Abstract
We present a new approach for performing Xuorescence immunoassay in whole blood using Xuorescently labeled anti-rabbit immunoglobulin G (IgG) on a silver surface. This approach, which is based on surface plasmon-coupled emission (SPCE), provides increased sensitivity and substantial background reduction due to exclusive selection of the signal from the fluorophores located near a bioaffinity surface. This article describes the effect of an optically dense sample matrix, namely human whole blood and serum, on the intensity of the SPCE. An antigen (rabbit IgG) was adsorbed to a slide covered with a thin silver metal layer, and the SPCE signal from the fluorophore-labeled anti-rabbit antibody, binding to the immobilized antigen, was detected. The effect of the sample matrix (buffer, human serum, or human whole blood) on the end-point immunoassay SPCE signal was studied. It was demonstrated that the kinetics of binding could be monitored directly in whole blood or serum. The results showed that human serum and human whole blood attenuate the SPCE end-point signal and the immunoassay kinetic signal only approximately two- and three fold, respectively, as compared with buffer, resulting in signals that are easily detectable even in whole blood. The high optical absorption of the hemoglobin can be tolerated because only fluorophores within a couple of hundred nanometers from the metallic film contribute to SPCE. Excited fluorophores outside the 200-nm layer do not contribute to SPCE, and their free space emission is not transmitted through the opaque metallic film into the glass substrate. We believe that SPCE has the potential of becoming a powerful approach for performing immunoassays based on surface-bound analytes or antibodies for many biomarkers directly in dense samples such as whole blood with no need for washing steps.
Keywords: Fluorescence immunoassay, Surface plasmon-coupled emission, Silver film, Whole blood, Background suppression
Fluorescence-based immunoassays are used extensively in medical diagnostics [1–5], particularly due to the fact that they are among the most sensitive assays known. Unfortunately, high background fluorescence often strongly interferes with the fluorescence signal of interest. Several approaches have been suggested to minimize the background signal caused by the sample matrix (e.g., serum or whole blood in clinical diagnostics), including fluorescence/polarization kinetics detection [6], time-gated detection based on long-lived lanthanide emission [7–9], and two-photon excitation [10,11]. Because of the high fluorescence background and optical density, immunoassays are rarely carried out in whole blood, and in cases when they are the procedure normally includes at least one washing step before the output signal is measured [9,12–15]. The development of methods for the direct analysis of whole blood samples remains in high demand, however, considering a number of relevant analytes that are bound to blood cells and proteins (e.g., cyclosporine) and for which the measured values will differ depending on whether plasma/serum or whole blood is used for the analysis.
In this article, we present a new approach for the detection of the fluorescence signal from whole blood. We performed a model immunoassay against rabbit immunoglobulin G (IgG),1 using fluorescently labeled anti-rabbit IgG on a silver surface, and measured binding directly in whole blood samples. This approach provides increased sensitivity and substantial background reduction due to exclusive collection of emitted light occurring only near the bioaffinity surface. This effect is based on the coupling between fluorophores and surface plasmons. The surface plasmons are collective oscillations of electrons that are confined to the interface between a thin metal film and the aqueous solution. The near field forms a wave that decreases rapidly with distance from the interface, a behavior that is common for the evanescent waves. In the case of a thin metal film on a substrate of a high refractive index, the plasmons in the metal can couple to the three-dimensional electromagnetic wave in the substrate medium.
A strong evanescent field induced by surface plasmons can excite a layer of fluorophores that extends up to approximately 200 nm above a thin metallic film into the liquid sample. We recently demonstrated that the reverse process is also possible; that is, excited fluorophores near the metallic layer may induce surface plasmons in the metallic film that radiate into the glass substrate [16,17]. This radiation occurs at a sharply defined angle and is nearly completely polarized. This phenomenon, which we call surface plasmon-coupled emission (SPCE), is closely related to surface plasmon resonance (SPR) [16,18–20]. The coupling between the field of the excited fluorophore and the thin metal film results in the creation of surface plasmons. For a thin metal layer and the high refractive index of the glass substrate, the surface plasmons can couple to photons in the glass substrate and a surface plasmon is converted to light.
