Abstract
Background
Pulmonary hypertension is a common complication of interstitial lung disease. This study was conducted to retrospectively analyze the incidence of pulmonary hypertension among interstitial lung disease patients and the correlation between systolic pulmonary artery pressure (PASP) and pulmonary functions. We also intended to investigate whether antinuclear antibody (ANA) could be an effective indicator of pulmonary hypertension.
Material/Methods
There were 182 patients diagnosed with interstitial lung disease through high-resolution computed tomography (HRCT). Pulmonary hypertension was defined as an increase of mean pulmonary arterial pressure (PAPm) ≥25 mmHg (~PASP ≥40 mmHg) at rest. Severe pulmonary hypertension was defined as PAPm ≥35 mmHg. There were 104 cases including in this study. There were 67 cases from the ANA positive (ANA+) group and 37 cases from the ANA negative (ANA−) group. All study patients had pulmonary function tests, which included the measurements of maximal voluntary ventilation (MVV), residual volume (RV), total lung capacity (TLC), forced expiratory volume (FVC), vital capacity (VC), and diffusing capacity of the lungs for carbon monoxide (DLCO).
Results
The pulmonary hypertension incidence in the study cohort was 25%, and the severe pulmonary hypertension incidence was 6.48%. The incidence of pulmonary hypertension in ANA+ cases was 22.22%. The incidence of pulmonary hypertension in the ANA− cases was 32.14%. The lung function test results showed moderate relationships between DLCO, FVC%, VC%, and PASP; no relationship between MVV, FEV1/FVC%, RV/TLC, and PASP; minimum relationship between FVC%, VC%, and PASP in the ANA+ group; and moderate relationship between FVC%, VC%, and PASP in the ANA− group.
Conclusions
Pulmonary hypertension occurred in 25% of the 182 interstitial lung disease patients and was negatively associated with deteriorated lung functions (specifically VC%, FVC%, and DLCO parameters). ANA level was not associated with the prognosis of pulmonary hypertension of patients with interstitial lung disease, and it did not significantly affect the correlation between PASP and pulmonary functions. Thus, ANA level did not seem to be a necessary indicator of pulmonary hypertension, and a more effective treatment method for pulmonary hypertension of patients with interstitial lung disease is urgently needed.
MeSH Keywords: Lung Diseases, Interstitial; Persistent Fetal Circulation Syndrome; Respiratory Function Tests
Background
The interstitium is a network of connective tissue and localizes between the alveolar and capillary basement membrane (parenchymal) around the bronchi and vasculature, and between the pleura and interlobular septae (sub pleural) [1,2]. Interstitial lung disease (ILD) is a panel of lung conditions that affect the interstitium and result in connective tissue fibrosis [3,4], which therefore leads to thickened interstitial tissue replacing healthy capillaries, alveolar and interstitium [5–8]. Due to similar clinical, radiologic, pathologic, and physiologic presentations, more than 200 connective tissue disorders, such as rheumatoid arthritis (RA), systemic sclerosis, and systemic lupus erythematosus (SLE), are classified together. These disorders occur as either a primary condition involving the lung parenchyma or a substrate of multiorgan involvement [9]. The diagnosis of ILD is commonly performed using high resolution computed tomography (HRCT) scan and biopsy. Lung function tests such as spirometry and diffusion capacity of carbon monoxide (DLCO) are also conducted to assess the severity of ILD [9].
An increase of mean pulmonary arterial pressure (PAPm) of ≥25 mmHg at rest defines pulmonary hypertension (PH). The occurrence of PH to those with ILD can worsen the impaired quality of lung functions especially when the PAPm exceeds 35 mmHg, which defines severe PH [10,11]. PH is a clinical manifestation of many lung diseases, including ILD and pulmonary fibrosis. The presentation of PH is similar; there is reduced exercise capacity and poor survival outcome [12–17]. Right heart catheterization (RHC) is the gold standard for PH diagnosis, while echocardiography is used for screening [18,19]. The most frequent cause of mortality of patients with ILD is due to respiratory failure; a previous study showed that PH is a marker of early mortality of ILD [20].
