Abstract
Hypertension is considered as the most common risk factor for cardiovascular disease (CVD). Inflammatory processes link hypertension and CVD, and participate in their pathophysiology. In recent years, there has been an increase in research focused on unraveling the role of inflammation and immune activation in development and maintenance of hypertension. Although inflammation is known to be associated with hypertension, whether inflammation is a cause or effect of hypertension remains to be elucidated. This review describes the recent studies that link inflammation and hypertension and demonstrate the involvement of oxidative stress and endothelial dysfunction—two of the key processes in the development of hypertension. Etiology of hypertension, including novel immune cell subtypes, cytokines, toll-like receptors, inflammasomes, and gut microbiome found to be associated with inflammation and hypertension are summarized and discussed. Most recent findings in this field are presented with special emphasis on potential of anti-inflammatory drugs and statins for treatment of hypertension.
Introduction
Hypertension (HTN) is a global health problem. Analysis of the data from 135 population-based studies involving 968,419 adults from 90 countries showed that about 31% have hypertension.[61] Hypertension remains an important public health problem in the US with 874 million adults having systolic BP ≥140 mmHg.[7] The prevalence of HTN among adults is 29.0%, with an increased prevalence in non-Hispanic black and older individuals.[24] Data from the National Vital Statistics System noted that there were 78,862 deaths primarily attributable to high blood pressure (HBP) in 2015. [42] Despite advances in therapy, the death rate attributable to HBP increased by 10.5% from 2005 to 2015. [7] A number of factors contribute to the pathogenesis of hypertension. Accumulating evidence suggest that altered immunity and inflammation is important in the genesis of HTN and also a mediator of its complications. In this review we will discuss how disturbed innate and adaptive immune responses mediate neuroendocrine disturbance and vascular inflammation leading to systemic hypertension.
Immunity and Inflammation
Innate and adaptive immunity are two important components of the immune system. Innate immunity provides a rapid antigen independent, nonspecific first line defense against exogenous and endogenous pathogens or injury. (Figure 1) In this, pattern-recognition receptors (PRRs) present on the cells of the innate immune system detect the pathogen-associated molecular patterns (PAMPs) and initiate the host responses,[74] which include (a) generation of cytokines, chemokines, complements and interferons through activation of transcriptional machinery, (b) induction of immune processes such as phagocytosis, autophagy and apoptosis,[17] and (c) initiation of a highly specific long lasting adaptive immune response. Antigen-presenting cells (APCs) such as dendritic cells (DC) take up the antigens, process them into short peptides and present them in the context of a major histocompatibility complex (MHC) leading to activation of T helper cells (CD4+) and cytotoxic (CD8+) T cells. Type 1 T helper (Th1) cells produce interferon-gamma, interleukin (IL)-2, and tumor necrosis factor (TNF)-β, which activate the macrophages. Type 2 T helper cells (Th2) generate IL-4, IL-5, IL-10, and IL-13, which facilitate antibody production. Depending on the micro-environment, macrophages may evolve into proinflammatory M1 or anti-inflammatory M2 sub-types. M1 macrophages release pro-inflammatory cytokines that induce CD4+ cell activaton.[64] On the other hand, M2 macrophages, generate anti-inflammatory cytokines such as IL-10 and promote activation of regulatory T cells (Tregs). Cytokines can recruit polymorphonuclear neutrophils (PMN) and monocytes to sites of injury and induce expression of adhesion molecules, integrin ligands, intercellular adhesion molecules (ICAM) and vascular cell adhesion molecules (VCAM) on vascular endothelial cells. The resulting vascular inflammation has been shown to cause endothelial dysfunction and hypertension. [75]
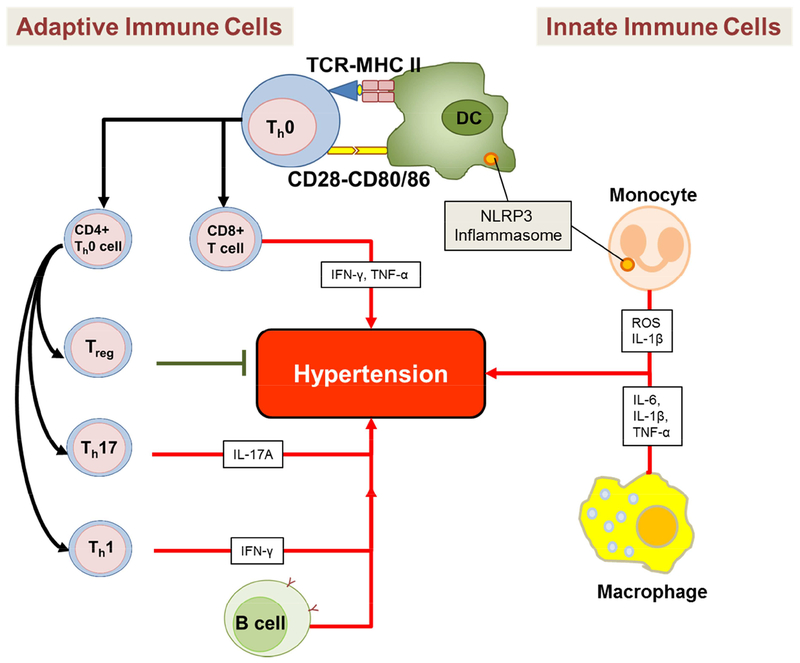
Figure 1. Innate and adaptive immune cells that play a role in hypertension.