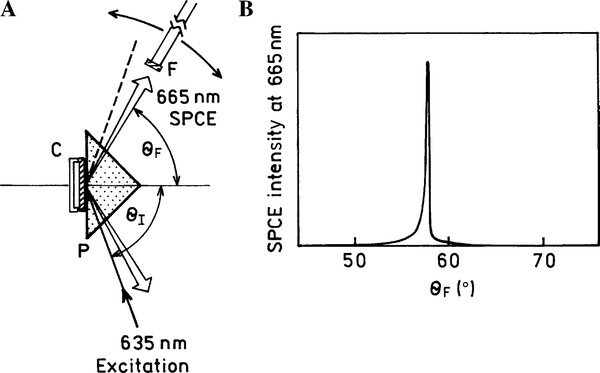
SPR occurs when light is impinging on a metal through a higher refractive index medium at a specific angle of incidence (θSP) that satisfies exactly the wave vector matching conditions, resulting in a sharp reflectivity decrease (Fig. 1). Similarly, excited plasmons may cause radiation into the glass substrate at the surface plasmon angle (θF) that satisfies the wave vector matching conditions for the emission wavelengths.
Fig. 1.
(A) Scheme of the SPCE measurement (left). The 635-nm beam excites the sample (AlexaFluor 647-labeled anti-rabbit antibodies bound to the rabbit IgG immobilized on the substrate surface) at the SPR angle θI (KR configuration). The SPCE of AlexaFluor 647 is emitted at the SPCE angle θF and is being detected with the fiber (F) equipped with a long wave pass filter and a 200-μm vertical air slit and connected to the SLM model 8000 spectrofluorometer. The fiber is positioned on the arm, which can be rotated around the stage. (B) Graph showing SPCE sharply concentrated around the θF angle (full width at half maximum [FWHM] ~0.5°).
There are two possible experimental configurations for SPCE excitation. First, the sample can be illuminated from the sample side, which is the so-called reverse Kretschmann (RK) configuration. In this case, the excitation cannot generate surface plasmons in the metal surface. The sample can also be illuminated through a prism at the plasmon resonance angle (θSP), which is called the Kretschmann (KR) configuration (Fig. 1A). If the incident angle θI = θSP, then the excited surface plasmons induce an evanescent Weld above the metal film surface. This evanescent field is strongly enhanced (up to 80-fold compared with the incident field) by the resonance interaction [21], and the evanescent field extends up to approximately 200 nm into the liquid sample. Hence, the KR illumination results in a strong selective excitation in close proximity to the metal surface. The enhanced excitation field is near the metal surface, effectively reducing the background from the sample volume matrix. Recently, we described a model immunoassay [22,23] and a myoglobin immunoassay [24] on a thin silver metal surface using SPCE. We tested two kinds of model background solution: a highly fluorescent one (nonbinding labeled antibodies) and a highly absorbing one (17% hemoglobin solution). We found that the use of SPCE provided very effective background suppression; the emission from labeled unbound antibodies was suppressed by more than 100-fold in the KR (vs. RK) configuration, and the highly absorbing hemoglobin solution reduced the detected SPCE fluorescence intensity by only approximately 50% [22].
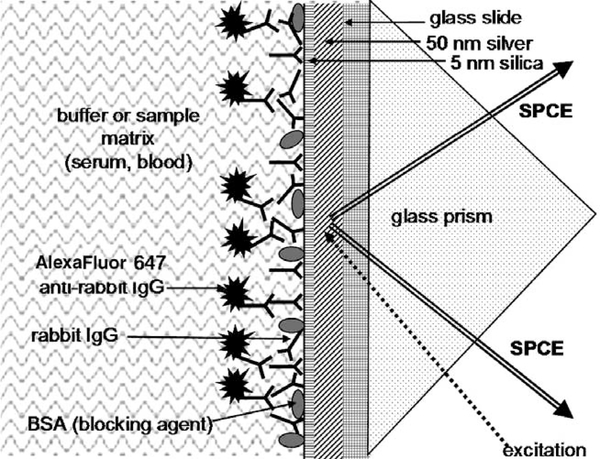
In this article, we report on the effect of human whole blood and human serum sample matrices on the SPCE signal in a model immunoassay on a silver-coated surface. Antigen (rabbit IgG) was adsorbed onto a slide covered with a thin silver metal layer, and an SPCE signal from fluorophore-labeled anti-rabbit antibody, binding to the immobilized antigen, was detected (Fig. 2).
