Abstract
Enhanced fluorescence on silver island films (SIFs) is utilized to develop a sandwich-format immunoassay for the cardiac marker myoglobin (Myo). Myoglobin was first captured on surfaces coated with anti-Myo antibodies; the surface was then incubated with fluorescently labeled anti-Myo antibodies. The system was examined on glass surfaces and on SIFs. We observed the enhancement of the signal from SIFs in the range of 10–15-fold if compared to the signal from the glass substrate not modified with a SIF. A kinetic immunoassay for Myo on SIF-modified surface results in a decreased background signal. The initial results show that it is possible to detect Myoglobin concentrations below 50 ng/mL, which is lower than clinical cut-off for Myoglobin in healthy patients. We suggest the use of SIF-modified substrates for increasing the sensitivity of surface assays with fluorescence detection.
Keywords: Myoglobin, Cardiac markers, Fluorescence immunoassay, Metal-enhanced fluorescence, Nano-size metallic particles, Silver island films
1. Introduction
Cardiovascular diseases are the one of the leading causes of mortality in developed countries. Cardiac markers are currently of clinical interest, with active debate on their use (Ellenius et al., 1997; Henderson, 1997; Malasky and Alpert, 2002; Panteghini, 2002; Gibler et al., 2003). Most authors do not recommend a single marker, but to use a combination of several markers (such as myoglobin, CK-MB, troponin I, and troponin T), and repeat the serum marker testing up to 12 h after the disease symptom (Fesmire, 2001; McCord et al., 2003; Newby et al., 2001; Storrow and Gibler, 1999). Sensitive and reliable methods of cardiac markers detection in an early development stage would have a fundamental impact on today’s preventative medicine, and myoglobin (Myo), although not cardiac specific, is one of the very early markers to increase after acute myocardial infarction (AMI) (Lim et al., 2002; Montague and Kircher, 1995; Storrow and Gibler, 1999; Zaninotto et al., 1999).
There is no clear agreement in the literature about the biomarker value distinguishing between normal and elevated level. The term “normal range” sometimes is replaced by “reference range”; “upper limit of normal” by “referent value,” “critical value,” “positive”, “decision limit”, or “cut-off point’ (see Table 1 and Galen, 1977). Some authors use a double “upper limit of normal” as a “cut-off”. Sometimes a “low” and “high” cut-off concentration are considered to distinguish illnesses of different severity, for example it was recommended use of two cut-off concentrations for cardiac troponin to differentiate normal from minor myocardial injury and AMI (Wu, 1999). The cut-off point for a biomarker considered as the upper limit of normal biomarker concentration in healthy people, is usually defined from the so-called receiver operating characteristics (ROC) analysis (Egan, 1975). For Myo (to exclude AMI) the cut-off value is usually approximately 100 ng/mL; some authors define even higher cut-off value, up to 200 ng/mL (see Table 1), due to the fact that myoglobin is less specific if compared to other cardiac markers, and its level can elevate not only because of AMI, but due to some other factors, such as muscular trauma or renal disorder (Woo et al., 1995).
Table 1.
Cut-off myoglobin values for the discrimination of AMI used by various authors
| [Myo] value, ng/mL |
Referred by authors as | Reference |
|---|---|---|
| 90 | Upper limit of normal |
de Winter et al., 1996; Laperche et al., 1995 |
| 92 | Abnormal | Mathew et al., 1999 |
| 100 | Cut-off | Castaldo et al., 1994 |
| 100 | Positive; Cut-off | Hillis et al., 1999 |
| 105 | Upper limit of normal | Newby et al., 2001 |
| 107 | Cut-off (ROC curve determined) | Apple et al., 1999 |
| 107 | Upper reference limit (95th percentile) | Di Serio et al., 2003 |
| 170 | Upper limit of normal | Ng et al., 2001 |
| 180 | ROC-curve determined decision limit | Apple et al., 2000 |
| 200 | Cut-off | McCord et al., 2001, 2003 |
Our recent studies of the metal-enhanced fluorescence showed that close proximity of the fluorophore to the metallic silver particles increases the brightness and photostability of the fluorophore (Lakowicz, 2001; Lakowicz et al., 2002). The possibility of an immunoassay development based on this enhancement effect was demonstrated for a model immunoassay on a silver island film (SIF) modified glass slide with an enhancement of about 5- to 10-fold (Matveeva et al., 2004), and for an insulin immunoassay on a microplate coated with silver nanoparticles with an enhancement of about 4-fold (Lochner et al., 2003). This enhancement phenomenon is the basis of our approach to the fluoroimmunoassay, and here we present the application of this technology to the immunoassay for myoglobin.
2. Materials and methods
2.1. Materials
Myoglobin (recombinant) and monoclonal antimyoglobin antibodies (capture anti-Myo antibodies clone 2mb-295, reporter anti-Myo antibodies clone 9mb-183r) were from “Spectral Diagnostics”, Canada. Buffer components and salts (such as poly-l-lysine, bovine serum albimun, glucose, sucrose, AgNO3) were from “Sigma-Aldrich”. Microscope glass slides were from “VWR”, 3 × 1 in. and 1 mm thick. Milli-Q purified water was used for all aqueous solutions.