Innate immune cells such as monocytes, macrophages, and dendritic cells (DC) inhibit or promote hypertension by producing various cytokines and reactive oxygen species (ROS). Monocytes and dentric cells (DC) also contain the NLRP3 inflammasome which also plays an important role in hypertension. Adaptive immune cells such as the B cells, CD4+ T cells (Treg, Th17, and Th1 cells), and CD8+ T cells also produce cytokines that inhibit or promote hypertension.
Pathogenesis of Essential Hypertension
The central mechanism of essential HTN is an increase in peripheral resistance to blood flow, especially in in the small resistant arteries.
BP is a heritable trait with 30 to 70% of the phenotypic variation attributable to genetic variation. [44;49] Initial “single gene locus theory”[76] was replaced by “polygenic theory” [1] that evolved into the “mosaic hypothesis” [73] that emphasized on 4gene-environmental interaction. The key determinants of BP are the sympathetic nervous system, the renin-angiotensin-aldosterone system, and the plasma volume. (Figure 2) Other contributing factors include excess production of hormones promoting sodium retention and vasoconstriction such as activation of renin-angiotensin-aldosterone system (RAS). Their effect is further enhanced by excess sodium intake and reduced potassium intake, deficiencies of vasodilators, such as prostacyclin and nitric oxide (NO), disturbed kallikrein–kinin system, alteration in vascular growth factors, exposure adverse environmental factors such as excess atmospheric particulate matter and stress. Increasing age, obesity and insulin resistance are well known risk factors for HTN. Guyton et al. [26] proposed a central role for kidney in the pathogenesis of hypertension. (Figure 3) He observed that most systems that would elevate BP, such as activation of the sympathetic nervous system (SNS), could only have a transient effect, and a sustained increase in BP would require a resetting of the pressure natriuresis curve. They further postulated that both salt-resistant and salt sensitive HTN were renal dependent, but that they exhibited different types of pressure natriuresis curves.
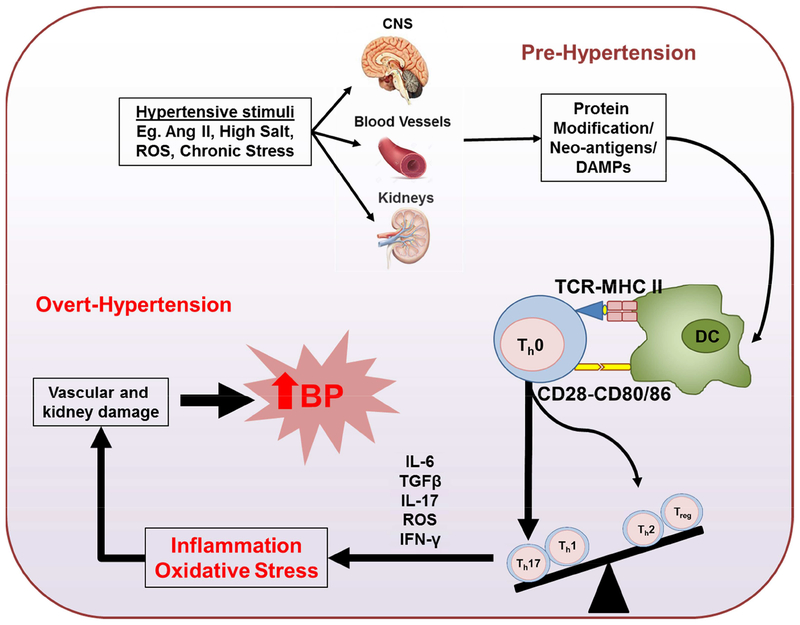
Figure 2. Immune cell activation and inflammation as critical mediators of hypertension.
Activation of central nervous system by hypertensive stimuli such as angiotensin II (Ang II), high salt intake, reactive oxygen species (ROS) and chronic stress will lead to an increase in sympathetic outflow, which causes a modest elevation in blood pressure (pre-hypertension). Elevation of pressure will lead to increase ROS production in the kidney and vasculature, and formation of neoantigens from endogenous proteins. These neoantigens are presented to T cells by DCs, causing T cell activation and cytokine production, including IL17. These cytokine productions will promote inflammation and oxidative stress in kidney and vascular smooth muscle leading to sodium retention, vasoconstriction and overt-hypertension.
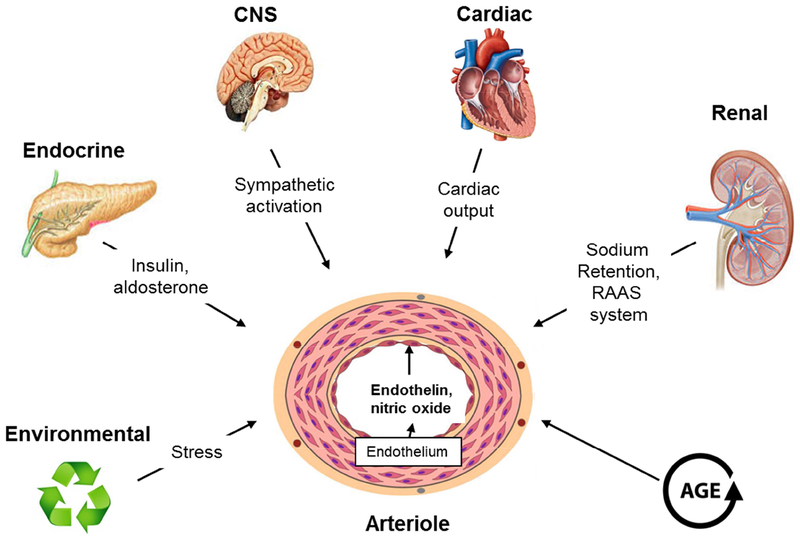
Figure 3. Some of the factors that play a key role in control of blood pressure by affecting the basic equation blood pressure = cardiac output (CO) × peripheral resistance (PR).