To date, the research regarding ILD-associated PH has been under slow development. Most published studies have been focused on lung transplant-, idiopathic pulmonary fibrosis (IPF)-, sarcoidosis- and systemic sclerosis-related PH. Different ILD types predict different PH incidence. To have a better view of the prevalence of PH among the patients with ILD, this research used a retrospectively study of the association of PH in patients with various types of ILD. We also grouped our participants into ANA+ and ANA− groups to see whether ANA played an important part in PH prognosis. In this study, we found that PH occurred in 25% of the 182 ILD patients and severe PH occurred in 6.48% of all the ILD patients. PASP was found negatively associated with deteriorated lung functions (specifically VC%, FVC%, and DLCO parameters). ANA level was not associated with the prognosis of PH in patients with ILD, and it did not significantly affect the correlation between PASP and pulmonary functions. Our outcome suggested that ANA level did not seem to be a useful indicator of ILD-related PH. And considering the high incidence rate of PH among ILD patients, a more effective treatment method for PH of ILD patients is urgently needed.
Material and Methods
Patient inclusion and exclusion criteria
Patients who were diagnosed as ILD through high-resolution computed tomography (HRCT) in Shandong Provincial Hospital and Taishan Medical College affiliated Laigang Hospital Respiratory Medicine Department during October 2012 and December 2016 were enrolled in this study. Patients with congenital heart disease, left heart failure, chronic thromboembolism, lung cancer, chronic lung diseases (e.g., bronchial asthma, chronic obstructive pulmonary disease, and bronchiectasia), pleura disease, as well as other cardiac, pulmonary, liver, and blood vessel diseases were excluded. Also, those with a history of thoracic operation, and human immunodeficiency virus (HIV) infection were excluded from this study. The study was approved by the ethics committee of the hospital (No. A20120919).
PH definition and characteristics of included patients
We used the diagnosis criteria of the 2015 European Society of Cardiology (ESC) and the European Respiratory Society (ESC/ERS) Guidelines for the diagnosis and treatment of PH, which defined PH as an increase of PAPm ≥25 mmHg (~PASP ≥40 mmHg) at rest as diagnosed by right heart catheterization (RHC). In this study, we were particularly interested in patients with PH due to ILD (classified into group 3.2 according to the 2015 ESC/ERS Guidelines). The baseline characteristics of the included patients are shown in Tables 1 and 2.
Table 1.
Baseline characteristics of the included patients.
| Characteristics | No. (%) |
|---|---|
| Age, years | 60.9±13.7 |
| Gender | |
| Male | 92 |
| Female | 90 |
| BMI, kg/m2 | 27.8±5.4 |
| Systolic pulmonary pressure, mmHg | 54.6±7.6 |
| ANA positive (n=126) | |
| Systemic lupus erythematosus (SLE) | 13 |
| Rheumatoid arthritis (RA) | 20 |
| Sjogren Syndrome | 10 |
| Overlap syndrome | 12 |
| Non-identified connective tissue disease | 5 |
| Cryptogenic organizing pneumonitis (COP) | 5 |
| Idiopathic pulmonary fibrosis (IPF) | 3 |
| Others | 58 |
| ANA negative (n=56) | |
| Suspected RA | 3 |
| Idiopathic pulmonary fibrosis (IPF) | 3 |
| Non-identified ILD | 50 |
Table 2.
Baseline characteristics of the patients who had lung function test (n=104).
| Characteristics | N0. (%) |
|---|---|
| Age, years | 51±10.5 |
| Gender | |
| Male | 54 |
| Female | 50 |
| BMI, kg/m2 | 27.2±4.8 |
| Systolic pulmonary pressure, mmHg | 46.2±5.1 |
| Lung function parameters | |
| MVV, L/min | 77.38±28.3 |
| VC% | 70.62±20.74 |
| RV/TLC% | 39.40±13.32 |
| FVC% | 67.66±19.93 |
| FEV1/FVC | 88.35±10.36 |
| DLCO | 50.96±23.11 |
| ANA positive (n=67) | |
| Systemic lupus erythematosus (SLE) | 5 |
| Rheumatoid arthritis (RA) | 11 |
| Sjogren Syndrome | 6 |
| Overlap syndrome | 9 |
| Non-identified connective tissue disease | 3 |
| Cryptogenic organizing pneumonitis (COP) | 5 |
| Idiopathic pulmonary fibrosis (IPF) | 2 |
| Others | 26 |
| ANA negative (n=37) | |
| Suspected RA | 2 |
| Idiopathic pulmonary fibrosis (IPF) | 3 |
| Non-identified ILD | 32 |
MVV – maximal voluntary ventilation; RV – residual volume; TLC – total lung capacity; FVC – forced expiratory volume; VC – vital capacity; DLCO – diffusing capacity of the lungs for carbon monoxide.