Reporter antibodies were labeled with Rhodamine Red-X or Alexa Fluor-647 using labeling kits from “Molecular Probes”; kits provided dyes with reactive succinimidyl ester moieties, which react effectively with the primary amines of proteins. We synthesized four conjugates with Rhodamine Red-X (with different labeling degree), and one conjugate with Alexa Fluor-647. Dye/protein ratios in conjugates (after chromatographic separation of unbound dye) were determined spectrophotometrically according to the kit instructions: dye concentrations were determined from the visible part of spectra, using the published molar extinction coefficients (ϵ570 = 120000 cm−1 M−1 for Rhodamine Red-X, and ϵ650 = 239000 cm−1 M−1 for Alexa Fluor-647), and antibody concentrations were determined from the UV part of spectra (ϵ280=203000 cm−1 M−1 for IgG), taking into account the UV absorbance contribution from the covalently bound dye (0.17 × A570 for Rhodamine Red-X, or 0.03 × A650 for Alexa Fluor-647, where A570 and A650 is absorbance at 570 or 650 nm). Dye/protein ratios used in conjugation reactions with Rhodamine Red-X, and resulting dye/protein ratios in corresponding purified conjugates, are summarized in Table 2. Dye/protein ratio used in conjugation reaction with Alexa Fluor-647 is unknown because the kit protocol does not give the amount of the portion of the activated dye to be used for one portion of 100 μg of IgG; kit protocol only indicated that it is optimized and one should get IgG labeled with 3–7 dyes. Our resulting dye/protein ratio for purified Alexa Fluor-647 conjugate was found to be 5.4.
Table 2.
Enhancement data for various Rhodamin Red-X modified anti-Myo antibodies
| Conjugate | (Reactive dye)/IgG used for conjugation, mol/mol |
Dye/IgG in purified conjugate, mol/mol |
SIF-modified surface |
Non-modified surface |
Enhancement | ||
|---|---|---|---|---|---|---|---|
| Average signal, a.u. |
SD (n =4), % |
Average signal, a.u. |
SD (n =4), % |
||||
| 1 (low-labeled) | 1.6 | 1 | 7.2 | 20 | 1 | 16 | 7.2 |
| 2 (medium-labeled) | 8 | 3.5 | 33 | 15 | 3.8 | 12 | 8.6 |
| 3 (high-labeled) | 40 | 8 | 0.69 | 41 | 0.30 | 22 | 2.3 |
| 4 (over-labeled) | 200 | ~50–100a | 0.46 | 44 | 0.35 | 19 | ~1 |
This value is inaccurate due to high error because of large contribution from the covalently bound dye (at high levels of labeling) into the UV absorbance which was used for estimation of IgG concentration, see Materials and methods for estimation of the dye/IgG ratio in purified conjugates.
2.2. Silver islands formation
Silver islands film (SIF) surface was formed according to the procedure described elsewhere (Lakowicz, 2001; Lakowicz et al., 2002; Matveeva et al., 2004). In particular, glass slides were first coated with poly-l-lysine (poly-l-lysine was used for coating glass substrate for two reasons: it resulted into better binding of capture antibodies to the glass surface, and it resulted into more efficient coating of the glass slide with SIF). Then half of the slide surface (coated with poly-l-lysine) was modified by depositing silver island film. Silver was deposited by chemical reduction of silver ions by wet-chemical process using D(+)glucose. After rinsing several times with water, slides were stored in water (milli-Q purified) at room temperature in closed tubes until use. For all data presented in this paper we used slides stored for a few days (1–10 days). However, we had slides stored for several months in these conditions, and we checked their performance in separate experiments, using both fresh (1–2 days old) and old (2–6 months old) slides for same immunoassay. Our data indicated that 2–6 months old slides perform same as fresh slides. We suppose silver particles could stabilize the aqueous medium against contamination.
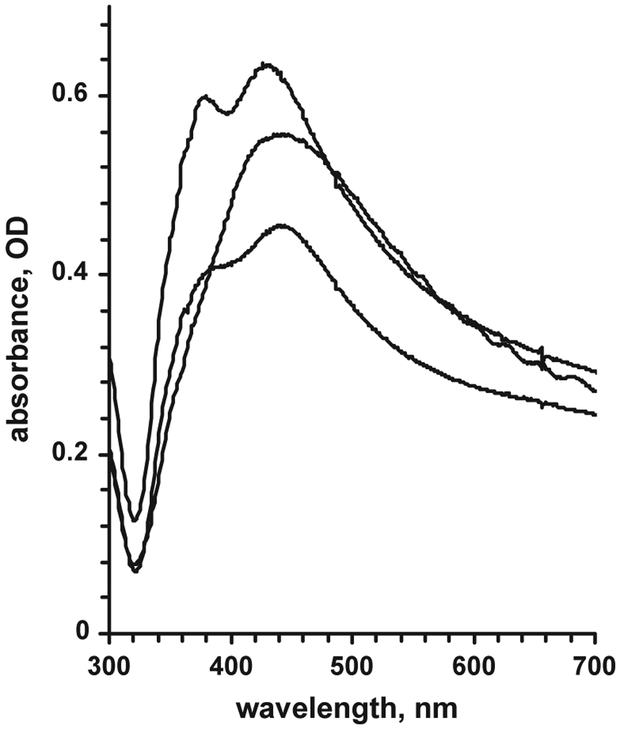
Several typical absorbance spectra of the SIF-coated glass slide are given in Fig. 1 (absorbance maximum was normally observed between 380 and 450 nm). Absorbance maximum wavelength and shape of the spectrum depend on the size and shape of the silver particles (Lakowicz, 2001; Lakowicz et al., 2002; Evanoff and Chumanov, 2004; Hao et al., 2004). Absorbance value at maximum indicates the density of the SIF layer. For our experiments we used slides with 420 nm absorbance maximum, and we kept the density of the SIF layer optimal (Matveeva et al., 2004), corresponding to the absorbance of the coated glass slides of 0.6–0.8 OD.
