Abstract
Calorie restriction or changes in dietary composition can enhance healthy aging, but the inability of most subjects to adhere to chronic and extreme diets, as well as potentially adverse effects, limits their application. We randomized 100 generally healthy participants from the United States into two study arms and tested the effects of a fasting-mimicking diet (FMD)—low in calories, sugars, and protein but high in unsaturated fats—on markers/risk factors associated with aging and age-related diseases. We compared subjects who followed 3 months of an unrestricted diet to subjects who consumed the FMD for 5 consecutive days per month for 3 months. Three FMD cycles reduced body weight, trunk, and total body fat; lowered blood pressure; and decreased insulin-like growth factor 1 (IGF-1). No serious adverse effects were reported. After 3 months, control diet subjects were crossed over to the FMD program, resulting in a total of 71 subjects completing three FMD cycles. A post hoc analysis of subjects from both FMD arms showed that body mass index, blood pressure, fasting glucose, IGF-1, triglycerides, total and low-density lipoprotein cholesterol, and C-reactive protein were more beneficially affected in participants at risk for disease than in subjects who were not at risk. Thus, cycles of a 5-day FMD are safe, feasible, and effective in reducing markers/risk factors for aging and age-related diseases. Larger studies in patients with diagnosed diseases or selected on the basis of risk factors are warranted to confirm the effect of the FMD on disease prevention and treatment.
INTRODUCTION
Metabolic syndrome is defined by co-occurrence of three of five of the following conditions: abdominal obesity, elevated fasting glucose, elevated blood pressure, high serum triglycerides, and low levels of high-density lipoprotein (HDL) cholesterol (1). Affecting 47 million Americans (2), it is associated with a major increase in the risk of cardiovascular disease (CVD) and all-cause mortality (3). Although prolonged fasting or very low calorie fasting-mimicking diets (FMDs) can ameliorate the incidence of diseases such as cancer and multiple sclerosis in mice (4-6), randomized trials to assess fasting’s ability to reduce markers/risk factors for aging and major age-related diseases have not been carried out (7-9). Prolonged fasting, in which only water is consumed for 2 or more days, reduces pro-growth signaling and activates cellular protection mechanisms in organisms ranging from single-cell yeast to mammals (10). In mammals, this is achieved in part by temporarily reducing glucose and circulating insulin-like growth factor 1 (IGF-1), a hormone well studied for its role in metabolism, growth, and development, as well as for its association with aging and cancer (11-16). Severe growth hormone receptor and IGF-1 deficiencies are associated with a reduced risk of cancer, diabetes, and overall mortality in humans (17, 18).
Mice fed periodically with the FMD show extended healthspan and multisystem regeneration, reduced inflammation and cancer incidence, and enhanced cognitive performance (5). Despite its potential for disease prevention and treatment, prolonged fasting is difficult to implement in human subjects and may exacerbate preexisting nutritional deficiencies, making it not feasible and/or safe for children, the elderly, frail individuals, and even most of the healthy adults. We have investigated whether a dietary intervention more practical and safer than fasting could affect markers or risk factors for aging and diseases. To this end, we developed an FMD based on a diet previously tested in animals and designed to achieve effects similar to those caused by fasting on IGF-1, insulin-like growth factor–binding protein 1 (IGFBP-1), glucose, and ketone bodies (17). To prevent nutrient deficiency, this FMD provided between 3000 and 4600 kJ per day, as well as high micronutrient nourishment, to each human subject (5). We also previously showed the safety and feasibility of this intervention in 19 study participants who consumed three monthly cycles of this FMD lasting 5 days each (5).
We now report the results of a randomized controlled trial of 100 subjects, 71 of whom completed three cycles of the FMD either in a randomized phase (n = 39) or after being crossed over from a control diet group to the FMD group (n = 32). We evaluated the effects of the FMD on risk factors and markers for aging, cancer, metabolic syndrome, and CVDs in generally healthy participants ranging from 20 to 70 years of age.
RESULTS
Baseline data for all subjects
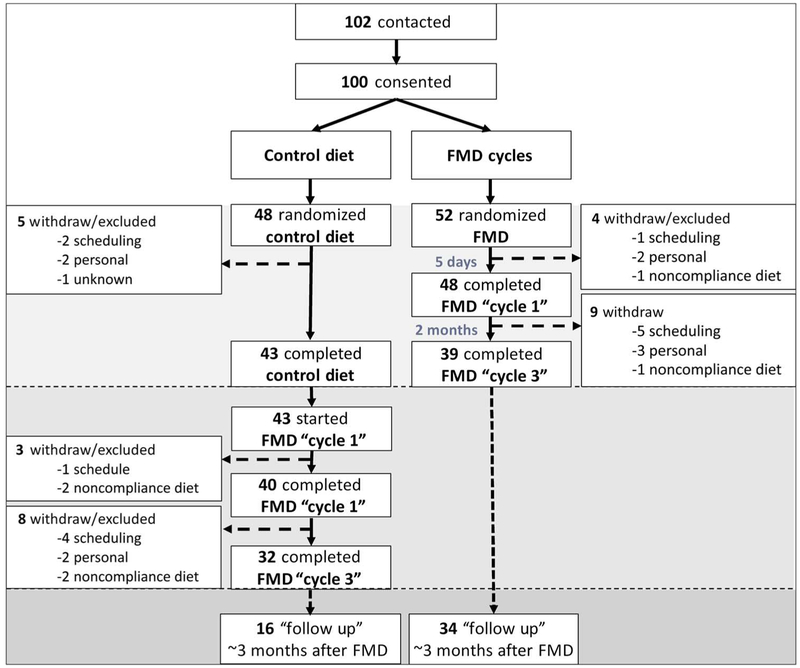
From April 2013 to July 2015, 100 study participants were randomized and assigned to either arm 1 (n = 48) or arm 2 (n = 52). At enrollment, independent of whether they completed the trial or not, subjects in the two arms were comparable for age, sex, race, and body weight (Table 1). Hispanics (27%) were underrepresented in the study population in comparison to their representation (~45%) in the greater Los Angeles area (California) (19). The participants in control arm 1 were asked to continue their normal diet for 3 months, whereas participants in arm 2 started the FMD intervention. Two participants withdrew from arm 1 because of scheduling conflicts before completion of the informed consent. In the randomized comparison (Fig. 1), 18 participants or 5 of 48 (10%) in the control arm and 13 of 52 participants in the FMD arm (25%) were excluded or withdrew from the study. Of the 48 subjects enrolled in the control arm, two withdrew because of scheduling conflicts, two because of unspecified personal issues, and one for unknown reasons. Six of the 52 subjects enrolled in the FMD arm withdrew from the study because of scheduling conflicts, five withdrew because of unspecified personal issues, and two participants were excluded from the study because of noncompliance to the FMD protocol.
Table 1. Characteristics of all subjects at enrollment.
Plus-minus values are means ± SD rounded to the nearest 10th.
| Characteristics | Arm 1 (n = 48) | Arm 2 (n = 52) |
|---|---|---|
| Sex, n (%) | ||
| Male | 18 (37.5) | 19 (36.5) |
| Female | 30 (62.5) | 33 (63.5) |
| Race or ethnic group, n (%)* | ||
| White | 26 (54.2) | 25 (48.1) |
| Black | 2 (4.2) | 5 (9.6) |
| Hispanic | 13 (27.1) | 14 (26.9) |
| Asian | 6 (12.5) | 7 (13.5) |
| Other | 1 (2.1) | 1 (1.9) |
| Age (years) | 42.2 ± 12.5 | 43.3 ± 11.7 |
| Weight (kg) | 77.0 ± 15.9 | 74.3 ± 16.6 |
| Education (years) | 16.7 ± 2.8 | 16.6 ± 2.3 |
| Smoking status, n (%) | ||
| Never smoked | 29 (60.4) | 39 (75.0) |
| Former smoker | 13 (27.1) | 9 (17.3) |
| Current smoker | 6 (12.5) | 4 (7.7) |
| BMI, n (%)† | ||
| Mean | 27.8 ± 5.1 | 26.6 ± 4.9 |
| <25 | 17 (35.4) | 20 (38.4) |
| 25–30 | 18 (37.5) | 21 (40.4) |
| >30 | 13 (27.1) | 11 (21.2) |
| Systolic blood pressure (mmHg) | 117.2 ± 12.3 | 117.2 ± 13.0 |
| Diastolic blood pressure (mmHg) | 75.6 ± 9.2 | 75.2 ± 7.8 |
| Triglycerides (mg/dl) | 104.0 ± 64.6 | 84.7 ± 37.2 |
| Cholesterol (mg/dl) | ||
| Total | 197.5 ± 39.6 | 185.7 ± 36.6 |
| LDL | 114.5 ± 36.1 | 110.3 ± 61.6 |
| HDL | 62.2 ± 16.4 | 65.2 ± 18.1 |
The race or ethnic group was assigned by the subjects themselves.