Echocardiography and RHC
The accuracy of echocardiography in diagnosis of PH is low in patients with advanced lung disease, however, it still is the most widely used tool for PH diagnosis [21,22]. The procedure for Doppler echocardiography of a previous research [11] was employed. Briefly, the maximal tricuspid regurgitation velocity was measured and the tricuspid pressure regurgitation gradient (TR) signal was obtained. TR >40 mmHg was screened out for RHC (a sterile catheter was used for each patient), the gold standard for PH diagnosis. The 182 diagnosed patients were divided into antinuclear antibody (ANA)+ and ANA− groups. There were 126 in the former group, and 56 in the latter group.
Pulmonary function tests
Tested pulmonary function parameters included maximal voluntary ventilation (MVV), residual volume (RV), total lung capacity (TLC), forced expiratory volume in 1 second (FEV1), vital capacity (VC)%, forced vital capacity (FVC)%, and diffusing capacity of the lungs for carbon monoxide (DLCO) following the American Thoracic Society guidelines. The pulmonary function parameters were measured using a Zan 100 PC-based diagnostic spirometer (nSpire Health Inc, KY, CO, USA).
Statistical analysis
SPSS 17.0 was used to analyze the correlation between PASP levels and pulmonary function parameters as well as other baseline characteristics. Normally distributed data are expressed as means ± standard deviation, non-parametric data are expressed as mean with range. P<0.05 was set as the statistical significance threshold. Chi-square test was used to compare the incidence rate of PH of different groups. Linear regression was performed with variables of interest.
Results
The incidence rate of PH among patients with ILD
Among the 182 patients diagnosed with ILD, 54 had PASP higher than 40 mmHg, suggesting a PH incidence rate of 29.67%. In addition, 14 of the 182 patients had PASP higher than 60 mmHg, indicating a 7.69% incidence rate of severe PH. Antinuclear antibodies (ANA) is one of the markers of autoimmunity commonly used in clinical practice [23]. A higher ANA level has been shown to predict a better prognosis [24]. We calculated the incidence rate of PH among the patients diagnosed with ILD to see whether a higher ANA level was associated with a better prognosis of PH. In our study, the PH incidence rate in the ANA+ group was 22.22% (28 out of 126 patients), whereas in the ANA− group, it was 32.14% (18 out of 56 patients, see Table 3). No significant difference of the PH incidence rate between the ANA+ group and the ANA− group was noted, suggesting that the ANA level was not associated with the prognosis of patients with ILD.
Table 3.
The incidence rate of PH among patients diagnosed with ILD that went through echocardiography in ANA+ and ANA− groups.
| Number | The number of patients with PH | PH incidence rate | |
|---|---|---|---|
| ANA+ | 126 | 28 | 22.22% |
| ANA− | 56 | 18 | 32.14% |
| ANA diagnosed cases | 182 | 46 | 25.30% |
PH – pulmonary hypertension; ILD – interstitial lung disease; ANA – antinuclear antibody.
The correlation between PASP and pulmonary function parameters
Among all the 104 patients who underwent pulmonary function tests, it was found that PASP was significantly negatively correlated with VC%, FVC%, and DLCO. PASP was found not significantly correlated with MVV, FEV1/FVC%, and RV/TLC in this case. Additionally, in neither the ANA+ group or the ANA− group was PASP found significantly negatively correlated with VC%, FVC% and DLCO (see Table 4 and Figures 1–3, data regarding MVV, FEV1/FVC% and RV/TLC are not shown).
Table 4.