Fig. 1.
Examples of absorbance spectra of SIF-modified glass slides.
2.3. Coating slides with anti-Myoglobin Ab/Myoglobin antigen
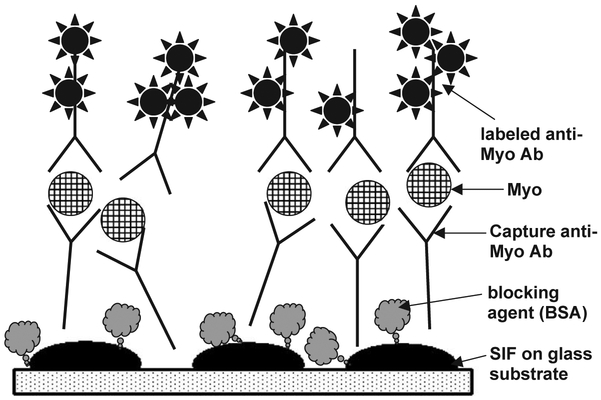
Myoglobin immunoassays were performed in a “sandwich” format (Fig. 2). Slides were non-covalently coated with capture anti-Myo antibody (Ab). First slides were dried in air and covered with the tape containing punched holes (regular size 6 mm diameter hole puncher) to form wells on the surface of the slides. Coating solution of anti-myoglobin immunoglobulin G (IgG) at 40 μg/mL (dissolved in Na-phosphate buffer, 50 mM, pH 7.4) was added to each well (25 μL per well), and slides were incubated for 2–4 h at room temperature in humid chamber. Slides then were rinsed with water, washing solution (0.05% Tween-20 in water), and water. Blocking was performed by adding blocking solution (1% BSA, 1% sucrose, 0.05% NaN3, 0.05% Tween-20 in 50 mM Tris–HCl buffer, pH 7.4; 35 μL per well) and incubation at room temperature for 2–4 h (or overnight at +4 °C) in humid chamber. Slides were rinsed with water, washing solution (0.05% Tween-20 in water) and water. Myoglobin antigen was added at various concentrations (0–1000 ng/mL, dissolved in blocking buffer, 25 μL per well) and slides were incubated at room temperature for 1 h and washed as described above, then used for end-point or kinetic measurements.
Fig. 2.
Scheme of the myoglobin immunoassay on the SIF-modified slide surface (sandwich format).
2.4. Detection measurements
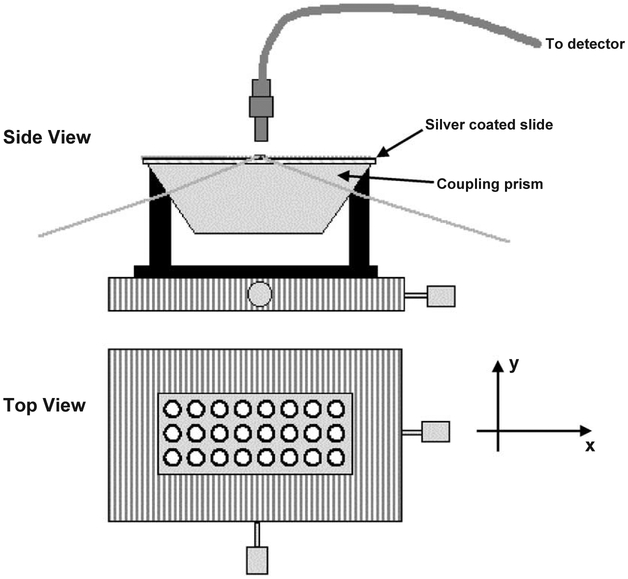
Emission spectra in solution were measured using a Varian Cary Eclipse fluorometer (“Varian Analytical Instruments”, USA). Absorption spectra in solution and on the surface of the slides were measured using a Hewlett Packard model 8543 spectrophotometer. Fluorescence measurements of the samples on glass slides were performed by placing the slides horizontally on the total internal reflection (TIR) stage as shown on the Fig. 3. For excitation we used the second harmonic (532 nm) of the diode pumped Nd:YVO4 laser (for Rhodamine Red-X or Alexa Fluor-647 labels) or 651 nm laser diode (for Alexa Fluor-647 label). Both lasers were compact laser pointer designs. Emission spectra were collected by fiber optics from the top using Fiber Optics Spectrometer (SD2000) from Ocean Optics, Inc. For observation we used cut-off plastic filters to attenuate excitation lines (filter with 50% transmittance at 550 nm for excitation of 532 nm, and filter with 50% transmittance at 665 nm for excitation of 651 nm).