The BMI is the weight in kilograms divided by the square of the height in meters.
Fig. 1. CONSORT diagram.
Consolidated Standards of Reporting Trials (CONSORT) diagram of 102 contacted subjects of which 100 were enrolled into the study two arms. Arm 1 (n = 48), the “control” group, maintained their normal caloric intake for a 3-month monitoring period. Data were collected at enrollment and again after 3 months. Participants in arm 2 (n = 52) started the FMD after randomization. The FMD is provided for 5 days per month for three consecutive cycles. Data were collected at enrollment, at the completion of the first FMD cycle but before resuming normal dietary intake, and also on average 5 days after subjects resumed their normal diet after the final FMD cycle. After the initial 3-month period, subjects in arm 1 also started the FMD. An optional follow-up visit in the clinic for analysis was offered to all participants about 3 months after the completion of the third FMD cycle.
Adverse effects and safety
Following the Common Terminology Criteria for Adverse Events (CTCAE; v4.0), 54 to 100% (depending on the adverse event) of the participants reported no adverse effects during the FMD cycles (fig. S1). The most common self-reported grade 1 (mild) or grade 2 (moderate) symptoms experienced by the participants were fatigue, weakness, and headaches. No adverse effects of grade 3 or higher were reported. A comprehensive metabolic panel that measured changes in metabolic markers and liver and kidney function showed no negative effects of three cycles of the FMD (table S1). In summary, after three cycles of the FMD, subjects reported only some mild and very few moderate side effects.
Baseline risk factors and metabolic markers: Comparison of randomized control and FMD subjects who completed the trial
At baseline, there were no significant differences in metabolic markers or risk factors for age-related diseases and conditions between the subjects who successfully completed the randomized trial in arm 1 (normal diet) and arm 2 (FMD), including body weight (P = 0.39), body mass index (BMI) (P = 0.24), total body fat (P = 0.11), trunk fat (P = 0.087), lean body mass (P = 0.15), waist circumference (P = 0.34), fasting glucose (P = 0.55), IGF-1 (P = 0.51), systolic and diastolic blood pressure (P = 0.60 and P = 0.91, respectively), triglycerides (P = 0.21), and C-reactive protein (CRP) (P = 0.28). The notable exception was that total cholesterol (P = 0.014) and low-density lipoprotein (LDL) (P = 0.024), but not HDL (P = 0.99), were significantly lower at baseline for subjects who were enrolled and completed arm 2 (Table 2). In summary, the values for disease markers and risk factors at baseline were comparable between the control diet and FMD groups, with the exception of total and LDL cholesterol.
Table 2. Biomarker/risk factor changes in subjects who completed the trial.
CI, confidence interval.
| Baseline* |
CTRL: 3 months after baseline |
Efficacy (comparing Δ) P§ |
||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | Mean ± SD | (95% CI) | FMD: 5 days after third FMD cycle |
||||
| Mean ± SD | (95% CI) | P† | Difference: Δ‡ | |||||
| Body weight (kg) | ||||||||
| Control diet, arm 1 | 43 | 77.2 ± 16.5 | (72.1–82.2) | 77.3 ± 17.0 | (72.0–82.5) | 0.72 | 0.1 ± 2.1 | <0.0001∥ |
| FMD, arm 2 | 39 | 74.1 ± 15.5 | (69.3–78.9) | 71.6 ± 14.6 | (67.0–76.1) | <0.0001 | −2.6 ± 2.5 | |
| BMI¶ | ||||||||
| Control diet, arm 1 | 43 | 27.4 ± 4.8 | (25.9–28.9) | 27.4 ± 5.0 | (25.9–28.9) | 0.82 | 0.0 ± 0.7 | <0.0001∥ |
| FMD, arm 2 | 39 | 26.2 ± 4.4 | (24.8–27.6) | 25.3 ± 4.3 | (24.0–26.5) | <0.0001 | −0.9 ± 0.9 | |
| Total body fat** (absolute volume) | ||||||||
| Control diet, arm 1 | 43 | 23,651 ± 8,155 | (21,142–26,161) | 23,607 ± 8,337 | (21,41–26173) | 0.83 | −44 ± 1,365 | 0.0002∥ |
| FMD, arm 2 | 38 | 20,643 ± 8,459 | (17,953–23,332) | 19,249 ± 7,792 | 16,772–21,726) | <0.0001 | −1,393 ± 1,786 | |
| Trunk fat** (absolute volume) | ||||||||
| Control diet, arm 1 | 43 | 8,429 ± 4,742 | (6,969–9,888) | 8,395 ± 4,776 | (6,925–9,865) | 0.83 | −33 ± 1,046 | 0.018 |
| FMD, arm 2 | 38 | 6,573 ± 4,877 | (5,022–8,124) | 5,938 ± 4,295 | (4,572–7,303) | 0.0023 | −636 ± 1,198 | |
| Lean body mass** (relative volume %) | ||||||||
| Control diet, arm 1 | 43 | 63.9 ± 8.2 | (61.4–66.4) | 64.0 ± 8.7 | (61.3–66.7) | 0.64 | 0.1 ± 1.5 | 0.070 |
| FMD, arm 2 | 38 | 66.8 ± 9.6 | (63.7–69.8) | 67.6 ± 9.4 | (64.6–70.6) | 0.016 0.8 ± 2.0 | −0.8 ± 25 | |
| Waist circumference (cm) | ||||||||
| Control diet, arm 1 | 28 | 95.4 ± 14.2 | (89.9–100.9) | 94.6 ± 14.5 | (88.9–100.2) | 0.10 | −0.8 ± 25 | 0.0035∥ |
| FMD, arm 2 | 28 | 92.1 ± 11.2 | (87.9–96.2) | 87.9 ± 120 | (83.5–92.4) | 0.0003 | −4.1 ± 5.2 | |
| Fasting glucose (mg/dl) | ||||||||
| Control diet, arm 1 | 41 | 88.1 ± 8.9 | (85.3–90.9) | 90.3 ± 9.7 | (87.3–93.4) | 0.14 | 2.2 ± 9.5 | 0.27 |
| FMD, arm 2 | 36 | 89.7 ± 8.5 | (86.5–92.1) | 89.0 ± 8.0 | (86.4–91.6) | 0.87 | −0.8 ± 9.9 | |
| IGF-1 (ng/ml) | ||||||||
| Control diet, arm 1 | 41 | 180.2 ± 84.5 | (153.5–2,069) | 188.9 ± 91.0 | (160.2–217.7) | 0.14 | 8.7 ± 36.9 | 0.0017∥ |
| FMD, arm 2 | 38 | 168.6 ± 69.1 | (146.6–190.5) | 146.9 ± 62.3 | (127.0–166.7) | 0.0063 | −21.7 ± 46.2 | |
| Systolic blood pressure (mmHg) | ||||||||
| Control diet, arm 1 | 43 | 116.5 ± 12.3 | (112.7–1,203) | 115.8 ± 13.6 | (111.6–120.0) | 0.60 | −0.7 ± 8.4 | 0.023 |
| FMD, arm 2 | 38 | 118.0 ± 13.4 | (113.7–1,222) | 113.5 ± 13.2 | (109.3–117.7) | <0.0001 | −4.5 ± 6.0 | |
| Diastolic blood pressure (mmHg) | ||||||||
| Control diet, arm 1 | 43 | 75.5 ± 9.6 | (72.5–78.5) | 74.8 ± 10.0 | (71.7–77.9) | 0.45 | −0.7 ± 6.2 | 0.053 |
| FMD, arm 2 | 38 | 75.7 ± 8.0 | (73.2–78.3) | 72.6 ± 8.7 | (70.5–76.0) | 0.0089 | −3.1 ± 4.7 | |
| Triglycerides (mg/dl) | ||||||||
| Control diet, arm 1 | 37 | 100.5 ± 68.2 | (77.7–123.2) | 101.5 ± 57.1 | (82.5–120.6) | 0.85 | 1.0 ± 35.0 | 0.27 |
| FMD, arm 2 | 30 | 83.0 ± 39.5 | (69.1–96.9) | 74.9 ± 37.6 | (61.7–88.2) | 0.19 | −8.1 ± 33.5 | |
| Total cholesterol (mg/dl) | ||||||||
| Control diet, arm 1 | 37 | 195.9 ± 38.9 | (182.9–2,089) | 183.9 ± 35.2 | (172.1–195.6) | 0.0015 | −12.0 ± 21.3 | 0.81 |
| FMD, arm 2 | 30 | 175.3 ± 25.3 | (166.4–1,842) | 164.4 ± 23.4 | (156.1–172.6) | 0.0012 | −10.9 ± 17.0 | |
| LDL cholesterol (mg/dl) | ||||||||
| Control diet, arm 1 | 37 | 111.2 ± 35.6 | (99.4–123.1) | 104.0 ± 31.8 | (93.4–114.6) | 0.018 | −7.2 ± 17.7 | 0.50 |
| FMD, arm 2 | 30 | 94.1 ± 23.0 | (86.0–102.2) | 89.7 ± 22.8 | (81.7–97.7) | 0.13 | −4.4 ± 16.0 | |
| HDL cholesterol (mg/dl) | ||||||||
| Control diet, arm 1 | 37 | 64.3 ± 16.1 | (5.9.2–69.9) | 59.3 ± 14.9 | (54.3–64.3) | 0.0002 | −5.3 ± 7.8 | 0.90 |
| FMD, arm 2 | 30 | 64.8 ± 17.2 | (58.6–70.6) | 59.6 ± 12.8 | (55.1–64.2) | 0.0097 | −5.0 ± 10.0 | |
| C-reactive protein (mg/liter) | ||||||||
| Control diet, arm 1 | 42 | 1.5 ± 1.9 | (0.92–2.11) | 1.9 ± 2.7 | (1.07–2.75) | 0.31 | 0.4 ± 2.5 | 0.27 |
| FMD, arm 2 | 38 | 1.1 ± 1.3 | (0.71–1.52) | 1.0 ± 1.2 | (0.61–1.37) | 0.61 | −0.1 ± 1.5 | |
No significant differences at baseline between arm 1 and arm 2 (FMD), with exception of total cholesterol (P = 0.014) and LDL (P = 0.024).
