Abstract
Articular cartilage injuries of the knee are common among young, active patients presenting with knee pain, swelling, and/or mechanical symptoms. These injuries have limited healing potential due to the avascular nature of hyaline cartilage. While several treatment options exist, osteochondral allograft (OCA) transplantation for the knee has been used successfully in primary management of large chondral or osteochondral defects and salvage of previously failed cartilage repair. OCA transplantation potentially yields a natural, matching contour of the native recipient surface anatomy and transplants mature, viable hyaline cartilage to the affected defect. Following OCA transplantation, strict compliance with a rehabilitation protocol is essential to enable optimal recovery. The outlined rehabilitation protocol is informed by the existing literature and incorporates current rehabilitation principles, the science of osteochondral incorporation, and adaptations based on an individual's readiness to progress through subsequent phases. The purpose of this clinical commentary is to discuss the diagnosis, surgical management, and post-operative rehabilitation following OCA transplantation and to assist the physical therapist in returning athletes to full sports participation.
Keywords: cartilage, knee, osteochondral allograft, rehabilitation
BACKGROUND AND PURPOSE
Chondral injuries of the knee manifest in a variety of sizes, depths, and locations and present across a spectrum of severity, ranging from small, isolated, shallow lesions to large, multifocal, full-thickness defects.1 Knee chondral injuries tend to occur in young, active patients, and have been identified in approximately 60% of patients undergoing knee arthroscopy.2–5 Articular cartilage defects may occur with acute trauma or repetitive overloading, resulting in softening of the cartilage, fissuring, flap tears or delamination.
Chondral injuries have limited healing potential due to the avascular nature of hyaline cartilage6,7 and present a complex treatment scenario. A number of factors must be considered when making treatment decisions, such as lesion location, defect size and depth, and individual patient characteristics, such as age and activity level.1,8–10 Current decision-making, based on defect geometry and patient characteristics, progresses linearly from non-operative to operative treatment, including palliative, reparative or restorative surgery.11
Small (<0.5-1 cm2) and/or superficial (grade I or II) lesions, as well as diffuse degenerative articular disease are generally treated non-operatively. Non-operative management consists of activity modification, maintenance of ideal body weight, over the counter non-steroidal anti-inflammatory medications, physical therapy, and occasionally intra-articular corticosteroid or viscosupplementation injections.1,12 Operative treatment ranges from simple arthroscopic lavage and debridement to marrow stimulation techniques (microfracture and subchondral drilling), cell based cartilage repair (autologous chondrocyte implantation) and whole tissue transfer (osteochondral autograft transfer and osteochondral allograft transplantation).
Although there are several operative treatment options for the treatment of articular pathology, each method has inherent limitations.11 For instance, marrow stimulation results in a hyaline-like fibrocartilage repair surface, which has been shown to be physiologically and biomechanically inferior to native hyaline cartilage.6,7 Osteochondral autograft transfer (OAT) is limited by donor site morbidity and the availability of autologous grafts from areas of lower contact pressures on the weight-bearing articular surfaces of the knee.13–15 Debridement, marrow stimulation techniques, and OAT have been reported to be ineffective or impractical for lesions >2 cm2.16–18 Staged cell-based cartilage repair such as autologous chondrocyte implantation (ACI) remains a viable option for large defects of the knee in young, active patients, though it necessitates two separate procedures and a prolonged recovery.
Osteochondral allograft (OCA) transplantation, however, is a single-stage technique that utilizes full thickness, viable hyaline cartilage, avoids donor site morbidity, permits resurfacing of large defects, and potentially yields a more natural, matching contour of the native recipient surface anatomy.19 Research conducted utilizing OCA transplantation for knee chondral repair demonstrated improved patient reported outcome scores (compared to pre-surgical scores)19–22 and graft survival rates of between 79% and 100% at a mean follow-up of two to four years,19–21,36 78% at 10 years,36 approximately 73% at 15 years,22,36 and 67% at 20 years.36 OCA transplantation has also been shown to be preferable in the setting of unshouldered lesions, extensive subchondral edema, or extensive bone loss that requires restoration.23,24
Following OCA transplantation, strict adherence to a rehabilitation protocol is critical to promote full recovery. The presented rehabilitation protocol is informed by the existing literature and incorporates current rehabilitation principles, the science of osteochondral incorporation, and adaptations based on an individual's readiness to progress through subsequent phases. The purpose of this clinical commentary is to discuss the diagnosis, surgical management, and post-operative rehabilitation following OCA transplantation and to assist the physical therapist in returning athletes to full sports participation.
