This 36th adult lung and heart-lung transplant report summarizes data from 69,200 adult lung and 4,128 adult heart-lung transplants performed through June 30, 2018 and reported to the International Thoracic Organ Transplant Registry. With each year’s report, we now provide more detailed analyses on a particular focus theme important to patient outcomes. Since 2013, these have been donor and recipient age; retransplantation; early graft failure; indication for transplant; allograft ischemic time; and multiorgan transplantation. Although widely accepted as critical to decision making at the time of receipt of an organ donor offer, there is surprisingly little literature outlining current practice and the impact of size (mis-)matching on outcomes. Hence, this year’s report focuses on an overall theme of donor and recipient size matching. In addition to reporting donor and recipient height and weight difference for all adult lung and heart-lung transplant recipients stratified by transplant type (bilateral or single) and indication, we report historical trends and associations between size match and survival. The Registry’s online slide sets include results from additional analyses and complementary information not included in this publication (see https://ishltregistries.org/registries/slides.asp).
Statistical methods
Data collection, conventions, and statistical methods
National and multinational organ and data exchange organizations and individual centers submit data to the International Society for Heart and Lung Transplantation International Thoracic Organ Transplant Registry. Since the Registry’s inception, 481 heart transplant centers, 260 lung transplant centers, and 184 centers that perform combined heart-lung transplants have reported data to the Registry.1–4 It is estimated that data submitted to the Registry represents approximately 75% of the worldwide thoracic transplant activity. Additional and extended analyses presented in the online slide sets (5 separate slide sets, named “Introduction/General Statistics”, “Overall Lung Transplantation Statistics”, “Adult Lung Transplantation Statistics”, “Overall Heart-Lung Transplantation Statistics”, and “Adult Heart-Lung Transplantation Statistics”, available at https://ishltregistries.org/registries/slides.asp) supplement the report. The International Society for Heart and Lung Transplantation website also contains slide sets for previous annual reports. This report references specific online e-slides when particular data are discussed but not shown because of space limitations; e-slide numbers refer to the online adult lung (shortened to L(a)) and adult heart-lung (HL(a)) transplant slides.
The International Thoracic Organ Transplant Registry website (http://ishlt.org/registries/ttx-registry) provides detailed spreadsheets of the data elements collected in the Registry. The Registry requires submission of core donor, recipient, and transplant procedure variables at baseline (i.e., around the time of transplantation) and at annual follow-up, and these variables therefore have low rates of missingness. Nevertheless, data quality depends on the accuracy and completeness of reporting. Rates of missingness may significantly increase for Registry variables that depend on voluntary reporting. The Registry uses various quality control measures to ensure acceptable data quality and completeness before including data for analyses.
Analytical conventions
Unless otherwise specified, analyses of lung or heart transplants do not include combined heart-lung transplant data. Recipients categorized as retransplanted include (1) those with a previously reported transplant of the same organ type, (2) those with the same organ type in combination with another solid organ, or (3) those with a retransplant diagnosis.5 It is possible that this approach slightly underestimates the number of retransplant events. The Registry does not capture the exact occurrence date for most secondary outcomes (e.g., renal dysfunction), but it does capture the window of occurrence (i.e., the event occurred between the first and the second-year annual follow-up visits). For the