 3.El-Sayed IH, Huang XH, El-Sayed MA: Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett 2005, 5:829–834. [DOI] [PubMed] [Google Scholar]
- 4.Raschke G, Kowarik S, Franzl T, Sonnichsen C, Klar TA, Feldmann J, Nichtl A, Kurzinger K: Biomolecular recognition based on single gold nanoparticle light scattering. Nano Lett 2004, 3:935–938. [Google Scholar]
- 5.Sonnichsen C, Alivisatos AP: Gold nanorods as novel nonbleaching plasmon-based orientation sensors for polarized single-particle microscopy. Nano Lett 2005, 5:301–304. [DOI] [PubMed] [Google Scholar]
- 6.Kalkbrenner T, Hakanson U, Sandoghdar V: Tomographic plasmon spectroscopy of a single gold nanoparticles. Nano Lett 2004, 4:2309–2314. [Google Scholar]
- 7.Su K-H, Wei Q-H, Zhang X, Mock JJ, Smith DR, Schultz S: Interparticle coupling effects on plasmon resonances of nanogold particles. Nano Lett 2003, 3:1087–1090. [Google Scholar]
- 8.Geddes CD, Lakowicz JR (Eds): Topics in Fluorescence Spectroscopy: Radiative Decay Engineering, vol 8 Kluwer Academic/Plenum Publishers; 2005. [Google Scholar]
- 9.•.Aslan K, Lakowicz JR, Geddes CD: Nanogold plasmon-resonance-based glucose sensing 2: wavelength-ratiometric resonance light scattering. Anal Chem 2005, 77:2007–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first practical report of wavelength ratiometric light scattering. In this paper, the authors show proof-of-concept and practical sensing scaffold/constructs.
- 10.••.Aslan K, Holley P, Davies L, Lakowicz JR, Geddes CD: Angular-ratiometric plasmon resonance light scattering for bioaffinity sensing. J Am Chem Soc 2005, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report for angular ratiometric plasmonic scatter.
- 11.Liu J, Lu Y: Colorimetric biosensors based on DNAzyme-assembled gold nanoparticles. J Fluoresc 2004, 14:343–354. [DOI] [PubMed] [Google Scholar]
- 12.Haes AJ, Stuart DA, Nie S, Van Duyne RP: Using solution-phase nanoparticles, surface-confined nanoparticle arrays and single nanoparticles as biological sensing platforms. J. Fluoresc 2004, 14:355–367. [DOI] [PubMed] [Google Scholar]
- 13.Nath N, Chilkoti A: Label free colorimetric biosensing using nanoparticles. J Fluoresc 2004, 14:377–389. [DOI] [PubMed] [Google Scholar]
- 14.Aslan K, Lakowicz JR, Geddes CD: Saccharide sensing using gold and silver nanoparticles - a review. J Fluoresc 2004, 14:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.••.Lakowicz JR: Radiative decay engineering 5: metal-enhanced fluorescence and plasmon emission. Anal Biochem 2005, 337:171–194. [DOI] [PMC free article] [PubMed] [Google Scholar]; First description of radiating plasmon model.
- 16.Yguerabide J, Yguerabide EE: Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications. I. Theory. Anal Biochem 1998, 262:137–156. [DOI] [PubMed] [Google Scholar]
- 17.Yguerabide J, Yguerabide EE: Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications. II. Experimental characterization. Anal Biochem 1998, 262:157–176. [DOI] [PubMed] [Google Scholar]
- 18.•.Roll D, Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR: Metallic colloid wavelength-ratiometric scattering sensors. Anal Chem 2003, 75:3440–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report of wavelength-ratiometric plasmon scatter.
- 19.••.Aslan K, Lakowicz JR, Geddes CD: Angular-dependent polarization-based plasmon light-scattering for bioaffinity sensing. Appl Phys Lett 2005, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; First report of angular-dependent polarized scatter.
