Abstract
Introduction
Automated insulin delivery for people with type 1 diabetes has been a major goal in the diabetes technology field for many years. While a fully automated system has not yet been accomplished, the MiniMed™ 670G artificial pancreas (AP) system is the first commercially available insulin pump that automates basal insulin delivery, while still requiring user input for insulin boluses. Determining the safety and efficacy of this system is essential to the development of future devices striving for more automation.
Areas Covered
This review will provide an overview of how the MiniMed 670G system works including its safety and efficacy, how it compares to similar devices, and anticipated future advances in diabetes technology currently under development.
Expert Opinion
The ultimate goal of advanced diabetes technologies is to reduce the burden and amount of management required of patients with diabetes. In addition to reducing patient workload, achieving better glucose control and improving hemoglobin A1c (HbA1c) values are essential for reducing the threat of diabetes-related complications further down the road. Current devices come close to reaching these goals, but understanding the unmet needs of patients with diabetes will allow future technologies to achieve these goals more quickly.
Keywords: artificial pancreas (AP), automated insulin delivery, continuous glucose monitor (CGM), hybrid closed loop, insulin pump, MiniMed 670G, Type 1 diabetes
1. INTRODUCTION
Type 1 diabetes (T1D) is an autoimmune disorder characterized by the destruction of insulin-producing beta cells in the pancreas leading to insulin deficiency [1]. Insulin deficiency prevents glucose from entering into the cells to be metabolized for energy, and instead, glucose builds up in the blood. This accumulation leads to hyperglycemia, or elevated blood glucose. As a result, patients with T1D require life-long, intensive insulin replacement to achieve glucose levels as close to normal as possible in order to decrease the threat of long-term diabetic complications (e.g., neuropathy and retinopathy) [2].
The likelihood of developing long-term complications commonly related to diabetes can be lessened by maintaining normal blood glucose levels. However, less than 30% of patients with T1D are able to consistently achieve normal glucose levels, despite the many advances in diabetes care [2]. T1D is one of the most common chronic diseases of children and adolescents, though it can arise at any age [3]. Both the incidence and prevalence of T1D has increased in recent years and are projected to continue to rise [1, 3].
Treatment for T1D can be varied, but always requires continuous replacement of insulin. Multiple daily injections (MDI) is one way that insulin is given. A long-acting insulin dose given once or twice per day provides the background insulin necessary for cellular metabolism of glucose, while rapid-acting insulin is given for each meal to cover carbohydrate intake and correct elevated glucose values. Insulin pumps are another way to deliver exogenous insulin. Insulin pumps are small, external devices that continuously deliver small amounts of rapid-acting insulin and, therefore, replace the need for multiple daily needle injections. The insulin is contained in a reservoir and infused through a cannula that is placed in the subcutaneous tissue and secured with adhesive-- creating what is referred to as an infusion site. Many insulin pumps contain tubing that connects the reservoir to the infusion site, while other pumps, called patch pumps, have no tubing, and sits directly on the skin. The infusion site is not surgically placed, and it must be removed and replaced every 3 days by the patient or a caregiver to prevent skin infection, lipohypertrophy, or scar tissue [6].
Insulin pumps have many advantages over MDI for both basal (continuous insulin) and bolus (large dose) delivery. First, insulin pumps allow for the basal insulin supply to be tailored to the needs of the patient based on variance in activity level, time of day, and physiologic trends of hypoglycemia or hyperglycemia. Another advantage of insulin pumps over MDI is that insulin pumps can deliver insulin to the nearest 0.01 units, whereas syringe injections can only deliver insulin to the nearest 0.5 units. Insulin pumps also provide the ability to administer multiple boluses a day without the additional discomfort of injections for the patient [7].
In addition to replacing insulin, individuals with T1D must also monitor their glucose levels. This can be done by blood glucose monitoring, or using a Continuous Glucose Monitor (CGM). CGMs are subcutaneous electrochemical sensors which measure the glucose levels of the interstitial fluid [8]. The sensor is connected to a transmitter that continuously transfers current blood glucose readings to a portable device or directly to the pump every five minutes, and will alert users if glucose levels exceed hyperglycemia or hypoglycemia thresholds [8]. Many types of CGM devices require multiple calibrations per day from a blood glucose meter value to improve accuracy [9]. More advanced models of insulin pumps also allow for integration with continuous glucose monitor (CGM) sensors providing patients with a real-time value of their current blood glucose on the pump [8]. CGM sensors are inserted under the skin by the patient and worn for 7–10 days before being replaced with a new sensor.
In hopes of minimizing the workload of managing T1D for individuals, research investigators are studying how to automate blood glucose control through the development of an artificial pancreas (AP) system, also referred to as a closed-loop system. This means that instead of patients monitoring glucose levels and responding with insulin dose calculations, an algorithm will calculate insulin doses automatically. While future devices may be able to automate all aspects of glycemic control, delays in insulin absorption and degradation and transfer of glucose between the intravenous and subcutaneous spaces mandate that early systems operate as “hybrid closed-loop” devices, meaning that some insulin delivery (e.g., basal insulin) will be automated, but bolus insulin will still require some amount of patient intervention (e.g., to bolus for meals, correct for hyperglycemia, or announce exercise) in order to achieve the best control.
AP systems operate from one of three main control algorithms. The model predictive control (MPC) algorithm predicts future blood glucose readings and adjusts insulin delivery based on that prediction [9]. The second algorithm is proportional-integral-derivative (PID) control which responds to the real-time glucose values [9] and adjusts basal insulin delivery to a target blood glucose (e.g., 120mg/dL) [11]. Last is the fuzzy logic (FL) control which bases insulin doses on CGM data [9] and attempts to imitate the reasoning of diabetes clinicians [12]. The aggressiveness of the system’s response to blood glucose deviations is determined by the tuning of these control algorithms and unique features of each algorithm [10].