Fig. 2.
SPCE model immunoassay scheme: binding of anti-rabbit anti-bodies (labeled with AlexaFluor 647) to the antigen, rabbit IgG, immobilized on the silver surface protected with a thin silica layer. The drawing is not to scale.
Materials and methods
Reagents and materials
Glass microscope slides (Corning, 3 × 1 in.) were coated by vapor deposition, first with continuous layers of 2 nm chromium, then 50 nm silver, and finally 5 nm silicon dioxide (EMF). Coated slides were manually cut to reduce the size to approximately 12 × 45 mm to better fit a demountable cuvette. Rabbit IgG, goat IgG, and human serum (from male AB plasma, sterile filtered) were obtained from Sigma, and AlexaFluor 647 anti-rabbit IgG conjugate (stock solution, 2mg/ml, dye/IgG=4.5 mol/mol) was obtained from Molecular Probes. Blood was drawn via venipuncture from one of the authors into tubes with sodium citrate as anticoagulant (tubes contained 3.2% solution of sodium citrate to provide a final mixture with whole blood of 1:4 [v/v]) and was kept at room temperature before use. Salts and buffer components (e.g., bovine serum albumin [BSA], Tween 20, sucrose) were obtained from Sigma–Aldrich. Absorbance spectra taken at different dilutions of the whole blood sample (used for background suppression check) showed that a 0.2-mm-thick layer of this blood sample had an optical density of approximately 3 at 670 nm, the emission maximum of the bound labeled antibodies.
Spectroscopic measurements
Absorption spectra were measured using a Hewlett–Packard model 8543 spectrophotometer and 0.2-mm pathway cuvettes. Fluorescence measurements on microscope slides were performed using index-matching fluid to attach the slides to a triangle prism made of SF-11 glass (refractive index 1.785) and positioned onto a precision rotation stage equipped with a fiber-optics mount on a 15-cm-long arm [17]. This configuration allowed fluorescence observation at varying angles relative to the incident angle. For collection of the angle-dependent emission intensity, a 200-μm air slit was placed onto the fiber input. The output of the fiber was connected to an SLM model 8000 spectrofluorometer. The fluorescence excitation was generated using a small solid-state red laser diode module (635 nm, maximum output 2 mW, powered by two 1.5-V AA batteries) similar to lasers used in commercial laser pointers.
Coating slides with antigen
Slides were noncovalently coated with rabbit IgG, with 2 ml of the IgG coating solution (diluted to 40 μg/ml in sodium phosphate buffer, 50 mM, pH 7.4) being added to the slide (0.5 ml/pre-cut slide) before the slide was incubated for 1 h at room temperature in a humid chamber. The slide was then rinsed with water, washing solution (0.05% Tween 20 in water), and water again. Blocking was performed by adding 0.6 ml of blocking solution (1% BSA, 1% sucrose, 0.05% NaN3, and 0.05% Tween 20 in 50 mM Tris-HCl buffer, pH 7.4), followed by incubation at room temperature for 1–2 h (or overnight at 4 °C) in the humid chamber. The slide was then rinsed with water, washing solution (0.05% Tween 20 in water), and water again; covered with blocking solution; and stored at 4 °C until use.