P values comparing within-group changes were calculated using paired two-tailed Student’s t test.
Plus-minus values are means ± SD rounded to the nearest tenth.
Between-arm comparison was calculated using two-tailed two-sample equal variance t tests. Using the Benjamini-Hochberg method for controlling the FDR of 0.05.
P values indicate that the null hypothesis of no difference between the control diet (arm 1) to FMD (arm 2) can be rejected.
The BMI is the weight in kilograms divided by the square of the height in meters.
Analyzed by DEXA.
Changes in risk factors and metabolic markers: Comparison of randomized control and FMD groups
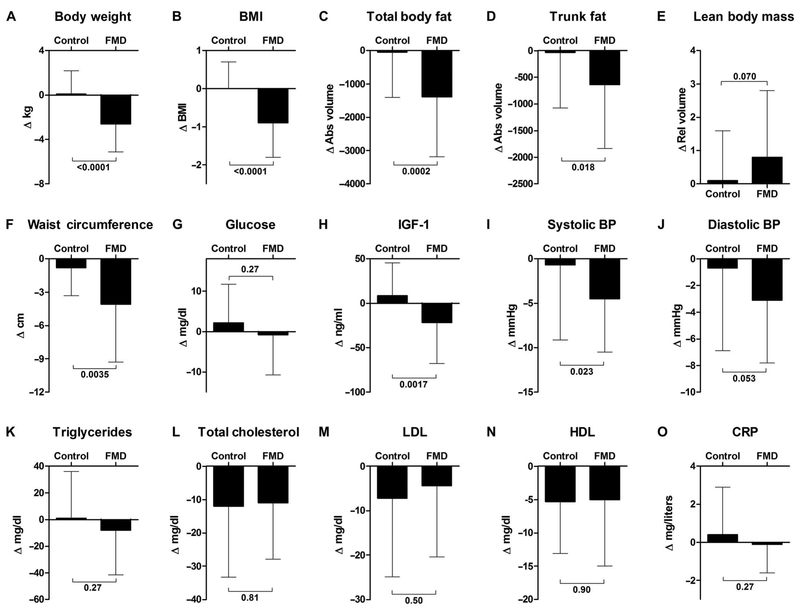
Next, we evaluated the effects of the FMD by assessing the changes in marker/risk factor values between baseline and 5 to 7 days after the end of the third cycle of the FMD and compared them to those occurring in the control arm within the same 3-month period (Fig. 2, Table 2, and table S2). Participants in the FMD arm (arm 2) lost on average 2.6 ± 2.5 kg (±SD) (P < 0.0001) of weight, which was due in part to a reduction in total body fat (absolute values and relative volume % of total mass) and trunk fat (absolute values) (Table 2 and table S2). Subjects on the control diet did not lose body weight (0.1 ± 2.1 kg). After controlling the false discovery rate (FDR) of 0.05 between the control and FMD groups, no change in the percentage of lean body mass was observed (relative to the total mass; P = 0.07), although absolute lean body mass was reduced in arm 2 (P = 0.004) (Table 2 and table S2). Waist circumference measured after three FMD cycles was reduced by 4.1 ± 5.2 cm (P = 0.0035 between groups). The FMD cycles also resulted in a decrease in IGF-1 concentrations of 21.7 ± 46.2 ng/ml (P = 0.0017 between groups). Systolic blood pressure was reduced by 4.5 ± 6.0 mmHg (P = 0.023 between groups), and diastolic blood pressure was reduced by 3.1 ± 4.7 mmHg (P = 0.053 between groups). Fasting glucose (P = 0.27), triglycerides (P = 0.27), cholesterol (total, P = 0.81; LDL, P = 0.50; HDL, P = 0.90), and the acute-phase inflammatory marker CRP (P = 0.27) did not differ significantly between groups. A graphical summary of these data is presented in Fig. 2. In conclusion, three cycles of the FMD reduced body weight, trunk and total body fat, blood pressure, and IGF-1 in comparison to a normal diet.
Fig. 2. Change analysis of metabolic variables during the randomization.
Effects on aging/disease markers and risk factors in all subjects who completed the randomized analysis in either the control arm or the FMD arm (5 to 7 days after the third cycle of FMD). (A) Body weight, (B) BMI, (C) total body fat, (D) trunk fat, (E) lean body mass, (F) waist circumference, (G) serum glucose level, (H) insulin-like growth factor 1, (I) systolic blood pressure, (J) diastolic blood pressure, (K) triglycerides, (L) total cholesterol, (M) LDL, (N) HDL, and (O) CRP were measured in both cohorts as described. The Δ change represents a comparison to baseline. All data are means ± SD. Between-arm comparisons were calculated using two-tailed two-sample equal variance t tests. For some of the 100 enrolled participants, the nurses were unable to collect all the samples/measurements from all subjects. We therefore excluded subjects with incomplete measurements from a particular marker group (see Table 2 for details). Abs, absolute; Rel, relative; BP, blood pressure.
Changes in risk factors and metabolic markers of age-related diseases and conditions: Observational pre-post FMD comparison
After 3 months, 43 subjects from the control arm were crossed over to the FMD intervention. Eleven (26%) of these subjects withdrew before completing three FMD cycles (Fig. 1). Five of these participants withdrew because of scheduling issues, and two subjects opted to leave the trial for unspecificpersonal reasons. We also excluded four participants based on nonadherence to the FMD protocol. The causes for withdrawal/exclusion were comparable between the arms. Considering both FMD treatment arms, 24 of the 95 participants (25%) were excluded or withdrew from the study before completion of the three FMD cycles (arm 2, n = 13 FMDs; arm 1, n = 11 after FMD crossover) because of scheduling conflicts (total, n = 11: arm 2, n = 6 FMDs; arm 1, n = 5 after FMD crossover), personal issues (total, n = 7: arm2, n = 5 FMDs; arm 1, n = 2 after FMD crossover), or dislike of the diet and/or nonadherence to the dietary protocol (total, n = 6: arm 2, n = 2 FMD; arm 1, n = 4 after FMD crossover) (Fig. 1). The 25% dropout rate for participants during the FMD is higher than the 10% dropout rate observed during control diet in arm 1, but this is expected considering that subjects in control diet group only dropped out because of scheduling conflicts because they were allowed to remain on their normal diet. Ninety-five (95%) subjects completed one cycle, and 71 (71%) subjects completed three cycles of the FMD. Compared to the 71 participants who completed the three FMD cycles in arms 1 and 2, the 24 subjects who dropped out were not different in age (42.5 ± 11.6 years versus 43.3 ± 13.1 years) or BMI (27.1 ± 4.9 versus 26.9 ± 4.7) but were mostly female (18% male versus 82% female; P = 0.0045, Fisher’s exact test; fig. S2).