The Medtronic MiniMed™ 670G system (Medtronic, Northridge, CA) is the first commercially available hybrid closed-loop system. It uses the MiniMed 670G insulin pump, the Guardian™ 3 CGM glucose sensor, and a modified PID algorithm called SmartGuard™ technology (Medtronic, Northridge, CA) [13]. The MiniMed 670G system responds to the sensor glucose value and automatically adjusts basal insulin delivery every five minutes [13] by either increasing, decreasing, or suspending insulin delivery [14]. Auto Mode, which is the proprietary name for the hybrid closed-loop functionality, targets a blood glucose of 120 mg/dL but can modify this target to 150 mg/dL for exercise as post-exercise hypoglycemia is common. The other parameters determined by the system are based on previous total daily insulin doses and fasting glycemic control [15]. This system has been shown to improve hemoglobin A1c levels and increase time in range [16] which are two of the main focuses of diabetes management.
2. REVIEW
2.1. OVERVIEW OF THE MARKET
The insulin pump market has evolved drastically over the years, with the MiniMed 670G system only being one of many systems available to individuals with T1D. Many insulin pumps work as stand-alone pumps, meaning that they are not integrated with a CGM but only deliver insulin. There are also three categories of insulin pump that work in conjunction with CGMs. With the first type, the CGM sends sensor glucose values to be displayed on the insulin pump, but no decisions for dosing are made from the CGM values. The second type involves the CGM sending sensor glucose values to the insulin pump which then can decide to suspend insulin before and when the blood glucose value reaches the preset low limit. This is called “suspend before low” and “suspend on low” [17]. The third type is the hybrid closed-loop system where the CGM sends sensor glucose values to the insulin pump and the pump can then make decisions on basal insulin delivery from those values.
There are currently three major companies that are producing insulin pumps in the United States: Medtronic Diabetes, Insulet Corporation, and Tandem Diabetes Care [9]. The most recent Medtronic insulin pump is the MiniMed 670G Hybrid Closed-loop, which can be integrated with the Guardian 3 CGM. The current Insulet insulin pump is the OmniPod DASH™ (Insulet Corporation, Acton, MA). The DASH is a patch pump that is often used in conjunction with the Dexcom G5® or G6® CGM (Dexcom, San Diego, CA) sensor but does not yet directly integrate with a CGM. It utilizes Bluetooth technology to communicate between the hand-held OmniPod Personal Diabetes Manager (PDM) and the insulin patch pump that is adhered to the skin [9]. Users can also access the insulin pump system data on a mobile device for remote monitoring. Finally, the Tandem insulin pump on the market at this time is the T:slim X2™ insulin pump (Tandem Diabetes Care, San Diego, CA) which can be integrated with the Dexcom G5 or G6 CGM systems. When integrated with the Dexcom G6, this pump uses Basal-IQ™ technology (Tandem Diabetes Care, San Diego, CA) to reduce occurrences of hypoglycemia through predictive low glucose suspend (PLGS), which is the same concept as suspend before low [17]. Another system comparable to the Tandem Basal-IQ system is the Medtronic 640G system, which is available outside the United States. This system also has the PLGS feature which greatly reduces the number of glucose values less than 70 mg/dL [18]. While full discussion of different PLGS systems is beyond the scope of this manuscript, a side-by-side comparison of the features of the MiniMed 630G, MiniMed 640G/670G PLGS, and Tandem Basal-IQ functions was recently published by Messer and colleagues.[19]
Many advances have been made with CGM devices as well over the past few years, which directly affects how well a closed-loop device will work. Currently, four different CGM systems are commercially available: the Medtronic Guardian 3 sensor, the Dexcom G6, the Senseonics Eversense® CGM (Senseonics, Inc., Germantown, MD) and the Abbott Freestyle® Libre (Abbott Laboratories, Chicago, IL). The Guardian Sensor 3 system is the only CGM that works in conjunction with the MiniMed 670G insulin pump to achieve hybrid closed-loop system functionality [9]. This CGM sends its data directly to the MiniMed 670G pump and requires at least 2 calibrations per day with a recommendation of 3–4 calibrations [9] to achieve the greatest accuracy. It is approved by the Food and Drug Administration (FDA) for use in patients down to age 7 years.
While insulin pumps and CGMs have many advantages, there are some limitations or concerns that patients have reported. For open loop pumps without automation, a large amount of user intervention is still required including careful monitoring of blood glucose and manual alterations to basal delivery and other various parameters [9]. Also, with any pump there is a certain increase to the risk of diabetic ketoacidosis due to a bent cannula under the skin which occludes insulin delivery, or other infusion site failures that are not recognized quickly [9]. CGM systems have offered patients the ability to know their real-time blood glucose and have ultimately helped improve the management of T1D [9]. However, the use of a CGM requires an additional insertion site on the body which may cause an added element of discomfort for the user. Also, CGM readings tend to lag behind blood glucose values to a certain degree during times of rapid glucose level changes [9]. Possible factors attributing to this lag include difference in the diffusion of glucose from blood to interstitial fluid and delayed sensor reaction time to glucose intake [20]. CGM time lag is most commonly seen during exercise.
There are other reasons that insulin pumps and CGMs fall short. Although the technological advancements and use of insulin pumps and CGMs have greatly increased in recent years, overall glycemic control has not improved significantly and only a minority of youth and adults with T1D meet the HbA1c goals for ideal diabetes control [21–23]. Many individuals additionally discontinue use of insulin pumps and CGM for a variety of reasons. In a survey of adults with T1D, the most common barriers to pump use were not liking to wear a device and finding them uncomfortable. For CGMs, the cost of supplies was the major reason for discontinuation followed by the bother of alarms and not being able to trust the device for accuracy [24]. This mistrust in CGM accuracy poses an additional problem in that it could reduce the number of patients desiring the MiniMed 670G and future AP systems, which rely on CGM data to make insulin dosing decisions. Properly addressing these psychosocial behaviors of T1D individuals can help to continue to increase the uptake of insulin pump and CGM use as well as ultimately improve glycemic control across all age groups.