report’s analyses, we use the midpoint between the annual follow-ups as a surrogate for the event date. Because deceased subjects no longer contribute to the secondary outcomes, to reduce the potential of underestimating event rates or other outcomes, we restrict some analyses to include only surviving recipients. For time-to-event and cumulative morbidity analyses, we censor the follow-up of recipients who have not yet experienced the event at the most recent annual follow-up or the time of retransplantation. We truncate time-to-event graphs (survival graphs) when the number of individuals at risk becomes <10. The Supplementary Material available online (www.jhltonline.org) includes additional information regarding the general statistical methods used for analyses and data interpretation. Previous Registry report themes provide more details regarding specific donor and recipient characteristics and outcomes.1,5–8
Focus theme methods: Donor and recipient size match
Although widely acknowledged as important in matching a donor with a recipient, there has been relatively little published on the impact of size matching on outcomes after lung and heart-lung transplantation. Most of the relatively small studies published so far suggest that recipients of oversized allografts experience either slightly better9–12 or equivalent13,14 short-term (primary graft dysfunction) and long-term (mortality) outcomes after lung transplantation. For this year’s report, size match has been assessed in terms of height and weight differences between donor and recipient. These parameters were selected because they are easily accessible and routinely used clinically, and because these Registry data fields have low rates of missingness. Weight is included because size mismatch, historically assessed using donor-recipient weight difference, is associated with important outcomes after heart transplantation including primary graft failure15 and mortality,16,17 and because lower donor-to-recipient weight ratio has been associated with shorter survival in a single study in lung transplantation.9 Intuitively, because lung transplantation involves placement of a donor organ into a thoracic cavity of relatively fixed volume, it is total lung capacity which should be matched between lung donors and recipients; however, as this parameter will not be known for lung donors, it must be estimated. The commonly used predictive equations to estimate total lung capacity include height, sex, and age; however, height is the most informative variable.18,19 In this report, for simplicity, we have elected to present only data concerning height rather than calculating total lung capacity. Because sex is also important when estimating total lung capacity, independent of height, data has been stratified for the 4 possible sex-match combinations. Much of this data is presented in the accompanying online slide sets. Donor and recipient height differences are presented as the difference (in cm) between donor and recipient. Throughout this report and the online slide set, data for oversized donors (i.e., donor-recipient height >5 cm) are presented in green shades and undersized donors (i.e., donor-recipient height <–5 cm) are presented in blue shades. Donor-recipient pairings where there is little height difference (−5 cm to 5 cm) are in the lightest shade of blue. It should be noted that the Registry does not capture data relating to donor lung resection (e.g., lobectomy or shave) at the time of transplant.
Lung transplantation
Centers and transplant activity
The Registry now contains data from 69,200 adult lung transplants performed through June 2018 reported by 260 participating transplant centers. Figure 1 (eSlide L(a) 4) shows the number of reported adult lung transplants each year stratified by procedure type. The proportion of all adult lung transplants which are single lung procedures continues to fall, with bilateral lung transplantation accounting for 81% of all procedures in 2017 (eSlides L(a) 4 and L(a) 7).
Figure 1.
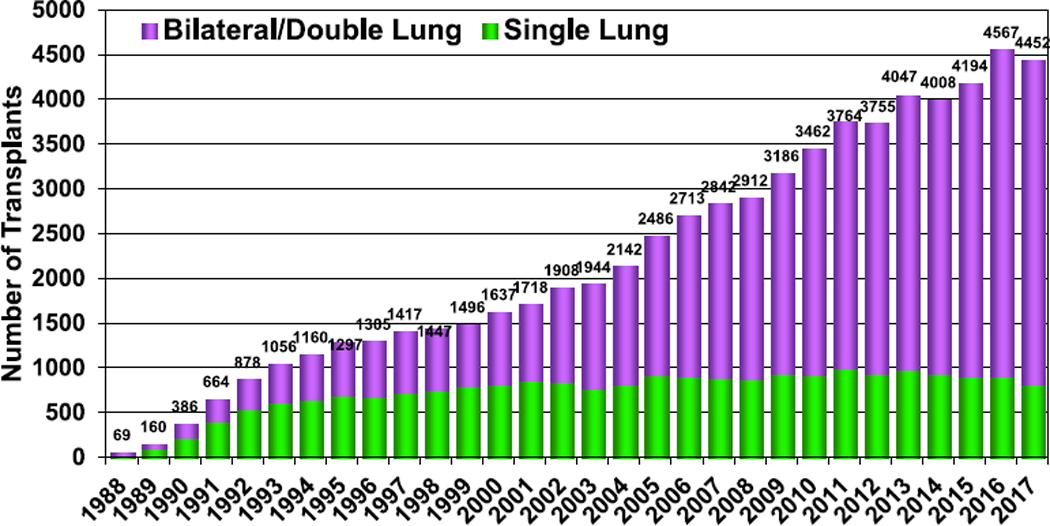
Number of adult lung transplants by year and procedure type (transplants: 1988–2017).