Because the differential dropout rate during the FMD treatment period (25% in FMD in the randomized arm 2 and/or after arm 1 crossover versus 10% in the randomized arm 1 control) may have caused biases in estimates of the FMD treatment effect, we compared the changes in trial outcomes between the two groups who completed three FMD cycles (n = 39 FMD randomized arm 2 and n = 32 after arm 1 crossover to FMD) using sensitivity analysis. Three FMD cycles had comparable effects between subjects in arm 1 (after crossover) and arm 2 (randomized) with the exception of HDL, which underwent a greater reduction in arm 2 (P = 0.03), and the decrease in absolute lean body mass, which was observed in arm 2 but not arm 1 (table S2). Because the FMD had similar effects in both arms, we combined the results from the two arms to assess the changes in metabolites and risk factors during the first FMD cycle (at day 5 of the FMD and before refeeding; table S3) and after completion of three FMD cycles (5 to 7 days after completing the third FMD cycle; table S2).
At the end of the first FMD cycle and before resuming the normal diet, body weight (P < 0.0001), BMI (P < 0.0001), absolute lean body mass (P < 0.0001), waist circumference (P < 0.0001), fasting glucose (P < 0.0001), IGF-1 (P < 0.0001), diastolic blood pressure (P < 0.0003), triglycerides (P < 0.0001), and LDL (P < 0.0026) were significantly reduced compared to baseline. In contrast, relative lean body mass (P = 0.02), β-hydroxybutyrate (P < 0.0001), and IGFBP-1 (P < 0.0001) were increased. Both absolute and relative total body fat (P = 0.075 and P = 0.047, respectively), systolic blood pressure (P = 0.076), as well as CRP (P = 0.75) were not significantly changed after completion of the first FMD cycle compared to baseline (table S3). These results indicate that subjects did follow the dietary changes imposed by the FMD and responded to them as anticipated.
In subjects who completed three FMD cycles (combining both FMD arms) and who returned to the normal diet for 5 to 7 days, body weight (P < 0.0001; n = 71), BMI (P < 0.0001; n = 71), total body fat (absolute, P < 0.0001; relative, P < 0.0001; n = 70), trunk fat (absolute, P < 0.001; relative, P = 0.0002; n = 70), absolute lean body mass (P = 0.0001; n = 70), waist circumference (P < 0.0001; n = 52), IGF-1 (P < 0.0001; n = 69), systolic and diastolic blood pressure (P < 0.0001 and P < 0.0004, respectively; n = 70), total cholesterol (P = 0.004; n = 55), LDL (P < 0.0011; n = 55), and HDL (P = 0.02; n = 55) were significantly reduced, and relative lean body mass (P = 0.0002; n = 70) was increased. Fasting glucose (P = 0.28; n = 66), β-hydroxybutyrate (P = 0.23; n = 69), IGFBP-1 (P = 0.84; n = 69), triglycerides (P = 0.16; n = 55), and CRP (P = 0.052; n = 69) were not significantly changed (table S2). In summary, the combined FMD groups from arms 1 and 2 confirmed that the FMD cycles promoted potent effects on many metabolic markers and disease risk factors, which are maintained after subjects return to their normal diet.
FMD effects stratified by baseline risk factor values: A post hoc observational pre-post FMD comparison
Age-related physiological changes that lead to increased risk factors occur before diseases can be diagnosed (20, 21). We used the aggregated FMD data of both study arms and performed a post hoc analysis of the FMD effect on risk factors for CVD and metabolic syndrome, defined as three of five of the following conditions: abdominal obesity, elevated fasting glucose, elevated blood pressure, high serum triglycerides, and low HDL cholesterol (1). We selected clinically relevant cutoffs and compared normal and at-risk subjects for each risk factor: total cholesterol >199 mg/dl and LDL cholesterol levels >130 mg/dl are associated with an increased risk for CVD (22), a fasting glucose >99 mg/dl indicates impaired fasting glucose/prediabetes (23), and triglyceride levels >100 mg/dl (24) as well as CRP >1 mg/liter are associated with increased risk for CVD (25). For serum IGF-1, no clinically relevant risk level has been established, but a number of epidemiological studies have associated IGF-1 levels above 200 ng/ml with various cancers (17, 26). We therefore compared the effect of FMD cycles on subjects in the highest quartile of IGF-1 expression (>225 ng/ml) with that on subjects with IGF-1 levels ≤225 ng/ml.
In a post hoc analysis, we tested how the changes in the FMD normal and at-risk subgroups compared to those in the control diet normal and at-risk subgroups, as defined by their baseline levels of various risk factors (Table 3). We observed a benefit of the FMD, but not in the control arm, on BMI in all BMI subgroups (P value for interaction = 0.03), although the FMD was particularly beneficial among subjects who were obese (BMI >30) at baseline. The FMD-dependent reduction in IGF-1 was also larger in participants with baseline IGF-1 ≥225 ng/ml (P value for interaction = 0.018).
Table 3. Post hoc comparisons for changes in risk factors for age-related diseases and conditions by baseline subgroups.
| SubGroup | Group differences (FMD – Control) Mean (95% CI) |
Within subgroup P |
Interaction P |
|---|---|---|---|
| BMI | |||
| <25 | −0.6 (−1.2 to −0.05) | 0.03 | 0.03 |
| 25–30 | −0.8 (−1.4 to −0.3) | 0.003 | |
| >30 | −1.9 (−2.6 to −1.1) | 0.0009 | |
| Systolic blood pressure (mmHg) | |||
| <120 | −3.4 (−7.2 to 0.5) | 0.086 | 0.80 |
| ≥120 | −4.3 (−10.4 to 1.8) | 0.17 | |
| Diastolic blood pressure (mmHg) | |||
| <80 | −2.5 (−5.3 to 0.3) | 0.08 | 0.87 |
| ≥80 | −3.0 (−8.2 to 2.3) | 0.26 | |
| Fasting glucose (mg/dl) | |||
| <99 | −0.8 (−5.2 to 3.6) | 0.72 | 0.12 |
| ≥99 | −11.7 (−25.0 to 1.5) | 0.08 | |
| IGF-1 (ng/ml) | |||
| <225 | −18.7 (−38.6 to 1.2) | 0.065 | 0.018 |
| ≥225 | −70.9 (−109.3 to −32.6) | 0.0004 | |
| Triglycerides (mg/dl) | |||
| <100 | −4.6 (−24.1 to 15.0) | 0.64 | 0.38 |
| ≥100 | −19.1 (−45.8 to 7.6) | 0.16 | |
| Cholesterol (mg/dl) | |||
| Total, <199 | −1.8 (−12.6 to 9.0) | 0.73 | 0.88 |