2.2. INTRODUCTION TO THE DEVICE
The MiniMed 670G insulin pump is the first hybrid closed-loop system to be approved by the FDA (see Figure 1). The MiniMed 670G can operate in two modes: Manual Mode and Auto Mode. In Manual Mode, the MiniMed 670G acts as a traditional pump working off insulin delivery settings programmed by the user. In this mode, the system may be integrated with the Guardian Sensor 3 to use the suspend before low feature which automatically suspends basal insulin delivery up to 30 minutes before the preset low glucose value is reached to avoid hypoglycemia [9]. In Auto Mode, the insulin pump is integrated with the Guardian Sensor 3 to provide fully automated basal insulin delivery based off the calculated total daily dose of the previous 2–6 days and present blood glucose values [15, 25].
Fig.1.
MiniMed 670G Insulin Pump and Guardian 3 CGM
The MiniMed 670G implements a modified PID algorithm [26]. This algorithm adjusts basal insulin delivery in response to the current glucose values, but it also takes into account insulin feedback which recognizes insulin delivery history [26]. It aims to achieve a glucose target of 120 mg/dl. The modified PID algorithm has some unique functionality. One advantage of the system is how it attempts to prevent postprandial hypoglycemia and hyperglycemia. The patient must initiate a properly calculated meal bolus prior to the meal; then the modified algorithm accounts for the insulin that is present at meal time and reduces the amount of insulin delivery after the meal to prevent hypoglycemia due to insulin stacking [26].
The Guardian Sensor 3 is a fourth-generation glucose sensor which is approved to be worn for up to 7 days [27]. The Guardian Sensor 3 is greatly improved from the previous Medtronic Enlite sensor in terms of sensor electrode design and enhanced algorithms that improve sensor accuracy and reliability [27]. The algorithm uses electrochemical impedance spectroscopy to detect sensor faults and glucose sensitivity changes that would require a new calibration [27]. A recent study analyzing the accuracy and precision of the Guardian Sensor 3 found that the mean absolute relative difference (MARD) between sensor glucose values and blood glucose meter values was smaller than with the Enlite sensor [28]. The MARD decreased an additional 1% when users calibrated 3–4 times per day as opposed to the system-required 2 calibrations [28].
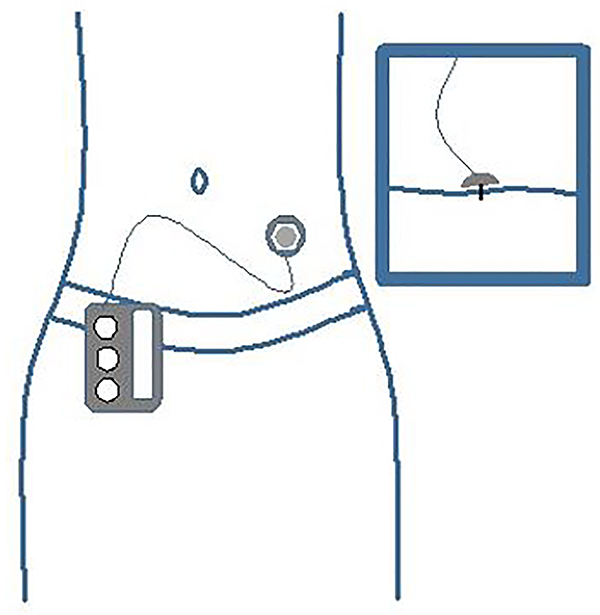
In addition to CGMs, infusion sets are another vital piece of equipment required for the use of the MiniMed 670G. Infusion sets connect the insulin reservoir in the pump to the body (see Figure 2). An infusion set includes a small cannula that is inserted under the skin and tubing that is detachable from the infusion site connected to a reservoir of insulin. There are different types of infusion sets that provide a wide range of benefits for all types of patients. The differences in infusion sets include the ability to have a soft or steel cannula, various angles at which the cannula can be inserted, and a variety of tubing lengths [29]
Fig.2.
Insulin Pump Infusion Site
Training for the MiniMed 670G is a somewhat lengthy process if the individual has no prior experience with an insulin pump and CGM. For experienced pump and CGM users, training may be quicker, as described by a large pediatric clinical center that published their training program which consisted of three steps [31]. The first step described by this group is a group or individual session where basic pump and CGM usage techniques are reviewed and instructions are given on how to use the Manual Mode features including suspend before low. The second step is initiating Auto Mode, which can occur in person or via a video conference call ~1 week after starting Manual Mode. During the visit, the trainer explains what Auto Mode is, how to use it, as well as how to troubleshoot any Auto Mode exits. After the MiniMed 670G user starts Auto Mode, follow-up phone calls from a trainer could occur at weeks 1, 2, and 4 after the start of Auto Mode to explain optimizing time in range and troubleshooting any problems or questions the patients may have [31]. There are likely many ways to train individuals on the MiniMed 670G, but all will need to include Manual Mode training, Auto Mode training, and follow-up troubleshooting.
2.3. CLINICAL PROFILE AND POST-MARKET FINDINGS
The MiniMed 670G system underwent phase 1, 2, and 3 clinical trials prior to its commercial approval by the FDA. This section will describe the clinical studies prior to commercial approval as well as various findings from post-market studies.
Phase I (Safety and Feasibility)
Safety and feasibility data are available for the MiniMed 670G AP system and were collected on laptop-based systems within a hospital research unit with close-supervision by healthcare providers. A feasibility study conducted by Steil et al. researched whether a fully automated closed-loop insulin delivery system could help achieve better glycemic control using a PID system [32]. The study consisted of 10 T1D patients using an insulin pump and 2 CGMs (1 used as a backup in case of sensor failure) that communicated with a laptop which ran the algorithms. The insulin delivery was based on the PID model. The P (proportion) factor delivered insulin in proportion to the difference between the sensor glucose value and the target glucose value of 120 mg/dL. The I (integral) factor delivered more insulin when there was a positive distance between the sensor glucose value and the target glucose value over time and gave less insulin when that distance was negative, and the glucose was below target. The D (derivative) factor increased insulin delivery when the glucose was rising and decreased insulin delivery when the glucose was falling. This component adjusts insulin delivery in proportion to the rate of change of glucose values. With the use of this system, the average glucose was similar to that achieved with traditional pumps while the glucose variability was greatly reduced. The study resulted in no occurrences of severe hypoglycemia, ~75% in target range, stable overnight glucose levels, and fasting glucose levels close to the target. Overall this study concluded that using an automated insulin delivery system to improve glycemic control is achievable.