For the 174 reporting centers with transplants during 2010 through June 2018, the distribution of transplant activity by center volume remained relatively stable, with 51 high-volume centers (30+ transplants per year) performing approximately two thirds of the transplant procedures. The number of centers reporting ≥50 transplants per year rose from 18 to 20 in the past year. These high-volume centers performed 39% of all transplants reported to the Registry (eSlide L(a) 5). These changes are consistent with a trend over the past 5 years for an increasing proportion of lung transplants to be performed at very high-volume centers. In 2015, for example, 14 centers reported performing 50 or more transplants, with these centers performing 33% of all adult lung transplants.6 Again, pediatric lung transplantation was much less common, with only 101 procedures (2% of total reported lung transplants) performed in children (defined in the Registry as <18 years old) in 2017 (eSlide L(p) 6).20
Indications
Indications for transplantation since January 1995, stratified by transplant type, are shown in Table 1 (eSlide L(a) 6). The trend for an increasing number of patients with interstitial lung disease (ILD) to undergo transplantation continues, with 32.4% and 8.1% of transplants reported to the Registry in the most recent year having been performed for patients with an idiopathic interstitial pneumonia (IIP) or non-IIP ILD, respectively (Figure 2 and eSlide L9(a) 8). These 2 indications together now significantly exceed the second most common indication for adult lung transplantation (chronic obstructive pulmonary disease [COPD]), having first surpassed COPD as the most common indication in 2007. This trend is particularly strong in North America (eSlides L(a) 11) where the proportion of all adult lung transplants performed for IIP or non-IIP ILD has increased from 38% to 47% over the past decade. In contrast, the proportion of patients transplanted for cystic fibrosis (CF) continues to slowly fall, now accounting for 13% of total adult lung transplants (eSlide L(a) 8), compared with over 15% five years ago.5
Table 1.
Adult Lung Transplant Procedure Type by Diagnosis (Transplants: January 1995–June 2018)
| Diagnosis | Single lung transplant (n = 19,958) | Bilateral lung transplant (n = 43,572) | All (n = 63,530) |
|---|---|---|---|
| COPD | 7,750 (38.8%) | 11,402 (26.2%) | 19,152 (30.1%) |
| IIP | 7,536 (37.8%) | 9,047 (20.8%) | 16,583 (26.1%) |
| Cystic fibrosis | 227 (1.1%) | 9,447 (21.7%) | 9,674 (15.2%) |
| Interstitial lung disease-not IIP | 1,123 (5.6%) | 2,486 (5.7%) | 3,609 (5.7%) |
| Alpha-1-antitrypsin deficiency | 814 (4.1%) | 2,155 (4.9%) | 2,969 (4.7%) |
| Retransplant | 1,003 (5.0%) | 1,553 (3.6%) | 2,556 (4.0%) |
| IPAH | 95 (0.5%) | 1,768 (4.1%) | 1,863 (2.9%) |
| Non-CF–bronchiectasis | 77 (0.4%) | 1,637 (3.8%) | 1,714 (2.7%) |
| Sarcoidosis | 343 (1.7%) | 1,197 (2.7%) | 1,540 (2.4%) |
| Pulmonary hypertension-not IPAH | 140 (0.7%) | 838 (1.9%) | 978 (1.5%) |
| LAM/tuberous sclerosis | 161 (0.8%) | 420 (1.0%) | 581 (0.9%) |
| Connective tissue disease | 169 (0.8%) | 395 (0.9%) | 564 (0.9%) |
| Obliterative bronchiolitis | 79 (0.4%) | 460 (1.1%) | 539 (0.8%) |
| Cancer | 8 (0.0%) | 30 (0.1%) | 38 (0.1%) |
| Other | 433 (2.2%) | 737 (1.7%) | 1,170 (1.8%) |
Abbreviations: CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; IIP, idiopathic interstitial pneumonia; IPAH, idiopathic pulmonary arterial hypertension; LAM, lymphangioleiomyomatosis
Figure 2.