Fig. 3.
TIR immunoassay measurement platform.
3. Results and discussion
A myoglobin immunoassay (Fig. 2) was chosen as an example of cardiac markers, and tested on SIF-modified glass slides using optimal SIF thickness (Matveeva et al., 2004) and two different fluorescent labels, Rhodamine Red-X or Alexa Fluor-647.
3.1. Enhancement of the signal from the SIF-coated glass versus non-coated glass
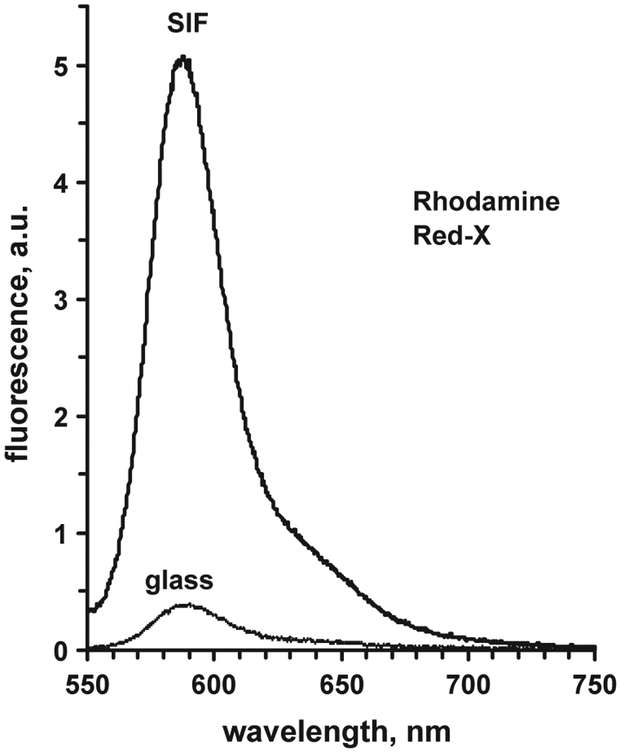
A typical example of the fluorescence spectra of labeled anti-Myo antibodies bound to the surface-immobilized (SIF-modified or non-modified glass substrate) antigen (Myo bound to the capture anti-Myo Ab’s) is presented in Fig. 4. The enhancement ratio is calculated as a ratio of the average signal measured from SIF-modified glass surface to the average signal measured from the non-modified glass surface. This enhancement may depend on numerous factors, such as density of the SIF, type of the fluorophore, wavelengths of the excitation and emission (Matveeva et al., 2004), and in our case of a sandwich myoglobin assay, it may also depend on the Myo concentration. We kept the density of the SIF layer optimal (Matveeva et al., 2004), corresponding to the absorbance of the coated glass slides of 0.6–0.8 OD. Typically, we observed an enhancement (for Myo 100 ng/mL) in the range of 10–15 for both labels, which is about same if compared to the enhancement for these same labels observed for a model immunoassay, labeled anti-rabbit antibodies binding to the antigen (rabbit IgG immobilized on the surface), that was 5–8 for Rhodamine Red-X, and 5–16 for Alexa Fluor-647 (Matveeva et al., 2004). The effect of the Myo concentration on the fluorescence enhancement value is rather small (if any), much below the signal variation due to non-homogeneity of the SIFs.
Fig. 4.
Example of the fluorescence spectra of the Rhodamin Red-X labeled anti-Myo Ab bound to the surface-immobilized Myo at [Myo] = 100 ng/mL measured from SIF-modified surface and non-modified glass surface.
In principle, the enhancement could be observed simply due to the better binding of the labeled Ab to the SIF-modified surface versus non-modified surface. Taking into account we have about 10–15 times enhancement, so about 10–15 fold more labeled Ab molecules should bind to the SIF-modified well surface if compared to non-modified glass well surface. We roughly estimated the binding by measuring the fluorescence of the labeled Ab supernatant solution before incubation in the reaction wells, and after incubation in SIF-modified and non-modified wells, assuming the binding efficiency was proportional to the loss in the supernatant intensity. We found the binding was more efficient on SIF-modified wells versus non-modified glass wells, but only for about 20–50% and not 10–15 times, so better binding alone can not explain the enhancement effect.
We tested several labeled reporter anti-Myo Abs with various degree of labeling (dye/IgG ratio) to find out if the overlabeling could in our case give better enhancement. We used Rhodamine Red-X with reactive succinimidyl ester moieties to synthesize four conjugates of this dye with antibodies with different labeling degree. Normally, a 2–50 molar excess of a dye-succinimidyl ester to a protein is used for labeling; we have used similar range for three conjugates to get a low-labeled, medium-labeled, and high-labeled conjugates, and we also synthesized one over-labeled conjugate using a 200-fold dye/protein excess for conjugation (see Table 2). We were expecting that high-labeled and over-labeled Ab’s may give better enhancement because of our earlier finding that most of the fluorophore self-quenching can be partially eliminated by proximity of the labeled protein to metallic silver particles (Lakowicz et al., 2003). However, for the Myo immunoassay high labeling and especially over-labeling of the reporter anti-Myo Ab’s did not result in higher enhancement (Table 2), probably due to the variations in the affinity of the labeled Ab at high labeling degree. Best enhancement of about 8–9 was obtained for medium-labeled reporter anti-Myo antibodies.