End-point SPCE experiment
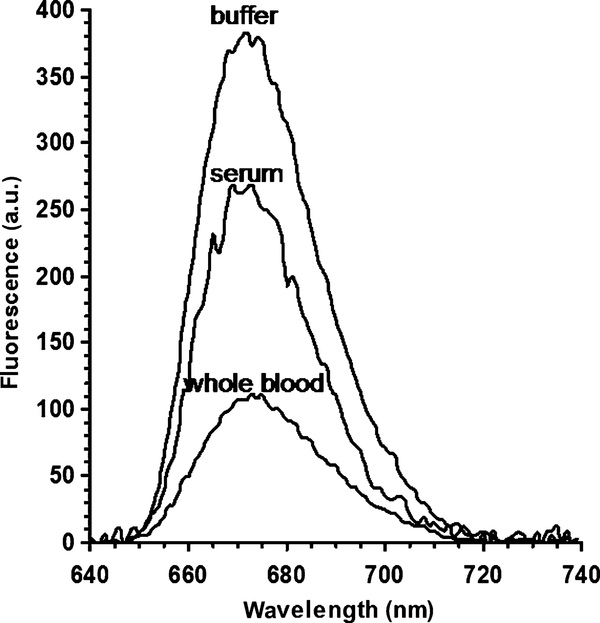
Dye-labeled AlexaFluor 647 anti-rabbit IgG (0.5 ml, diluted to [IgG] = 10 μg/ml with blocking solution) was added to the slide (coated with antigen as described above) and incubated at room temperature in a humid chamber for 1 h. Then the slide was rinsed with water, washing solution (0.05% Tween 20 in water), and water again. Then a 0.2-mm-thick demountable quartz cuvette was mounted onto the metallic side of the slide. Approximately 40 μl of the blocking solution or a sample background solution (serum or whole blood) was added to the cuvette using a syringe, and fluorescence measurements were performed in the KR optical configuration (Fig. 1A). The data shown in Fig. 3 represent averages of two fluorescence spectra (taken on different days) for the sample matrices (buffer, serum, and whole blood).
Fig. 3.
Fluorescence spectra (SPCE) of the AlexaFluor 647-labeled anti-rabbit antibodies bound to the rabbit IgG immobilized on a 50-nm silver mirror surface observed in buffer, human serum, and human whole blood (KR configuration). a.u., arbitrary units.
Kinetic binding experiment
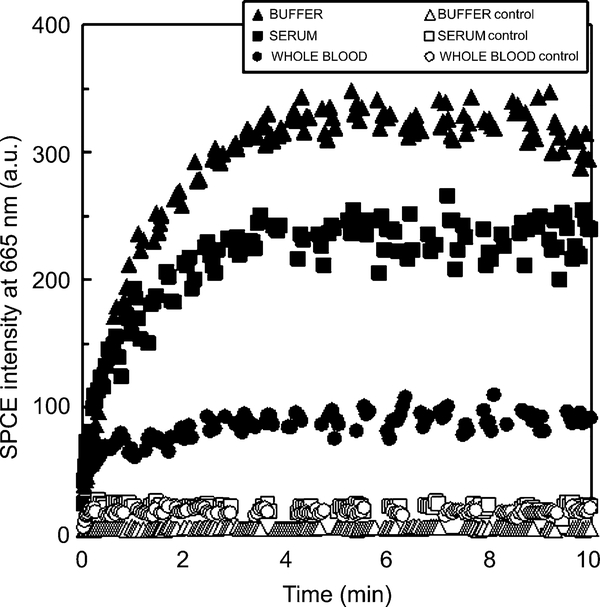
A 0.2-mm-thick demountable cuvette was mounted on the metallic side of the slide (coated with antigen as described above). Approximately 40 μl of the Alexa-Fluor 647 anti-rabbit IgG (diluted to [IgG]=10 μg/ml with blocking solution, serum, or whole blood) was added to the cuvette using a syringe. The kinetics was immediately monitored at room temperature (~20 °C). The data shown in Fig. 4 represent averages of two experiments (performed on different days) for the sample matrices (buffer, serum, and whole blood).
Fig. 4.
Kinetics of the binding of the AlexaFluor 647-labeled anti-rabbit antibodies to the rabbit IgG immobilized on a 50-nm silver mirror surface observed in buffer (blocking solution, ▲), human serum (■), and human whole blood (●) with the SPCE/KR configuration. Nonspecific kinetics (nonspecific binding of the AlexaFluor 647-labeled anti-rabbit antibodies to the immobilized goat IgG) is shown as corresponding open symbols unfilled triangles, squares, and circles. a.u., arbitrary units.
Limit of detection
Each limit of detection (LOD) value for blocking solution, serum, or whole blood was estimated as the concentration of labeled IgG (binding to the immobilized antigen) corresponding to the SPCE signal of the blank measurement (zero concentration of labeled antibodies) plus three times the standard deviation, assuming linearity between 0 and 70 nM (10 μg/ml) of labeled IgG. The linearity was checked for labeled IgG (0–10 μg/ml) in blocking solution (n=4) for the SPCE signal at 40 μl sample volume (see above) 30–40 min after sample injection. Labeled IgG signals (SPCE fluorescence intensity at 670 nm) were measured at 40 μl sample volume (see above) 30–40 min after sample injection and were averaged for two slides (3–5 measurements/slide). Blank signal measurements (SPCE fluorescence intensity at 670 nm) were averaged for two slides (10 measurements/slide) for each type of sample (blocking solution, serum, and whole blood).