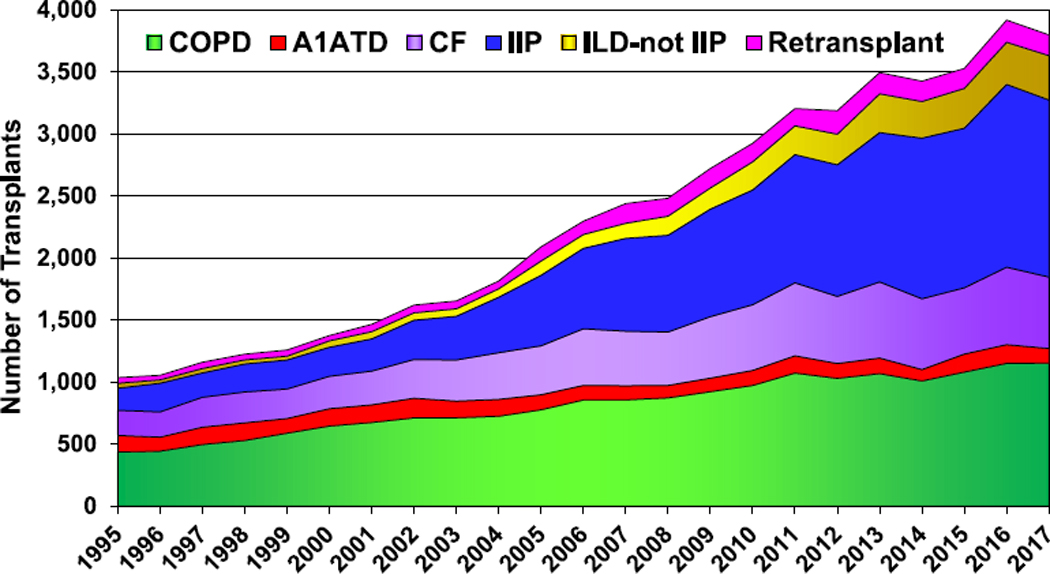
Major indications for adult lung transplantation by year (absolute number; transplants: 1990–2017). A1ATD, alpha-1-antitrypsin deficiency; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; IIP, idiopathic interstitial pneumonia; ILD-not IIP, interstitial lung disease-not idiopathic interstitial pneumonia.
Immunosuppression
The proportion of patients receiving induction therapy has continued to rise; over 80% of adult lung transplant recipients transplanted in 2017 received some form of induction, up from 76% in 2016 (Figure 3, eSlides L(a) 39 and L(a) 40). Previous trends in choice of induction therapy have been sustained, with ongoing growth in the proportion receiving an interleukin-2 receptor antagonist and decline in the proportion receiving anti-lymphocyte or anti-thymocyte globulin or alemtuzumab (Figure 3, eSlide L(a) 40). After induction, the proportion of patients receiving tacrolimus plus mycophenolate mofetil or mycophenolic acid has plateaued in the past few years (eSlide L(a) 44). On average, 62% of patients transplanted between 2005 and June 2018 were receiving tacrolimus, mycophenolate mofetil or mycophenolic acid, and prednisone at 1-year post-transplant (eSlides L(a) 45); however, the proportion in most recent years is higher. The use of cyclosporine and azathioprine continues to gradually decline (eSlide L (a) 44).
Figure 3.