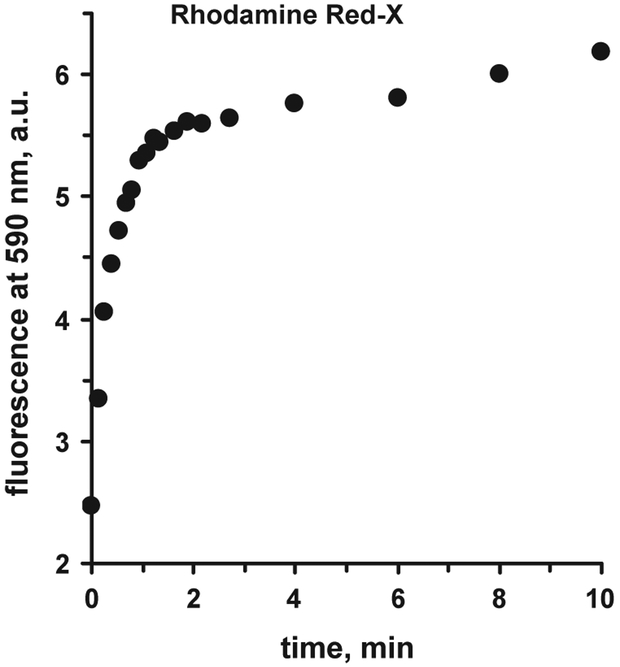
3.2. Kinetics of binding
The important characteristic of the immunoassay is kinetics of binding. Fig. 5 shows the binding kinetics for Rhodamine Red-X labeled anti-myoglobin antibody to the myoglobin bound to the capture antibody on the silver island film surface. The kinetics was monitored starting immediately after addition of the labeled Ab solution to the well. For the first 0.5 min the signal increase is very fast; within about 3 min the signal approaches the plateau, and later, next 10–15 min, only a slight increase can be monitored.
Fig. 5.
Kinetics of binding of Rhodamine Red-X labeled anti-Myo Ab to Myo on SIF-modified glass surface ([Myo] = 200 ng/mL, excitation 532 nm).
3.3. Myoglobin immunoassay on SIF: end-point and kinetic approach
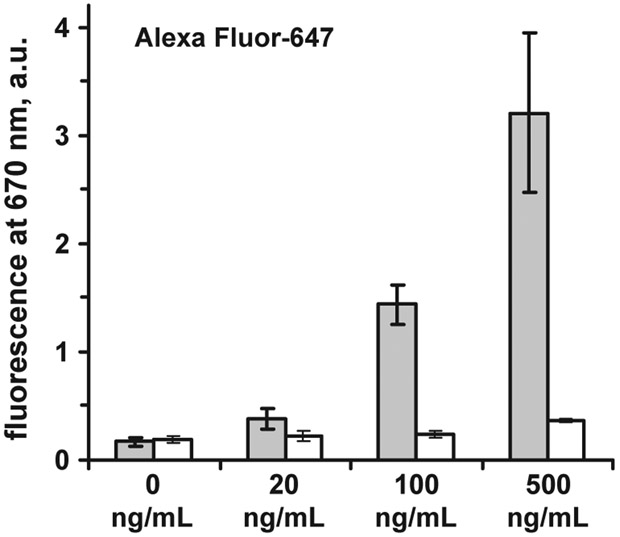
We tested the effect of Myoglobin concentration on the measured signal in the end-point assay experiment. Fig. 6 shows detected signal for SIF-modified substrate and non-modified glass. Although the assay was not optimized according to the antibody pairs and incubation times neither for SIF-modified surface nor for glass surface, we can see that the sensitivity is improved on SIF surface as compared to glass. The signal for a myoglobin concentration of 100 ng/mL (which is equal or lower then the clinical cut-off value for myoglobin, see Table 1) on SIF-modified substrate is already higher than the background signal at 0 ng/mL (Fig. 6). For glass substrate, a concentration of 500 ng/mL is still comparable to the background.
Fig. 6.
Fluorescence of Alexa Fluor-647 labeled anti-Myo Ab at different myoglobin concentrations on SIF-modified glass substrate (grey); white–non-modified glass (excitation 532 nm). Error bars represent ± 1 SD.