Elevation in blood pressure is medicated by the collective contribution of a number of genetic, environmental (including diet) and physiological factors. Both excess sodium intake and renal sodium retention would presumably work primarily on increasing fluid volume and cardiac output. Various hormonal mediators, including angiotensin II, nitric oxide and endothelin may initiate the increased peripheral resistance.
Historical perspective of Immunity and HTN
In the 1960s, investigators showed that immunosuppression attenuates hypertension in rats with partial renal infarction, [101] and transfer of lymph node cells from rats with renal infarction causes HTN in normal rats.[69] However, the enthusiasm for the immune mechanism for hypertension was lost when depressed T cell function was reported in spontaneously hypertensive rats (SHR), [3;90] and that restoration of T cell function by engraftment with normal thymus reduced BP. [3] In 1980s, Olson showed that injection of splenic cells from Deoxycorticosterone acetate (DOCA)-salt hypertensive and renal hypertensive rats to normotensive rats renders them hypertensive. [70] Advances in molecular methods has enabled our understanding of the immune mechanisms underpinning the pathogenesis of hypertension. Recently, investigators have shown that T cells in the renal interstitium contribute to HTN through augmented angiotensin II (ANG II) and oxidant generation and reduction in local nitric oxide.[23;81] Rodriguez-Iturbe et al. [80] showed that the reduction in intrarenal T cells and macrophages could also block salt sensitive HTN in the ANG II model.
Immune cells in Hypertension
Here we will discuss the cell type that are involved in immune regulation and play an important role in hypertensive response.
Monocyte and macrophages
Cells of the monocyte/macrophage lineage are important in vascular remodeling. Ang II was incapable of inducing endothelial dysfunction, vascular remodeling or hypertension in homozygous osteopetrotic mice (Op/Op), which is deficient in macrophage colony-stimulating factor (m-CSF). [15] Similar finding was noted in DOCA-salt challenge in the same mouse model. [41] Wenzel et al [100] showed that infiltrating monocytes with a pro-inflammatory phenotype and macrophages are essential for AGT II-induced vascular dysfunction and HTN. Mice with severe combined immunodeficiency (lacking both T and B lymphocytes) exhibit a blunted BP response and reduced sodium retention in response to ANG II.[13] Guzik et al [27] reported that RAG-1−/− mice, also lacking T and B cells, have blunted BP response and do not develop vascular dysfunction during ANG II infusion or DOCA-salt exposure. They further showed that adoptive transfer of T cells, but not of B cells, restored the hypertensive effect of ANG II in these mice.[27]
Lymphocytes
Increased proportion of immunosenescent, pro-inflammatory, cytotoxic CD8+ T cells that are CD28 null and positive for CD57 is seen in patients with HTN.[105] T cells express angiotensinogen, angiotensin converting enzyme and renin and produce ANG II. [31;34] Infiltrating lymphocytes in renal sections of experimental models of salt sensitive hypertension stained positive for ANG II.[45] Th17 cells are a subset of T-cells characterized by the expression of retinoic acid-related orphan receptor (ROR)γt and by the production of interleukin 17 (IL-17). Madhur et al [53] showed that ANG II increased IL-17 production from T lymphocytes and that ANG II-induced elevations in BP were not sustained in IL-17−/−mice. Once thought to be generated only by Th17 cells, IL-17 is now known to be secreted by macrophages, dendritic cells, natural killer T cells, and γδ T cells in response to immune system activation. [72] Administration of recombinant IL-17 in C57BL/6 mice decreased NO-dependent vascular relaxation via Rho-kinase signaling and caused HTN.[66] ANG II mediated HTN associated vascular dysfunction, vascular inflammation, oxidative stress and aortic stiffening were reduced in IL-17A null mice (IL-17A−/−). [53] Similarly, IL-17 has been demonstrated to have deleterious cardiovascular effects in rodent models of DOCA-salt dependent hypertension. [2] Interaction of the T-cell receptor with the processed peptide presented by the APC, and simultaneous interaction of receptors on the T-cell surface with B7 ligands on the APC surface are required for T cell activation. Vinh et al. [97] demonstrated that in an experimental model of hypertension, blockade of B7-dependent co-stimulation by CTLA4-Ig reduces the development of hypertension in response to AngII and DOCA, opening a new promising avenue for the development of therapies against the disease.