Results and discussion
The scheme of our SPCE immunoassay is shown in Fig. 1A. The sample (protein-coated silver slide) with assembled cuvette was illuminated in the KR configuration. SPCE was observed on the prism side of the sample, at the plasmon resonance angle, and through a long-pass filter. The intensity observed through the prism was sharply concentrated near the θF angle of approximately 58° (Fig. 1B).
In the end-point experiments, AlexaFluor 647-labeled anti-rabbit IgGs were first bound to immobilized rabbit IgG near the silver surface (Fig. 2). Then the excess of nonbound antibodies was washed away, a sample matrix was added (serum, whole blood, or just blocking solution for comparison), and the fluorescence signal and spectrum were monitored. We found the emission to be strongly directional and focused near an angle of 58° (Fig. 1B, signal measured in blocking solution). The spectrum of the SPCE (in the KR configuration) was characteristic of the AlexaFluor 647 probe and was not corrupted by scattered light at the excitation wavelength for all tested sample matrices (Fig. 3). The whole blood sample used for this measurement had an optical density of approximately 3 at 665 nm (emission maximum of the tested dye AlexaFluor 647) at the optical path length of 0.2 mm (as used for the SPCE experiment). This optical density in a 0.2-mm-thick blood sample would attenuate the fluorescence signal approximately 103-fold under free-space conditions. As shown in Fig. 3, using SPCE generation and detection, the signal was attenuated less than 2-fold in a human serum sample, and approximately 3-fold in a whole blood sample, as compared with the transparent buffer medium (blocking solution).
The sample matrix (serum or whole blood) can affect not only the final immunoassay signal detection but also the binding process itself. We tested the effect of human serum and human whole blood directly in the sample matrix by measuring the SPCE signal of the AlexaFluor 647-labeled antibodies (Fig. 4). The SPCE intensity change with time is clearly detectable for serum and even for whole blood due to localization of the probe volume in the evanescent field range near the silver metal surface. The increase of the signal can be correlated with specific binding, as shown in Fig. 4. Nonspecific binding of the labeled anti-rabbit IgG to the wrong antigen (goat IgG) results in an SPCE signal that does not change with time.
To this point, we had not tested in detail common analytical assay characteristics such as precision, accuracy, specificity, limits of quantization, and linearity. Our purpose was to demonstrate the applicability of the SPCE technology to an immunoassay performed in an extremely dense optical sample consisting of whole blood. Our model SPCE immunoassay conditions (e.g., temperature, incubation time) and other characteristics, such as slide substrate properties (metal layer coverage and protective silica layer), have not been optimized and are the topic of further investigations. SPCE is a relatively new approach, and we had not yet built a dedicated instrument for SPCE signal detection in our lab. We currently use a very simple, manually assembled assay chamber. Our optical stage for SPCE detection requires upright orientation of the slide, and this is not very convenient for liquid samples. Despite the fact that SPCE detection itself requires only a very short recording time, assembling the cuvette, inserting the sample, and manually adjusting the SPCE angle are rather time-consuming. Our current experimental assembly is not very effective and should be converted to adapt smaller size slides and a matching size cuvette. Furthermore, microfluidic devices with replaceable bottoms might allow for sample volume minimization and efficient high-throughput studies.
To characterize the effect of serum and whole blood on the SPCE signal, we have estimated the LOD. LOD values have been determined as the concentration of labeled IgG (binding to the immobilized antigen) corresponding to the SPCE signal of the blank measurement (zero concentration of labeled antibodies) plus three times the standard deviation, assuming linearity between 0 and 70nM (10 μg/ml) of labeled IgG. We consider these data to be only an estimation of LOD because in this case the binding molecule is labeled IgG, which is a model (labeled) protein rather than a real antigen of interest. However, data from Table 1 show quantitatively the difference in SPCE detection directly in whole blood or serum versus a clear buffer solution. We may expect a 3- to 5-fold increase in the LOD in serum and a 5- to 10-fold increase in the LOD in whole blood.