CLINICAL PRESENTATION
Among athletes, the mean age of patients with knee chondral injuries has been reported to be 33 years (range 26-47).25 Males (62%) have been reported to be affected more commonly than females (38%).26 The patellofemoral joint is reported to be affected most frequently (37%), of which patellar defects have been reported to be more common than trochlear (64% and 36%, respectively) followed by the femoral condyles (35% overall; 68% medial vs. 32% lateral), and tibial plateau (25%).25
Patients with knee chondral injuries may report insidious or traumatic onset of symptoms. They often experience pain with weight bearing, swelling exacerbated by activity, limping, and/or feelings of instability.10 Patients may also report mechanical symptoms such as locking or catching, which may be indicative of a chondral flap tear, a large defect, or a loose intra-articular body. Prior non-operative and operative management should also be noted.
A thorough knee examination should include assessment of tenderness to palpation, passive and active motion, strength, ligamentous stability, lower extremity alignment and gait. The presence of knee effusion, quadriceps inhibition, and restricted passive and active motion may suggest intra-articular pathology. Tenderness to palpation at the joint line or just above may be indicative of a meniscal injury or chondral lesion of the femoral condyle.27 Patellar apprehension, patellofemoral crepitus, or a positive patellar grind test may raise suspicion for a patellofemoral defect. Atrophy of the quadriceps, especially the vastus medialis, is often indicative of a chronic injury.27
In addition to examination of the involved knee, a complete examination of the patient's lower extremity and core is necessary to assess for concomitant strength and flexibility deficits, as well as gait dysfunction. If tolerated by the patient, pre-operative functional testing to develop a baseline for future testing may also be performed. Multiple functional tests and maneuvers exist of varying levels of complexity. The Vail Sport TestTM has been found to have excellent intra-rater reliability and includes the following individual components: single leg squats, lateral bounding, forward and backward jogging.28
The patient history and physical exam findings associated with knee chondral injury are often non-specific and may also be found in patients with patellar instability, meniscal tears, cruciate ligament injuries, and other intra-articular pathology. Therefore, it is important to keep a broad differential and proceed with diagnostic imaging.
IMAGING
Radiographic evaluation should include bilateral weight bearing anteroposterior, lateral, 45-degree flexion posteroanterior, and patellofemoral (sunrise) views of the knee.7 Sizing markers should be used to facilitate allograft size matching to the recipient site.29 Standing long axis views of the lower extremities should also be obtained to assess the mechanical axis and determine if corrective osteotomy is needed.9,10,29 The mechanical axis should fall centrally between the tibial eminences, as genu varum or valgum malalignment is thought to cause compartment overload and premature graft failure.1,9,30,31