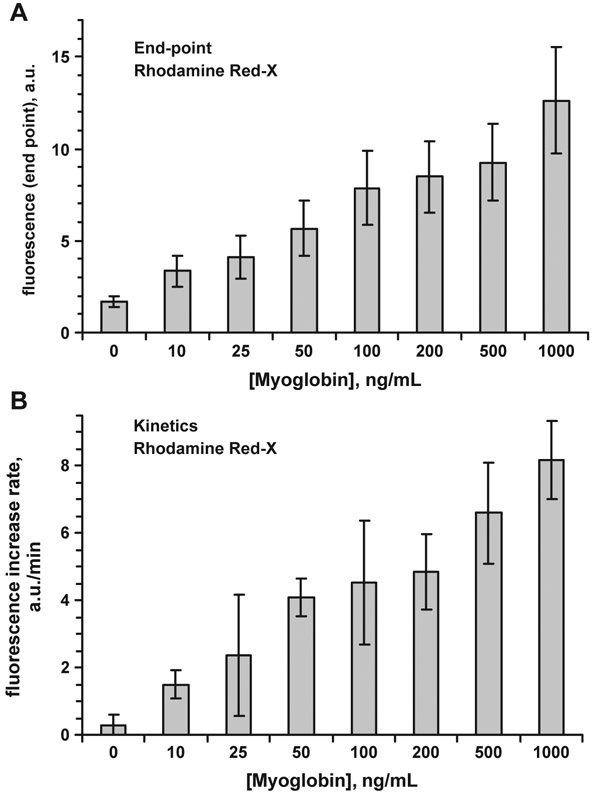
Kinetic measurements can result in improved myoglobin immunoassays on SIF. Fluorescence signals for different myoglobin concentrations measured in the end-point and kinetic modes are shown in Fig. 7. The kinetic data were calculated as the initial reaction rate (linear approach) within the first 0.5 min. The kinetic set of experimental data shows much lower contribution of background signal. It is probably because the non-specific binding occurs with much slower rate, and fluorescent background grows much slower. At the kinetic mode the sufficient signal level is obtained with the concentrations much below 100 ng/mL.
Fig. 7.
Fluorescence of Rhodamine Red-X labeled anti-Myoglobin Ab at different Myoglobin concentrations on SIF-modified glass (excitation 532 nm); A—end-point measurements; B—kinetic measurements (measured within first 0.5 min of the binding). Error bars represent ±1 SD.
3.4. Labels and excitation wavelengths comparison
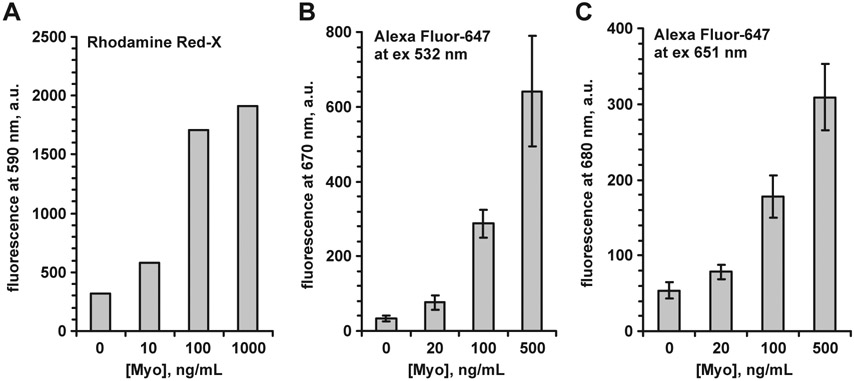
We tested two labels, Rhodamine Red-X and Alexa Fluor-647, having different excitation and emission maxima, for the Myo immunoassay on SIF-modified surface. We excited Rhodamine Red-X-labeled Ab at 532 nm, and for Alexa Fluor-647 we have been able to use both 532 and 651 excitation wavelengths, since the fluorescence signal was high enough to be detected even when exciting far from optimum wavelength. Examples of Myo immunoassays for different labels and excitation wavelengths are presented in Fig. 8. The effect of the label color and extinction wavelength on the enhancement value was small, below of the variability due to the SIF layer non-homogeneity.
Fig. 8.
Myoglobin immunoassay on SIF-modified surface: A—Rhodamine Red-X label at excitation 532 nm; B—Alexa Fluor-647 label at excitation 532 nm; C—Alexa Fluor-647 label at excitation 651 nm. Error bars represent ±1 SD.
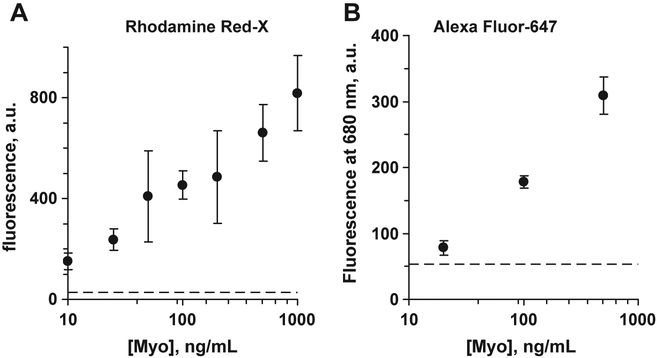
Fig. 9 presents quantization of the immunoassay data. Calibration curve was mostly consisting of two parts (at linear Myo scale) with linear response part at low Myo concentrations and saturation of the response signal at high Myo concentrations. Again, high deviations do not allow good quantization at this point, but calibration curve becomes linear within the whole range of Myo concentrations at logarithmic Myo concentration scale.
Fig. 9.
Fluorescence of Rhodamin Red-X labeled anti-Myoglobin Ab (A, excitation 532 nm), and Alexa Fluor-647 labeled anti-Myo Ab (B,excitation 651 nm), at various Myoglobin concentrations (logarithmic myoglobin concentration scale) on SIF-modified glass; end-point measurements. Error bars represent ±1 SD.