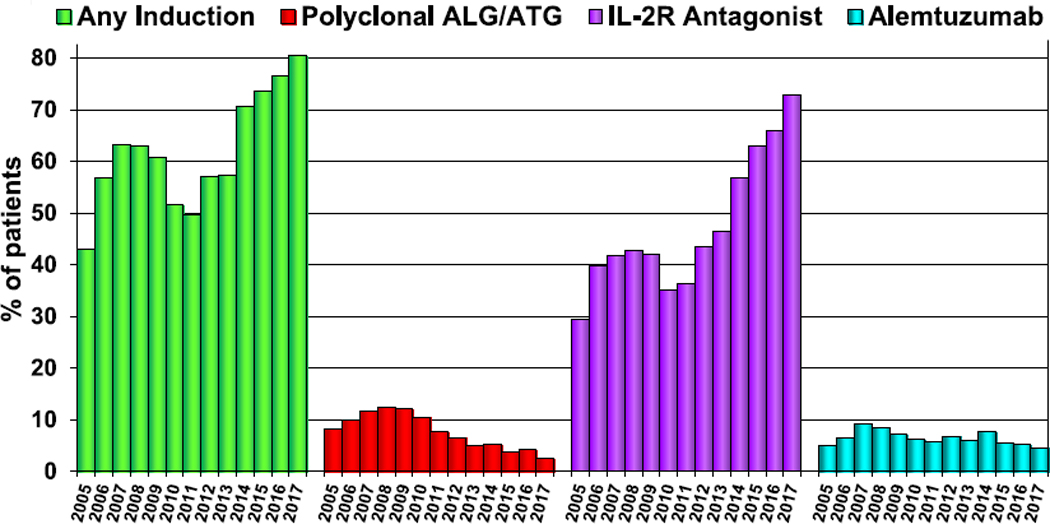
Induction immunosuppression for adult lung transplant recipients (transplants: January 2005–December 2017). ALG, antilymphocyte globulin; ATG, anti-thymocyte globulin; IL-2R, interleukin-2 receptor.
Morbidity
The Registry collects relatively limited data regarding post-transplant morbidities, with most focus on chronic lung allograft dysfunction, renal dysfunction, diabetes, and malignancies. The prevalence of severe renal dysfunction (indicated by a creatinine >2.5 mg/dl [221 μmol/L], chronic dialysis, or renal transplant) was 4.8% (3.4%, 1.4%, and 0%, respectively) at 1 year post-transplant in the most recent era, down from 7.6% pre-2004 (eSlide L(a) 53). The prevalence of diabetes mellitus also fell over this period (24.7% to 17.0%); however, the prevalence of bronchiolitis obliterans syndrome (BOS) remained frustratingly static at 8.5% (eSlide L(a) 53). At 10 years post-transplant, cumulative incidence of severe renal dysfunction was 24.6%, with 6.4% receiving chronic dialysis and 3.5% having received a renal transplant (eSlide L(a) 55). The prevalence of BOS was 67.1% (eSlide L(a) 55). It should be kept in mind that there may be many explanations for these changes over time, including, for example, changes in the most common indications for transplantation and an increase in recipient age over time.
Acute rejection and BOS
Between 2005 and June 2018, 26.6% of patients experienced at least 1 episode of treated acute rejection between discharge and 1 year after transplant (eSlide L(a) 26). There has been a small but steady decline in the proportion of patients experiencing rejection year-on-year (29% in 2014, 28% in 2017, and 27.3% in 2018).1,5,8 This potential trend will be monitored closely in the coming years. Younger recipients are more likely to be treated for acute rejection in the first post-transplant year (31.2% of those aged 18–34 years experienced at least 1 episode compared with 25.8% of those aged 35–49 years [eSlide L(a) 26]). Acute rejection was not more common in retransplant recipients (eSlide L(a) 28). Unfortunately, the previously noted impact of BOS on post-transplant outcomes has not changed in this report. For over 3 decades now, obliterative bronchiolitis and its clinical equivalent BOS have been the leading cause of post-transplant mortality. BOS continues to affect approximately 10% of patients each year, with the annual incidence highest in the first 5 years post-transplant (eSlide L(a) 56), before declining to some extent. The annual incidence is even higher in retransplant recipients (eSlide L(a) 58).