Table 1.
LODs of SPCE signals of labeled antibody (model immunoassay) in various sample media
| Sample medium | LODa (nM) | LODb (pmol/sample) |
|---|---|---|
| Buffer | 1.1 | 0.045 |
| Serum 1 | 4.1 | 0.16 |
| Serum 2 | 3.6 | 0.14 |
| Serum 3 | 4.9 | 0.20 |
| Blood 1 | 5.1 | 0.21 |
| Blood 2 | 10.0 | 0.41 |
LOD values in this column refer to the concentration of labeled antibody (labeled anti-rabbit IgG) binding to the immobilized antigen (rabbit IgG).
LOD values in this column refer to the amount of labeled antibody (labeled anti-rabbit IgG) binding to the immobilized antigen (rabbit IgG) per sample (sample volume 40 μl).
The accuracy part of the SPCE immunoassay that is related solely to the signal detection was estimated previously for the buffer solution [24] and was found to be greater than 2% for low analyte concentration and greater than 10% for high analyte concentration. We would expect that the accuracy in measuring the SPCE signal in whole blood will be lower than that in buffer solution, and improving the SPCE immunoassay parameters might require some modifications in the assay protocol, including the addition of some additives (e.g., surfactants) into the whole blood sample to reduce nonspecific binding.
SPCE-based detection has many advantages over existing surface assay formats. Standard enzyme-linked immunosorbent assay (ELISA) protocols allow high sensitivity to be achieved, but they require multiple incubation and washing steps. LOD values for IgG detection using ELISA vary from as low as 0.02ng/ml [25] (IgG in buffer with fluorescence detection) to as high as 1000ng/ml [26] (IgG in diluted milk with colorimetric detection). When IgG is detected by ELISA in serum, serum is normally diluted (despite the washing steps), and even though a low LOD value (e.g., 5ng/ml IgG [27], 60ng/ml IgG [28,29]) can be achieved in such diluted samples, ELISA yields a higher whole serum LOD value (e.g., 3000ng/ml IgG [28,29]). Our estimated LOD value for IgG in buffer is approximately 160ng/ml (1.1 nM) (Table 1), which is higher than that using ELISA. However, for serum samples our estimated LOD is approximately 600ng/ml (4nM), which is lower than that using ELISA, and for whole blood samples our estimated LOD is 700–1500ng/ml (ELISA data for whole blood samples were not available).
The closest analogue to the SPCE approach is SPR (Biacore). SPR-based sensors are very sensitive and well developed and are able to detect nanomolar (LOD of 400 nM IgG in 100% serum [30]) to subnanomolar concentrations of IgG in serum [31]. However, because SPR is label free, it detects any binding occurring on the surface; hence, selectivity is an issue, especially in samples such as whole blood with many components that may cause nonspecific binding. SPR protocol includes dilution of serum samples (100 times in [31]), and there currently are no data on SPR immunoassays in whole blood samples [32].
The SPCE approach allows immunoassays to be performed directly in the optically dense samples, such as whole blood, without washing steps. We expect that SPCE will be widely used for the detection of clinically important biomolecules binding to surfaces in whole blood samples.
Conclusions
SPCE-based fluorescence generation and detection was used to perform a model immunoassay against rabbit IgG using fluorescently labeled (with AlexaFluor 647) anti-rabbit IgG on a silver-coated surface in an optically dense sample matrix, namely human whole blood and human serum. The SPCE-specific signal was easily detected in this sample matrix, and the kinetics of binding was monitored directly in whole blood or serum. Hence, the SPCE approach appears to allow immunoassays to be performed directly in extremely dense optical samples. SPCE has a high potential of becoming widely used for the detection of clinically important biomarkers in whole blood samples.
Acknowledgments
This work was supported by the National Center for Research Resources (RR-08119), the National Institute of Biomedical Imaging and Bioengineering (EB-1690), Philip Morris USA and Philip Morris International, and the Biomolecular Interaction Technology Center (University of New Hampshire).