Dendritic cells
DCs are bone marrow-derived, antigen-presenting cells. A complex pattern of interlinked DCs are present in most human and animal tissues, such as arteries, kidneys and brain, which are involved in BP regulation. An amiloride-inhibitable sodium channel regulates sodium entry in DCs, leading to intracellular calcium influx and activation of protein kinase C. [4] This results in increased superoxide production and accumulation of immunogenic isolevuglandins (IsoLGs) in DCs. [4] IsoLGs can cross-link lysine residues on proteins, rendering them immunogenic, which are presented by DCs to T-lymphocytes, triggering T-cell activation and hypertension. [40] Furthermore, IsoLGs containing DCs generate excess IL-6, IL-1β, and IL-23, which promote differentiation of T cells into pro-inflammatory IL-17 producing cells. [53] Importantly, the use of IsoLGs scavengers prevented DC activation and ameliorated HTN, presenting a therapeutic potential. [40]
Regulatory T cells
Tregs are characterized by the expression of CD4+CD25+ and the transcription factor Foxp3. These cells play a crucial role in maintaining immunologic self-tolerance and protection from autoimmune diseases. Tregs release soluble factors such IL-10, IL-35, and TGF-β, which have anti-inflammatory effect. [86] Adoptive transfer of Tregs prevented ANG II-induced hypertension, endothelial dysfunction, vascular stiffness and vascular inflammation. [5] Similarly, aldosterone induced increase in BP, endothelial dysfunction, oxidative stress and infiltration of aorta and kidney with immune cells were attenuated by adoptive transfer of Treg cells. [35] Transfer of Tregs isolated from normotensive mice to hypertensive mice significantly reduced BP and improved endothelium-dependent relaxation in coronary arterioles. [36]
Inflammasomes
The inflammasomes are multiprotein intracellular innate immune system receptors that induce inflammation in response to PAMP and damage-associated molecular patterns (DAMPs). The nucleotide-binding oligomerization domain (Nod)-like receptor containing pyrin domain 3 (NLRP3) inflammasome is the most characterized member of the pattern recognition receptors (PRRs). Activation of the NLRP3 inflammasome involves triggering of caspases that facilitate the maturation of inactive pro-inflammatory cytokine precursors. Murine ATP-induced hypertension is accompanied by increased caspase-1 activity, IL-1β production and CD43+ T-cell infiltration in the renal medulla.[103] An intronic 42 base pair variable number of tandem repeat (VNTR) polymorphism in the CIAS1 gene that encodes for NLRP3 has been linked to essential hypertension in humans.[71] Interestingly, the CIAS1 gene is part of the CATERPILLER gene family, which also contains PYRIN-containing Apaf-1-like protein 5 (PYPAF5) that encodes the ANG II/vasopressin receptor implicated in salt-sensitive hypertension in the Dahl SS model. [82] Pharmacological inhibition and genetic modulation of NLRP3 activation result in potent therapeutic effects in a wide variety of experimental inflammatory diseases. [54] Caspase-1 inhibitor WEHD, attenuated inflammasome activation and blocked ATP-induced hypertension. [103]
Toll-Like Receptors in Hypertension
Toll-like receptors (TLR) are major components of the innate immune system that recognize DAMP to initiate inflammatory signaling. About twelve TLRs have been identified in mammals, which are expressed in immune as well as nonimmune cells in specific combinations.[43;99] All TLRs contain a cytoplasmic Toll/Interleukin-1 Receptor (TIR) domain, which, upon activation, recruits adaptor proteins such as myeloid differentiation factor 88 (MyD88), MyD88-adapter-like (Mal), IL-1 receptor associated kinase-4 (IRAK4) and TIR-containing adaptor molecule (TICAM). Different signaling cascades are activated by TLRs depending on the adaptor protein binding. The cytokine cascade initiated by TLR is essential for the transition from nonspecific innate to targeted adaptive immunity. TLRs could modulate vascular function, induce low grade vascular inflammation and thus contributing to hypertension. [8;8;51;91] ANG II upregulated TLR-4 expression and increased TNF-α in rat vascular smooth muscle cells. [33] TLR signaling from the neuro-immuno-sympathetic connection from the CNS to the immune system is altered in spontaneously hypertensive rats. [28] These rats have increased expression of TLR-4 protein in mesenteric arteries and treatment with anti-TLR-4 antibody lowers BP. [8]
Oxidative Stress in Hypertension
The reactive oxygen species (ROS) including superoxide (O2−), hydroxyl radical (HO•), hydrogen peroxide (H2O2), peroxynitrite (ONOO−), nitric oxide (NO•), and hypochlorous acid (HOCl) are produced during cellular metabolism and scavenged by antioxidants. ROS are important signaling molecules that mediate activation of transcription factors, induction of immune response genes and phosphorylation of kinases.[18] They regulates endothelial function and vascular tone and vascular remodeling through proliferation and apoptosis.[96] Chronic inflammation is known to promote oxidative stress and vice versa by activating transcription factors such as nuclear factor (NF)-κB. [9] Nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) is a major source of ROS in immune cells and also in the vasculature.[94] NO and prostacyclin released from endothelial cells inhibit platelet aggregation, the neutrophils attachment to endothelial cells, expression of adhesion molecules and inhibition of smooth muscle cell proliferation.[25] ANG II also contributes to the production of ROS through increased activity of NADPH oxidase.[92] Oxidative stress is an important cause of endothelial dysfunction, primarily through reducing NO bioavailability through chemical reaction of superoxide with NO, resulting in the formation of peroxynitrite. [6] Peroxynitrite may further increase oxidative stress by inhibiting eNOS activity through oxidation of 4-tetrahydrobiopterin (BH4), a cofactor of eNOS. This leads to eNOS uncoupling, where eNOS produces superoxide instead of NO.[95] Inhibition of NO induced ROS generation using pharmacological and gene-targeted approaches result in regression of vascular remodeling, improved endothelial function, and lowering of BP. [11] Despite the consistent and promising findings from experimental studies clinical studies investigating beneficial effects of antioxidants have largely been disappointing. [37;62] Currently, researchers are advocating for the use of more disease specific, target-directed, and highly bioavailable antioxidants instead of nonspecific antioxidant vitamins. [22]
Microbiome and Hypertension