In summary, the main disadvantage of the method is the non-homogeneous nature of SIFs: deviations are significant, and this method in its current format would be difficult to use as analytical method for precise detection of Myoglobin. To improve the precision, we suggest changing the method of SIF deposition in order to obtain more homogeneous structures. For example, vapor deposition, or some kind of nanolithography could be used for homogeneous SIF coatings, and this is a topic for future investigations. Our current data, however, demonstrate applicability of this method to clinically relevant Myoglobin concentration range, and show the need and usefulness of development of other (time-consuming and costly) ways for homogeneous SIF-modification of the surfaces.
4. Conclusions
In this report we present our results that show high potential of metal enhanced fluorescence for developing a universal platform for cardiac markers detection. The initial results for the sandwich myoglobin immunoassay (on SIF-modified surface using wet chemistry) show that it is possible to detect myoglobin concentrations below 50 ng/mL, which is lower than clinical cut-off for Myoglobin in healthy patients. To improve the analytical parameters of the assay (such as precision), we suggest changing the method of SIF deposition in order to obtain more homogeneous SIF structures.
Acknowledgements
This work was supported by the National Institute of Biomedical Imaging and Bioengineering, EB-1690, the National Center for Research Resource, RR-08119, and Philip Morris USA, Inc. The authors are grateful to Dr. Garth Styba and the company “Spectral Diagostics, Inc.” (Canada) for kind gift of the recombinant Myoglobin and anti-Myoglobin antibodies.
Abbreviations:
- Ab
antibodies
- AMI
acute myocardial infarction
- a.u.
arbitrary units
- IgG
immunoglobulin G
- Myo
myoglobin
- SIF
silver island film
- TIR
total internal reflection
- SD
standard deviation
References
- Apple FS, Christenson RH, Valdes R Jr., Andriak AJ, Berg A, Duh SH, Feng YJ, Jortani SA, Johnson NA, Koplen B, Mascotti K, Wu AH, 1999. Simultaneous rapid measurement of whole blood myoglobin, creatine kinase MB, and cardiac troponin I by the triage cardiac panel for detection of myocardial infarction. Clin. Chem 45 (2), 199. [PubMed] [Google Scholar]
- Apple FS, Anderson FP, Collinson P, Jesse RL, Kontos MC, Levitt MA, Miller EA, Murakami MM, 2000. Clinical evaluation of the first medical whole blood, point-of-care testing device for detection of myocardial infarction. Clin. Chem 46 (10), 1604. [PubMed] [Google Scholar]
- Castaldo AM, Ercolini P, Forino F, Basevi A, Vrenna L, Castaldo P, D’Ambrosio V, Castaldo A, 1994. Plasma myoglobin in the early diagnosis of acute myocardial infarction. Eur. J. Clin. Chem. Clin. Biochem 32 (5), 349. [DOI] [PubMed] [Google Scholar]
- Di Serio F, Antonelli G, Trerotoli P, Tampoia M, Matarrese A, Pansini N, 2003. Appropriateness of point-of-care testing (POCT) in an emergency department. Clin. Chim. Acta 333 (2), 185. [DOI] [PubMed] [Google Scholar]
- Egan JP, 1975. Signal Detection Theory and ROC Analysis. Academic Press, New York, pp. 277. [Google Scholar]
- Ellenius J, Groth T, Lindahl B, Wallentin L, 1997. Early assessment of patients with suspected acute myocardial infarction by biochemical monitoring and neural network analysis. Clin. Chem 43 (10), 1919. [PubMed] [Google Scholar]
- Evanoff DD Jr., Chumanov G, 2004. Size-controlled synthesis of nanoparticles: 1. “Silver-Only” aqueous suspensions via hydrogen reduction. J. Phys. Chem., B 108 (37), 13948. [DOI] [PubMed] [Google Scholar]
- Fesmire FM, 2001. Improved identification of acute coronary syndromes with delta cardiac serum marker measurements during the emergency department evaluation of chest pain patients. Cardiovasc. Toxicol 1 (2), 117. [DOI] [PubMed] [Google Scholar]
- Galen RS, 1977. The normal range: a concept in transition. Arch. Pathol. Lab. Med 101 (11), 561. [PubMed] [Google Scholar]
- Gibler WB, Blomkalns AL, Collins SP, 2003. Evaluation of chest pain and heart failure in the emergency department: impact of multimarker strategies and B-type natriuretic peptide. Rev. Cardiovasc. Med 4 (Suppl. 4), S47. [PubMed] [Google Scholar]
- Hao E, Schatz GC, Hupp JT, 2004. Synthesis and optical properties of anisotropic metal nanoparticles. J. Fluoresc 14 (4), 331. [DOI] [PubMed] [Google Scholar]
- Henderson AR, 1997. An overview and ranking of biochemical markers of cardiac disease. Strengths and limitations. Clin. Lab. Med 17 (4), 625. [PubMed] [Google Scholar]