Footnotes
Abbreviations used: IgG, immunoglobulin G; SPCE, surface plasmon-coupled emission; SPR, surface plasmon resonance; RK, reverse Kretschmann; KR, Kretschmann; BSA, bovine serum albumin; LOD, limit of detection; ELISA, enzyme-linked immunosorbent assay; FWHM, full width at half maximum.
References
- [1].Tetin SY, Stroupe SD, Antibodies in diagnostic applications, Curr. Pharm. Biotechnol 5 (2004) 9–16. [DOI] [PubMed] [Google Scholar]
- [2].Luppa PB, Sokoll LJ, Chan DW, Immunosensors: principles and applications to clinical chemistry, Clin. Chim. Acta 314 (2001) 1–26. [DOI] [PubMed] [Google Scholar]
- [3].Borrebaeck CA, Antibodies in diagnostics: From immunoassays to protein chips, Immunol. Today 21 (2000) 379–382. [DOI] [PubMed] [Google Scholar]
- [4].Vo-Dinh T, Sepaniak MJ, Griffin GD, Alarie JP, Immunosensors: principles and applications, Immunomethods 3 (1993) 85–92. [Google Scholar]
- [5].Hemmila IA, Applications of Fluorescence in Immunoassays, John Wiley, New York, 1992. [Google Scholar]
- [6].Gomez-Hens A, Aguilar-Caballos MP, Stopped-flow fluorescence polarization immunoassay, Comb. Chem. High Throughput Screen 6 (2003) 177–182. [DOI] [PubMed] [Google Scholar]
- [7].Lövgren T, Pettersson K, Time-resolved fluoroimmunoassay: advantages and limitations, in: Van Dyke K, Van Dyke R (Eds.), Luminescence Immunoassay and Molecular Applications, CRC Press, Boca Raton, FL, 1990, pp. 233–253. [Google Scholar]
- [8].Mathis G, Rare earth cryptates and homogeneous fluoroimmunoassays with human sera, Clin. Chem 39 (1993) 1953–1959. [PubMed] [Google Scholar]
- [9].von Lode P, Rosenberg J, Pettersson K, Takalo H, A europium chelate for quantitative point-of-care immunoassays using direct surface measurement, Anal. Chem 75 (2003) 3193–3201. [DOI] [PubMed] [Google Scholar]
- [10].Baker GA, Pandey S, Bright FV, Extending the reach of immunoassays to optically dense specimens by using two-photon excited Xuorescence polarization, Anal. Chem 72 (2000) 5748–5752. [DOI] [PubMed] [Google Scholar]
- [11].Soini E, Meltola NJ, Soini AE, Soukka J, Soini JT, Hanninen PE, Two-photon fluorescence excitation in detection of biomolecules, Biochem. Soc. Trans 28 (2000) 70–74. [DOI] [PubMed] [Google Scholar]
- [12].Choi S, Choi EY, Kim DJ, Kim JH, Kim TS, Oh SW, A rapid, simple measurement of human albumin in whole blood using a Xuorescence immunoassay, Clin. Chim. Acta 339 (2004) 147–156. [DOI] [PubMed] [Google Scholar]
- [13].von Lode P, Rainaho J, Pettersson K, Quantitative, wide-range, 5-minute point-of-care immunoassay for total human chorionic gonadotropin in whole blood, Clin. Chem 50 (2004) 1026–1035. [DOI] [PubMed] [Google Scholar]
- [14].Ahn JS, Choi S, Jang SH, Chang HJ, Kim JH, Nahm KB, Oh SW, Choi EY, Development of a point-of-care assay system for high-sensitivity C-reactive protein in whole blood, Clin. Chim. Acta 332 (2003) 51–59. [DOI] [PubMed] [Google Scholar]
- [15].Tarkkinen P, Palenius T, Lovgren T, Ultrarapid, ultrasensitive one-step kinetic immunoassay for C-reactive protein (CRP) in whole blood samples: measurement of the entire CRP concentration range with a single sample dilution, Clin. Chem 48 (2002) 269–277. [PubMed] [Google Scholar]
- [16].Lakowicz JR, Radiative decay engineering: III. Surface plasmoncoupled directional emission, Anal. Biochem 324 (2004) 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gryczynski I, Malicka J, Gryczynski Z, Lakowicz JR, Radiative decay engineering: IV. Experimental studies of surface plasmoncoupled directional emission, Anal. Biochem 324 (2004) 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Raether H, Surface plasma oscillations and their applications, in: Hass G, Francombe MH, Hoffman RW (Eds.), Physics of Thin Films: Advances in Research and Development, vol. 9, Academic Press, New York, 1977, pp. 145–261. [Google Scholar]