| Total, ≥199 | −0.2 (−18.2 to 17.7) | 0.98 | |
| LDL, <199 total cholesterol | 1.0 (−8.8 to 10.8) | 0.84 | 0.60 |
| LDL, ≥199 total Cholesterol | 6.2 (−11.2 to 23.6) | 0.48 | |
| HDL, <50 | −1.2 (−9.0 to 0.5) | 0.75 | 0.70 |
| HDL, ≥50 | 0.5 (−4.2 to 5.2) | 0.83 | |
| CRP (mg/liter) | |||
| <1 | −0.4 (−1.5 to 8.7) | 0.47 | 0.59 |
| ≥1 | −0.9 (−2.4 to 0.6) | 0.24 | |
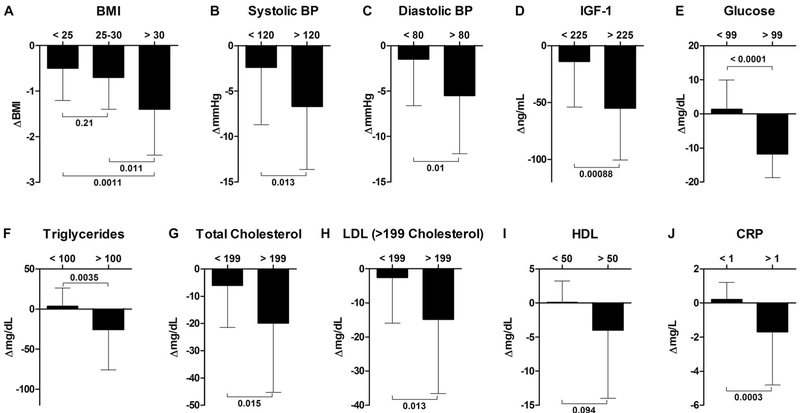
Next, we evaluated the effect size, that is, efficacy in normal and at-risk subjects (Table 4) in subjects stratified by risk factor. Subjects with a BMI of greater than 30 (obese) experienced a greater reduction in BMI by the end of the three FMD cycles than those with a BMI of less than 25 (P = 0.011 between groups) and BMI of 25 to 30 (P = 0.0011 between groups). Systolic blood pressure was reduced by 2.4 ± 6.3 mmHg in subjects with baseline systolic blood pressure ≤120 mmHg but by 6.7 ± 6.9 mmHg in subjects with systolic blood pressure >120 mmHg (P = 0.013 between groups), and diastolic blood pressure was reduced by 1.5 ± 5.1 mmHg in subjects with diastolic blood pressure ≤80 mmHg but by 5.5 ± 6.4 mmHg in those with baseline levels above 80 mmHg (P = 0.01 between groups). Fasting glucose did not change for participants with baseline levels ≤99 mg/dl but was reduced by 11.8 ± 6.9 mg/dl in participants with baseline fasting glucose >99 mg/dl (P < 0.0001 between groups); notably, this reduction brought glucose in these subjects within the healthy range. IGF-1 levels in subjects with baseline levels above 225 ng/ml were reduced by 55.1 ± 45.6 ng/ml, nearly four times more (P < 0.001 between groups) than the 14.1 ± 39.9 ng/ml reduction observed in participants with IGF-1 concentrations below 225 ng/ml. Triglyceride levels were reduced more in participants with baseline levels >100 mg/dl (P = 0.0035 between groups). Total cholesterol was reduced significantly more in participants with total cholesterol higher than 199 mg/dl at baseline (P = 0.015 between groups). LDL was reduced by 14.9 ± 21.7 mg/dl in those with total cholesterol baseline levels above 199 mg/dl but was not reduced by FMD cycles in normal-range subjects (P = 0.013 between groups). There was no reduction (P = 0.094 between groups) in HDL levels for those study participants with HDL levels below or above 50 mg/dl at baseline. CRP was not reduced for subjects with levels below 1 mg/liter but was reduced by 1.6 ± 1.3 mg/liter and returned to the normal levels in most subjects with baseline CRP higher than 1 mg/liter (P = 0.0003 between groups). A graphical summary of these data is presented in Fig. 3; before-after dot plots of individual subjects in the control cohort as well as in normal and at-risk subjects in the FMD cohort are presented in fig. S3.
Table 4. Post-hoc analysis of risk factors for age-related diseases and conditions in at-risk subjects who completed the FMD trial.
| Variable (at baseline) |
n | Baseline |
FMD: 5 days after third FMD |
Efficacy (comparing Δ) P‡ |
||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | (95% CI) | Mean ± SD | (95% CI) | P* | Δ† | |||
| BMI§ | ||||||||
| <25 | 27 | 22.4 ± 1.7 | (21.68–23.03) | 21.9 ± 1.6 | (21.24–22.52) | 0.0014 | −0.5 ± 0.7 | 0.0020∥ |
| 25–30 | 30 | 27.1 ± 1.4 | (26.58–27.60) | 26.4 ± 1.6 | (25.76–26.99) | <0.0001 | −0.7 ± 0.7 | 0.21 |
| >30 | 14 | 34.4 ± 3.5 | (32.37–36.36) | 33.0 ± 3.5 | (30.95–34.95) | 0.0001 | −1.4 ± 1.0 | 0.011 |
| Systolic blood pressure (mmHg) | ||||||||
| <120 | 49 | 110.3 ± 6.9 | (108.5–112.4) | 107.9 ± 7.7 | (105. 7–110.1) | 0.0089 | −2.4 ± 6.3 | 0.013 |
| >120 | 21 | 133.4 ± 9.0 | (129.3–137.5) | 126.7 ± 11.3 | (121.5–131.8) | 0.0002 | −6.7 ± 6.9 | |
| Diastolic blood pressure (mmHg) | ||||||||
| <80 | 53 | 71.8 ± 5.0 | (70.43–73.21) | 70.3 ± 5.9 | (68.64–71.89) | 0.032 | −1.5 ± 5.1 | 0.010 |
| >80 | 17 | 87.7 ± 6.9 | (84.20–91.27) | 82.2 ± 10.0 | (77.09–87.38) | 0.0026 | −5.5 ± 6.4 | |
| Fasting glucose (mg/dl) | ||||||||
| <99 | 53 | 87.7 ± 6.4 | (85.97–89.50) | 89.0 ± 8.2 | (86. 75–91.25) | 0.29 | 1.3 ± 8.6 | <0.0001 |
| >99 | 13 | 104.2 ± 4.4 | (101.5–106.9) | 92.4 ± 8.2 | (87.42–97.35) | <0.0001 | −11.8 ± 6.9 | |
| IGF-1 (ng/ml) | ||||||||
| <225 | 52 | 145.1 ± 38.4 | (134.4–155.8) | 131.0 ± 46.8 | (118.0–144.0) | 0.014 | −14.1 ± 39.9 | 0.00088 |
| >225 | 17 | 286.6 ± 47.0 | (261.6–311.7) | 231.5 ± 64.7 | (197.0–265.9) | 0.0002 | −55.1 ± 45.6 | |
| Triglycerides (mg/dl) | ||||||||
| <100 | 34 | 65.8 ± 18.4 | (59.45–72.09) | 69.5 ± 28.9 | (59.58–79.45) | 0.33 | 3.7 ± 22.3 | 0.0035 |
| >100 | 21 | 149.3 ± 41.2 | (127.9–160.8) | 123.7 ± 52.7 | (97.54 –148.0) | 0.058 | −25.6 ± 50.2 | |
| Cholesterol (mg/dl) | ||||||||
| Total, <199 | 40 | 170.3 ± 18.3 | (164.4–176.2) | 164.2 ± 20.2 | (157. 7–170.6) | 0.016 | −6.1 ± 15.3 | 0.015 |
| Total, >199 | 15 | 228.1 ± 23.0 | (215.4–240.9) | 208.2 ± 26.3 | (193.6–222.8) | 0.0088 | −19.9 ± 25.4 | |
| LDL, <199 total cholesterol | 40 | 91.4 ± 20.2 | (85.0 –97.73) | 88.8 ± 21.0 | (82.13–95.38) | 0.22 | −2.6 ± 13.4 | 0.013 |
| LDL, >199 total cholesterol | 15 | 141.1 ± 29.1 | (125.0–157.2) | 126.2 ± 24.7 | (112.2–139.9) | 0.018 | −14.9 ± 21.7 | |
| HDL, <50 | 17 | 44.1 ± 4.1 | (41.94–46.17) | 44.2 ± 4.6 | (41.87–46.6) | 0.82 | 0.1 ± 3.1 | 0.094 |
| HDL, >50 | 38 | 69.6 ± 14.1 | (65.06–74.17) | 65.6 ± 11.6 | (61.81 –69.32) | 0.015 | −4.0 ± 10.0 | |
| CRP (mg/liter) | ||||||||
| <1 | 43 | 0.4 ± 0.3 | (0.28–0.46) | 0.6 ± 0.9 | (0.27–0.85) | 0.20 | 0.2 ± 1.0 | 0.0003 |
| >1 | 26 | 3.3 ± 2.8 | (2.22–4.44) | 1.6 ± 1.2 | (1.06–2.15) | 0.0085 | −1.7 ± 3.1 | |
P values comparing within-group changes were calculated using paired two-tailed Student’s t test.
Plus-minus values are means ± SD rounded to the nearest tenth.
Between-group comparison was calculated using two-tailed two-sample equal variance t tests.
The BMI is the weight in kilograms divided by the square of the height in meters.
One-way analysis of variance for the BMI groups.
Fig. 3. Post hoc analysis of metabolic variables in subgroups identified by severity of risk factors.
