Abstract
Objectives
Despite changes to brain integrity with aging, some functions like basic language processes remain remarkably preserved. One theory for the maintenance of function in light of age-related brain atrophy is the engagement of compensatory brain networks. This study examined age-related changes in the neural networks recruited for simple language comprehension.
Methods
Sixty-five adults (native English-speaking, right-handed, and cognitively normal) aged 17–85 years underwent a functional magnetic resonance imaging (fMRI) reading paradigm and structural scanning. The fMRI data were analyzed using independent component analysis to derive brain networks associated with reading comprehension.
Results
Two typical frontotemporal language networks were identified, and these networks remained relatively stable across the wide age range. In contrast, three attention-related networks showed increased activation with increasing age. Furthermore, the increased recruitment of a dorsal attention network was negatively correlated to gray matter thickness in temporal regions, whereas an anterior frontoparietal network was positively correlated to gray matter thickness in insular regions.
Conclusions
We found evidence that older adults can exert increased effort and recruit additional attentional resources to maintain their reading abilities in light of increased cortical atrophy.
Keywords: Aging, compensation, language, frontal attention, atrophy, functional MRI
INTRODUCTION
Typical Language Network
In aging individuals, language comprehension is critical for maintaining a high quality of life. Reading comprehension requires more than just understanding single words and sentences but additionally calls for constructing and understanding representations of language at its phonological, semantic, syntactic, and thematic levels. The neural basis of these aspects of language comprehension have been extensively explored in healthy, younger adults using functional magnetic resonance imaging (fMRI) (Binder et al., 2000; Friederici, 2012; Hickok & Poeppel, 2007; Price, 2012; Rauschecker & Scott, 2009; Tyler & Marslen-Wilson, 2008). However, since age-related atrophy is well-documented (Fjell et al., 2009; Raz & Rodrigue, 2006; Raz et al., 2004; Storsve et al., 2014), investigating how language comprehension networks may be altered in aging may articulate ways to improve everyday functioning of older adults.
One method of studying comprehension on a neural level is by using fMRI tasks that involve reading or listening to sentences and narratives. Meta-analyses of narrative comprehension tasks in younger, healthy adults report activation of a left-dominant frontotemporal network along the Sylvian fissure that includes the superior and middle temporal gyri, temporoparietal junction, anterior temporal lobes, frontal operculum, and inferior frontal gyrus when compared to a resting baseline (Ferstl et al., 2008; Mar, 2011). Because the focus of reading-based comprehension studies has been on younger adults and developmental studies, it is unclear how brain aging may affect reading comprehension on a neural level.
Age-Related Brain and Cognitive Changes
Structural and functional brain changes during aging are well-established and include gray matter volume reductions with preferential loss in the prefrontal cortex (PFC), temporal and parietal lobes, subcortical gray matter, and the cerebellum (Alexander et al., 2012, 2006; Bergfield et al., 2010; Fjell et al., 2009; Raz & Rodrigue, 2006; Raz et al., 2004; Walhovd et al., 2005). Selective declines in cognitive functions are also associated with the aging process, most notably in working memory, attention, inhibition, and processing speed (Drag & Bieliauskas, 2010; Glisky, 2007; Salthouse, 2010). Despite these changes, older adults’ implicit memory, knowledge storage, and some language abilities, like vocabulary, remain relatively intact, with performance declines in these domains predominately related to increasing difficulty and effortful processing (Grossman et al., 2002; Park & Reuter-Lorenz, 2009; Park et al., 2002; Shafto & Tyler, 2014; Wingfield & Stine-Morrow, 2000).
Age-related behavioral changes in reading comprehension have been reported (Caplan & Waters, 2005; Caplan et al., 2011; DeDe, 2014; Meyer, 1987; Wingfield et al., 2003). At the single word level, older adults read and recognize individual words slower than younger adults (Kliegl et al., 2004; Rayner et al., 2006; Stine-Morrow et al., 2001), likely reflecting overall cognitive slowing (Salthouse, 2010), and make more errors recognizing words with greater phonological neighborhood densities compared to younger adults (Taler et al., 2010). In single word comprehension using predictability paradigms, older adults are generally worse than younger adults at predicting upcoming words in a sentence unless given ample time for processing (DeDe & Flax, 2016). Other studies find that for self-paced reading or listening paradigms at the sentence level, comprehension is slower, but find no age-related decline in syntactic processing (Burke & Shafto, 2008; Caplan & Waters, 2005; Norman et al., 1992). Declines in reading or listening comprehension performance using probes (offline measures) are likely due to declines in working memory (Caplan et al., 2011; Kemtes & Kemper, 1997; Norman et al., 1992; Wingfield & Grossman, 2006). However, comprehension of higher-level sentence meaning remains relatively intact until very old age (Mackenzie, 2000).
To delineate brain activity during language comprehension in older adults, the few existing studies use aurally presented, single-sentence fMRI paradigms. In a study investigating the neural networks engaged in levels of syntactic complexity (Peelle et al., 2009), both young and older adults showed increased activation for syntactic complexity in areas which are typically associated with language processing (Binder et al., 2000; Friederici, 2012; Hickok & Poeppel, 2007; Price, 2012; Rauschecker & Scott, 2009; Tyler & Marslen-Wilson, 2008). However, as the sentences became more syntactically complex, older adults exhibited reduced activation in left inferior frontal regions relative to younger participants, and also recruited middle and superior frontal areas that were not included in the sentence-processing network seen in younger adults (Peelle et al., 2009). A comprehension study using visually presented sentences also found increased activation for complex sentences in dorsal regions of the left inferior frontal gyrus in older compared to younger adults, despite similar behavioral performance (Grossman et al., 2002). These findings suggest that as age-related atrophy affects brain functioning, older adults may recruit additional and specifically frontal brain regions in response to language, in order to maintain similar performance levels to younger adults (Wingfield & Grossman, 2006). These findings are consistent with models of compensatory mechanisms of cognitive aging that propose increases in neural recruitment to compensate for atrophic changes (Cabeza, 2002; Park & Reuter-Lorenz, 2009). Neither of these studies of compensation incorporated atrophic changes in their analyses, which limits the extent to which structure–function relationships can be made.
Changes in Language Network Activation and Age-Related Atrophy
A small body of literature suggests atrophy within left language-related temporal regions in older adults contributes to behavioral difficulties in auditory comprehension behavior, and is also associated with increased neural activity in right temporal and bilateral frontal regions (Eckert et al., 2008; Tyler et al., 2009). Increased prefrontal cortical activation is proposed to be a compensatory mechanism by which the brain maintains, or attempts to maintain, behavior despite declining structural integrity. The Scaffolding Theory of Aging and Cognition (STAC) suggests that as age-related neural declines occur, additional neural pathways are more strongly developed to accomplish particular cognitive goals (Park & Reuter-Lorenz, 2009). This model specifically posits that language abilities are sufficiently maintained with age by supplementary and compensatory brain regions. Thus, the observed relationship between atrophy in language regions and increased frontal cortex activation in response to language processing can provide insight into the possible mechanism by which language abilities are maintained with age.
The Present Study
It is unclear whether age-related structural changes within the language network relate to increases in frontal cortex recruitment in regions outside the frontotemporal language network during language comprehension, as proposed by the STAC model. We aimed to fill this gap in knowledge by examining cortical atrophy and brain activity in a large age range of individuals. We used a passive narrative reading fMRI paradigm, thus minimizing extra-language systems that are present when responses are required, to determine the relationship between age-related atrophy and the recruitment of frontal brain networks. Passive reading or listening paradigms may provide a more accurate representation of the neural resources recruited for language comprehension, as recent evidence suggests response-based language paradigms elicit additional networks that are not recruited during natural comprehension (Campbell et al., 2016; Davis et al., 2014; Hasson et al., 2006; Zhang et al., 2014).