High-resolution magnetic resonance imaging (MRI) of the knee is a reliable means with which to evaluate articular cartilage defects.32 The area of the lesion may be measured on MRI by determining the largest defect diameter on two orthogonal views and multiplying them.1 However, MRI alone has been shown to underestimate the mean defect area by up to 60%.33,34 Cartilage-specific MRI sequences including T1rho (also referred to as T1ρ or “spin lock”) and T2 mapping permit a more detailed evaluation of a lesion's size and location.10 MRI also enables evaluation for concomitant pathology, such as ligamentous or meniscal injury, and identification of subchondral edema. Computed tomography (CT) arthrography may also be useful in patients unable to undergo MRI or after failed prior cartilage repair to assess changes in the subchondral bone.9
SURGICAL INDICATIONS
OCA transplantation is indicated for primary surgical management of large (>2-3 cm2), full thickness (grade 3 or 4) chondral or osteochondral lesions of the femoral condyles,29 as well as second-line treatment of patellofemoral35 and tibial defects in symptomatic patients refractory to nonsurgical treatment.1,8–10,24,36 Idiopathic and posttraumatic lesions, as well as defects associated with osteochondritis dissecans (OCD), osteonecrosis, and failed prior cartilage repair may be treated with OCA transplantation.8–10
Contraindications include current tobacco use, body mass index (BMI) > 35 kg/m2, inflammatory conditions, diffuse degenerative articular changes, or uncorrected knee pathology such as knee malalignment, abnormal patellar tracking, meniscal deficiency, or ligamentous insufficiency.8,9
Articular or peri-articular pathology should be corrected prior to, or at the time of, OCA transplantation. To prevent overload of the diseased compartment, genu varum may be corrected with medial high tibial opening wedge osteotomy; genu valgum may be corrected with a lateral distal femoral opening wedge osteotomy.1,8,9 Meniscal transplantation may be considered for meniscal deficiency, and a tibial tubercle osteotomy or patellar retinacular procedure are options to address pathologic patellar tracking.1,8,9
SURGICAL MANAGEMENT
OCA transplantation is performed under general anesthesia with the patient in the supine position. Once satisfactory anesthesia is administered, a thorough examination of the knee is completed. If planning to use platelet-rich plasma (PRP) or other biologic adjunct, blood should be drawn from the iliac crest or other site for later use.35 A well-padded thigh tourniquet is applied, and diagnostic arthroscopy may be performed.
Depending on the location of the lesion, a medial or lateral parapatellar arthrotomy is carried out. The size of the chondral defect is measured using templates of various sizes, matching the size that will completely cover the defect. A guide pin is placed in the center of the defect and the defect edges are scored. The defect is reamed to create a socket for the OCA transfer, while irrigation is applied to prevent heat necrosis of the adjacent articular cartilage and subchondral bone. Reaming is continued until bleeding, healthy bone is encountered with care taken not to exceed a maximum of 7 or 8 mm of overall bone depth.29 The reamed defect is then measured carefully to ensure proper donor plug trimming.
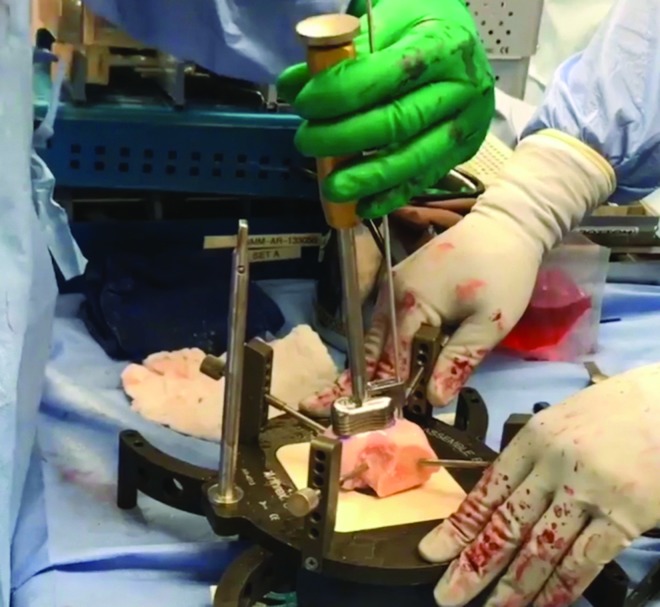
The fresh (15-28 days) allograft is warmed by soaking it in room temperature saline. Once warmed, the allograft specimen is secured in an allograft workstation and the osteochondral donor plug is harvested with the use of a coring reamer (Figure 1). Again, irrigation is used to prevent heat necrosis. The plug is trimmed to match the desired depth and diameter of the defect.