Subjects from both study arms who completed three FMD cycles were post hoc stratified on the basis of being in either normal-risk or at-risk subgroups for factors associated with age-related diseases and conditions. The Δ change shown represents comparisons to baseline. All data are means ± SD. Between-arm comparisons were calculated using two-tailed two-sample equal variance t tests. One-way analysis of variance was used for the BMI groups (see Table 4 for details).
This post hoc analysis indicates that the FMD had more pronounced effects in at-risk participants than in those subjects with risk factor values within the normal range, with the exception of HDL. Larger randomized trials are necessary to confirm the results on the efficacy of the FMD in the treatment of patients at risk for diseases.
Voluntary follow-up 3 months after FMD
We invited participants to return on a voluntary basis about 3 months (actual mean follow-up time, 3.2 ± 1.3 months; n = 50) after their third and final FMD cycle. In these subjects, the FMD’s effects on body weight, BMI, waist circumference, glucose (in at-risk subjects), IGF-1, and systolic (in at-risk subjects) and diastolic blood pressure persisted for at least 3 months after the final FMD cycle (table S4). Subjects with low HDL levels at baseline displayed increased HDL levels at the 3-month follow-up, whereas CRP levels remained significantly lower in study participants with baseline CRP levels above 1 mg/liter. Notably, some of the at-risk groups include only a few subjects, and thus, larger studies are needed to establish long-term effects of the FMD on disease risk factors.
These results indicate that some of the beneficial effects of multiple cycles of the FMD may last for several months. Although subjects were not advised to change their diet or exercise regimen after the FMD cycles ended, we cannot rule out that some of the changes after the additional 3 months may be a result of lifestyle changes such as healthier diets and/or improved physical activity after the completion of this trial.
DISCUSSION
This randomized phase 2 trial indicates that three cycles of a 5-day FMD per month are feasible, safe, and effective in reducing body weight, waist circumference and BMI, absolute total body and trunk fat, systolic blood pressure, as well as IGF-1. Metabolic markers such as fasting glucose, triglycerides, and CRP, as well as total, HDL, and LDL cholesterol, which were within the normal range at baseline, were not significantly affected in the randomized comparison after three FMD cycles. After 3 months, subjects from the control arm were crossed over to the FMD intervention. Our post hoc analysis of the aggregated data from all 71 subjects who completed three FMD cycles confirmed the effects of the FMD on trunk and total body fat, blood pressure, and IGF-1. A post hoc analysis also allowed us to analyze subjects with elevated risk factors or metabolic markers associated with metabolic syndrome and age-related diseases, such as high BMI, blood pressure, fasting glucose, triglycerides, CRP, cholesterol, and IGF-1. The FMD had more pronounced effects on all these markers in at-risk participants than in those subjects who had risk factor values within the normal range. Some of these metabolic markers, namely, CRP, systolic/diastolic blood pressure, and serum lipids, have been proposed as markers of biological aging (27). However, other markers affected by the FMD, including IGF-1 and glucose, have been strongly implicated in aging and age-related diseases (5, 18, 28).
Study participants were instructed not to alter their lifestyle for the duration of the trial and were allowed to consume food of their choice during the normal diet periods, that is, subjects were not placed on a prespecified or calorie-restricted diet. We observed changes that were both positive (total cholesterol and LDL) and negative (HDL) in arm 1 subjects during the control diet period, potentially explained by dietary habit changes in anticipation for the FMD, despite no change in weight, BMI, body fat, or lean mass. Similarly, the persistent effects of the FMD observed 3 months after study completion may be affected by changes in dietary habits and/or physical activity. The composition of the diet tested in this trial was based on the FMD that is known to extend healthspan in mice. Similarly to the study in mice (5), we expect the FMD effects to be mostly independent of an overall caloric restriction, because both groups likely consumed similar levels of calories per month: For example, estimating a 9200 kJ diet for each of the 25 to 26 nonrestricted days and about 19,200 kJ kcal for the 5 days of FMD per month, the between-group difference in consumed calories is expected to be about 10%. In addition, this difference may be overestimated because it is likely that subjects have an elevated calorie intake after the FMD period, as we have shown for mice (5). Day 1 of the FMD supplies ~4600 kJ (11% protein, 46% fat, and 43% carbohydrate), whereas days 2 to 5 provide ~3000 kJ per day (9% protein, 44% fat, and 47% carbohydrate); thus, fat and complex carbohydrates are the major source of calories in the FMD.
Our studies in cells and mice indicate that both glucose and proteins interfere with the protective and regenerative effects of fasting (29). Because our previous data indicate that dietary composition can be equally or more important than calorie restriction, it will be important to test the effects of a similarly restricted diet that provides higher proportions of carbohydrates and/or proteins. It remains to be established whether part of the effects of FMD that we observed are mediated by stem cell–based regeneration or rejuvenation, as indicated by our mouse studies (5).
The FMD-induced reduction in serum glucose and IGF-1 is of interest given their role in pro-aging signaling pathways and cancer (17, 30-33). In addition to a marker for insulin resistance and a metabolic input for cancer cells, glucose is associated with cellular sensitization to toxins and senescence (28, 34, 35). Growth hormone receptor deficiency, resulting in reduced IGF-1 levels, is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans (18). The observed reduction in IGF-1 in our study, but not either after 6 months of intermittent energy restriction (IER) (36) or after 6 years of 20% caloric restriction (37), is probably related to the long-lasting effects of the low protein/amino acid content of the FMD (average 5 days of FMD; 11.5% versus 21% IER or 24% long-term CR). In fact, twenty eight vegans consuming a moderately protein-restricted (10%) diet for about 5 years had reduced IGF-1 levels compared to a group that consumed a chronic 20% calorie-restricted diet (37). We also previously showed that reduced IGF-1 levels and reduced cancer risk were associated with low protein consumption in participants of the National Health and Nutrition Examination Survey cohort (17). Specific ingredients, for example, high levels of unsaturated fats and micronutrients, may also positively contribute to some of the beneficial effects of the FMD.
Note that 25% of the subjects who tested the FMD dropped out of the trial, whereas 10% of the participants opted out of the control arm. This indicates that, despite our efforts to reduce the burden of low-calorie/protein diets, adherence to this dietary regimen requires committed study participants. Further, compared to the control diet arm, the FMD arm imposed an additional daylong visit to the clinic, which may have contributed to reduced compliance. Compliance with prescribed therapies, even placebo, may be an identifiable marker for an overall healthy behavior of study participants (38). Thus, this kind of volunteer, who is observing a benefit and thus not dropping out, could introduce potential bias into the analysis of our trial. The overall comparability at baseline between the control and both FMD arms, as well as the comparable response to the FMD (arm 2 and arm 1 after crossover) suggests no major differences in response for those subjects who completed the trial. Further, those subjects who dropped out of this trial were not different in age or BMI compared to those who completed the trial. It remains to be established why we experienced a gender difference (82% of dropouts were female). The 25% overall dropout rate (all causes) of study participants before the completion of the third FMD cycle is in the range observed in other trials aimed at evaluating dietary interventions in adults. For example, 16 weeks of dieting in combination with physical exercise yielded a discontinuation rate of about 30% (39), and a hypocaloric diet in 28 overweight/obese women resulted in a dropout rate of 40% after 6 months (40). In a trial assessing the effect of intermittent energy/carbohydrate restriction and daily energy restriction on weight loss and metabolic disease risk markers in overweight women, Harvie et al. reported a 23% dropout rate (41). Nonetheless, there are limitations of our trial that should be considered: (i) A relatively small number of subjects in the randomized comparison; (ii) despite providing nourishment and calories for the duration of the FMD, we experienced a higher dropout rate during the FMD intervention than in the control arm; (iii) the findings that the FMD reduced metabolic markers more effectively in at-risk subjects are based on a non-randomized post hoc analysis of the individual factors in generally healthy participants, and thus, further evaluation in subjects with diagnosed disease is needed.
Other less restrictive diets such as those requiring a very low calorie intake twice a week would impose 8 days per month of a severe restriction compared to the 5 days per month or per several months of a less restrictive intervention tested here (41). However, an advantage of these diets is that they may not require as much medical supervision as the longer FMD. FMDs or any type of prolonged fasting interventions lasting more than 12 hours, particularly those lasting several days, require supervision, preferably from a health care professional familiar with prolonged fasting. Although our results suggest that cycles of the plant-based FMD might be safe for elderly individuals, additional studies are necessary to determine its safety for subjects who are 70 years and older.