In healthy adults aged 17–85 years using independent component analysis (ICA) techniques to identify neural networks, we sought to expand the Parks and Reuter-Lorenz (2009) scaffolding model of aging to language functioning. ICA methods were used in this study because this approach has been shown to reveal more brain regions showing task-related activation compared to general linear model (GLM)-based techniques (Xu et al., 2013). Further, in ICA the time course of the onset and offset of each task condition is correlated to the time course associated with each functionally connected brain network. Sometimes, regions within one identified network overlap with regions in a separate network, and it has been argued that this likely reflects the occurrence of more than one neural process within the shared regions (Xu et al., 2013). Thus, this technique allows you investigate the dynamic changes in network involvement between task conditions, as opposed to individual voxels or regions that significantly differ in activation in one condition compared to another. Therefore, we predicted similar recruitment of classic frontotemporal language networks across ages, but in response to increasing atrophic changes, older adults would recruit additional frontal lobe networks to support language comprehension. Our study combines whole-brain structural and functional imaging analyses to expand the compensatory aging model to the critical cognitive function of language.
METHODS
Participants
Sixty-five adults aged 17–85 years [43 women; mean (SD) age = 52.57 (20.49); mean (SD) education = 15.91 (3.03) years] participated in this study. These participants represent a convenience sample of participants who were recruited for several neuroimaging longitudinal studies of Alzheimer’s disease across several institutions in the Arizona Alzheimer’s Consortium (Barrow Neurological Institute (BNI) and Banner Sun Health Research Institute). All participants were native English-speaking, right-handed, and cognitively normal with no clinical diagnosis of mild cognitive impairment or dementia. Eligibility for older adult participants included a Mini Mental Status Exam (Folstein et al., 1975) score of ≥25 and no indicators of depression as measured by either the Geriatric Depression Scale (Yesavage et al., 1982) or the Beck Depression Inventory-II (Beck et al., 1996). Older participants were in the parent studies for more than 5 years, and all remained cognitively intact, suggesting that none had clinical symptoms of degenerative disease at the time of data collection. Written informed consent was obtained from the participant in accordance with the individual institutions’ Institutional Review Board guidelines.
fMRI Task Design
The passive reading paradigm consisted of alternating blocks of sentences that formed short stories and blocks of repeating letter strings. Letter strings were used as an active control condition, as opposed to a “rest” block wherein only a visual crosshair is presented, to control for early visual processing and single-letter orthographic representations (Fernandez et al., 2003; Noppeney & Price, 2004; Robertson et al., 2000). Thus, modeling the dynamic network fluctuations to the presentation of the reading blocks compared to the letter blocks should identify networks engaged in processing meaningful language stimuli at the semantic, syntactic, and thematic levels. Five stories, each presented for 24 s in duration, and five baseline letter blocks, each presented for 18 s, were displayed for a total duration of 3 min and 36 s. The stories contained 6.7 sentences with 10.5 words per sentence on average (e.g. “Mr. Jones had to go to New York on business. He lives in Philadelphia … ”). The letter blocks consisted of strings of consonants arranged in similar lengths as the words and sentences in the reading blocks (e.g. “Hhhh ggggg d ddd”). The words and sentence structures within the stories were on average at a Flesch–Kincaid third grade reading level in order to minimize the need for working memory. No responses were required during scanning. To ensure task adherence, all participants were informally asked content questions (e.g. names of characters and thematic content) about the stories after scanning. All participants could recall major story themes; these data were not recorded. The stimuli were presented through Neurolab’s MR-compatible high-resolution goggles and synchronization system in order to sync stimulus delivery with the start of image acquisition. Presentation® software (www.neurobs.com) was used for the creation and delivery of stimuli.
MRI Procedures and Analysis
All scans were performed on a 3T GE Signa HDX scanner (General Electric, Milwaukee, WI, USA) located at BNI in Phoenix,AZ, and using an eight-channel head coil. Echo-planar imaging (EPI), T2*-weighted scans were acquired axially (TE = 30 ms, TR = 3000, field of view = 24 cm, matrix = 64 × 64, 4-mm-thick slices, 0 skip, covering the entire brain, for near isotropic resolution = 3.75 × 3.75 × 4 mm3). High-resolution three-dimensional (3D) T1-weighted anatomical scans were collected using a 3D spoiled gradient recalled echo pulse sequence (TE = Min/Full, Preptime = 300, flip angle = 13, EX = 2, slice thickness = 2 mm, 0 skip between slices, Field of view = 24 cm, in plane resolution = 0.9375 mm2 voxels). Images were acquired in the axial plane. T1 scan duration measured 6 min.
In order to determine the pattern of brain atrophy associated with aging, we generated whole-brain cortical thickness from the T1-weighted images using the Freesurfer 5.3.0 software (http://surfer.nmr.mgh.harvard.edu/) (Dale et al., 1999; Fischl, 2012; Fischl & Dale, 2000). Briefly, this process includes motion correction, skull stripping, intensity normalization, segmentation of the gray matter, and transformation to the Talairach template. Statistical maps were generated using FreeSurfer’s Query, Design, Estimate, Contrast (QDEC) interface. To explore the effects of age on gray matter thickness, we conducted a GLM with participant age as a predictor and gender as a covariate. False-discovery correction at p ≤ .05 was applied to the results to control for multiple comparisons. Mean gray matter thickness was calculated within regions of interest (ROIs) that showed significant thickness decreases with age localized to regions of left hemisphere language network. Mean thickness within ROIs were then used in correlation analyses of gray matter thickness and ICA network analysis.
FMRI data sets were preprocessed using the software package Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm, Wellcome Department of Imaging Neuroscience, London, UK) implemented in MATLAB (MathWorks Inc., Natick, MA, USA). Briefly, all images were spatially realigned to remove motion-related signal changes, normalized to the Montreal Neurological Institute (MNI) EPI template to ensure all images were in a common space, and smoothed with a Gaussian kernel of 4 mm3. All scans were confirmed to have less than 2-mm translational displacement and less than 1° rotational displacement prior to undergoing post-processing. Next, a group ICA was performed using the GIFT toolbox (http://icatb.sourceforge.net) as described by Kim et al. (2009), with each extracted component representing spatial patterns of brain regions that share a similar time course of BOLD (blood oxygen level-dependent) signal change (i.e. functional brain networks). ICA was performed on the participants as a group and second-level analyses were performed to examine age-related differences. The optimal number of networks was determined to be 25 using a modified minimum description length algorithm (Li et al., 2007). A one-sample t-test at an FWE (family-wise error)-corrected threshold (p < .001) was performed on each resulting network to determine the significant regions involved with each identified time course.
To identify artifactual networks (i.e. those related to motion, physiological noise, white matter, etc.), each network spatial map was correlated with SPM8 MNI prior probabilistic maps of white matter and cerebrospinal fluid (CSF) to identify maps related to ventricle signal or other sources of signal artifacts. If the spatial correlation for white matter was greater than r2= .02 or if the correlation for CSF was greater than r2= .05, then the network was discarded (Kim et al., 2009; Stevens et al., 2007). Similarly, networks were discarded if they had less than r2= .025 association with the SPM8 MNI gray matter prior probability map (Stevens et al., 2007). A visual inspection was also performed to identify and remove networks associated with motion artifacts.
To identify networks associated with the experimental design, regressions were performed on the remaining 16 ICA networks’ time courses with the SPM8 GLM design matrix of the reading and letter active control condition onsets convolved with a canonical hemodynamic response function. The beta weights from these regressions reveal the degree to which each network time course correlated with the task time course, alternating between the reading and letter conditions (i.e. a high beta weight indicating a task-related component). Two-tailed, one-sample t-tests [p < .003 with Bonferroni correction (.05/16 = .003)] were performed on the average network beta weights across all participants to identify networks that were significantly and either positively or negatively related to the onset of the reading condition. The resulting significant networks’ beta weights were entered into a partial correlational analysis with participant age, controlling for gender, using bias-corrected and accelerated bootstrapping 95% confidence intervals (CIs) at 1000 iterations (p < .05, reported in brackets) to identify reading-related networks that differed with age. Networks were visually identified by comparing significant network region location and extent to previously described brain networks (Power et al., 2011; Yeo et al., 2011).