Phase II (Safety and Efficacy)
Ruiz et al. conducted a study on the effect of the insulin feedback feature of AP systems on the glycemic control of closed-loop users [26]. Insulin feedback was added to the original PID model explained by Steil et al. in their feasibility study [32]. This new study found that the overall blood glucose levels were significantly higher when the insulin feedback was taken into account. However, the insulin feedback eliminated postprandial hypoglycemia, whereas 8 hypoglycemic events (6 directly after meal time) occurred in systems that did not use insulin feedback. The balance of reduction in hypoglycemia versus reduction in hyperglycemia was a major focus of the early development of automated systems, with the consensus of the field and regulators focusing on maximizing hypoglycemia reduction. The system also returned the patient to normal glucose levels more quickly after meals compared to the other system designs. The overall result and benefit of incorporating insulin feedback into the algorithm for the MiniMed 670G system was that it enhanced the timing of insulin delivery at meals preventing postprandial hypoglycemia which is vital to maintaining good glycemic control.
Phase III (Large Scale Safety and Efficacy)
Two multicenter pivotal studies published by Garg et al. and Forlenza et al. analyze the safety of the MiniMed 670G system for use in T1D patients [14, 16]. Garg et al. reported on initial system use in adolescents (ages 14–21 years) and adults (ages 22–75 years) with T1D. The study consisted of a 2-week run-in phase in Manual Mode followed by a 3-month study phase in Auto Mode. The data collected during the 2-week run-in phase was used as a basis for determining the Auto Mode parameters. During this study, Auto Mode was enabled 75.8% of the time by adolescents and 88% of the time by adults. For both age groups, the percent of sensor glucose values within the target range increased during the day and night. There were no episodes of severe hypoglycemia or diabetic ketoacidosis during the study.
Forlenza et al. reported on a similar multicenter study in children (ages 7–13 years) with T1D. This study consisted of a 2-week run-in phase in Manual Mode followed by a 3-month study phase in Auto Mode, as well. During this study, Auto Mode was in use 81% of the time and the overall time in target range was 65%. The results of both studies showed increased time in range, less time in hypoglycemia and hyperglycemia, reduced glycemic variability, and lower HbA1c values. These outpatient clinical trial data are what led to the commercial approval of the MiniMed 670G system by the FDA. Based on these two studies, the MiniMed 670G system was determined to be safe for use in patients ages 7 and older and to improve the overall management of T1D.
Phase IV (Ongoing Surveillance)
Phase IV data on the MiniMed 670G system are being published now that the MiniMed 670G device has been commercially available for a few years. Stone et al. conducted a study on the real-world glucose outcomes after 3-months of MiniMed 670G use [13]. Data were extracted from the CareLink™ system (Medtronic, Northridge, CA) which is the database to which Medtronic users upload their pumps and/or CGMs to view their diabetes management history over a given time period. After analyzing the CareLink data, the study showed increased time in range from an average across all age groups of ~62.5% in Manual Mode to ~70.8% in Auto Mode. There was also a decrease in the percent of sensor glucose values in the hypoglycemia and hyperglycemia ranges.
Another study followed fifty-one youth through the first 6 months of MiniMed 670G hybrid closed-loop use [33]. This study found a 37% discontinuation rate and the patients who continued to use the system showed a decrease in the amount of time spent in Auto Mode by ~10% as well as a decrease in sensor wear. As a result, these patients experienced an increase in HbA1c values and a decrease in the time in target glucose range. The proposed solution to this dilemma is to provide patients with additional support after the start of Auto Mode to ensure sensor wear and help patients learn how to address Auto Mode exits to increase the overall hybrid closed-loop use.
The studies demonstrating discontinuation of around a third of users starting the MiniMed 670G along with attrition in Auto Mode use among those continuing to use the system display a concerning trend in need of further research. Several large academic groups have begun longitudinal studies of these patients investigating predictors of discontinuation, behaviors around system use, and glycemic control in continuers and discontinuers. These studies are currently ongoing without published conclusions at this time. While not specific to the MiniMed 670G, Tanenbaum investigated barriers to device uptake among adults with T1D.[24] They found that hassle of wearing devices and dislike of having devices on one’s body were frequently endorsed modifiable barriers. Other studies focused on CGM have found that “alarm fatigue” may play a major role in device discontinuation.[34] It is likely that similar on-body device burdens and issues with alarms have played role in discontinuation and attrition among users of the MiniMed 670G.
2.4. ALTERNATIVE DEVICES
While the MiniMed 670G is the only commercially available hybrid closed-loop insulin pump at this time, other AP systems have been designed and are being tested in clinical trials for possible future commercialization. The Tandem Control-IQ AP system uses the Tandem t:slim X2 insulin pump with the Dexcom G6 CGM. This pump uses a hybrid closed-loop algorithm which automates correction boluses, a hypoglycemia safety system which stops insulin delivery to avoid low blood glucose, and targets a tighter range of glucose levels overnight [35]. Early studies of the Control IQ system algorithm found an increase in the time in range (especially overnight), mean glycemic control much closer to target range without increasing hypoglycemia, and time in hyperglycemia was reduced [35–37]. The Control IQ device is currently in phase 3 clinical trials.
The OmniPod Horizon™ Automated Glucose Control System (Insulet Corporation, Acton, MA) is another hybrid closed-loop system under development. It consists of an OmniPod patch pump, a personalized MPC algorithm, and a Dexcom CGM [38]. A handheld PDM device transmits commands, such as meal boluses or changes to settings to the OmniPod patch pump. The personalized MPC algorithm adjusts insulin dosing based on CGM values to reduce the difference between the predicted blood glucose over 60 minutes and the target blood glucose value [38]. The safety and feasibility studies of this device concluded that the system improved glycemic control, reduced exercise-related hypoglycemia with pre-exercise announcement, and improved postprandial glycemic control in the event of missed boluses or overestimated boluses [38–40].