In summary, and with the limitations outlined above, these results indicate that the periodic FMD cycles are effective in improving the levels of an array of metabolic markers/risk factors associated with poor health and aging and with multiple age-related diseases. As suggested by preclinical studies, interventions that promote longevity should also extend healthspan. Further investigations in larger clinical trials focused on subjects with diagnosed metabolic syndrome, diabetes, and CVDs as well as subjects at high risk for developing cancer and other age-related diseases are needed.
MATERIALS AND METHODS
Subjects
One-hundred participants without a diagnosed medical condition in the previous 6 months were enrolled (ClinicalTrials.gov; ). All participants provided written informed consent, and the University of Southern California (USC) Institutional Review Board (IRB) approved the protocol. Recruitment of subjects was based on fliers, the ClinicalTrials.gov and usc.com websites, and/or word of mouth. Because this was a dietary intervention study, it was not possible for participants or all study personnel to be blinded to group assignment. However, study personnel involved in data collection and specimen analysis were blinded to group assignments.
Study design
Flow of participant enrollment and participation was prepared following the CONSORT standards for randomized clinical trials with crossover design. All data were collected at the USC Diabetes and Obesity Research Institute. Subjects were recruited from April 2013 to July 2015 under protocols approved by the USC IRB (HS-12-00391)based on established inclusion (generally healthy adult volunteers and 18 to 70 years of age; BMI, 18.5 and up) and exclusion [any major medical condition or chronic diseases, mental illness, drug dependency, hormone replacement therapy (dehydroepiandrosterone, estrogen, thyroid, and testosterone), pregnant or nursing female, special dietary requirements or food allergies, alcohol dependency, and medications known to affect body weight] criteria. Intention to treat analysis was performed by including all available observations. Eligible participants were randomly assigned using a random-number generator to either arm 1 or arm 2 of the study. All participants completed a health habits questionnaire. Prespecified outcome measures included safety and feasibility, and evaluation of changes in metabolic risk factors for diabetes and CVD and metabolic markers associated with age-related diseases and mortality; these outcomes were measured at baseline during and after completion of the intervention. Laboratory examinations included height, weight, body composition (including total and trunk body fat, soft lean tissue, and bone mineral content) measured by dual-energy x-ray absorptiometry (DEXA), oscillometric blood pressure measurements, and overnight fasting blood draw through venipuncture.
Arm 1 (control).
Participants completed anthropometric measurements and blood collection at enrollment and after 3 months to provide an estimate of non–diet-related changes (Fig. 1). Participants were instructed to maintain their regular eating habits. After 3 months, subjects were crossed over to the experimental FMD group (Fig. 1).
Arm 2 (FMD).
Participants were instructed to consume the FMD, which was provided in a box, for 5 continuous days, and to return to their normal diet after completion until the next cycle that was initiated about 25 days later. Participants completed three cycles of this 5-day FMD (Fig. 1). Participants completed baseline and follow-up examinations at the end of the first FMD (before resuming normal diet to measure the acute FMD effects) and after a washout period of 5 to 7 days of normal caloric intake after the third FMD cycle. An optional follow-up assessment 3 months after the third FMD cycle was offered.
Experimental FMD
The FMD is a plant-based diet designed to attain fasting-like effects on the serum levels of IGF-1, IGFBP-1, glucose, and ketone bodies while providing both macro- and micronutrients to minimize the burden of fasting and adverse effects (5). Day 1 of the FMD supplies ~4600 kJ (11% protein, 46% fat, and 43% carbohydrate), whereas days 2 to 5 provide ~3000 kJ (9% protein, 44% fat, and 47% carbohydrate) per day. The FMD comprises proprietary formulations belonging to USC and L-Nutra (www.prolonfmd.com) of vegetable-based soups, energy bars, energy drinks, chip snacks, tea, and a supplement providing high levels of minerals, vitamins, and essential fatty acids (fig. S4). All items to be consumed per day were individually boxed to allow the subjects to choose when to eat while avoiding accidentally consuming components of the following day.
Common Terminology Criteria for Adverse Events
Study participants were asked about adverse events at each study visit; events were graded according to the general CTCAE guidelines (see the Supplementary Materials for details).
Blood tests and serum markers
Complete metabolic and lipid panels (overnight fasting) were completed at the Clinical Laboratories at the Keck Medical Center of USC and analyzed immediately after the blood draw of each visit (see the Supplementary Materials for details).
Statistical analysis
The primary comparisons of randomized groups involved changes in outcomes observed in the control period of arm 1 versus the changes observed in the FMD group (arm 2) after completion of three FMD cycles. Secondary observational analyses involved (i) comparing the FMD effects in arm 2 (randomized to FMD) versus arm 1 (receiving FMD after completion of the randomized control period) and (ii) summarizing the changes for arms 1 and 2 combined after completion of the first and third FMD cycles. Changes from baseline were normally distributed. Comparison of changes from baseline within the treatment arms was performed using paired two-tailed Student’s t tests, and P values <0.05 were considered significant. The between-arm comparison of treatment changes from baseline was performed using two-tailed two-sample equal variance t tests, and P values <0.05 were considered significant. To control for multiple testing, we used the Benjamini-Hochberg FDR method. All reported P values are nominal two-sided P values; those that met the FDR criteria and remained “significant” at P < 0.05 are indicated with an asterisk.
M.W. generated the random allocation sequence and enrolled and assigned participants to interventions. M.W. was not involved in outcome assessments. For this initial randomized trial, the sample size of 100 total subjects was based on detection of a 25% reduction in mean IGF-1, with a two-sided a of 0.05 and 70% power. The estimated control group mean (SD) IGF-1 of 194 (97) used published data on males and females aged 26 to 40 years (42). Statistical analyses were performed on deidentified data. Baseline information and changes from baseline were summarized using means ± SDs for subjects randomized to the control (arm 1, n = 48) and the diet group (arm 2, n = 52). All subjects are included in the arm assigned regardless of treatment adherence (intention to treat); no attempt was made to impute missing values (primarily because if data after completion of the third FMD cycle were not available, then other measurement time points were usually unavailable).
In post hoc subgroup analyses, we compared FMD-control group differences over the randomized trial period (three FMD cycles versus control) within high/lower-risk subgroups and tested whether those treatment effects differed in the higher-risk versus lower-risk groups. This subgroup analysis was completed using analysis of variance, with main effects of treatment (FMD and control) and risk group (high and low); the interaction of treatment-by-risk group tested whether the randomized FMD effect differed in high-risk versus low-risk groups. In observational analyses of the pre-post FMD changes combining the two treatment arms, pre-post changes in markers within risk subgroups were tested using paired t test; pre-post changes over risk subgroups were compared using two-sample t test or analysis of variance.
Supplementary Material
Table S1. Complete metabolic panel.
Table S2. Arm-specific markers of adherence and changes in risk factors, including arm 1 after crossover to FMD, and summary of FMD arms 1 and 2.
Table S3. Changes in risk factors and metabolic markers of adherence after the first FMD.
Table S4. Changes in risk factors and metabolic markers of adherence 3 months after intervention.
Fig. S1. Subject self-reported adverse effects based on CTCAE.
Fig. S2. Comparison of participants who completed the trial versus dropouts.
Fig. S3. Baseline to 3 months before/after comparison of individual subjects in the control cohort and all subjects who completed the FMD.
Fig. S4. Nutritional information of the FMD.
Acknowledgments:
We thank Y. Guan for help in data curation and acknowledge the technical expertise of R. Buono (USC Leonard Davis School of Gerontology).
Funding:
Funding was provided by the USC Edna Jones chair fund to V.D.L. W.J.M.’s contributions were provided through the Southern California Clinical and Translational Science Institute supported by NIH UL1TR001855.
Footnotes
Competing interests: The experimental FMD was provided by L-Nutra Inc. The funding sources had no involvement in study design; collection, analysis, and interpretation of data; writing of the report; or decision to submit the article for publication. The USC has licensed intellectual property to L-Nutra that is under study in this research.As part of this license agreement, the University has the potential to receive royalty payments from L-Nutra. V.D.L. and T.E.M., who have equity interest in L-Nutra, did not participate in the collection and analysis of the data. One-hundred percent of V.D.L.’s equity will be assigned to the nonprofit foundation Create Cures. U.S. patents 20140227373A1 (“Methods and diets to protect against chemotoxicity and age related illnesses”) and 20140112909A1 (“Methods and formulations promoting tissue regeneration, longevity and healthspan”) related to the work described here have been filed.