Partial correlations were used to compare average gray matter thickness within ROIs and individual participants’ reading network beta weights. All analyses controlled for age and gender and were also bias corrected with accelerated bootstrapping 95% CIs at 1000 iterations (p < .05). Statistical analyses on the network beta weights and average gray matter thickness were performed using IBM SPSS Statistics (Windows version 22.0, Chicago, IL, USA).
RESULTS
Structural Gray Matter Analysis
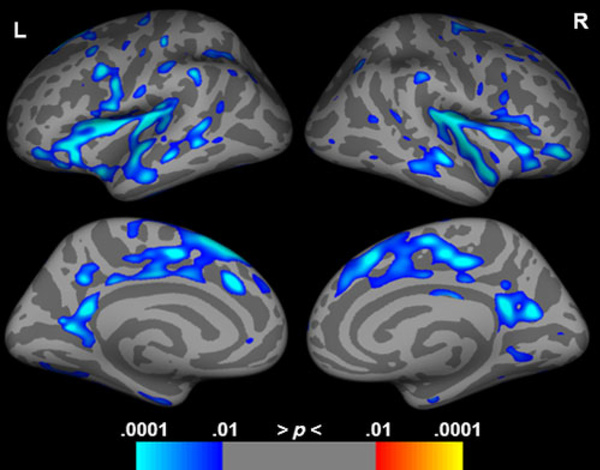
Cortical thickness measurements were obtained from 50 of the participants (age mean (SD) = 48.48 (19.15), age range 17–84 years, 35 females) who had sufficiently high-quality T1 scans, and revealed declines in gray matter thickness associated with advancing age in bilateral insula, portions of the inferior frontal cortices, middle to anterior temporal cortices, and middle to posterior cingulate cortices (Figure 1; Supplementary Table S1), with posterior temporal, parietal, and occipital regions relatively spared.
Fig. 1.
Correlation of gray matter thickness with age, controlling for gender. Cool colors indicate regions of gray matter thickness decreases with increasing age. Results are shown FDR corrected at p < .05.
Network Analysis
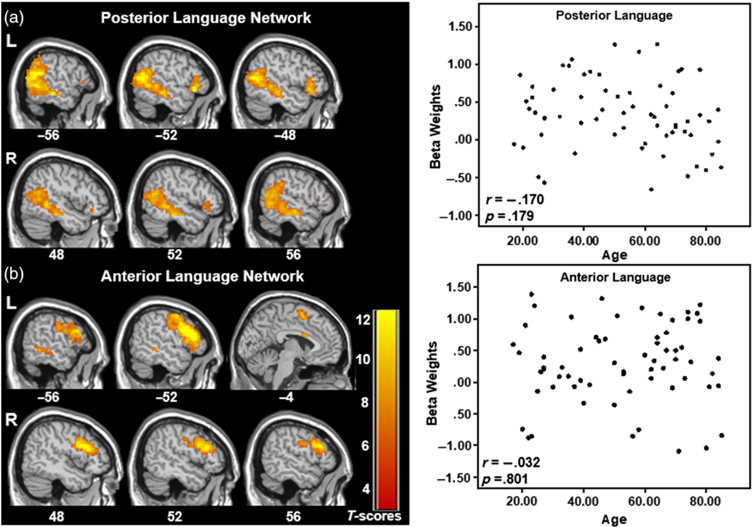
There were two identifiable language comprehension networks that were significantly and positively related to the reading condition but were not significantly correlated with age. The “Posterior Language Network” consisted of a network including bilateral posterior temporal lobe as well as smaller portions of the left and right inferior frontal cortex, with greater and more extensive involvement of the left hemisphere (see Figure 2 top and Table 1). The partial correlation analysis between age and this network’s reading condition beta weights controlling for gender was not significant (r =−.170, CI [−.422, .112], p = .179). The “Anterior Language Network” included bilateral frontal regions, medial superior frontal gyrus, and a region of the left middle temporal gyrus, with greater and more extensive involvement of the left hemisphere (see Figure 2 bottom and Table 1). This network was also not significantly correlated with age, controlling for gender (r = .032, CI [−.250, .336], p = .801). No significant correlations were found when gender was removed as a covariate.
Fig. 2.
Two networks comprising posterior (a) and anterior (b) aspects of typical language brain networks and their corresponding scatterplots of the network’s beta weights with participant age. No significant differences in language network expression with age are observed. Select slices are displayed for each network. The data are shown FWE corrected at p < .001 with 20-voxel cluster threshold. MNI Z-coordinates are displayed beneath each slice.
Table 1.
Regions and peak coordinates for language networks.
| MNI coordinates |
|||||
|---|---|---|---|---|---|
| Region (Brodmann area) | Cluster size | X | Y | Z | Peak T-score |
| Posterior language network | |||||
| L superior temporal gyrus (39) | 1327 | −63 | −55 | 10 | 14.12 |
| L inferior frontal gyrus (47) | 131 | −51 | 23 | −5 | 12.36 |
| L inferior frontal gyrus (45) | −54 | 26 | 10 | 8.79 | |
| R middle temporal gyrus (37) | 1219 | 63 | −55 | 4 | 11.58 |
| R inferior frontal gyrus (47) | 42 | 54 | 20 | −5 | 9.26 |
| L precuneus (31) | 34 | −3 | −58 | 43 | 7.57 |
| R superior frontal gyrus (6) | 25 | 9 | 23 | 63 | 7.49 |
| Anterior language network | |||||
| L inferior frontal gyrus (44) | 1432 | −48 | 20 | 19 | 15.80 |
| L precentral gyrus (6) | −45 | 2 | 31 | 14.70 | |
| R inferior frontal gyrus (44) | 615 | 48 | 20 | 22 | 13.23 |
| L inferior parietal lobule (39) | 247 | −30 | −67 | 43 | 10.50 |
| L medial frontal gyrus (6) | 78 | −3 | 5 | 58 | 9.89 |
| L caudate | 98 | −12 | 5 | 13 | 9.87 |
| L cingulate gyrus (24) | −3 | 8 | 25 | 9.11 | |
| L middle temporal gyrus (21) | 83 | −57 | −43 | −5 | 8.82 |
| R caudate | 45 | 9 | −1 | 13 | 8.72 |
All results presented with FWE-corrected p ≤ .001
In a conventional GLM-based analysis comparing activation in the reading condition to the letter control condition, greater activation to the stories was observed predominately along the left middle temporal gyrus and bilateral calcarine sulci (Supplementary Figure S1 and Supplementary Table S2). Although some differences in the regions identified by each methodology were observed (e.g. left frontal gyrus and primary visual cortex), both ICA and GLM methodologies identified left middle temporal gyrus. The ICA approach identified a greater set of regions related to story reading than the conventional GLM. The differences between these methodologies are not surprising. Since ICA uses a data-driven and hypothesis-free method to determine the spatial structure of underlying networks, ICA differs from GLM approaches such as SPM which adopt a confirmatory analysis method that tests an a priori hypothesis based on a predetermined behavioral response with corrections for violations of multiple comparisons that arise in GLM voxel-based analyses. Because of these differences in assessing BOLD responsivity, ICA will identify regions that may be difficult to hypothesize a priori. Further, ICA is more robust to motion and other artifacts that could make it more sensitive for identifying regions that show a regular but weak BOLD response, resulting in a greater set of regions related to a task. For further information on ICA and its differences to conventional GLM, see Calhoun and de Lacy (2017), Calhoun et al. (2008), James et al. (2014), McKeown et al. (2003), and Xu et al. (2013). Furthermore, regressions of the reading versus letter control GLM contrast with participant age exhibited no voxels that survived multiple comparison corrections (FWE p < .05).