The correlation between PASP and pulmonary function indexes.
| Group | The correlation coefficient r | ||
|---|---|---|---|
| VC% | FVC% | DLCO | |
| ANA+ (N=67) | −0.482 | −0.506 | −0.676 |
| ANA− (N=37) | −0.783 | −0.725 | −0.713 |
| Total | −0.645 | −0.614 | −0.681 |
Figure 1.
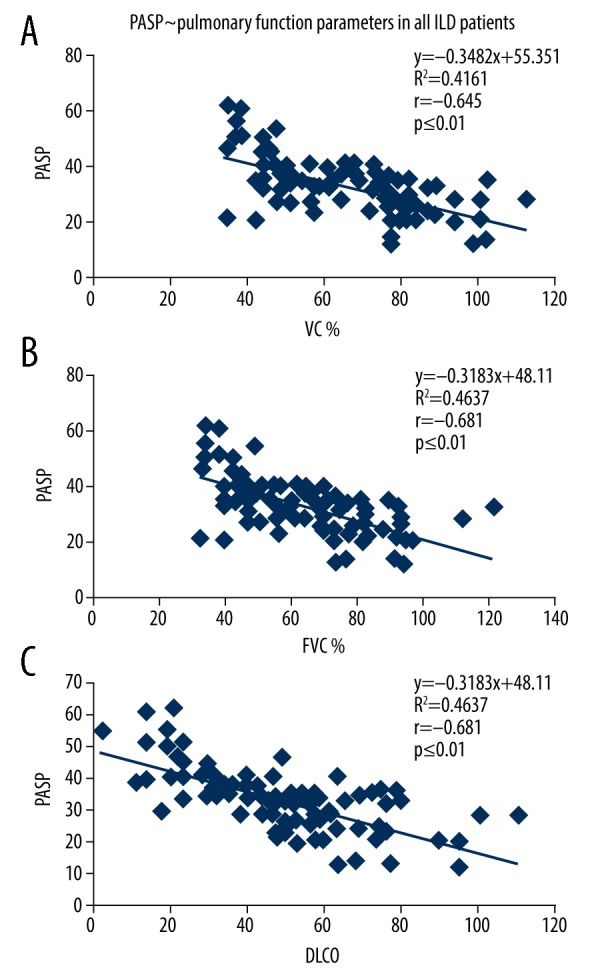
The correlation between the PASP of ILD patients and the pulmonary function parameters. (A) The correlation between the PASP (x-axis) of ILD patients and VC% (y-axis). (B) The linear correlation between the PASP (x-axis) of ILD patients and FVC% (y-axis). (C) The linear regression analysis of the PASP (x-axis) of ILD patients and DLCO (y-axis). PASP – pulmonary artery systolic pressure; ILD – interstitial lung disease; VC – vital capacity; FVC – forced expiratory volume; DLCO – diffusing capacity of the lungs for carbon monoxide.
Figure 2.
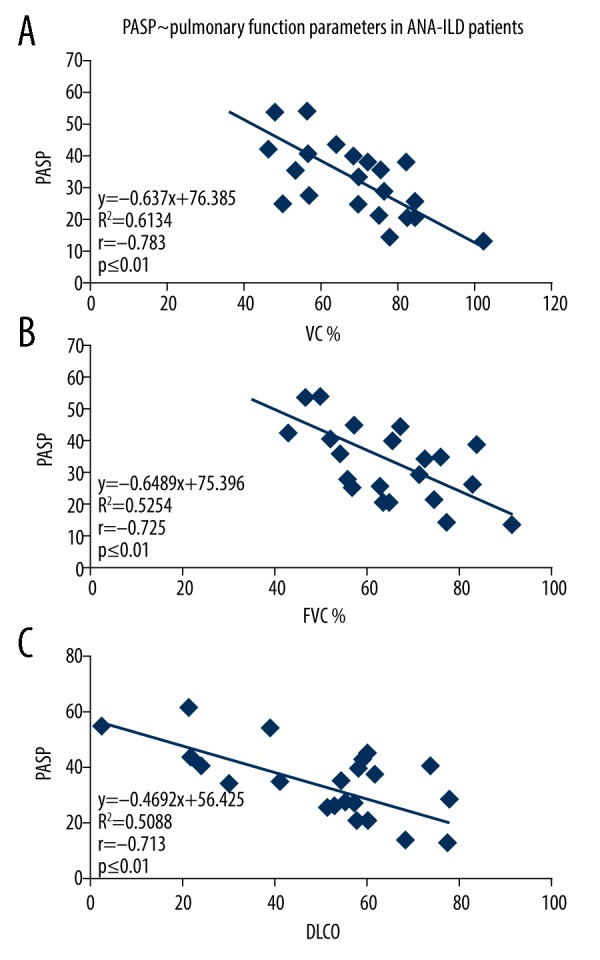
The correlation between the PASP of ANA negative ILD patients and pulmonary function parameters. (A) The correlation between the PASP (x-axis) of ANA– ILD and VC% (y-axis). (B) The linear regression analysis between the PASP of ANA– ILD (x-axis) and FVC% (y-axis). (C) The correlation between the PASP of ANA– ILD (x-axis) and DLCO (y-axis). PASP – pulmonary artery systolic pressure; ANA – antinuclear antibody; ILD – interstitial lung disease; VC – vital capacity; FVC – forced expiratory volume; DLCO – diffusing capacity of the lungs for carbon monoxide.