Figure 1.
The allograft specimen is secured in an allograft workstation and the osteochondral donor plug is harvested.
Preparing for implantation, the donor plug is cleansed with saline using pulsed lavage to remove any remaining bone marrow elements from the subchondral bone. This is thought to minimize the chance of immune reaction by the recipient.10,29 If blood was drawn to use as a biologic adjunct, the graft may be soaked in platelet-rich plasma (PRP) or other biologic medium.35 The bone plug is then inserted via a “press fit” technique into the recipient socket to match the exact height of the surrounding articular cartilage. If the fit is satisfactory, the knee is copiously irrigated and the wound is closed in a layered fashion.
REHABILITATION
The post-operative rehabilitation protocol for osteochondral allograft transplantation utilized at the senior authors’ (MTP, RFL) institution emphasizes early motion with a supervised rehabilitation program commencing immediately post-operatively (See Appendix 1).19 Progression of the protocol should be performed under careful supervision by the rehabilitation team, with attention to proper progression. The number of repetitions and sets performed for each exercise, as well as the amount of time holding for each stretch should be tailored to the individual. General guidelines are provided in Appendix 1. Improper phase progression may often present with persistent or recurrent pain and/or swelling; if this occurs, the patient should rest, decrease the number of repetitions and sets performed, and slowly progress as tolerated. The overall principles guiding OCA transplantation rehabilitation include protection of the knee joint from load and sheer forces, with the safe and step-wise return to weight bearing activities and sport.
Progression of Rehabilitation Phases
Phase I: Early Recovery (Weeks 0 to 8)
Early goals include pain and effusion reduction. The graft must be protected from load and shear forces, and the patient should remain non–weight bearing for the first eight weeks. Immediate range of motion is permitted, with emphasis on attaining full knee extension. Passive and active knee range of motion is not restricted, except for patients who have undergone OCA transplantation for a focal patellar osteochondral lesion; these patients remain limited to 90 degrees of knee flexion during the first two weeks after surgery to protect the allograft. Scar and adhesions along the incision site or arthroscopy portal sites may lead to loss of patellar mobility, and immediate patellar mobilization in all directions is recommended. These mobilization exercises should be a component of the patient's home therapy program, performed for five minutes, three to five times every day.37 Additionally, in the early stage of recovery is restoration of quadriceps control: quadriceps setting exercises, terminal knee extensions and straight-leg raises with the patient wearing a knee immobilizer (or locked hinged knee brace) should be performed four times daily.37
The proposed benefits of cryotherapy are well established and include edema control, local vasoconstriction and pain reduction.38 Multiple cryotherapy modalities, including crushed ice or cold packs, have also been shown to be effective for short-term pain reduction.39 A prospective randomized study analyzed an active compression cold therapy system following anterior cruciate ligament reconstruction and reported significant increased range of motion and functional knee scores compared to cold therapy alone.40 In the authors’ treatment protocol, patients are prescribed an active compression cold therapy device (Game Ready®, Concord, Georgia, U.S.A.) on low compression for thirty minutes followed by sixty minutes off to allow for skin temperature recovery. This cycle repeats itself at least four times each day.
Phase II: Weeks 8 to 12
The next phase of rehabilitation focuses on gait normalization and early strengthening (Appendix 1). Full active and passive motion should be achieved by initiation of this phase and is obtained through both manual therapy and independent patient exercises, such as supine wall slides, supine active knee flexion, quad sets and prone knee extension with gravity. Patients may begin weaning from crutches, progress weight bearing and initiate a series of balance exercises (Figures 2a, 2b; 3a, 3b). A step-wise advancement of weight bearing is implemented to incrementally increase load to the joint with care to avoid a rapid increase in stress to the graft site. A persistent or recurrent knee effusion may inhibit quadriceps firing and lead to a gait abnormality,41 and a limp or gait abnormality is a contraindication to advancement of rehabilitation phases. Pool walking, and assistive ambulation systems or treadmills, provide a useful option to advance weight bearing tolerance in a controlled environment. Once full weight bearing is accomplished, restoration of a normal gait pattern is emphasized.