Advances in sequencing techniques have revealed that the human gut microbiome plays a vital role in human health and disease. Experimental as well as and human studies suggest that gut microbiome is altered and possibly etiologically linked to hypertension. Compared to the healthy controls, hypertensive individuals have decreased microbial richness and diversity, with abundance in Prevotella enterotype.[50] Furthermore, fecal microbial transplant from hypertensive humans to germfree mice resulted in increased blood pressure.[50] The microbiota of salt sensitive rats has a distinct gut microbiome profile with abundance in phylum Bacteroidetes.[60] Spontaneously hypertensive rats (SHRs) have higher Firmicutes to Bacteroidetes ratio and reduced Actinobacteria compared to normotensive Wistar Kyoto (WKY) control rats.[104] ANG II-treated rats also have increased Firmicutes/Bacteroidetes ratio.[104] Short chain fatty acids (SCFAs) are byproducts of gut microbial metabolism that have been known to influence several aspects of host physiology, including blood pressure regulation.[77] SCFAs have an anti-inflammatory effect on both colonic epithelium and immune system,[55;58;84] which is mediated through activation of the G protein receptor family.[48] SCFAs bind to the aryl hydrocarbon receptor (AHR) to increase transcription of IL-10R and reduce gut inflammation.[47;83] This is significant because about 70% of the body’s immune cells reside in the gut associated lymphatic tissue (GALT). As discussed above, excess IL-17A production is associated with hypertension.[53;68] Th17 producing cells are known to be affected by the abundance of specific commensal bacteria.[32] High salt intake depletes Lactobacillus murinus and treatment of mice with L. murinus prevented salt-sensitive hypertension by modulating TH17 cells.[102] Interestingly, some symbiotic bacteria produce ACE inhibitors, renin inhibitors, and antioxidant molecules during the digestion of mucin.[14] A recent meta-analysis provided preliminary support that Lactobacillus-rich probiotics might affect blood pressure in hypertensive subjects.[38]
Salt Sensitivity and Inflammation
Sodium is the major cation that maintains extracellular fluid volume. Epidemiological, clinical, and experimental studies have demonstrated that excess dietary salt intake contributes to hypertension and reductions in salt intake lowers blood pressure. [29;63] However, the effect of salt intake on cardiovascular disease and death is non-linear. [93] Salt sensitivity is defined as a change in blood pressure of 5 to10% or at least 5 mm Hg, in response to a change in NaCl intake. [89] In salt-sensitive HTN, the accumulation of sodium in tissue is accompanied by retention of water in order to maintain the isotonicity. High-salt diet in rats leads to sodium accumulation in interstitium of skin, resulting in increased density of lymphatics mediated by activation of tonicity-responsive enhancer binding protein in mononuclear phagocyte system cells infiltrating the interstitium of the skin.[52] Both Dahl Salt sensitive rats fed high salt as well as hypertensive humans are found to have infiltration of macrophages and CD4+ and CD8+ T cells in the kidneys.[56] Mycophenolate mofetil, an immunosuppressive agent was able to prevent the infiltration of T cells into the kidneys and reduce the development of salt sensitive HTN and kidney damage.[16] It has been suspected that apart from the classical systemic RAAS an independently functioning RAAS exists within the kidney that regulate sodium excretion and BP.[12] As noted above T cells express angiotensinogen, angiotensin converting enzyme and renin and produce ANG II.[31;34] This could explain the intra-renal activation of RAS in salt sensitive hypertension.
Brain inflammation in Hypertension
Sympathetic pre-motor neurons controlling vasomotor activity are located predominantly in the rostral ventrolateral medulla (RVLM). SNS stimulation elevates BP by increasing cardiac output, vascular resistance and fluid retention. Recent findings suggest that SNS also acts as an integrative interface between the brain and the immune system.[19] Perivascular macrophages in the blood brain barrier can transfer peripheral inflammatory signals to the brain.[106] Inflammation of forebrain and hindbrain nuclei controlling the SNS efferent output from the brain is important in the development of neurogenic HTN. Interestingly, lymphoid organs have an abundant supply of sympathetic innervation and nor-epinephrine released into the lymphoid tissue by the autonomic nerves have an immunomodulatory function.[65] Furthermore, ANG II-induced hypertension is dependent upon the activation of NF-kB in the paraventricular nucleus (PVN),[10] and direct injection of IL-1β into the PVN or via the intracerebroventricular route increases BP.[10;87] Thus inflammation in specific regions of the brain is linked to systemic hypertension.
Treatment strategies
A number of clinical studies have reported a strong association between inflammation and hypertension. In a prospective cohort study, baseline levels of C-Reactive protein was found to be independently associated with an increased risk for incident hypertension.[85] Also, elevated levels of inflammation-sensitive plasma proteins fibrinogen, α1-antitrypsin, haptoglobin, ceruloplasmin, and orosomucoid are associated with a future increase in BP among healthy middle-aged men.[20] Despite the evidence for immune activation, anti-inflammatory drugs are not in general used treat hypertension. Experimental evidence supporting use of anti-inflammatory agents in the treatment of HTN is described throughout the review. In the clinical setting, preliminary finding suggest that minocycline, a centrally penetrating anti-inflammatory antibiotic produces sustained blood pressure reduction in a hypertensive individual.[78] Mycophenolate mofetil, which blocks T cell and B cell proliferation reduce hypertension in experimental models of hypertension. [57;80] Preliminary reports show that treatment with mycophenolate mofetil resulted in a significant reduction in BP in patients with psoriasis and rheumatoid arthritis patients. [30] Another observational study suggested that long-term use of immunosuppressive medication may reduce arterial stiffness and improve BP control in patients with CKD. [21] Statins are known to have anti-inflammatory properties, which results in modest reduction in BP in hypercholesterolemic patients.[88] Monoclonal antibodies against IL-17A or IL-17RA, the IL-17A receptor have shown promising results in in patients with psoriasis.[46;59] However, a meta-analysis examining the effect of anti-TNFα agents in patients with rheumatoid arthritis is associated with increased risk of developing hypertension.[59;107] Similarly, clinical trials have not demonstrated beneficial effect of vitamin antioxidants in hypertension.[39;98] Despite convincing evidence linking inflammation to hypertension, reducing inflammation and has not been uniformly successful in reducing blood pressure.