Figure 3.
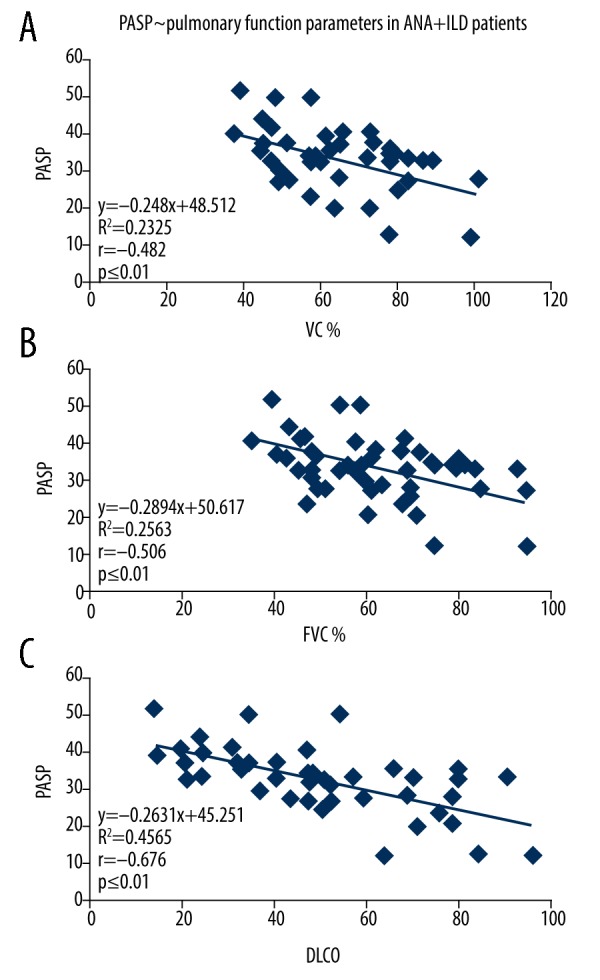
The correlation between the PASP of ANA positive ILD patients and pulmonary function parameters. (A) The correlation between the PASP of ANA positive ILD (x-axis) and VC% (y-axis). (B) The linear regression analysis between the PASP of ANA positive ILD (x-axis) and FVC% (y-axis). (C) The correlation between the PASP of ANA positive ILD (x-axis) and DLCO (y-axis). PASP – pulmonary artery systolic pressure; ANA – antinuclear antibody; ILD – interstitial lung disease; VC – vital capacity; FVC – forced expiratory volume; DLCO – diffusing capacity of the lungs for carbon monoxide.
Discussion
In this research, we studied the prevalence of PH among patients with ILD and the relationship between ILD-associated PH and pulmonary functions. Patients with various types of ILD were included in this research. The prevalence of PH in our study cohort was 25% and severe PH was 6.48%. PASP was demonstrated to have significant correlations with VC%, FVC%, and DLCO in despite of ANA level.