- [19].Pockrand I, Surface plasma oscillations at silver surfaces with thin transparent and absorbing coatings, Surf. Sci 72 (1978) 577–588. [Google Scholar]
- [20].Lakowicz JR, Radiative decay engineering: V. Metal-enhanced Xuorescence and plasmon emission, Anal. Biochem 337 (2005) 171–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liebermann T, Knoll W, Surface-plasmon field-enhanced fluorescence spectroscopy, Colloids Surf. A 171 (2000) 115–130. [Google Scholar]
- [22].Matveeva E, Gryczynski Z, Gryczynski I, Lakowicz JR, Immunoassays based on directional surface plasmon-coupled emission, J. Immunol. Methods 286 (2004) 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Matveeva E, Malicka J, Gryczynski I, Gryczynski Z, Lakowicz JR, Multi-wavelength immunoassays using surface plasmon-coupled emission, Biochem. Biophys. Res. Commun 313 (2004) 721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Matveeva E, Gryczynski Z, Gryczynski I, Malicka J, Lakowicz JR, Myoglobin immunoassay utilizing directional surface plasmon-coupled emission, Anal. Chem 76 (2004) 6287–6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].FluoroNunc Plates and Modules: A Solid Phase for Fluorescent Immunoassays, NUNC Tech Note, vol. 5, No. 12, 2004. (http://www.nuncbrand.com/page/en/644.aspx). [Google Scholar]
- [26].Hurley IP, Coleman RC, Ireland HE, Williams JHH, Measurement of bovine IgG by indirect competitive ELISA as a means of detecting milk adulteration, Dairy Sci. 87 (2004) 543–549. [DOI] [PubMed] [Google Scholar]
- [27].Lal G, Balmer P, Stanford E, Martin S, Warrington R, Borrow R, Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes, J. Immunol. Methods 296 (2005) 135–147. [DOI] [PubMed] [Google Scholar]
- [28].Quinn CP, Semenova VA, Elie CM, Romero-Steiner S, Greene C, Li H, Stamey K, Steward-Clark E, Schmidt DS, Mothershed E, Pruckler J, Schwartz S, Benson RF, Helsel LO, Holder PF, Johnson SE, Kellum M, Messmer T, Thacker WL, Besser L, Plikaytis BD, Taylor TH Jr., Freeman AE, Wallace KJ, Dull P, Sejvar J, Bruce E, Moreno R, Schuchat A, Lingappa JR, Marano N, Martin SK, Walls J, Bronsdon M, Carlone GM, Bajani-Ari M, Ashford DA, Stephens DS, Perkins BA, Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen, Emerg. Infect. Dis 8 (2002) 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Biagini RE, Sammons DL, Smith JP, MacKenzie BA, Striley CA, Semenova V, Steward-Clark E, Stamey K, Freeman AE, Quinn CP, Snawder JE, Comparison of a multiplexed fluorescent covalent microsphere immunoassay and an enzyme-linked immunosorbent assay for measurement of human immunoglobulin G antibodies to anthrax toxins, Clin. Diagn. Lab. Immunol 11 (2004) 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mason S, La S, Mytych D, Swanson SJ, Ferbas J, Validation of the Biacore 3000 platform for detection of antibodies against erythropoietic agents in human serum samples, Curr. Med. Res. Opin 19 (2003) 651–659. [DOI] [PubMed] [Google Scholar]
- [31].Ritter G, Cohen LS, Williams C Jr., Richards EC, Old LJ, Welt S, Serological analysis of human anti-human antibody responses in colon cancer patients treated with repeated doses of humanized monoclonal antibody A33, Cancer Res. 61 (2001) 6851–6859. [PubMed] [Google Scholar]
- [32].Pol E, Biacore AB, private communication, Biacore seminar, Baltimore, MD, 13 May 2005. [Google Scholar]