Conclusion
Evidence from human and animal studies strongly suggest an association between inflammation and hypertension. It could very well be that hypertension could be etiologically linked to abnormal immune response, which also plays a vital role in hypertension-associated end organ damage. The cause-effect relationship is further confounded by the fact that a number of factors that are associated with hypertension, such as obesity, insulin resistance, ageing, SNS activation are also associated with inflammation. Evidence from experimental models of hypertension support the beneficial effect of immunomodulatory agents in the treatment of hypertension. Further studies are required to identify the target for intervention and most effective agent with optimal risk benefit ratio. Furthermore, whether such therapies could be effective in all types of hypertension irrespective of underlying etiology remains to be elucidated. Encouraging results from the Canakinumab Antiinflammatory Thrombosis Outcome study (CANTOS)[79] and the Low Dose Colchicine for Secondary Prevention of Cardiovascular Disease (LoDoCo) [67] study in reducing cardiovascular mortality and morbidity support the safety and efficacy of anti-inflammatory therapies in human subjects. At the present time, however, anti-inflammatory therapy for hypertension remains an attractive concept, but not ready for prime time.
Highlight.
Experimental studies show that immune activation and inflammation are involved in the pathogenesis of hypertension
T cells express angiotensinogen, angiotensin converting enzyme and produce angiotensin II
Increased generation of reactive oxygen species and the associated decrease in nitric oxide (NO) cause endothelial dysfunction leading to hypertension
Laboratory research and association studies in humans support the role of anti-inflammatory therapy for hypertension.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The nature of essential hypertension. Lancet 2:1027–1030, 1959 [PubMed] [Google Scholar]
- 2.Amador CA, Barrientos V, Pena J et al. : Spironolactone decreases DOCA-salt-induced organ damage by blocking the activation of T helper 17 and the downregulation of regulatory T lymphocytes. Hypertension 63:797–803, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Ba D, Takeichi N, Kodama T, Kobayashi H: Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immunol 128:1211–1216, 1982 [PubMed] [Google Scholar]
- 4.Barbaro NR, Foss JD, Kryshtal DO et al. : Dendritic Cell Amiloride-Sensitive Channels Mediate Sodium-Induced Inflammation and Hypertension. Cell Rep 21:1009–1020, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barhoumi T, Kasal DA, Li MW et al. : T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57:469–476, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Beckman JS, Koppenol WH: Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol 271:C1424–C1437, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Virani SS, Callaway CW et al. : Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 137:e67–e492, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Bomfim GF, Echem C, Martins CB et al. : Toll-like receptor 4 inhibition reduces vascular inflammation in spontaneously hypertensive rats. Life Sci 122:1–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canty TG Jr., Boyle EM Jr., Farr A et al. : Oxidative stress induces NF-kappaB nuclear translocation without degradation of IkappaBalpha. Circulation 100:II361–II364, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Cardinale JP, Sriramula S, Mariappan N et al. : Angiotensin II-induced hypertension is modulated by nuclear factor-kappaBin the paraventricular nucleus. Hypertension 59:113–121, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cave A: Selective targeting of NADPH oxidase for cardiovascular protection. Curr Opin Pharmacol 9:208–213, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Crowley SD, Gurley SB, Oliverio MI et al. : Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115:1092–1099, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowley SD, Song YS, Lin EE et al. : Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 298:R1089–R1097, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dave LA, Hayes M, Montoya CA et al. : Human gut endogenous proteins as a potential source of angiotensin-I-converting enzyme (ACE-I)-, renin inhibitory and antioxidant peptides. Peptides 76:30–44, 2016 [DOI] [PubMed] [Google Scholar]
- 15.De CC, Amiri F, Brassard P et al. : Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 25:2106–2113, 2005 [DOI] [PubMed] [Google Scholar]
- 16.De MC, Das S, Lund H, Mattson DL: T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298:R1136–R1142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deretic V, Saitoh T, Akira S: Autophagy in infection, inflammation and immunity. Nat Rev Immunol 13:722–737, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Droge W: Free radicals in the physiological control of cell function. Physiol Rev 82:47–95, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES: The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52:595–638, 2000 [PubMed] [Google Scholar]
- 20.Engstrom G, Janzon L, Berglund G et al. : Blood pressure increase and incidence of hypertension in relation to inflammation-sensitive plasma proteins. Arterioscler Thromb Vasc Biol 22:2054–2058, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Ferro CJ, Edwards NC, Hutchison C et al. : Does immunosuppressant medication lower blood pressure and arterial stiffness in patients with chronic kidney disease? An observational study. Hypertens Res 34:113–119, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Firuzi O, Miri R, Tavakkoli M, Saso L: Antioxidant therapy: current status and future prospects. Curr Med Chem 18:3871–3888, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Franco M, Martinez F, Quiroz Y et al. : Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 293:R251–R256, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Fryar CD, Ostchega Y, Hales CM et al. : Hypertension Prevalence and Control Among Adults: United States, 2015–2016. NCHS Data Brief 1–8, 2017 [PubMed] [Google Scholar]
- 25.Garg UC, Hassid A: Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 83:1774–1777, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyton AC, Coleman TG, Cowley AV Jr. et al. : Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52:584–594, 1972 [DOI] [PubMed] [Google Scholar]