- 20.Liang HP, Wan LJ, Bai CL, Jiang L: Gold hollow nanospheres: tunable surface plasmon resonance controlled by interior cavity sizes. J Phys Chem B 2005, 109:7795–7800. [DOI] [PubMed] [Google Scholar]
- 21.Chen MMY, Katz A: Synthesis and characterization of gold-silica nanoparticles incorporating a mercaptosilane core-shell interface. Langmuir 2002, 18:8566–8572. [Google Scholar]
- 22.Olderburgh SJ, Westscott SL, Averitt RD, Halas NJ: Surface enhanced Raman scattering in the near infrared using metal nanoshell substrates. J Phys Chem B 1999, 111:4729–4735. [Google Scholar]
- 23.Aslan K, Gryczynski I, Malicka J, Matveeva E, Lakowicz JR, Geddes CD: Metal-enhanced fluorescence: an emerging tool in biotechnology. Curr Opin Biotechnol 2005, 16:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geddes CD, Aslan K, Gryczynski I, Malicka J, Lakowicz JR: Noble metal nanostructures for metal-enhanced fluorescence. In Review Chapter for Annual Reviews in Fluorescence 2004. Edited by Geddes CD, Lakowicz JR. Kluwer Academic/Plenum Publishers; 2004:365–401. [Google Scholar]
- 25.•.Lakowicz JR: Radiative decay engineering: biophysical and biomedical applications. Anal Biochem 2001, 298:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]; First description of radiative decay engineering (RDE).
- 26.Lakowicz JR, Shen Y, D’Auria S, Malicka J, Fang J, Grcyzynski Z, Gryczynski I: Radiative decay engineering. 2. Effects of silver island films on fluorescence intensity, lifetimes, and resonance energy transfer. Anal Biochem 2002, 301:261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geddes CD, Cao H, Gryczynski I, Gryczynski Z, Fang J, Lakowicz JR: Metal-enhanced fluorescence (MEF) due to silver colloids on a planar surface: Potential applications of indocyanine green to in vivo imaging. J Phys Chem A 2003, 107:3443–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aslan K, Leonenko Z, Lakowicz JR, Geddes CD: Fast and slow deposition of silver nanorods on planar surfaces: application to metal-enhanced fluorescence. J Phys Chem B 2005, 109:3157–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parfenov A, Gryczynski I, Malicka J, Geddes CD, Lakowicz JR: Enhanced fluorescence from fluorophores on fractal silver surfaces. J Phys Chem B 2003, 107:8829–8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.•.Geddes CD, Lakowicz JR: Metal-enhanced fluorescence. J Fluoresc 2002, 12:121–129. [Google Scholar]; First description of metal-enhanced fluorescence (MEF).
- 31.Aslan K, Lakowicz JR, Geddes CD: Annealed silver island films for metal-enhanced fluorescence. J Fluoresc 2005, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enderlein J: Theoretical study of single molecule fluorescence in a metallic nanocavity. Appl Phys Lett 2002, 80:315–317. [Google Scholar]
- 33.Enderlein J: Spectral properties of a fluorescing molecule within a spherical metallic nanocavity. Phys Chem Chem Phys 2002, 4:2780–2786. [Google Scholar]
- 34.Lakowicz JR, Malicka J, D’Auria S, Gryczynski I: Release of the self-quenching of fluorescence near silver metallic surfaces. Anal Biochem 2003, 320:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pawley JB (Ed): Handbook of Biological and Confocal Miscroscopy 2nd Edn Plenum Press; 1995. [Google Scholar]
- 36.Xu C, Webb WW: Multiphoton excitation of molecular fluorophores and nonlinear laser microscopy In Topics in Fluorescence Spectroscopy, Vol 5 Edited by Lakowicz JR. Plenum Press, 1997. [Google Scholar]
- 37.Lakowicz JR: Principles of Fluorescence Spectroscopy. Kluwer; 1999 [Google Scholar]