Figures 2a and 2b.
Weight shifts may be performed as patients progress to full weight bearing. Begin by standing with feet hip-width apart. Initially, weight is distributed more to the non-operative leg with progression towards equal weight distribution to both feet (2a). Progress to shifting weight to one side and holding for 30 seconds (2b). Return to the starting position and repeat on the opposite side.
Figures 3a and 3b.
Single leg stands may be performed to increase balance. Begin by standing with feet hip-width apart. Initially, weight is equally distributed on both legs. Lift one leg off the floor and balance in the upright position for 30 seconds (3a). Return to the standing position and switch sides. As balance and strength improve, the elevated leg may extend back as the torso bends forward (3b). A walking stick or ski pole may be used initially to assist with balance.
More recent attention40 has been given to the significant emotional disturbance, anxiety and depression associated with significant knee injuries in athletes, which may be associated with loss of their athletic identity. Evidence for the use of aerobic exercise multiple times per week to lower these symptoms has been demonstrated.42 Cardiovascular fitness training may be introduced as early as week 8 post-operatively with no resistance biking and aqua jogging. Biking with resistance and swimming with fins may begin at post-operative week 10.
Phase III and Phase IV: Weeks 12 to 20
Phases III and IV emphasize the increase in functional strength of the lower extremity with weight training and advancing cardiovascular fitness exercises. The progression of these phases is outlined in Appendix 1. Therapists should implement a schedule of training variables, including increasing load, sets and repetitions (periodization) to maximize training adaptations and to prevent overtraining.43 Combined balance and strength exercises are performed (Figures 3 through 6). Fully weight bearing cardiovascular exercises, including inclined treadmill walking and the elliptical trainer begin 16 weeks post-operatively. As with earlier phases, an increase in swelling or pain indicates inappropriate phase progression.
Figures 6a and 6b.
Double leg bridges with heels on ball with added hamstring curl. To perform double leg bridges with heels on ball (6a), lie supine and place both heels on a medicine ball. Lift hips off the floor and hold with back straight. Return to resting position. Add a hamstring curl (6b) by flexing the knees as heels roll the ball towards the glutes with hips off the floor and back straight. Extend the knees as heels roll the ball back to starting position.
Phase V: Week 20+
Low-impact activities are recommended for the first 12 months to allow complete healing and incorporation of the graft. All patients are advised to perform low-impact activities and avoid high-impact activities as much as possible. Upon achievement of full lower extremity strength, range of motion and proprioception, readiness for return to activity may be determined with functional sport testing. A simple assessment of adequate balance and control during performance of a single-leg squat to 90-degrees of flexion is a reliable test to evaluate frontal plane motion of the knee.44 When adequate balance and control is demonstrated, a progression of running, cutting and agility skills may commence. Principles of safe progression are essential, including using flat surfaces before incline or decline, even surfaces used followed by uneven surfaces, straight line maneuvers prior to cutting, and simple followed by complex agility tasks. As throughout the protocol, attention is paid to observe for a return of pain and/or 242 swelling, which may indicate improper advancement.
Further readiness testing may be performed on a sport-specific basis. The Vail Sport Test™ has excellent intra-rater reliability when used as a measure of physical performance following anterior cruciate ligament reconstruction. The test variables, including single leg squats, lateral bounding, as well as forward and backward jogging, provide useful information in the determination of readiness to return to play.28 Sport-specific exercises may also be assessed to further delineate readiness. LaPrade45 and colleagues reported improved patient-reported outcomes following OCA transplantation, with a survival rate of 78.8% at 10 years. Those with worse survival rates included revision cases, patellar chondral lesions and bipolar lesions, indicating proper patient selection is key to improving results.