- 27.Guzik TJ, Hoch NE, Brown KA et al. : Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204:2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harwani SC, Chapleau MW, Legge KL et al. : Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ Res 111:1190–1197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He FJ, Li J, MacGregor GA: Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 346:f1325, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B: Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol 17:S218–S225, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Hoch NE, Guzik TJ, Chen W et al. : Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 296:R208–R216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivanov II, Atarashi K, Manel N et al. : Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139:485–498, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji Y, Liu J, Wang Z, Liu N: Angiotensin II induces inflammatory response partly via toll-like receptor 4-dependent signaling pathway in vascular smooth muscle cells. Cell Physiol Biochem 23:265–276, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Jurewicz M, McDermott DH, Sechler JM et al. : Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol 18:1093–1102, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Kasal DA, Barhoumi T, Li MW et al. : T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 59:324–330, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Kassan M, Galan M, Partyka M et al. : Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol 31:2534–2542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly RP, Poo YK, Isaac HB et al. : Lack of effect of acute oral ingestion of vitamin C on oxidative stress, arterial stiffness or blood pressure in healthy subjects. Free Radic Res 42:514–522, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Khalesi S, Sun J, Buys N, Jayasinghe R: Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension 64:897–903, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Kim MK, Sasaki S, Sasazuki S et al. : Lack of long-term effect of vitamin C supplementation on blood pressure. Hypertension 40:797–803, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Kirabo A, Fontana V, de Faria AP et al. : DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 124:4642–4656, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ko EA, Amiri F, Pandey NR et al. : Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol 292:H1789–H1795, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B: Deaths: Final Data for 2014. Natl Vital Stat Rep 65:1–122, 2016 [PubMed] [Google Scholar]
- 43.Kumar H, Kawai T, Akira S: Toll-like receptors and innate immunity. Biochem Biophys Res Commun 388:621–625, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Kupper N, Willemsen G, Riese H et al. : Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension 45:80–85, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Kurokawa K: Kidney, salt, and hypertension: how and why. Kidney Int Suppl 55:S46–S51, 1996 [PubMed] [Google Scholar]
- 46.Langley RG, Elewski BE, Lebwohl M et al. : Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med 371:326–338, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Lanis JM, Alexeev EE, Curtis VF et al. : Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol 10:1133–1144, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le PE, Loison C, Struyf S et al. : Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278:25481–25489, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Levy D, DeStefano AL, Larson MG et al. : Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension 36:477–483, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Li J, Zhao F, Wang Y et al. : Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5:14, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang CF, Liu JT, Wang Y et al. : Toll-like receptor 4 mutation protects obese mice against endothelial dysfunction by decreasing NADPH oxidase isoforms 1 and 4. Arterioscler Thromb Vasc Biol 33:777–784, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Machnik A, Neuhofer W, Jantsch J et al. : Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15:545–552, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Madhur MS, Lob HE, McCann LA et al. : Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55:500–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mangan MSJ, Olhava EJ, Roush WR et al. : Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov 17:588–606, 2018 [DOI] [PubMed] [Google Scholar]
- 55.Maslowski KM, Vieira AT, Ng A et al. : Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461:1282–1286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mattson DL: Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol 307:F499–F508, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mattson DL, James L, Berdan EA, Meister CJ: Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48:149–156, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL: An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122:107–118, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Mease PJ, Genovese MC, Greenwald MW et al. : Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 370:2295–2306, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Mell B, Jala VR, Mathew AV et al. : Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics 47:187–197, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mills KT, Bundy JD, Kelly TN et al. : Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 134:441–450, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mishra GD, Malik NS, Paul AA et al. : Childhood and adult dietary vitamin E intake and cardiovascular risk factors in mid-life in the 1946 British Birth Cohort. Eur J Clin Nutr 57:1418–1425, 2003 [DOI] [PubMed] [Google Scholar]
- 63.Mozaffarian D, Fahimi S, Singh GM et al. : Global sodium consumption and death from cardiovascular causes. N Engl J Med 371:624–634, 2014 [DOI] [PubMed] [Google Scholar]
- 64.Murray PJ: Macrophage Polarization. Annu Rev Physiol 79:541–566, 2017 [DOI] [PubMed] [Google Scholar]
- 65.Nance DM, Sanders VM: Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun 21:736–745, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nguyen H, Chiasson VL, Chatterjee P et al. : Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res 97:696–704, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL: Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 61:404–410, 2013 [DOI] [PubMed] [Google Scholar]
- 68.Norlander AE, Saleh MA, Kamat NV et al. : Interleukin-17A Regulates Renal Sodium Transporters and Renal Injury in Angiotensin II-Induced Hypertension. Hypertension 68:167–174, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Okuda T, GROLLMAN A: Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med 25:257–264, 1967 [PubMed] [Google Scholar]