Another series of approaches to automated insulin delivery which must be considered are the so-called do-it-yourself or DIY systems. [41, 42]. DIY systems are considered closed-loop systems as they automate insulin delivery and use CGM data to make future insulin dosing decisions. The users of these systems consist of those who have been frustrated with the time it has taken for newer, more automated systems to become commercially available. DIY systems consist of a controller such as a cell phone or mini-computer which runs an open-source algorithm and then communicates with an insulin pump and CGM using hacked device communication protocols. The control algorithm runs on an app that must be built by the patient which carries out the insulin delivery initiated by the user. Many patients have switched over to this form of diabetes management as they feel it removes much of the burden during day-to-day life. However, the DIY systems have not undergone clinical trials, and the FDA has actively discouraged their use in patients with TID due to safety concerns. Patients who choose to switch to the DIY system must take full responsibility for their care and any technological or diabetes-related issues they experience as a result.
A valuable resource for clinicians to understand the many details of advanced diabetes technologies is the CARES paradigm, which helps clarify how systems CALCULATE insulin doses, how users can ADJUST system settings, when to REVERT to non-automated functionality, how to EDUCATE patients on the system, and relevant SENSOR/SHARING characteristics [19, 43]. This concept was created with the goal of educating clinicians about the various diabetes technologies that are available in a way that is practical and re-teachable to patients. It may also be beneficial to T1D patients who wish to compare their options for technologies and to discover which choice would best suit their needs. An example of how the MiniMed 670G system would be explained using the CARES paradigm is provided in Table 1.
Table 1:
Explanation of MiniMed 670G Hybrid Closed Loop System Using a CARES Paradigm Example
| MiniMed 670G | |
|---|---|
| CALCULATION | HCL system --Uses TDI calculated from last 2–6 days --Automated basal calculated by system q 5 minutes --HCL set point = 120 mg/dl --No automated correction doses. Manual correction doses based on HCL algorithm and not on programmed sensitivity factors |
| ADJUSTMENT (for HCL mode) | Can modify: I:C ratios (for meal boluses), AIT (for subsequent correction doses), Temp target of 150 mg/dl (to change HCL set point) Cannot modify: Basal rates, ISF, HCL set point of 120 mg/dl (except when using temp target of 150 mg/dl) |
| REVERT | -- Will revert to OL if persistent hyperglycemia, maximum or minimum delivery thresholds, loss of CGM data, sensor integrity concerns --Must turn off HCL in order to use temporary basal rates and/or combo boluses --Consider turning off for illness/ketones as system may suspend insulin. If insulin needs temporarily increase during illness, HCL may not be able to respond quickly enough. Use temp basals in OL during illness if persistent hyperglycemia. --Consider turning off for dramatic change in insulin sensitivity (e.g. steroid use) due to system taking days to adjust TDI calculations |
| EDUCATION | --Consider treating hypoglycemia with less CHO (e.g. 5–10g) if system has not delivered insulin (been suspended) for period of time prior to low glucose --Important to pre-bolus for optimal mealtime management (similar to traditional insulin pump) --System may display “BG required” for HCL functioning, when user is required to enter a fingerstick BG value into the pump. This is different from a sensor calibration. Users should understand difference between sensor calibration and BG required alerts. ----Follow system prompts for “BG required” --For dosing adjustments, change I:C ratios (10–25%) and active insulin time --Cannot use temp basals and/or combo boluses in HCL mode (‘temp target’ feature will allow for temporary reduction in basal insulin delivery in HCL mode) |
| SENSOR/SHARING | MiniMed Guardian 3 --Requires 2–4 calibrations for optimal use --6–7 day sensor life --Perform SMBG for diabetes management decisions --Important to calibrate when glucose is stable ( i.e. before meals, bedtime, or when no sensor trend arrows) to prevent calibration errors |
| AIT= Active Insulin Time, BG= Blood glucose, CGM= Continuous glucose monitor, CHO = carbohydrates, HCL= hybrid closed loop, I:C = Insulin to carbohydrate, ISF = Insulin sensitivity factor, OL= open loop, PLGS = Predictive Low Glucose Suspend, SMBG = Self-Monitoring Blood Glucose, TDI= total daily insulin, TS= Threshold suspend |
2.5. HOW THE TECHNOLOGY FITS INTO THE FIELD
The MiniMed 670G system was first approved for use in the United States in the fall of 2016 [25]. It is FDA approved for use in patients ages 7 and older, but studies are underway to test the safety and efficacy of the device down to age 2 years [44]. The MiniMed 670G subsequently received CE (Conformité Européenne) mark approval in Europe in 2018 for ages 7 years and older [45]. Other countries including Canada, South Africa, Australia, have also been approved for commercial use of 670G for ages 7 and older, with global expansion continuing to date [46].
3. Conclusion
T1D is a chronic autoimmune disease that requires intense time and effort to maintain good blood glucose control. With the consistent and rapid growth of both the incidence and prevalence of T1D, the development of advanced technologies with the goal of reducing the burden for patients is essential. The MiniMed 670G hybrid closed-loop system has made significant strides in improving automation for T1D management with its use of the modified PID control algorithm with insulin feedback. The automatic basal insulin delivery allows for more accurate and specialized basal insulin dosing and, therefore, more consistent blood glucose trends from day to day.
Use of the MiniMed 670G is shown to improve HbA1c values and to increase the amount of time spent in the target blood glucose range while not increasing occurrences of hypoglycemia which will ultimately result in a decreased risk of developing diabetes-related complications. While the MiniMed 670G still requires calibrations, meal boluses, and correction boluses, the overall user intervention is much less compared to other devices. Future advances in diabetes technology seek to remove these requirements leading to even less user involvement.