SUMMARY
Chondral injuries of the knee are common among young, active patients presenting with knee pain, swelling, and/or mechanical symptoms. These injuries have limited healing potential due to the avascular nature of hyaline cartilage. While several treatment options exist, OCA transplantation for the knee has been used successfully in primary management of large chondral or osteochondral defects, and salvage of previously failed cartilage repair. OCA transplantation potentially yields a natural, matching contour of the native recipient surface anatomy and transplants mature, viable hyaline cartilage to the affected defect. Following OCA transplantation, strict compliance with a rehabilitation protocol is essential to enable optimal recovery. The outlined rehabilitation protocol is informed by the existing literature and incorporates current rehabilitation principles, the science of osteochondral incorporation, and adaptations based on an individual's readiness to progress through progressive phases. The rehabilitation protocol provides a safe and effective step-wise approach to return patients to their preferred sport or activity.
Appendix 1: Osteochondral Allograft Transplantation Protocol
| Phase I | |
| 0 to 8 weeks after surgery | |
Precautions:
|
|
Goals:
|
|
Therapeutic Exercises:
|
|
| Phase II | |
| 8 to 12 weeks after surgery | |
Precautions:
|
|
Goals:
|
|
Therapeutic Exercises
Cardiovascular Exercises (Weeks 8-10):
Cardiovascular Exercises (Weeks 10-12):
Low load prolonged stretching
|
|
| Phase III | |
| 12 to 16 weeks after surgery | |
Precautions:
|
|
Goals:
|
|
Therapeutic Exercises
Cardiovascular Exercises
|
|
| Phase IV | |
| 16 to 20 weeks after surgery | |
Precautions:
|
|
Goals:
|
|
Therapeutic Exercises
Cardiovascular Exercises
Low load prolonged stretching
|
|
| Phase V | |
| 20 + weeks after surgery | |
Precautions:
|
|
Goals:
|
|
Therapeutic Exercises
Cardiovascular Exercises
Low load prolonged stretching
|
|
Activities of Daily Living (ADL):
|
|
Patients status post osteochondral allograft transplantation for a focal patella lesion are restricted from 0 to 90 degrees of motion for weeks 0 to 2 post-operatively.
Figure 4.
Reverse lunge with static hold. Stand with feet hip to shoulder-width apart. Take a step back with one leg while keeping the torso erect and bending both knees. Hold for an increasing amount of time as strength improves. Return to starting stance; repeat and alternate sides.
Figures 5a and 5b.
Squat to calf raise. Stand with feet hip to shoulder-width apart. Begin with traditional body-weight squat. As emerge from the squatting position, progress to a standing calf raise.
REFERENCES
- 1.Camp CL Stuart MJ Krych AJ. Current concepts of articular cartilage restoration techniques in the knee. Sports Health. 2014;6(3):265-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aroen A Loken S Heir S, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32(1):211-215. [DOI] [PubMed] [Google Scholar]
- 3.Curl WW Krome J Gordon ES Rushing J Smith BP Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997 Aug;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 4.Hjelle K Solheim E Strand T Muri R Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002 Sep;18(7):730-4. [DOI] [PubMed] [Google Scholar]
- 5.Widuchowski W Widuchowski J Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007 Jun;14(3):177-82. [DOI] [PubMed] [Google Scholar]
- 6.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982 Mar;64(3):460-6. [PubMed] [Google Scholar]
- 7.Alford JW Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005 Feb;33(2):295-306 [DOI] [PubMed] [Google Scholar]
- 8.Cole BJ Pascual-Garrido C Grumet RC. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009 Jul;91(7):1778-90. [PubMed] [Google Scholar]