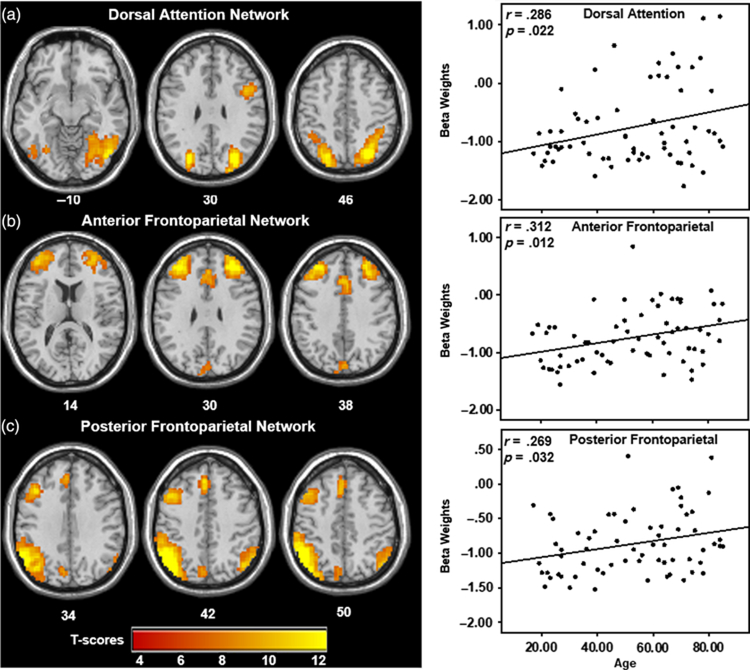
There were three networks that were significantly and negatively related to the reading condition that showed significant positive correlations with age, including gender as a covariate. This indicates that there was increased use of these networks with increased age. Adding education as a covariate did not substantially change the findings below. The dorsal attention network (DAN) showed significant involvement in left and right superior parietal lobe as well as a region in the right inferior frontal gyrus, and was significantly correlated with age, controlling for gender [Figure 3(a) and Table 2; r = .286, CI [.070, .480], p = .022]. The frontoparietal network (FPN) consisted of bilateral prefrontal regions and anterior cingulate cortex [Figure 3(b) and Table 2], corresponding to the anterior regions of the FPN, and was significantly correlated with age, controlling for gender (r = .312, CI [.122, .517], p = .012). The posterior FPN demonstrated involvement predominately in the left inferior parietal lobe extending into the supramarginal gyrus, left middle frontal gyrus, and left medial superior frontal gyrus, and was significantly correlated with age, controlling for gender [Figure 3(c) and Table 2; r = .269, CI [.002, .496], p = .032]. It should be noted that these effects are marginal, in the small to moderate range, and when corrected for multiple comparisons for the five tested networks [p < .01 (Bonferroni .05/5 = .01)], only the relationship between the anterior FPN and age remains significant.
Fig. 3.
Networks significantly and positively correlated with participant age, indicating increased recruitment of three attention networks (a) the DAN, (b) the anterior, and (c) posterior aspects of the FPN with age during the reading paradigm. Scatterplots of the beta weights for the three attention networks significantly and positively correlated with participant age. Plots indicate that older adults recruit attention-related networks during reading to a greater degree than younger adults. Select slices are displayed for each network FWE corrected at p < .001 with 20-voxel cluster threshold. MNI Z-coordinates are displayed beneath each slice.
Table 2.
Regions and peak coordinates for networks significantly correlated with age.
| MNI coordinates |
|||||
|---|---|---|---|---|---|
| Region (Brodmann area) | Cluster size | X | Y | Z | Peak T-score |
| Dorsal attention network | |||||
| R superior occipital gyrus (39) | 2141 | 30 | −76 | 28 | 17.10 |
| L cuneus (39) | 957 | −27 | −76 | 28 | 15.70 |
| R inferior frontal gyrus (44) | 143 | 45 | 8 | 28 | 10.73 |
| R superior frontal gyrus (6) | 42 | 27 | −4 | 52 | 8.95 |
| L fusiform gyrus (19) | 60 | −30 | −67 | −14 | 8.82 |
| Anterior frontoparietal network | |||||
| R middle frontal gyrus (10) | 731 | 33 | 47 | 25 | 15.47 |
| L middle frontal gyrus (10) | 790 | −27 | 44 | 25 | 14.46 |
| R cingulate gyrus (8) | 224 | 6 | 20 | 34 | 11.97 |
| L cuneus (19) | 190 | 3 | −82 | 40 | 9.29 |
| Posterior frontoparietal network | |||||
| L inferior parietal lobule (39) | 1202 | −54 | −61 | 43 | 16.29 |
| L middle frontal gyrus (9) | 871 | −39 | 23 | 37 | 12.80 |
| R inferior parietal lobule (39) | 231 | 57 | −61 | 40 | 11.19 |
All results presented with FWE-corrected p ≤ .001
Structure–Function Correlations
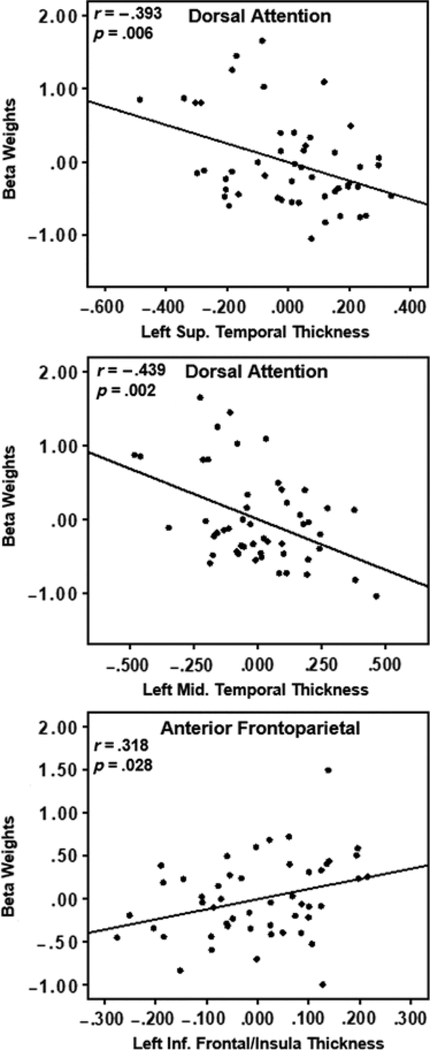
Gray matter atrophy was correlated with the beta weights of reading networks showing age-related changes. The average gray matter thickness within both the left superior temporal and middle temporal ROIs were significantly and negatively correlated with the DAN involvement to the language paradigm (r =−.393, CI [−.575, −.161], p = .006 and r =−.439, CI [−.633, −.181], p = .002, respectively) controlling for age and gender (Figure 4). Additionally, the average gray matter thickness in the left inferior frontal/insula ROI was significantly and positively correlated to the anterior FPN involvement after controlling for age and gender: r = .318, CI [.016, .562], p = .028 (Figure 4). Posterior FPN involvement in the language paradigm was not correlated to gray matter thickness in any ROI (superior temporal ROI: r = .062, CI [−.164, .298], p = .674; middle temporal ROI: r = .129, CI [−.192, .436], p = .383; and inferior frontal/insula ROI: r = .103, CI [−.142, .355], p = .486). Adding education as a covariate did not alter these trends.
Fig. 4.
Partial correlation scatterplots of average gray matter thickness and network beta weights after controlling for participant age and gender. The top two plots show that increasing atrophy in left temporal regions is correlated with increased recruitment of the anterior aspect of the FPN. The bottom plot shows that increasing atrophy in a left inferior frontal region is correlated with decreased recruitment of the posterior aspect of the FPN.
DISCUSSION
Our findings demonstrate that the classically defined language network remains stable with age but that additional networks are recruited to maintain sufficient function. These findings strongly support the STAC (Park & Reuter-Lorenz, 2009). This model posits that cognitive functioning is largely maintained during aging despite significant biological changes through the engagement of secondary neural circuitry. The model specifically addresses the maintenance of language skills through life and posits that this function may have an extensive and overlearned scaffolding network. This is in line with our findings that older adults are still able to engage frontotemporal language networks similar to younger adults in a passive reading fMRI paradigm. However, older adults utilize additional brain networks that are associated with guiding and focused attention while reading to a greater degree than younger adults. Critically, we find that structural declines within portions of the language network may necessitate the recruitment of additional brain networks, like the DAN and FPN, likely to maintain sufficient reading comprehension.