4. EXPERT OPINION
The idea of an artificial pancreas or closed-loop system for use in diabetes management has been an exciting concept for many years. However, it is important to consider that there may be a large gap between a patient’s expectation versus reality of the current technology [47]. Many patients and even clinicians have a concept of what an artificial pancreas should be and, while researchers are working hard to make those wishes become reality, a fully closed-loop artificial pancreas system has still not been fully attained. The MiniMed 670G system, along with other hybrid closed-loop systems that are currently undergoing clinical trials, still requires the user to input meal boluses, as neither the sensor nor the insulin is able to react quickly enough to counteract the rapid rise in blood glucose caused by carbohydrate intake. Additionally, hybrid closed-loop systems will not keep patients in the ideal blood glucose range 100% of the time. While this is the ideal, occurrences of both hypoglycemia and hyperglycemia are possible and somewhat inevitable, requiring users to intervene in order to return to normal glucose levels.
However, even though the patients’ expectations of the MiniMed 670G AP system may not always be met, use of this system will likely ultimately lead to better glucose control. It is currently the only hybrid closed-loop system commercially available, making it an appropriate choice for patients and clinicians who desire automated insulin delivery in conjunction with the CGM sensor.
Despite the shortcomings of the current MiniMed 670G AP system, the field of diabetes technology is rapidly changing, adding new techniques to lessen diabetes burden and management. Some ways in which the current system may be improved in the future are listed. First, a major goal in the design of future devices is to remove nearly all exits from Auto Mode as that is one of the main complaints of the current MiniMed 670G system. Automated correction boluses, in addition to the automated basal insulin delivery, are being researched, as well as a possible new blood glucose target of 110 mg/dL which will hopefully lower HbA1c values to an even greater extent. The next-generation enhanced-hybrid closed loop system will incorporate changes to the current MiniMed 670G system to accomplish these improvements such as automated correction boluses and reduced alarms and closed loop exits. In a 1-week feasibility study completed by Lee et al. [48], use of the system resulted in greater time in range, lower average sensor glucose, and a much higher amount of time (99.98%) spent in closed loop mode. The participants did experience an increased time below 70 mg/dL; however, they all were satisfied overall with the system and its steps toward optimizing glucose control in T1D patients.
Further goals include the addition of Bluetooth technology which would allow for remote monitoring on smartphones and has been a notable request of both patients and caregivers of MiniMed 670G users. Other future improvements could include the ability for users to change more of the parameters of the pump that are not currently able to be changed. The last improvement could include a better sensor requiring less calibrations per day and achieving greater accuracy than past models. With a more accurate sensor, Auto Mode would be more precise in its delivery, as well.
Another large step leading to decreased user intervention is the development of factory calibration for all CGM sensors. While this has been achieved in the Dexcom G6 sensor, the Medtronic sensors necessary for the use of Auto Mode do not provide this. Factory calibration would allow for greater sensor accuracy leading to the greater overall accuracy of the hybrid closed-loop system in addition to the removal of finger sticks.
Further advances with insulin pump technology may include the commercialization of a dual hormone pump. This type of pump would contain insulin to manage carbohydrate intake and correct for hyperglycemia. However, it would also contain glucagon, which is a hormone that increases the level of glucose in the blood to inhibit hypoglycemia from occurring [49]. Current pumps that contain only insulin must suspend insulin delivery to prevent the patient from having hypoglycemia, but the dual hormone pump would be able to both suspend insulin delivery and begin delivering glucagon to automatically bring the blood glucose level up into a safe range without the need to consume sugar.
Another hope for future advancements is the ability of the AP system to automatically detect when the user is eating. Meal detection would remove one of the largest areas of user error by incorrect carbohydrate counting and would potentially remove many occurrences of postprandial hyperglycemia without the need for carbohydrate counting. Some studies have already been completed testing current AP systems on how they react to unannounced meals and the results were promising; however, the studies did not result in the blood glucose values that would be most desirable after a meal [50]. Meal announcement was still required for larger meals as the sensor reaction time falls even further behind with greater carbohydrate intake [51].
The achievement of these many advances in future diabetes technologies will ultimately lead to a fully closed-loop system requiring very minimal to no user interaction. Researchers do not know exactly how close a fully closed-loop system is to development, but bigger steps are being made with each new device that make the dream of a fully closed-loop system seem like it could become reality someday.
ARTICLE HIGHLIGHTS.
Type 1 diabetes is an autoimmune disorder causing an insulin deficiency where complications arise when hypoglycemia (low blood glucose) or hyperglycemia (high blood glucose) go untreated.
The MiniMed 670G System is the first hybrid closed-loop artificial pancreas system that automates basal insulin delivery based on past and present blood glucose values when used in conjunction with the Guardian 3 continuous glucose monitor sensor.
The MiniMed 670G uses a modified proportional-integral-derivative (PID) control algorithm which takes insulin feedback into account to prevent postprandial hypoglycemia and to achieve a target blood glucose of 120 mg/dL.
Many studies have been completed verifying the safety of the system and have concluded that the use of the MiniMed 670G results in increased time in the target blood glucose range as well as improved HbA1c values.
Future advances to diabetes technology are being made in hopes of reducing diabetes burden and management on patients with type 1 diabetes by improving glycemic control and reducing patient intervention.
Acknowledgments
FUNDING
This paper was funded by an NIH K12 award (NIDDK 2K12DK094712-06).
DECLARATION OF INTEREST
L.H. Messer has served as a consultant for Tandem Diabetes Care, Clinical Sensors, and Capillary Biomedical, and is a contracted trainer for Medtronic. G.P. Forlenza has received research funding from Medtronic, Dexcom, Abbott, Tandem, Insulet, Bigfoot, Beta Bionics, and TypeZero; he has served as a consultant/speaker for Medtronic, Dexcom, Abbott, Tandem, Insulet, DreaMed, and Lilly. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
REVIEWER DISCLOSURES
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
INFORMATION RESOURCES
The Medtronic website, https://www.medtronicdiabetes.com/products/minimed-670g-insulin-pump-system, provides detailed explanations of what the MiniMed 670G system is, how it works, and why people like using it.
Diabetes technology resources can be found at https://bdcpantherdiabetes.org/ which provides information for clinicians and patients with diabetes about the various advanced diabetes technologies that have been recently released.