- 9.Gomoll AH Farr J Gillogly SD Kercher J Minas T. Surgical management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010 Oct. 20;92(14):2470-90. [PubMed] [Google Scholar]
- 10.Sherman SL Garrity J Bauer K Cook J Stannard J Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014 Feb;22(2):121-33. [DOI] [PubMed] [Google Scholar]
- 11.McNickle AG Provencher MT Cole BJ. Overview of existing cartilage repair technology. Sports Med Arthrosc Rev. 2008 Dec;16(4):196-201. [DOI] [PubMed] [Google Scholar]
- 12.Poddar SK Widstrom L. Nonoperative Options for Management of Articular Cartilage Disease. Clin Sports Med. 2017 Jul;36(3):447-456. [DOI] [PubMed] [Google Scholar]
- 13.Jakob RP Franz T Gautier E Mainil-Varlet P. Autologous osteochondral grafting in the knee: indication, results, and reflections. Clin Orthop Relat Res. 2002 Aug;(401):170-84. [DOI] [PubMed] [Google Scholar]
- 14.LaPrade RF Botker JC. Donor-site morbidity after osteochondral autograft transfer procedures. Arthroscopy. 2004 Sep;20(7):e69-73. [DOI] [PubMed] [Google Scholar]
- 15.Garretson RB 3rd Katolik LI Verma N Beck PR Bach BR Cole BJ. Contact pressure at osteochondral donor sites in the patellofemoral joint. Am J Sports Med. 2004 Jun;32(4):967-74. [DOI] [PubMed] [Google Scholar]
- 16.Gudas R Kalesinskas RJ Kimtys V Stankevicius E Toliusis V Bernotavicius G Smailys A. A prospective randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint in young athletes. Arthroscopy. 2005 Sep;21(9):1066-75. [DOI] [PubMed] [Google Scholar]
- 17.Gudas R Gudaite A Pocius A Gudiene A Cekanauskas E Monastyreckiene E Basevicius A. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012 Nov;40(11):2499-508. [DOI] [PubMed] [Google Scholar]
- 18.Knutsen G Drogset JO Engebretsen L Grøntvedt T Isaksen V Ludvigsen TC Roberts S Solheim E Strand T Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007 Oct;89(10):2105-12. [DOI] [PubMed] [Google Scholar]
- 19.LaPrade RF Botker J Herzog M Agel J. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. A prospective outcomes study. J Bone Joint Surg Am. 2009 Apr;91(4):805-11. [DOI] [PubMed] [Google Scholar]
- 20.Williams RJ 3rd Ranawat AS Potter HG Carter T Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007 Apr;89(4):718-26. [DOI] [PubMed] [Google Scholar]
- 21.McCulloch PC Kang RW Sobhy MH Hayden JK Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle: minimum 2-year follow-up. Am J Sports Med. 2007 Mar;35(3):411-20. [DOI] [PubMed] [Google Scholar]
- 22.Levy YD Görtz S Pulido PA McCauley JC Bugbee WD. Do fresh osteochondral allografts successfully treat femoral condyle lesions?. Clin Orthop Relat Res. 2013 Jan;471(1):231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gortz S Bugbee WD. Allografts in articular cartilage repair. Instructional course lectures. 2007;56:469-480. [PubMed] [Google Scholar]
- 24.Harris JD Siston RA Pan X Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flanigan DC Harris JD Trinh TQ Siston RA Brophy RH. Prevalence of chondral defects in athletes’ knees: a systematic review. Med Sci Sports Exerc. 2010 Oct;42(10):1795-801. [DOI] [PubMed] [Google Scholar]
- 26.Bachmann GF Basad E Rauber K Damian MS Rau WS. Degenerative joint disease on MRI and physical activity: a clinical study of the knee joint in 320 patients. Eur Radiol. 1999;9(1):145-52. [DOI] [PubMed] [Google Scholar]
- 27.Moyad TF. Cartilage Injuries in the Adult Knee: Evaluation and Management. Cartilage. 2011;2(3):226-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrison JC Shanley E Thigpen C Geary R Osler M Delgiorno J. The reliability of the vail sport test as a measure of physical performance following anterior cruciate ligament reconstruction. Int J Sports Phys Ther. 2012;7(1):20-30 [PMC free article] [PubMed] [Google Scholar]