Data and materials availability: Use of the FMD may require a material transfer agreement.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/9/377/eaai8700/DC1
Materials and Methods
CONSORT checklist
Trial protocols
REFERENCES AND NOTES
- 1.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM, Smith SC Jr.; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity, Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Giles WH, Dietz WH, Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 287, 356–359 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM, Metabolic syndrome and risk of incident cardiovascular events and death: A systematic review and meta-analysis of longitudinal studies. J. Am. Coll. Cardiol 49, 403–414 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Choi IY, Piccio L, Childress P, Bollman B, Ghosh A, Brandhorst S, Suarez J,Michalsen A, Cross AH, Morgan TE, Wei M, Paul F, Bock M, Longo VD, A diet mimicking fasting promotes regeneration and reduces autoimmunity and multiple sclerosis symptoms. Cell Rep. 15, 2136–2146 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandhorst S, Choi IY, Wei M, Cheng CW, Sedrakyan S, Navarrete G, Dubeau L,Yap LP, Park R, Vinciguerra M, Biase SD, Mirzaei H, Mirisola MG, Childress P, Ji L,Groshen S, Penna F, Odetti P, Perin L, Conti PS, Ikeno Y, Kennedy BK, Cohen P,Morgan TE, Dorff TB, Longo VD, A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 22, 86–99 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longo VD, Panda S, Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 23, 1048–1059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruce-Keller AJ, Umberger G, McFall R, Mattson MP, Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann. Neurol 45, 8–15 (1999). [PubMed] [Google Scholar]
- 8.Hartman AL, Rubenstein JE, Kossoff EH, Intermittent fasting: A “new” historical strategy for controlling seizures? Epilepsy Res. 104, 275–279 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Müller H, de Toledo FW, Resch KL, Fasting followed by vegetarian diet in patients with rheumatoid arthritis: A systematic review. Scand. J. Rheumatol 30, 1–10 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Longo VD, Mattson MP, Fasting: Molecular mechanisms and clinical applications. Cell Metab. 19, 181–192 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng C-W, Adams GB, Perin L, Wei M, Zhou X, Lam BS, Sacco SD, Mirisola M, Quinn DI, Dorff TB, Kopchick JJ, Longo VD, Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 14, 810–823 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonntag WE, Lynch D, Cefalu WT, Ingram RL, Bennett SA, Thornton PL,Khan AS, Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: Inferences from moderate caloric-restricted animals. J. Gerontol. A Biol. Sci. Med. Sci 54, B521–B538 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A, Delayed occurrence of fatal neoplastic diseases in Ames dwarf mice: Correlation to extended longevity. J. Gerontol. A Biol. Sci. Med. Sci 58, 291–296 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Flurkey K, Papaconstantinou J, Miller RA, Harrison DE, Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc. Natl. Acad. Sci. U.S.A 98, 6736–6741 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, Barrett JC, Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res. 57, 4667–4672 (1997). [PubMed] [Google Scholar]
- 16.Bonkowski MS, Dominici FP, Arum O, Rocha JS, Al Regaiey KA, Westbrook R,Spong A, Panici J, Masternak MM, Kopchick JJ, Bartke A, Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLOS ONE 4, e4567 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng C-W, Madia F,Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM, Longo VD, Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 19, 407–417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng C-W, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD, Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med 3, 70ra13 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. www.census.gov.
- 20.Gillman MW, Developmental origins of health and disease. N. Engl. J. Med 353, 1848–1850 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontana L, Kennedy BK, Longo VD, Seals D, Melov S, Medical research: Treat ageing. Nature 511, 405–407 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Nayor M, Vasan RS, Recent update to the US cholesterol treatment guidelines:A comparison with international guidelines. Circulation 133, 1795–1806 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes Association, Diagnosis and classification of diabetes mellitus. Diabetes Care 37 (suppl. 1), S81–S90 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, Lennie TA, Levi M, Mazzone T,Pennathur S, Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 123, 2292–2333 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M,Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F; Centers for Disease Control and Prevention, American Heart Association, Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107, 499–511 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Pollack MN, Insulin, insulin-like growth factors, insulin resistance, and neoplasia. Am. J. Clin. Nutr 86, s820–s822 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Levine ME, Modeling the rate of senescence: Can estimated biological age predict mortality more accurately than chronological age? J. Gerontol. A Biol. Sci. Med. Sci 68, 667–674 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fontana L, Partridge L, Longo VD, Extending healthy life span—From yeast to humans. Science 328, 321–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandhorst S, Wei M, Hwang S, Morgan TE, Longo VD, Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp. Gerontol 48, 1120–1128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M, Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 363, 1346–1353 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M, Giovannucci E, Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J. Natl. Cancer Inst 94, 1099–1106 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Allen NE, Roddam AW, Allen DS, Fentiman IS, dos Santos Silva I, Peto J,Holly JMP, Key TJ, A prospective study of serum insulin-like growth factor-I (IGF-I), IGF-II, IGF-binding protein-3 and breast cancer risk. Br. J. Cancer 92, 1283–1287 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fletcher O, Gibson L, Johnson N, Altmann DR, Holly JMP, Ashworth A, Peto J,dos Santos Silva I, Polymorphisms and circulating levels in the insulin-like growth factor system and risk of breast cancer: A systematic review. Cancer Epidemiol. Biomarkers Prev. 14, 2–19 (2005). [PubMed] [Google Scholar]
- 34.Rapp K, Schroeder J, Klenk J, Ulmer H, Concin H, Diem G, Oberaigner W,Weiland SK, Fasting blood glucose and cancer risk in a cohort of more than 140,000 adults in Austria. Diabetologia 49, 945–952 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Stocks T, Rapp K, Bjørge T, Manjer J, Ulmer H, Selmer R, Lukanova A, Johansen D,Concin H, Tretli S, Hallmans G, Jonsson H, Stattin P, Blood glucose and risk of incident and fatal cancer in the metabolic syndrome and cancer project (Me-Can): Analysis of six prospective cohorts. PLOS Med. 6, e1000201 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J,Jebb SA, Martin B, Cutler RG, Son TG, Maudsley S, Carlson OD, Egan JM,Flyvbjerg A, Howell A, The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes 35, 714–727 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO, Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 7, 681–687 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hays RD, Kravitz RL, Mazel RM, Sherbourne CD, DiMatteo MR, Rogers WH,Greenfield S, The impact of patient adherence on health outcomes for patients with chronic disease in the medical outcomes study. J. Behav. Med 17, 347–360 (1994). [DOI] [PubMed] [Google Scholar]
- 39.Wycherley TP, Noakes M, Clifton PM, Cleanthous X, Keogh JB, Brinkworth GD, A high-protein diet with resistance exercise training improves weight loss and body composition in overweight and obese patients with type 2 diabetes. Diabetes Care 33, 969–976 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Florakis D, Diamanti-Kandarakis E, Katsikis I, Nassis GP, Karkanaki A, Georgopoulos N, Panidis D, Effect of hypocaloric diet plus sibutramine treatment on hormonal and metabolic features in overweight and obese women with polycystic ovary syndrome: A randomized, 24-week study. Int. J. Obes 32, 692–699 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Harvie M, Wright C, Pegington M, McMullan D, Mitchell E, Martin B, Cutler RG, Evans G, Whiteside S, Maudsley S, Camandola S, Wang R, Carlson OD, Egan JM, Mattson MP, Howell A, The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr 110, 1534–1547 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brabant G, von zur Mühlen A,Wüster C, Ranke MB, Kratzsch J, Kiess W, Ketelslegers J-M, Wilhelmsen L, Hulthén L, Saller B, Mattsson A, Wilde J, Schemer R, Kann P, Serum insulin-like growth factor I reference values for an automated chemiluminescence immunoassay system: Results from a multicenter study. Horm. Res 60, 53–60 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Complete metabolic panel.
Table S2. Arm-specific markers of adherence and changes in risk factors, including arm 1 after crossover to FMD, and summary of FMD arms 1 and 2.
Table S3. Changes in risk factors and metabolic markers of adherence after the first FMD.
Table S4. Changes in risk factors and metabolic markers of adherence 3 months after intervention.
Fig. S1. Subject self-reported adverse effects based on CTCAE.
Fig. S2. Comparison of participants who completed the trial versus dropouts.
Fig. S3. Baseline to 3 months before/after comparison of individual subjects in the control cohort and all subjects who completed the FMD.
Fig. S4. Nutritional information of the FMD.