REFERENCES
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Pettitt DJ, et al. , Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care, 2014. 37(2): p. 402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faulds ER, Zappe J, and Dungan KM, REAL-WORLD IMPLICATIONS OF HYBRID CLOSE LOOP (HCL) INSULIN DELIVERY SYSTEM. Endocr Pract, 2019. 25(5): p. 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaccardi F, et al. , Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J, 2016. 92(1084): p. 63–9.*Detailed explanation of what diabetes is and what causes it
- 4.DiMeglio LA, Evans-Molina C, and Oram RA, Type 1 diabetes. Lancet, 2018. 391(10138): p. 2449–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katsarou A, et al. , Type 1 diabetes mellitus. Nat Rev Dis Primers, 2017. 3: p. 17016. [DOI] [PubMed] [Google Scholar]
- 6.Messer LH, et al. , Preserving Skin Integrity with Chronic Device Use in Diabetes. Diabetes Technol Ther, 2018. 20(S2): p. S254–s264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pala L, Dicembrini I, and Mannucci E, Continuous subcutaneous insulin infusion vs modern multiple injection regimens in type 1 diabetes: an updated meta-analysis of randomized clinical trials. Acta Diabetol, 2019. [DOI] [PubMed] [Google Scholar]
- 8.Vettoretti M and Facchinetti A, Combining continuous glucose monitoring and insulin pumps to automatically tune the basal insulin infusion in diabetes therapy: a review. Biomed Eng Online, 2019. 18(1): p. 37.**Valuable review on the safety and effectiveness of integrating CGM sensors with insulin pumps to provide automation in basal insulin delivery
- 9.Allen N and Gupta A, Current Diabetes Technology: Striving for the Artificial Pancreas. Diagnostics (Basel), 2019. 9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doyle FJ 3rd, et al. , Closed-loop artificial pancreas systems: engineering the algorithms. Diabetes Care, 2014. 37(5): p. 1191–7.**Extensive description of how the artificial pancreas system is designed and how it works
- 11.Ly TT, et al. , Automated hybrid closed-loop control with a proportional-integral-derivative based system in adolescents and adults with type 1 diabetes: individualizing settings for optimal performance. Pediatr Diabetes, 2017. 18(5): p. 348–355. [DOI] [PubMed] [Google Scholar]
- 12.Mauseth R, et al. , Use of a “fuzzy logic” controller in a closed-loop artificial pancreas. Diabetes Technol Ther, 2013. 15(8): p. 628–33. [DOI] [PubMed] [Google Scholar]
- 13.Stone MP, et al. , Retrospective Analysis of 3-Month Real-World Glucose Data After the MiniMed 670G System Commercial Launch. Diabetes Technol Ther, 2018. 20(10): p. 689–692. [DOI] [PubMed] [Google Scholar]
- 14.Garg SK, et al. , Glucose Outcomes with the In-Home Use of a Hybrid Closed-Loop Insulin Delivery System in Adolescents and Adults with Type 1 Diabetes. Diabetes Technol Ther, 2017. 19(3): p. 155–163.*One of the 670G pivotal trials that influenced FDA approval and determines safety and efficacy of the device
- 15.Messer LH, et al. , Optimizing Hybrid Closed-Loop Therapy in Adolescents and Emerging Adults Using the MiniMed 670G System. Diabetes Care, 2018. 41(4): p. 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forlenza GP, et al. , Safety Evaluation of the MiniMed 670G System in Children 7–13 Years of Age with Type 1 Diabetes. Diabetes Technol Ther, 2019. 21(1): p. 11–19.*One of the 670G pivotal trials that influenced FDA approval and determines safety and efficacy of the device
- 17.Forlenza GP, et al. , Predictive Low-Glucose Suspend Reduces Hypoglycemia in Adults, Adolescents, and Children With Type 1 Diabetes in an At-Home Randomized Crossover Study: Results of the PROLOG Trial. Diabetes Care, 2018. 41(10): p. 2155–2161. [DOI] [PubMed] [Google Scholar]
- 18.Zhong A, et al. , Effectiveness of Automated Insulin Management Features of the MiniMed((R)) 640G Sensor-Augmented Insulin Pump. Diabetes Technol Ther, 2016. 18(10): p. 657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messer LH, Berget C, and Forlenza GP, A Clinical Guide to Advanced Diabetes Devices and Closed-Loop Systems Using the CARES Paradigm. Diabetes Technol Ther, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaharieva DP, et al. , Lag Time Remains with Newer Real-Time Continuous Glucose Monitoring Technology During Aerobic Exercise in Adults Living with Type 1 Diabetes. Diabetes Technol Ther, 2019. 21(6): p. 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster NC, et al. , State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther, 2019. 21(2): p. 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck RW, Miller KM, and Foster NC, The T1D Exchange Clinic Network and Registry: 10 Years of Enlightenment on the State of Type 1 Diabetes in the United States. Diabetes Technol Ther, 2019. 21(6): p. 310–312. [DOI] [PubMed] [Google Scholar]
- 23.Rodbard D, State of Type 1 Diabetes Care in the United States in 2016–2018 from T1D Exchange Registry Data. Diabetes Technol Ther, 2019. 21(2): p. 62–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanenbaum ML, et al. , Diabetes Device Use in Adults With Type 1 Diabetes: Barriers to Uptake and Potential Intervention Targets. Diabetes Care, 2017. 40(2): p. 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aleppo G and Webb KM, INTEGRATED INSULIN PUMP AND CONTINUOUS GLUCOSE MONITORING TECHNOLOGY IN DIABETES CARE TODAY: A PERSPECTIVE OF REAL-LIFE EXPERIENCE WITH THE MINIMED() 670G HYBRID CLOSED-LOOP SYSTEM. Endocr Pract, 2018. 24(7): p. 684–692. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz JL, et al. , Effect of insulin feedback on closed-loop glucose control: a crossover study. J Diabetes Sci Technol, 2012. 6(5): p. 1123–30.*An interesting explanation of the modified PID algorithm the 670G system uses
- 27.Christiansen MP, et al. , Accuracy of a Fourth-Generation Subcutaneous Continuous Glucose Sensor. Diabetes Technol Ther, 2017. 19(8): p. 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slover RH, et al. , Accuracy of a Fourth-Generation Continuous Glucose Monitoring System in Children and Adolescents with Type 1 Diabetes. Diabetes Technol Ther, 2018. 20(9): p. 576–584. [DOI] [PubMed] [Google Scholar]
- 29.Medtronic. Comparing Infusion Sets. [cited 2019 11 Jun]; Available from: https://www.medtronicdiabetes.com/customer-support/infusion-set-support/compare-set.