Age-Related Changes in Network Recruitment
As expected, we found no significant age-related differences in the recruitment of two frontotemporal brain networks associated with reading comprehension: a posterior language network and an anterior language network. Our findings agree with previous studies of the language comprehension neural networks across the life span, with the anterior language network overlapping largely with the described dorsal language stream and the posterior language network overlapping with the ventral language stream (Friederici, 2012; Hickok & Poeppel, 2007; Price, 2012; Rauschecker & Scott, 2009; Saur et al., 2010). Both ICA and GLM approaches identified the left middle temporal gyrus, a key region for lexical–semantic processing, as more involved in the reading condition over the letter control condition (Binder et al., 2009; Démonet et al., 2005; Turken & Dronkers, 2011). However, some differences in the regions constituting the language network were observed between ICA and GLM methodologies, namely that the GLM analysis found increased activation in visual cortex and not inferior frontal regions. The inclusion of inferior frontal cortex in the ICA analysis mirrors previous studies that illustrate functional connectivity between major hubs of the language network, particularly the middle temporal and inferior frontal cortical regions (Tomasi & Volkow, 2012b; Turken & Dronkers, 2011). The exclusion of inferior frontal regions within the GLM analysis is to be expected; inferior frontal cortex is typically only observed in fMRI activation comparisons of complex versus simple grammatical structures, conditions which require domaingeneral resources to aid in reading comprehension (e.g. working memory or cognitive control), or paradigms that require a response (Narain et al., 2003; Rogalsky & Hickok, 2011; Rogalsky et al., 2008). In this study, the story condition consisted of grammatically simple sentences with relatively low working memory demands and did not require a response. Further, theGLMcontrast included activation in visual cortex while the ICA approach did not, likely reflecting the particular contrast in the GLM analysis and not traditional language network involvement per se. Previous studies comparing coherent text to letter strings also observe increased activation in visual cortical areas, including calcarine sulcus, reflecting increased visual analysis of words (Choi et al., 2014; Henderson et al., 2015). Therefore, it is likely that activation outside of inferior frontal and superior temporal/inferior parietal regions in response to language comprehension as seen in older adults reflects the need for additional, domain-general cognitive resources.
These findings provide further support for the compensation model that older adults require additional neural resources in an attempt to maintain comparable function to younger adults (Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Cappell, 2008), further suggesting that additional resources are needed for even relatively simple tasks. In the third-grade-level, narrative-based reading paradigm used here, advancing age was associated with increasing recruitment of three additional networks that spatially overlap with brain networks that together comprise the well-defined, task-positive network (Fox et al., 2006). The regions within this network have been characterized as the Multiple-Demand system (Duncan, 2013) or more recently, the Extrinsic Mode Network (EMN; Hugdahl et al., 2015). The ICA technique applied here divide this network into three subnetworks: the DAN and the FPN divided into anterior and posterior aspects of this network. Previous work has also separated these distinct networks from the EMN (Power et al., 2011; Yeo et al., 2011). Important for the current study, the EMN is frequently observed during fMRI tasks associated with higher-order cognitive functions like attention, working memory, and executive control (i.e. allocation of domain-general cognitive resources in response to a challenge) in addition to resting-state fMRI paradigms (Cole & Schneider, 2007; Duncan, 2013; Fox et al., 2006; Hugdahl et al., 2015; Niendam et al., 2012; Owen et al., 2005; Seeley et al., 2007; Vincent et al., 2008). This is consistent with our hypothesis that older adults engage non-language networks during language processing and supports the STAC model.
Age-Related Changes in the DAN
Our finding of age-related differences in engagement of the DAN, which includes bilateral intraparietal sulcus and the right superior frontal gyrus/frontal eye fields (Corbetta & Shulman, 2002), is one of the first reported for reading. Greater activation in bilateral superior frontal gyri or intraparietal sulci has been reported for aurally presented sentences or single words in older adults compared to younger adults (Peelle et al., 2009; Tyler et al., 2009; Wong et al., 2009). However, in a study by Jobard et al. (2007), the authors directly contrast reading text to listening to speech and report activation in the left precentral gyrus and superior parietal lobe, regions within the DAN, as well as bilateral occipital regions, thus suggesting that the DAN engages during reading rather than auditory presentation of language. A recent meta-analysis of word reading and eye movements during fMRI support this notion, demonstrating overlapping activation for reading and eye movements in left superior parietal and precentral regions (Zhou & Shu, 2017). It is possible that the recruitment of the DAN in this study reflects the use of visually presented text and increased use of this network during aging is used to help maintain and orient visual attention to the text.
Alternatively, eye-tracking studies of reading reveal that older adults produce more word fixations, more frequent and longer saccades, and skip words more often than younger adults, and that was not fully explained by visual acuity declines (Gordon et al., 2016; Kliegl et al., 2004; Rayner et al., 2006). Further, older adults are more likely to make regressive eye movements toward initially skipped words, suggesting that their initial interpretation of the passage meaning was incorrect, reflecting a riskier reading strategy (Gordon et al., 2016). The increased recruitment of the DAN during reading with age may instead reflect these differences in reading strategy.
Additionally, this network is reportedly involved in top-down biasing of attention toward goal-related stimuli or events (Corbetta & Shulman, 2002; Fox et al., 2006; Vossel et al., 2014), which may be more important in older individuals who must exert greater effort to perform the task. Madden (2007) has suggested that activation of frontal and parietal regions within theDANin response to cognitive tasks reflects an increased reliance on top-down attentional control in older adults. Functional connectivity within the DAN network decreases with age, which may explain age-related difficulties with sustained attention (Tomasi & Volkow, 2012a). This study adds to the growing literature and suggests aging requires additional brain network engagement to orient one’s locus of attention to and maintain attention on even simple, written text.
Age-Related Changes in the FPN
In our study, the anterior and posterior portions of the FPN, including bilateral prefrontal regions and anterior cingulate cortex in the anterior portions and inferior parietal lobe and left middle and medial superior frontal gyri in the posterior portions, also showed a positive relationship with participant age (Seeley et al., 2007; Vincent et al., 2008). Similar findings of increased FPN activation to visually presented sentences in older adults have been reported for the left dorsal inferior frontal cortex and right posterior temporal-parietal regions (Grossman et al., 2002). Younger adults only expressed this pattern when sentences were long or syntactically complex, reflecting the highest working memory load (Wingfield & Grossman, 2006). Considering our findings of increased FPN involvement, this suggests that older adults may rely upon increased working memory resources while reading even simple passages compared to young adults. In auditory paradigms, older adults were found to maintain increased activation in the anterior cingulate in both clear and degraded speech, overlapping with our reported anterior FPN (Erb & Obleser, 2013). These findings indicate that older adults rely upon attention networks to a greater degree than younger adults regardless of the perceptual difficulty, and that the effortful aspect of language processing is not simply a reflection of the degradation of primary auditory or visual processing. Erb and Obleser (2013) also demonstrated that greater activation in the right middle frontal gyrus (a region within the FPN) was associated with better comprehension of degraded auditory sentences in older adults but not in younger adults. Our study extends these findings further by suggesting that, even when auditory processing is not required, older adults still recruit regions within the FPN.
Many studies have shown similar age-related increases in FPN activity in response to a variety of stimuli and tasks not related to language processing. Many studies have linked activation of the FPN to working memory performance, with activation increasing within the network with increasing task difficulty (i.e. item set size; for reviews see Nee et al., 2013; Owen et al., 2005). In a study that compared network activation in an n-back task between younger and older adults, older adults were reported to exhibit greater activation within ventrolateral PFC and the right FPN relative to younger adults (Saliasi et al., 2014). These findings indicate that the involvement of the FPN in our reading task may suggest that older adults rely upon working memory processes to a greater degree than younger adults, even in response to relatively simple short stories.