- Hillis GS, Zhao N, Taggart P, Dalsey WC, Mangione A, 1999. Utility of cardiac troponin I, creatine kinase-MBmass, myosin light chain 1, and myoglobin in the early in-hospital triage of “high risk” patients with chest pain. Heart 82(5), 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz JR, 2001. Radiative decay engineering: biophysical and biomedical applications. Anal. Biochem 298 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz JR, Shen Y, D’Auria S, Malicka J, Gryczynski Z, Gryczynski I, 2002. Radiative decay engineering: 2. Effects of silver island films on fluorescence intensity lifetimes and resonance energy transfer. Anal. Biochem 301 (2), 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz JR, Malicka J, D’Auria S, Gryczynski I, 2003. Release of the self-quenching of fluorescence near silver metallic surfaces. Anal. Biochem 320 (1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laperche T, Steg PG, Dehoux M, Benessiano J, Grollier G, Aliot E, Mossard J-M, Aubry P, Coisne D, Hanssen M, Iliou M.-Ch., 1995. A study of biochemical markers of reperfusion early after thrombolysis for acute myocardial infarction. Circulation 92, 2079. [DOI] [PubMed] [Google Scholar]
- Lim J, Hawkins RC, Ng K, Chan SP, Cheng A, Ng KS, 2002. A preliminary study of the utility of combined cardiac markers in the evaluation of patients presenting early with suspected acute coronary syndrome. Ann. Acad. Med. Singap 31 (6), 772. [PubMed] [Google Scholar]
- Lochner N, Lobmaier C, Wirth M, Leitner A, Pittner F, Gabor F, 2003. Silver nanoparticle enhanced immunoassays: one step real time kinetic assay for insulin in serum. Eur. J. Pharm. Biopharm 56 (3), 469. [DOI] [PubMed] [Google Scholar]
- Malasky BR, Alpert JS, 2002. Diagnosis of myocardial injury by biochemical markers: problems and promises. Cardiol. Rev 10, 306. [DOI] [PubMed] [Google Scholar]
- Mathew T, Menown I, Smith B, Smye M, Nesbitt S, Young I, Adgey AAJ, 1999. Diagnosis and risk stratification of patients with anginal pain and non-diagnostic electrocardiograms. Q. J. Med 92 (10), 565. [DOI] [PubMed] [Google Scholar]
- Matveeva E, Gryczynski Z, Malicka J, Gryczynski I, Lakowicz JR, 2004. Metal-enhanced fluorescence immunoassays using total internal reflection and silver island-coated surfaces. Anal. Biochem 334 (2), 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J, Nowak RM, McCullough PA, Foreback C, Borzak S, Tokarski G, Tomlanovich MC, Jacobsen G, Weaver DW, 2001. Ninety-minute exclusion of acute myocardial infarction by use of quantitative point-of-care testing of myoglobin and troponin I. Circulation 104 (13), 483. [DOI] [PubMed] [Google Scholar]
- McCord J, Nowak RM, Hudson MP, McCullough PA, Tomlanovich MC, Jacobsen G, Tokarski G, Khoury N, Weaver WD, 2003. The prognostic significance of serial myoglobin, troponin I, and creatine kinase-MB measurements in patients evaluated in the emergency department for acute coronary syndrome. Ann. Emerg. Med 42 (3), 343. [DOI] [PubMed] [Google Scholar]
- Montague C, Kircher T, 1995. Myoglobin in the early evaluation of acute chest pain. Am. J. Clin. Pathol 104 (4), 472. [DOI] [PubMed] [Google Scholar]
- Newby LK, Storrow AB, Gibler WB, Garvey JL, Tucker JF, Kaplan AL, Schreiber DH, Tuttle RH, McNulty SE, Ohman EM, 2001. Bedside multimarker testing for risk stratification in chest pain units: the chest pain evaluation by creatine kinase-MB, myoglobin, and troponin I (CHECKMATE) study. Circulation 103 (14), 1832. [DOI] [PubMed] [Google Scholar]
- Ng SM, Krishnaswamy P, Morissey R, Clopton P, Fitzgerald R, Maisel AS, 2001. Ninety-minute accelerated critical pathway for chest pain evaluation. Am. J. Cardiol 88 (6), 611. [DOI] [PubMed] [Google Scholar]
- Panteghini M, 2002. The measurement of cardiac markers: where should we focus? Am. J. Clin. Pathol. 118 (3), 354. [DOI] [PubMed] [Google Scholar]
- Storrow AB, Gibler WB, 1999. The role of cardiac markers in the emergency department. Clin. Chim. Acta 284 (2), 187. [DOI] [PubMed] [Google Scholar]
- de Winter RJ, Koster RW, Schotveld JH, Sturk A, Straalen JP, Sanders GT, 1996. Prognostic value of troponin T, myoglobin, and CK-MB mass in patients presenting with chest pain without acute myocardial infarction. Heart 75 (3), 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Lacbawan FL, Sunheimer R, LeFever D, McCabe JB, 1995. Is myoglobin useful in the diagnosis of acute myocardial infarction in the emergency department setting? Am. J. Clin. Pathol 103 (6), 725. [DOI] [PubMed] [Google Scholar]
- Wu AH, 1999. Biochemical markers of cardiac damage: from traditional enzymes to cardiac-specific proteins. IFCC subcommittee on standardization of cardiac markers (S-SCM). Scand. J. Clin. Lab. Invest, Suppl. 230, 74. [PubMed] [Google Scholar]
- Zaninotto M, Altinier S, Lachin M, Celegon L, Plebani M, 1999. Strategies for the early diagnosis of acute myocardial infarction using biochemical markers. Am. J. Clin. Pathol 111 (3), 399. [DOI] [PubMed] [Google Scholar]