The prevalence of PH has varied in different study cohorts. For instance, 212 patients with mixed types of ILD were included in a study by Anderson et al., and 14% of these patients had PH with 25 mmHg and 35 mmHg defined as the threshold for mild and severe PH, respectively [9]. In the Andersen et al. study 18 patients went through RHC to eliminate false positives and negatives produced from echocardiography diagnosis for PH. In a study by Nadrous et al. in a tertiary center, 88 patients with IPF, in whom PASP were assessed with transthoracic echocardiography, the mean PASP ranged from 28 mmHg to 116 mmHg [25]. In the Nadrous et al. study, RHC, which is used to confirm the presence and degree of PH, was not conducted. A study by Handa et al. of 246 consecutive Japanese sarcoidosis patients who were evaluated for PH (defined as PASP ≥40 mmHg) by Doppler echocardiography found that 5.7% of the participants had PH [26]. Another retrospective study analyzing a large number of RHC data from patients registered with the ultrasound lung transplant registry showed that 46.1% of patients with IPF waiting for lung transplantation had PH [27]. During 2004 and 2005, David et al. performed a retrospective study on 454 patients with IPF who were registered for lung transplantation with the United Network for Organ Sharing; 376 patients underwent RHC and 36% of them had PH [28]. Our analysis revealed that PH affected ~25% of the included participants, and severe PH was relatively infrequent at 6.48%. The incidence rate of PH in patients with ILD can be very different due to the type of ILD, the severity of ILD, and the diagnostic method of PH. We hypothesized that the most possible reason for the difference between our result and the result of the Anderson et al. study, was because of the different physiological conditions of the study cohorts. Both studies both enrolled mixed types of ILDs, and our cohort included in-patients, suggesting a severe condition of disease in these patients. Compared to other studies, our patient cohort had mixed types of ILD, whereas most of the other studies included cohorts with only one type. Lastly, it is notable that the incidence rate of PH of our cohort was 25%, whereas normally it has been reported that 30% to 40% of patients with ILD have PH. This is primarily because we used the internationally recognized definition whereas PASP ≥30/35 mmHg as this value has been defined as the criteria for PH in China.
The correlation between lung function parameters and PH might not be as clinically meaningful as expected, and appears to be speculative rather than causative, i.e., this correlation might help with the prediction of PH but not with the understanding of the mechanism of PH. Caminati et al. pointed out that pulmonary function parameters, such as FVC, are useless as a routine assessment of ILD severity [10]. No significant positive correlation was noted between FVC and PH in studies by Nadrous et al. [29] and Shorr et al. [30], suggesting that ILD with PH might not be attributable to FVC but other causes. However, in another study, PASP correlated best with DLCO, to which it was inversely related and had a significant adverse impact on survival. Also, when PASP was >50 mmHg, the patients were more prone to require oxygen supplementation [29]. Similarly, another study concluded that a reduced DLCO should raise the suspicion of undiagnosed PH. The authors pointed out that patients with the need for supplemental oxygen in conjunction with a DLCO of less than 40% were much more likely to have PH than those without either of these 2 features [31]. Nadrous et al. [29] also reported that the prevalence of PH in those with late-stage IPF was significantly correlated with DLCO. In our study, we found that in all patients with ILD, PASP was found significantly correlated with FVC%, VC%, and DLCO despite the ANA grouping. Among all the patients with ILD, PASP was most significantly correlated with DLCO; in the ANA+ group, PASP was found significantly correlated with DLCO; and in the ANA− group, PASP was found significantly correlated with VC%. And no significant difference of the correlation was noted between the ANA+ group and the ANA− group. A reduction of VC% alone is not considered to be direct evidence of a restrictive ventilation, and reduced FVC% is considered to be an indicative of a restrictive defect in ILD. DLCO, on the other hand, represents the diffusing capacity of the lung [32]. Thus, VC%, FVC%, and DLCO together can reflect a relatively accurate lung functioning state. Our results suggested that PASP had a significant association with lung function parameters, i.e., PH has a significant correlation with ILD. ANA is one of the markers of autoimmunity commonly used in clinical practice [23,33,34]. Those with a higher ANA titer level are reported to have a better prognosis [24]. However, in our study, we found that ANA did not show a significant correlation with PH because no significant difference of PH incidence was noted between the ANA+ group and the ANA− group, suggesting that ANA cannot be a useful biomarker of PH. Also, in neither the ANA+ group or the ANA− group, was PASP found to be significantly correlated with lung functions, and there was no significance between the 2 groups, suggesting that ANA did not directly link to ILD, either.