Comparatively, in a visuospatial reasoning task, older adults exhibited increased activation in medial prefrontal and supramarginal/parietal regions compared to younger adults, with increased activation positively correlating with enhanced task performance in older adults, suggesting a similar compensatory role of this network for supporting sustained visual attention (Drag et al., 2016). Increased recruitment of the anterior and posterior aspects of the FPN in our study, similar to the DAN, may reflect increased effort toward maintaining visual attention.
Relationship Between Atrophy and Network Recruitment
In our sample, age-related atrophy spared Wernicke’s area (i.e. posterior temporal and inferior parietal regions) and frontal and parietal regions of the attention networks. Notably, recruitment of the network we identify as the posterior language network, including ventral language stream regions such as the posterior superior and middle temporal lobes and the left inferior frontal gyrus (Hickok & Poeppel, 2007; Saur et al., 2010), did not differ with age in response to the reading paradigm. Instead, increased recruitment of the DAN correlated with reduced gray matter thickness in middle to anterior temporal areas. This finding differs from previous studies which showed reduced gray matter volume in middle and superior temporal gyri was associated with increased activation in left superior temporal gyrus and middle frontal gyrus, as well as right hemisphere homologues of the language network, all regions which fall outside the DAN (Eckert et al., 2008; Tyler et al., 2009). Differences in the relationship between atrophy and network recruitment between these studies and the one reported here may reflect differences in the type of language stimuli used (i.e. auditory versus visual). This study did not include diffusion tensor imaging, which would help determine whether concurrent white matter integrity in the temporal lobes also show similar relationships with age-related network recruitment.
Similar results of atrophy and increased fMRI activity associations more generally have been reported, such that older adults with increased atrophy in superior frontal, superior temporal, and inferior parietal areas engaged portions of the FPN the most (Marstaller et al., 2015). During aging, language networks may have less efficient processing or comprehension of language stimuli, either due to the structural degradation of neurons within the language network or of white matter tracts connecting regions of the language network, and therefore must engage additional brain networks to maintain function.
Alternative Hypotheses and Limitations
There are alternative hypotheses to explain why older adults show increases in attention-related network recruitment compared to younger adults. Some suggest this observation reflects age-related dysregulation in brain networks that support cognition (Andrews-Hanna et al., 2007; Damoiseaux et al., 2008; Morcom & Henson, 2018), while others suggest it reflects overall decline in brain structural integrity with age, which equally impacts sensory and cognitive processes (Baltes & Lindenberger, 1997; Park et al., 2004). Our findings do not support these hypotheses because we found no age differences in the use of the classic frontotemporal language brain regions, demonstrating that there was not a generalized dysfunction between all brain regions and networks. However, as reported by Andrews-Hanna et al. (2007), it may be the case that only certain brain networks (e.g. the DMN and, critically, the DAN) exhibit age-related dysregulation. Although in our study, recruitment of the DMN did not differ with age (data not shown). Considering our results related to the anterior and posterior FPNs, they could also be interpreted as demonstrating a lack of network disengagement in response to reading, or a lack of network specification with advanced age, given that the beta weights of each network are negative. Ithas been suggested that loss of specificity with brain regions and networks reflects reduced neural efficiency, rather than compensation (Morcom & Henson, 2018).
Furthermore, the increases in attention-related network recruitment with age should be interpreted with some caution, given that only the relationship between the anterior FPN and age survived multiple comparison corrections. However, since the involvement of the language networks did not change with age, we believe that these small-to-moderate effect sizes do not detract from the overall findings of increased age-related attentional network involvement and that additional studies with a larger cohort (e.g. around 120 participants determined by a post-hoc power analysis) would further support our findings.
Since this study was cross-sectional, we are unable to determine if age-related gray matter atrophy in language processing networks has a causal relationship with increasing recruitment of attention-related networks, or if there is an underlying mechanism(s) contributing to both observations. Similarly, the cross-sectional design of this study did not allow us to compare lifestyle differences as potential contributing factors to age-related neural changes, as proposed in the revised STAC model (STAC-r; Reuter-Lorenz & Park, 2014).
For this study, we chose not to require responses during our reading paradigm since response-based comprehension designs have been shown to elicit additional, attention-related networks that are not observed in passive paradigms (Davis et al., 2014; Hasson et al., 2006; Zhang et al., 2014), and thus we do not have a quantitative measure of performance during the scan. We confirmed with each participant that they understood the main theme of the stories to ensure that they were actively reading during the task. Engagement during the task was further confirmed through identifying activation in typical language processing networks in each participant’s data. Future studies utilizing a large age range like our studies with a task that captures performance would be helpful to confirm our findings. Collectively, our finding of consistent activity in the “classic” frontotemporal network across the group supports that participants engaged in the task.
CONCLUSION
In summary, our study reveals that age-related increases in the recruitment of brain networks associated with focused attention occur even for simple narrative texts. Gray matter atrophy within language processing regions (anterior and middle superior and middle temporal gyri) is related to increasing recruitment of the DAN, and decreasing recruitment of the FPN, with age. These findings contribute to a comprehensive understanding of age-related changes in brain networks and their differential engagement during language processing. This identifies potential intervention targets for older adults that reduce cognitive demands during communication or improve attention-driven brain network function to maintain quality of life and independence.
Supplementary Material
Acknowledgments
FUNDING
This work was supported in part by the State of Arizona Alzheimer’s Consortium, the Barrow Neurological Foundation, and by the National Institute on Aging NIA 5 P30 AG019610–03.
Footnotes
CONFLICTS OF INTEREST
The authors have nothing to disclose.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/S1355617719000274.