- 30.Jendle J, et al. , Cost-Effectiveness Analysis of the MiniMed 670G Hybrid Closed-Loop System Versus Continuous Subcutaneous Insulin Infusion for Treatment of Type 1 Diabetes. Diabetes Technol Ther, 2019. 21(3): p. 110–118. [DOI] [PubMed] [Google Scholar]
- 31.Berget C, et al. , A Clinical Training Program for Hybrid Closed Loop Therapy in a Pediatric Diabetes Clinic. J Diabetes Sci Technol, 2019: p. 1932296819835183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steil GM, et al. , Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes, 2006. 55(12): p. 3344–50. [DOI] [PubMed] [Google Scholar]
- 33.Berget C, et al. , Real World Use of Hybrid Closed Loop Therapy in Pediatric Patients with Type 1 Diabetes: A Clincial Observation Study (abstract). Diabetes Technol Ther, 2019. In Press. [Google Scholar]
- 34.Pickup JC, Ford Holloway M, and Samsi K, Real-time continuous glucose monitoring in type 1 diabetes: a qualitative framework analysis of patient narratives. Diabetes Care, 2015. 38(4): p. 544–50. [DOI] [PubMed] [Google Scholar]
- 35.Brown S, et al. , First Look at Control-IQ: A New-Generation Automated Insulin Delivery System. Diabetes Care, 2018. 41(12): p. 2634–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekhlaspour L, et al. , Closed Loop Control in Adolescents and Children During Winter Sports: Use of the Tandem Control-IQ AP System. Pediatr Diabetes, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forlenza GP, et al. , Successful At-Home Use of the Tandem Control-IQ Artificial Pancreas System in Young Children During a Randomized Controlled Trial. Diabetes Technol Ther, 2019. 21(4): p. 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buckingham BA, et al. , Safety and Feasibility of the OmniPod Hybrid Closed-Loop System in Adult, Adolescent, and Pediatric Patients with Type 1 Diabetes Using a Personalized Model Predictive Control Algorithm. Diabetes Technol Ther, 2018. 20(4): p. 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckingham BA, et al. , Performance of the Omnipod Personalized Model Predictive Control Algorithm with Meal Bolus Challenges in Adults with Type 1 Diabetes. Diabetes Technol Ther, 2018. 20(9): p. 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forlenza GP, et al. , Performance of Omnipod Personalized Model Predictive Control Algorithm with Moderate Intensity Exercise in Adults with Type 1 Diabetes. Diabetes Technol Ther, 2019. 21(5): p. 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hng TM and Burren D, Appearance of Do-It-Yourself closed-loop systems to manage type 1 diabetes. Intern Med J, 2018. 48(11): p. 1400–1404. [DOI] [PubMed] [Google Scholar]
- 42.Marshall DC, et al. , Do-It-Yourself Artificial Pancreas Systems in Type 1 Diabetes: Perspectives of Two Adult Users, a Caregiver and Three Physicians. Diabetes Ther, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Messer LH, et al. , The dawn of automated insulin delivery: A new clinical framework to conceptualize insulin administration. Pediatr Diabetes, 2018. 19(1): p. 14–17. [DOI] [PubMed] [Google Scholar]
- 44.Salehi P, Roberts AJ, and Kim GJ, Efficacy and Safety of Real Life Usage of MiniMed 670G Automode in Children with Type 1 Diabetes Less than 7 Years Old. Diabetes Technol Ther, 2019. [DOI] [PubMed] [Google Scholar]
- 45.GlobeNewswire. Medtronic’s MiniMed 670G Hybrid Closed Loop System Earns EU Approval. 2018. [cited 2019 Jun 21]; Available from: https://www.mpo-mag.com/contents/view_breaking-news/2018-06-25/medtronics-minimed-670g-hybrid-closed-loop-system-earns-eu-approval.
- 46.Idlebrook C Medtronic 670G and Tandem t:slim X2 approved in Canada. 2018. [cited 2019 Jun 21]; Available from: https://myglu.org/articles/medtronic-670g-and-tandem-t-slim-x2-approved-in-canada.
- 47.Messer LH, Why Expectations Will Determine the Future of Artificial Pancreas. Diabetes Technol Ther, 2018. 20(S2): p. S265–s268. [DOI] [PubMed] [Google Scholar]
- 48.Lee MH, et al. , Glucose Control in Adults with Type 1 Diabetes Using a Medtronic Prototype Enhanced-Hybrid Closed-Loop System: A Feasibility Study. Diabetes Technol Ther, 2019. 21(9): p. 499–506. [DOI] [PubMed] [Google Scholar]
- 49.Payne FW, Ledden B, and Lamps G, Capabilities of Next-Generation Patch Pump: Improved Precision, Instant Occlusion Detection, and Dual-Hormone Therapy. J Diabetes Sci Technol, 2019. 13(1): p. 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramkissoon CM, et al. , Unannounced Meals in the Artificial Pancreas: Detection Using Continuous Glucose Monitoring. Sensors (Basel), 2018. 18(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samadi S, et al. , Automatic Detection and Estimation of Unannounced Meals for Multivariable Artificial Pancreas System. Diabetes Technol Ther, 2018. 20(3): p. 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paldus B, et al. , Glucose Control Using a Standard Versus an Enhanced Hybrid Closed Loop System: A Randomized Crossover Study. Diabetes Technol Ther, 2019. 21(1): p. 56–58. [DOI] [PubMed] [Google Scholar]