A major weakness of our study was that it was a retrospective study. A retrospective study is designed to use pre-existing data, making it subjected to numerous biases, such as selection bias, because the control group was recruited by convenience sampling, and thus did not represent the general population. Another limitation of our study was that other risk factors might have been present that were not measured. Only the association but not the causation could be determined. Compared to prospective studies, retrospective studies usually have an inferior level of evidence. The correlation between PASP and pulmonary functions in this study was statistically significant, meaning it was unlikely to have occurred by chance; however, to determine the clinical significance, we believe a clinical practice study is needed.
Conclusions
This was a retrospective study that aimed to analyze the correlation between ILD and PH by analyzing the correlations between lung function parameters and PSAP. ILD was diagnosed by HRCT. PH was diagnosed using echocardiography and RHC. The incidence rate of PH in patients with mixed types of ILD was 25%. We found that significant relationships existed between PASP and DLCO, VC%, FVC% in the patients with ILD, suggesting that PASP was correlated with impaired lung functions, and thus can be used to predict ILD. In addition, no significant difference of PH prevalence was seen between the ANA+ group and the ANA− group. In light of the prevalence of PH in ILD and the correlation between PH and ILD, coupled with a limited therapeutic option for PH in ILD, clinical trials of agents directed at controlling the PASP in ILD seem warranted.
Footnotes
Source of support: Departmental sources
References
- 1.Verschakelen JA, De Wever W. Computed Tomography of the Lung. Springer; Berlin Heidelberg: 2018. Basic anatomy and CT of the normal lung; pp. 3–19. [Google Scholar]
- 2.Zhang G, Zheng X. Practical reviews on the anatomy of the chest. Practical Reviews. 2018:33–54. [Google Scholar]
- 3.Antoniou KM, Margaritopoulos GA, Tomassetti S, et al. Interstitial lung disease. Eur Respir Rev. 2014;23:40–54. doi: 10.1183/09059180.00009113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burchell RK. In: Interstitial Lung Diseases. Gram DW, Milner RJ, Lobetti R, editors. Chapter 32. 2017. [Google Scholar]
- 5.Studer SM, Kaminski N. Towards systems biology of human pulmonary fibrosis. Proc Am Thorac Soc. 2007;4:85–91. doi: 10.1513/pats.200607-139JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ley K, Zarbock A. From lung injury to fibrosis. Nat Med. 2008;14:20–21. doi: 10.1038/nm0108-20. [DOI] [PubMed] [Google Scholar]
- 7.Strieter RM, Mehrad B. New mechanisms of pulmonary fibrosis. Chest. 2009;136:1364–70. doi: 10.1378/chest.09-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sisson TH, Mendez M, Choi K, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:254–63. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen CU, Mellemkjær S, Hilberg O, et al. Pulmonary hypertension in interstitial lung disease: Prevalence, prognosis and 6 min walk test. Respir Med. 2012;106:875–82. doi: 10.1016/j.rmed.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Caminati A, Cassandro R, Harari S. Pulmonary hypertension in chronic interstitial lung diseases. Eur Respir Rev. 2013;22:292–301. doi: 10.1183/09059180.00002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen CU, Mellemkjaer S, Hilberg O, et al. Pulmonary hypertension in interstitial lung disease: Prevalence, prognosis and 6 min walk test. Respir Med. 2012;106:875–82. doi: 10.1016/j.rmed.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Cottin V, Le Pavec J, Prévot G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35(1):105–11. doi: 10.1183/09031936.00038709. [DOI] [PubMed] [Google Scholar]
- 13.Cottin V, Harari S, Humbert M, et al. Pulmonary hypertension in lymphangioleiomyomatosis: characteristics in 20 patients. Eur Respir J. 2012;40:630–40. doi: 10.1183/09031936.00093111. [DOI] [PubMed] [Google Scholar]