REFERENCES
- Alexander GE, Bergfield KL, Chen K, Reiman EM, Hanson KD, Lin L, Bandy D, Caselli RJ, & Moeller JR (2012). Gray matter network associated with risk for Alzheimer’s disease in young to middle-aged adults. Neurobiology of Aging, 33(12), 2723–2732. doi: 10.1016/j.neurobiolaging.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Chen K, Merkley TL, Reiman EM, Caselli RJ, Aschenbrenner M, Santerre-Lemmon L, Lewis DJ, Pietrini P, Teipel SJ, & Moeller JR (2006). Regional network of magnetic resonance imaging gray matter volume in healthy aging. NeuroReport, 17(10), 951. doi: 10.1097/01.wnr.0000220135.16844.b6. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME, & Buckner RL (2007). Disruption of large-scale brain systems in advanced aging. Neuron, 56(5), 924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes PB & Lindenberger U (1997). Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychology and Aging, 12(1), 12–21. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck depression inventory-II. San Antonio, 78(2), 490–498. [Google Scholar]
- Bergfield KL, Hanson KD, Chen K, Teipel SJ, Hampel H, Rapoport SI, Moeller JR, & Alexander GE (2010). Age-related networks of regional covariance in MRI gray matter: reproducible multivariate patterns in healthy aging. NeuroImage, 49(2), 1750–1759. doi: 10.1016/j.neuroimage.2009.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, & Possing ET (2000). Human temporal lobe activation by speech and nonspeech sounds. Cerebral Cortex, 10(5), 512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, & Conant LL (2009). Where is the semantic system? A critical review and metaanalysis of 120 functional neuroimaging studies. Cerebral Cortex, 19(12), 2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM & Shafto MA (2008). Language and aging In The handbook of aging and cognition. Informa UK Limited; Retrieved from doi: 10.4324/9780203837665.ch8. [DOI] [Google Scholar]
- Cabeza R (2002). Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging, 17(1), 85–100. http://dx.doi.org.ezproxy1.lib.asu.edu/10.1037/0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Calhoun VD & de Lacy N (2017). Ten key observations on the analysis of resting-state functional MR imaging data using independent component analysis. Neuroimaging Clinics of North America, 27(4), 561–579. doi: 10.1016/j.nic.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, & Pearlson GD (2008). Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Human Brain Mapping, 29(7), 828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Samu D, Davis SW, Geerligs L, Mustafa A, Tyler LK, & others. (2016). Robust resilience of the frontotemporal syntax system to aging. The Journal of Neuroscience, 36(19), 5214–5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, DeDe G, Waters G, Michaud J, & Tripodis Y (2011). Effects of age, speed of processing, and working memory on comprehension of sentences with relative clauses. Psychology and Aging, 26(2), 439–450. doi: 10.1037/a0021837. [DOI] [PubMed] [Google Scholar]
- Caplan D & Waters G (2005). The relationship between age, processing speed, working memory capacity, and language comprehension. Memory, 13(3–4), 403–413. doi: 10.1080/09658210344000459. [DOI] [PubMed] [Google Scholar]
- Choi W, Desai RH, & Henderson JM (2014). The neural substrates of natural reading: a comparison of normal and nonword text using eyetracking and fMRI. Frontiers in Human Neuroscience, 8. doi: 10.3389/fnhum.2014.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW & Schneider W (2007). The cognitive control network: integrated cortical regions with dissociable functions. NeuroImage, 37(1), 343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Corbetta M & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience, 3(3), 201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage, 9(2), 179–194. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, Smith SM, & Rombouts SARB (2008). Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex, 18(8), 1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Davis SW, Zhuang J, Wright P, & Tyler LK (2014). Age-related sensitivity to task-related modulation of language-processing networks. Neuropsychologia, 63, 107–115. doi: 10.1016/j.neuropsychologia.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeDe G (2014). Sentence comprehension in older adults: evidence for risky processing strategies. Experimental Aging Research, 40(4), 436–454. doi: 10.1080/0361073X.2014.926775. [DOI] [PubMed] [Google Scholar]
- DeDe G & Flax JK (2016). Language comprehension in aging In Cognition, language and aging (pp. 107–133). Amsterdam, TheNetherlands: John Benjamins Publishing Company. [Google Scholar]
- Démonet J-F, Thierry G, & Cardebat D (2005). Renewal of the neurophysiology of language: functional neuroimaging. Physiological Reviews, 85(1), 49–95. doi: 10.1152/physrev.00049.2003. [DOI] [PubMed] [Google Scholar]
- Drag LL & Bieliauskas LA (2010). Contemporary review 2009: cognitive aging. Journal of Geriatric Psychiatry and Neurology, 23(2), 75–93. doi: 10.1177/0891988709358590. [DOI] [PubMed] [Google Scholar]
- Drag LL, Light SN, Langenecker SA, Hazlett KE, Wilde EA, Welsh R, Steinberg BA, & Bieliauskas LA (2016). Patterns of frontoparietal activation as a marker for unsuccessful visuospatial processing in healthy aging. Brain Imaging and Behavior, 10(3), 686–696. doi: 10.1007/s11682-015-9428-y. [DOI] [PubMed] [Google Scholar]
- Duncan J (2013). The structure of cognition: attentional episodes in mind and brain. Neuron, 80(1), 35–50. doi: 10.1016/j.neuron.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Walczak A, Ahlstrom J, Denslow S, Horwitz A, & Dubno JR (2008). Age-related effects on word recognition: reliance on cognitive control systems with structural declines in speech-responsive cortex. JARO, 9(2), 252–259. doi: 10.1007/s10162-008-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb J & Obleser J (2013). Upregulation of cognitive control networks in older adults’ speech comprehension. Frontiers in Systems Neuroscience, 7. doi: 10.3389/fnsys.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, Specht K, Weis S, Tendolkar I, Reuber M, Fell J, Klaver P, Ruhlmann J, Reul J, & Elger CE (2003). Intrasubject reproducibility of presurgical language lateralization and mapping using fMRI. Neurology, 60(6), 969–975. doi: 10.1212/01.WNL.0000049934.34209.2E. [DOI] [PubMed] [Google Scholar]
- Ferstl EC, Neumann J, Bogler C, & von Cramon DY (2008). The extended language network: a meta-analysis of neuroimaging studies on text comprehension. Human Brain Mapping, 29(5), 581–593. doi: 10.1002/hbm.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B (2012). FreeSurfer. NeuroImage, 62(2), 774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97(20), 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, & Walhovd KB (2009). High consistency of regional cortical thinning in aging across multiple samples. Cerebral Cortex, 19(9), 2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Minimental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, & Raichle ME (2006). Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences, 103(26), 10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD (2012). The cortical language circuit: from auditory perception to sentence comprehension. Trends in Cognitive Sciences, 16(5), 262–268. doi: 10.1016/j.tics.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Glisky E (2007). Changes in cognitive function in human aging In Brain aging (pp. 3–20). Informa UK Limited; Retrieved from 10.1201/9781420005523.sec1. [DOI] [PubMed] [Google Scholar]
- Gordon PC, Lowder MW, & Hoedemaker RS (2016). Reading in normally aging adults In Wright HH (Ed.), Cognition, language and aging (pp. 165–191). Amsterdam: John Benjamins Publishing Company. doi: 10.1075/z.200.07gor. [DOI] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Chen W, & Gee J (2002). Age-related changes in working memory during sentence comprehension: an fMRI study. NeuroImage, 15(2), 302–317. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, & Small SL (2006). Repetition suppression for spoken sentences and the effect of task demands. Journal of Cognitive Neuroscience, 18(12), 2013–2029. doi: 10.1162/jocn.2006.18.12.2013. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Choi W, Luke SG, & Desai RH (2015). Neural correlates of fixation duration in natural reading: evidence from fixation-related fMRI. NeuroImage, 119, 390–397. doi: 10.1016/j.neuroimage.2015.06.072. [DOI] [PubMed] [Google Scholar]
- Hickok G & Poeppel D (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Raichle ME, Mitra A, & Specht K (2015). On the existence of a generalized non-specific task-dependent network. Frontiers in Human Neuroscience, 9. doi: 10.3389/fnhum.2015.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James PJ, Krishnan S, & Aydelott J (2014). Working memory predicts semantic comprehension in dichotic listening in older adults. Cognition, 133(1), 32–42. doi: 10.1016/j.cognition.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Jobard G, Vigneau M, Mazoyer B, & Tzourio-Mazoyer N (2007). Impact of modality and linguistic complexity during reading and listening tasks. NeuroImage, 34(2), 784–800. doi: 10.1016/j.neuroimage.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Kemtes KA & Kemper S (1997). Younger and older adults’ online processing of syntactically ambiguous sentences. Psychology and Aging, 12(2), 362–371. http://dx.doi.org.ezproxy1.lib.asu.edu/10.1037/0882-7974.12.2.362. [DOI] [PubMed] [Google Scholar]
- Kim DI, Mathalon DH, Ford JM, Mannell M, Turner JA, Brown GG, Belger A, Gollub R, Lauriello J, Wible C, & Calhoun VD (2009). Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophrenia Bulletin, 35(1), 67–81. doi: 10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegl R, Grabner E, Rolfs M, & Engbert R (2004). Length, frequency, and predictability effects of words on eye movements in reading. European Journal of Cognitive Psychology, 16(1–2), 262–284. [Google Scholar]
- Li Y-O, Adalı T,&Calhoun VD(2007).Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping, 28(11), 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie C (2000). The relevance of education and age in the assessment of discourse comprehension. Clinical Linguistics & Phonetics, 14(2), 151–161. [Google Scholar]