- 29.Dean CS Chahla J, Serra Cruz R LaPrade RF. Fresh Osteochondral Allograft Transplantation for Treatment of Articular Cartilage Defects of the Knee. Arthrosc Tech. 2016;5(1):e157-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaver RJ Mahomed M Backstein D Davis A Zukor DJ Gross AE. Fresh osteochondral allografts for post-traumatic defects in the knee. A survivorship analysis. J Bone Joint Surg Br. 1992;74(1):105-110. [DOI] [PubMed] [Google Scholar]
- 31.Ghazavi MT Pritzker KP Davis AM Gross AE. Fresh osteochondral allografts for post-traumatic osteochondral defects of the knee. J Bone Joint Surg Br. 1997;79(6):1008-1013. [DOI] [PubMed] [Google Scholar]
- 32.Potter HG Chong le R. Magnetic resonance imaging assessment of chondral lesions and repair. The J Bone Joint Surg Am. 2009;91 Suppl 1:126-131. [DOI] [PubMed] [Google Scholar]
- 33.Gomoll AH Yoshioka H Watanabe A Dunn JC Minas T. Preoperative Measurement of Cartilage Defects by MRI Underestimates Lesion Size. Cartilage. 2011;2(4):389-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell AB Knopp MV Kolovich GP, et al. Preoperative MRI underestimates articular cartilage defect size compared with findings at arthroscopic knee surgery. Am J Sports Med. 2013;41(3):590-595. [DOI] [PubMed] [Google Scholar]
- 35.Ferrari MB Sanchez G Chang A Sanchez A, Ellera Gomes JL Provencher MT. Osteochondral Allograft Transplantation for Treatment of Focal Patellar Osteochondral Lesion. Arthrosc Tech. 2017;6(4):e907-e912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gortz S Bugbee WD. Fresh osteochondral allografts: graft processing and clinical applications. J Knee Surg. 2006;19(3):231-240. [DOI] [PubMed] [Google Scholar]
- 37.Manske RC Prohaska D Lucas B. Recent advances following anterior cruciate ligament reconstruction: rehabilitation perspectives: Critical reviews in rehabilitation medicine. Curr Rev Musculoskelet Med. 2012;5(1):59-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bleakley C McDonough S MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261. [DOI] [PubMed] [Google Scholar]
- 39.Malanga GA Yan N Stark J. Mechanisms and efficacy of heat and cold therapies for musculoskeletal injury. Postgrad Med. 2015;127(1):57-65. [DOI] [PubMed] [Google Scholar]
- 40.Logan CA O’Brien LT LaPrade RF. Post-Operative Rehabilitation of Grade Iii Medial Collateral Ligament Injuries: Evidence Based Rehabilitation and Return to Play. Int J Sports Phys Ther. 2016;11(7):1177-1190. [PMC free article] [PubMed] [Google Scholar]
- 41.Rutherford DJ Hubley-Kozey CL Stanish WD. Knee effusion affects knee mechanics and muscle activity during gait in individuals with knee osteoarthritis. Osteoarthritis Cartilage. 2012;20(9):974-981. [DOI] [PubMed] [Google Scholar]
- 42.Stanton R Reaburn P. Exercise and the treatment of depression: a review of the exercise program variables. J Sci Med Sport. 2014;17(2):177-182 [DOI] [PubMed] [Google Scholar]
- 43.Buford TW Rossi SJ Smith DB Warren AJ. A comparison of periodization models during nine weeks with equated volume and intensity for strength. J Strength Cond Res. 2007;21(4):1245-1250. [DOI] [PubMed] [Google Scholar]
- 44.Ageberg E Bennell KL Hunt MA Simic M Roos EM Creaby MW. Validity and inter-rater reliability of medio-lateral knee motion observed during a single-limb mini squat. BMC Musculoskelet Disord. 2010;11:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Familiari F Cinque ME Chahla J Godin JA Olesen ML Moatshe G LaPrade RF. Clinical Outcomes and Failure Rates of Osteochondral Allograft Transplantation in the Knee: A Systematic Review. Am J Sports Med. 2017 Oct. 1:363546517732531. [DOI] [PubMed] [Google Scholar]