- 14.Le Pavec J, Lorillon G, Jais X, et al. Pulmonary Langerhans cell histiocytosis-associated pulmonary hypertension: Clinical characteristics and impact of pulmonary arterial hypertension therapies. Chest. 2012;142:1150–57. doi: 10.1378/chest.11-2490. [DOI] [PubMed] [Google Scholar]
- 15.Nathan SD, Cottin V. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Eur Respir Monogr. 2012;57:148–60. [Google Scholar]
- 16.Shlobin OA, Nathan SD. Management of end-stage sarcoidosis: Pulmonary hypertension and lung transplantation. Eur Respir J. 2012;39:1520–33. doi: 10.1183/09031936.00175511. [DOI] [PubMed] [Google Scholar]
- 17.Cottin V. Treatment of pulmonary hypertension in interstitial lung disease: Do not throw out the baby with the bath water. Eur Respir J. 2013;41:781–83. doi: 10.1183/09031936.00024113. [DOI] [PubMed] [Google Scholar]
- 18.Margaritopoulos GA, Antoniou KM, Wells AU. Comorbidities in interstitial lung diseases. Eur Respir Rev. 2017;26 doi: 10.1183/16000617.0027-2016. pii: 160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habash F, Gurram P, Almomani A, et al. Correlation between echocardiographic pulmonary artery pressure estimates and right heart catheterization measurement in liver transplant candidates. J Cardiovasc Imaging. 2018;26:75–84. doi: 10.4250/jcvi.2018.26.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shorr AF, Wainright JL, Cors CS, et al. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J. 2007;30(4):715–21. doi: 10.1183/09031936.00107206. [DOI] [PubMed] [Google Scholar]
- 21.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:615–21. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan SD, Shlobin OA, Barnett SD, et al. Right ventricular systolic pressure by echocardiography as a predictor of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med. 2008;102:1305–10. doi: 10.1016/j.rmed.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunez B, Sauleda J, Anto JM, et al. Anti-tissue antibodies are related to lung function in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1025–31. doi: 10.1164/rccm.201001-0029OC. [DOI] [PubMed] [Google Scholar]
- 24.Medford ARL. Interstitial lung disease and antinuclear antibody: Consider nonspecific interstitial pneumonia histology and newer antibodies. Chest. 2012;141:1360–61. doi: 10.1378/chest.11-3116. [DOI] [PubMed] [Google Scholar]
- 25.Nadrous HF, Pellikka PA, Krowka MJ, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128:2393–99. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 26.Handa T, Nagai S, Miki S, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. 2006;129:1246–52. doi: 10.1378/chest.129.5.1246. [DOI] [PubMed] [Google Scholar]
- 27.Shorr AF, Wainright JL, Cors CS, et al. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J. 2007;30:715–21. doi: 10.1183/09031936.00107206. [DOI] [PubMed] [Google Scholar]
- 28.Lederer DJ, Arcasoy SM, Wilt JS, et al. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:659–64. doi: 10.1164/rccm.200604-520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL. The impact of pulmonary hypertension on survival in patients with idiopathic pulmonary fibrosis. Chest. 2005;128:616–17S. doi: 10.1378/chest.128.6_suppl.616S-a. [DOI] [PubMed] [Google Scholar]
- 30.Shorr AF, Wainright J, Lettieri C, Helman D. The impact of pulmonary hypertension on survival in patients with idiopathic pulmonary fibrosis listed for lung transplant. Chest. 2007;132:428A. [Google Scholar]
- 31.Lettieri CJ, Nathan SD, Barnett SD, et al. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–52. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 32.Behr J, Furst DE. Pulmonary function tests. Rheumatology (Oxford) 2008;47(Suppl 5):v65–67. doi: 10.1093/rheumatology/ken313. [DOI] [PubMed] [Google Scholar]
- 33.Stevenson B, Thompson G, Watson M, et al. A breath of fresh air: investigating autoantibodies in interstitial lung disease. Pathology. 2019;51:S132. doi: 10.1016/j.pathol.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Rojas-Serrano J, Mejia M, González-Pérez M, et al. Long-term pulmonary function in a cohort of interstitial lung disease patients positive to anti-synthetase antibodies. ATS Journal. 2018;25:197. [Google Scholar]