- Madden DJ (2007). Aging and visual attention. Current Directions in Psychological Science, 16(2), 70–74. doi: 10.1111/j.1467-8721.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar RA (2011). The neural bases of social cognition and story comprehension. Annual Review of Psychology, 62(1), 103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Marstaller L, Williams M, Rich A, Savage G, & Burianová H (2015). Aging and large-scale functional networks: white matter integrity, gray matter volume, and functional connectivity in the resting state. Neuroscience, 290, 369–378. doi: 10.1016/j.neuroscience.2015.01.049. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Hansen LK, & Sejnowski TJ (2003). Independent component analysis of functional MRI: what is signal and what is noise? Current Opinion in Neurobiology, 13(5), 620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BJ (1987). Reading comprehension and aging. Annual Review of Gerontology and Geriatrics, 7(1), 93–115. [PubMed] [Google Scholar]
- Morcom AM & Henson RNA (2018). Increased prefrontal activity with aging reflects nonspecific neural responses rather than compensation. Journal of Neuroscience, 38(33), 7303–7313. doi: 10.1523/JNEUROSCI.1701-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narain C,Scott SK,Wise RJS,Rosen S,Leff A,Iversen SD, & Matthews PM (2003). Defining a left-lateralized response specific to intelligible speech using fMRI. Cerebral Cortex (New York, N.Y.: 1991), 13(12), 1362–1368. [DOI] [PubMed] [Google Scholar]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, & Jonides J (2013). A meta-analysis of executive components of working memory. Cerebral Cortex, 23(2), 264–282. doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, & Carter CS (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive, Affective, & Behavioral Neuroscience, 12(2), 241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U & Price CJ (2004). An fMRI Study of Syntactic Adaptation. Journal of Cognitive Neuroscience, 16, 702–713. doi: 10.1162/089892904323057399. [DOI] [PubMed] [Google Scholar]
- Norman S, Kemper S, & Kynette D (1992). Adults’ reading comprehension: effects of syntactic complexity and working memory. Journal of Gerontology, 47(4), P258–P265. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, & Bullmore E (2005). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Human Brain Mapping, 25(1), 46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, & Smith PK (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and Aging, 17(2), 299. [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, & Smith MR (2004). Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 101(35), 13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC & Reuter-Lorenz P (2009). The adaptive brain: aging and neurocognitive scaffolding. Annual Review of Psychology, 60(1), 173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Wingfield A, & Grossman M (2009). Neural processing during older adults’ comprehension of spoken sentences: age differences in resource allocation and connectivity. Cerebral Cortex, 20(4), 773–782. doi: 10.1093/cercor/bhp142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, & Petersen SE (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ(2012).A review and synthesis of the first 20yearsofPET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62(2), 816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP & Scott SK (2009). Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nature Neuroscience, 12(6), 718–724. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner K, Reichle ED, Stroud MJ, Williams CC, & Pollatsek A (2006). The effect of word frequency, word predictability, and font difficulty on the eye movements of young and older readers. Psychology and Aging, 21(3), 448. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, & Acker JD (2004). Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiology of Aging, 25(3), 377–396.doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N & Rodrigue KM (2006). Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews, 30(6),730–748.doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA & Cappell KA (2008). Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17(3), 177–182. doi: 10.1111/j.1467-8721.2008.00570.x. [DOI] [Google Scholar]
- Reuter-Lorenz PA & Park DC (2014). How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychology Review, 24(3), 355–370. doi: 10.1007/s11065-014-9270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DA, Gernsbacher MA, Guidotti SJ, Robertson RRW, Irwin W, Mock BJ, & Campana ME (2000). Functional neuroanatomy of the cognitive process of mapping during discourse comprehension. Psychological Science, 11(3), 255–260. doi: 10.1111/1467-9280.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C & Hickok G (2011). The Role of Broca’s area in sentence comprehension. Journal of Cognitive Neuroscience, 23(7), 1664–1680. doi: 10.1162/jocn.2010.21530. [DOI] [PubMed] [Google Scholar]
- Rogalsky C, Matchin W, & Hickok G (2008). Broca’s area, sentence comprehension, and working memory: an fMRI study. Frontiers in Human Neuroscience, 2, 14. doi: 10.3389/neuro.09.014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliasi E, Geerligs L, Lorist MM, & Maurits NM (2014). Neural correlates associated with successful working memory performance in older adults as revealed by spatial ICA. PLoS ONE, 9(6), e99250. doi: 10.1371/journal.pone.0099250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2010). Selective review of cognitive aging. Journal of the International Neuropsychological Society: JINS, 16(5), 754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Schelter B, Schnell S, Kratochvil D, Küpper H, Kellmeyer P, Kümmerer D, Klöppel S, Glauche V, Lange R, & Weiller C (2010). Combining functional and anatomical connectivity reveals brain networks for auditory language comprehension. NeuroImage, 49(4), 3187–3197. doi: 10.1016/j.neuroimage.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, & Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience, 27(9), 2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA&Tyler LK(2014). Language in the aging brain: The network dynamics of cognitive decline and preservation. Science, 346(6209), 583–587. doi: 10.1126/science.1254404. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson G, & Calhoun VD (2007). Functional neural circuits for mental timekeeping. Human Brain Mapping, 28(5), 394–408. doi: 10.1002/hbm.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine-Morrow EA, Milinder L-A, Pullara O, & Herman B (2001). Patterns of resource allocation are reliable among younger and older readers. Psychology and Aging, 16(1), 69. [DOI] [PubMed] [Google Scholar]
- Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, & Walhovd KB (2014). Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: Regions of accelerating and decelerating change. Journal of Neuroscience, 34(25), 8488–8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taler V, Aaron GP, Steinmetz LG, & Pisoni DB (2010). Lexical neighborhood density effects on spoken word recognition and production in healthy aging. Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 65(5), 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D & Volkow ND (2012a). Aging and functional brain networks. Molecular Psychiatry, 17(5), 549–558. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D& Volkow ND (2012b). Resting functional connectivity of language networks: Characterization and reproducibility. Molecular Psychiatry,17(8),841–854.doi: 10.1038/mp.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU & Dronkers NF (2011). The neural architecture of the language comprehension network: Converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience, 5. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK & Marslen-Wilson W (2008). Fronto-temporal brain systems supporting spoken language comprehension. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 363(1493), 1037–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Shafto MA, Randall B, Wright P, Marslen-Wilson WD,&Stamatakis EA(2009).Preserving syntactic processing across the adult life span: The modulation of the frontotemporal language system in the context of age-related atrophy. Cerebral Cortex, 20(2), 352–364. doi: 10.1093/cercor/bhp105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, & Buckner RL (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100(6), 3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, & Fink GR (2014). Dorsal and ventral attention systems. The Neuroscientist, 20(2), 150–159. doi: 10.1177/1073858413494269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, & Fischl B (2005). Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging, 26(9), 1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Wingfield A & Grossman M (2006). Language and the aging brain: Patterns of neural compensation revealed by functional brain imaging. Journal of Neurophysiology, 96(6), 2830–2839. doi: 10.1152/jn.00628.2006. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Peelle JE, & Grossman M (2003). Speech rate and syntactic complexity as multiplicative factors in speech comprehension by young and older adults. Aging, Neuropsychology, and Cognition, 10(4), 310–322. doi: 10.1076/anec.10.4.310.28974. [DOI] [Google Scholar]
- Wingfield A & Stine-Morrow EA (2000). Language and speech In The Handbook of Aging and Cognition (2nd ed., pp. 359–416). Mahwah, NJ, USA: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Wong PCM, Jin JX, Gunasekera GM, Abel R, Lee ER, & Dhar S (2009). Aging and cortical mechanisms of speech perception in noise. Neuropsychologia, 47(3), 693–703. doi: 10.1016/j.neuropsychologia.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Potenza MN, & Calhoun VD (2013). Spatial ICA reveals functional activity hidden from traditional fMRI GLM-based analyses. Frontiers in Neuroscience, 7. doi: 10.3389/fnins.2013.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, & Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(3), 1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, & Leirer VO (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17(1), 37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zhang H-Y, Chen W-X, Jiao Y, Xu Y, Zhang X-R, & Wu J-T (2014). Selective vulnerability related to aging in large-scale resting brain networks. PloS One, 9(10), e108807. doi: 10.1371/journal.pone.0108807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W & Shu H (2017). A meta-analysis of functional magnetic resonance imaging studies of eye movements and visual word reading. Brain and Behavior, 7(5), e00683. doi: 10.1002/brb3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.