- 70.Olsen F: Transfer of arterial hypertension by splenic cells from DOCA-salt hypertensive and renal hypertensive rats to normotensive recipients. Acta Pathol Microbiol Scand C 88:1–5, 1980 [DOI] [PubMed] [Google Scholar]
- 71.Omi T, Kumada M, Kamesaki T et al. : An intronic variable number of tandem repeat polymorphisms of the cold-induced autoinflammatory syndrome 1 (CIAS1) gene modifies gene expression and is associated with essential hypertension. Eur J Hum Genet 14:1295–1305, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Onishi RM, Gaffen SL: Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129:311–321, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Page IH: The mosaic theory of arterial hypertension--its interpretation. Perspect Biol Med 10:325–333, 1967 [DOI] [PubMed] [Google Scholar]
- 74.Palm NW, Medzhitov R: Pattern recognition receptors and control of adaptive immunity. Immunol Rev 227:221–233, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Peyster E, Chen J, Feldman HI et al. : Inflammation and Arterial Stiffness in Chronic Kidney Disease: Findings From the CRIC Study. Am J Hypertens 30:400–408, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Platt R: Heredity in hypertension. Q J Med 16:311, 1947 [PubMed] [Google Scholar]
- 77.Pluznick JL, Protzko RJ, Gevorgyan H et al. : Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A 110:4410–4415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qi Y, Aranda JM, Rodriguez V et al. : Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension - A case report. Int J Cardiol 201:157–158, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ridker PM, Everett BM, Thuren T et al. : Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 377:1119–1131, 2017 [DOI] [PubMed] [Google Scholar]
- 80.Rodriguez-Iturbe B, Pons H, Quiroz Y et al. : Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59:2222–2232, 2001 [DOI] [PubMed] [Google Scholar]
- 81.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ: Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286:F606–F616, 2004 [DOI] [PubMed] [Google Scholar]
- 82.Ruiz-Opazo N, Lopez LV, Herrera VL: The dual AngII/AVP receptor gene N119S/C163R variant exhibits sodium-induced dysfunction and cosegregates with salt-sensitive hypertension in the Dahl salt-sensitive hypertensive rat model. Mol Med 8:24–32, 2002 [PMC free article] [PubMed] [Google Scholar]
- 83.Schiering C, Wincent E, Metidji A et al. : Feedback control of AHR signalling regulates intestinal immunity. Nature 542:242–245, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Segain JP, Raingeard dl B, Bourreille A et al. : Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut 47:397–403, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sesso HD, Buring JE, Rifai N et al. : C-reactive protein and the risk of developing hypertension. JAMA 290:2945–2951, 2003 [DOI] [PubMed] [Google Scholar]
- 86.Shevach EM: Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity 30:636–645, 2009 [DOI] [PubMed] [Google Scholar]
- 87.Shi Z, Gan XB, Fan ZD et al. : Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf) 203:289–297, 2011 [DOI] [PubMed] [Google Scholar]
- 88.Strazzullo P, Kerry SM, Barbato A et al. : Do statins reduce blood pressure?: a meta-analysis of randomized, controlled trials. Hypertension 49:792–798, 2007 [DOI] [PubMed] [Google Scholar]
- 89.Sullivan JM: Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertension 17:I61–I68, 1991 [DOI] [PubMed] [Google Scholar]
- 90.Takeichi N, Hamada J, Takimoto M et al. : Depression of T cell-mediated immunity and enhancement of autoantibody production by natural infection with microorganisms in spontaneously hypertensive rats (SHR). Microbiol Immunol 32:1235–1244, 1988 [DOI] [PubMed] [Google Scholar]
- 91.Tang PC, Qin L, Zielonka J et al. : MyD88-dependent, superoxide-initiated inflammation is necessary for flow-mediated inward remodeling of conduit arteries. J Exp Med 205:3159–3171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taniyama Y, Griendling KK: Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension 42:1075–1081, 2003 [DOI] [PubMed] [Google Scholar]
- 93.Taylor RS, Ashton KE, Moxham T et al. : Reduced dietary salt for the prevention of cardiovascular disease: a meta-analysis of randomized controlled trials (Cochrane review). Am J Hypertens 24:843–853, 2011 [DOI] [PubMed] [Google Scholar]
- 94.Touyz RM, Schiffrin EL: Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens 19:1245–1254, 2001 [DOI] [PubMed] [Google Scholar]
- 95.Vasquez-Vivar J, Kalyanaraman B, Martasek P et al. : Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A 95:9220–9225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vaziri ND, Rodriguez-Iturbe B: Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol 2:582–593, 2006 [DOI] [PubMed] [Google Scholar]
- 97.Vinh A, Chen W, Blinder Y et al. : Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 122:2529–2537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ward NC, Wu JH, Clarke MW et al. : The effect of vitamin E on blood pressure in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. J Hypertens 25:227–234, 2007 [DOI] [PubMed] [Google Scholar]
- 99.Watters TM, Kenny EF, O’Neill LA: Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunol Cell Biol 85:411–419, 2007 [DOI] [PubMed] [Google Scholar]
- 100.Wenzel P, Knorr M, Kossmann S et al. : Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124:1370–1381, 2011 [DOI] [PubMed] [Google Scholar]
- 101.WHITE FN, GROLLMAN A: AUTOIMMUNE FACTORS ASSOCIATED WITH INFARCTION OF THE KIDNEY. Nephron 1:93–102, 1964 [DOI] [PubMed] [Google Scholar]
- 102.Wilck N, Matus MG, Kearney SM et al. : Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551:585–589, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xia M, Abais JM, Koka S et al. : Characterization and Activation of NLRP3 Inflammasomes in the Renal Medulla in Mice. Kidney Blood Press Res 41:208–221, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang T, Santisteban MM, Rodriguez V et al. : Gut dysbiosis is linked to hypertension. Hypertension 65:1331–1340, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Youn JC, Yu HT, Lim BJ et al. : Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62:126–133, 2013 [DOI] [PubMed] [Google Scholar]
- 106.Yu Y, Zhang ZH, Wei SG et al. : Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension 55:652–659, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao Q, Hong D, Zhang Y et al. : Association between anti-TNF therapy for rheumatoid arthritis and hypertension: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 94:e731, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]