Survival
For adults who underwent primary lung transplantation in the most recent era (2010–June 2017) (n = 29,872), the median survival was 6.7 years (Figure 4, eSlide L(a) 14). For adult recipients who survived to 1 year after primary transplant, the median survival in the most recent cohort where computation was feasible was 8.9 years (eSlide L(a) 15). In unadjusted Kaplan-Meier analyses, female recipients (eSlide L(a) 16), recipients with CF as the indication for transplantation (eSlide L(a) 18), and recipients of bilateral lung transplants (eSlides L(a) 21–24) continue to experience longer survival. Although survival after transplantation is significantly greater in the most recent era (2010–June 2017), the survival curves for this and the preceding era (2002–2009) have not diverged as impressively as in previous eras (Figure 4, eSlide L(a) 14). In contrast, the superior survival for CF recipients in the most recent era appears more robust (eSlide L(a) 25). Again, it should be kept in mind that these analyses have not been adjusted for potential confounders that may explain some of the reported differences.
Figure 4.
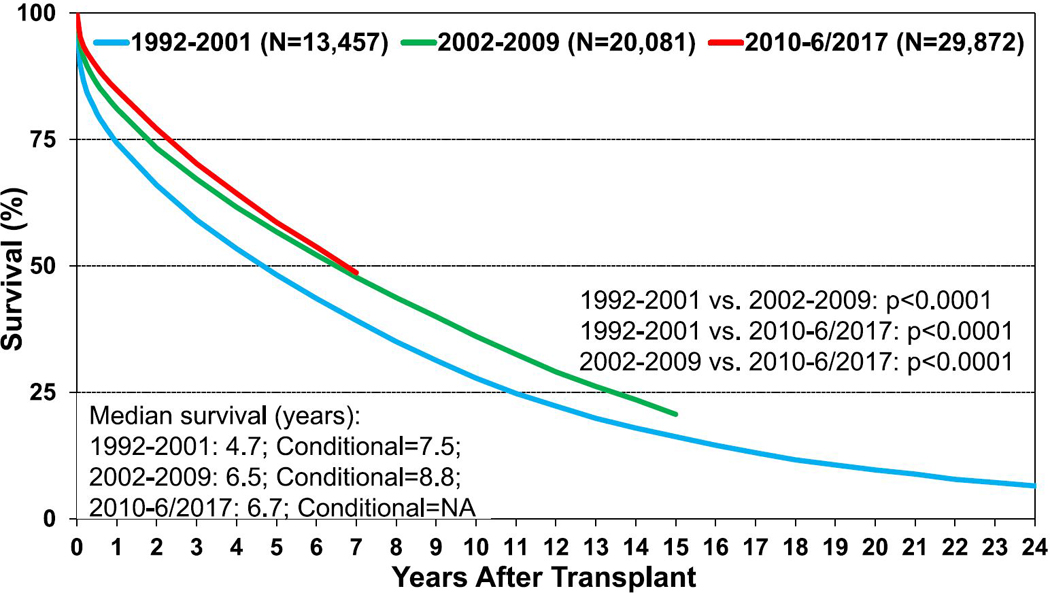
Kaplan-Meier survival for adult lung transplant recipients by transplant era (transplants: 1992–June 2017). NA, not applicable.
Causes of death after adult lung transplantation showed similar trends to previous reports and are presented in Table 2. Unadjusted mortality and morbidity rates are described above. We performed multivariable Cox proportional hazards regression analyses to identify independent factors associated with post-transplant mortality and morbidity. These analyses establish independent associations between risk factors and outcomes but cannot establish causality. The results of multivariable analyses for statistically significant categorical risk factors for 10-year mortality for adult lung transplant recipients with 95% confidence limits (transplants: 2000–June 2008) are presented in Figure 5 (eSlide L(a) 77). Of note, this analysis did not include the focus theme variables concerning size matching between donor and recipient (height and weight differences); however, the 5-year multivariable model presented later in this report as part of the focus theme (eSlide L(a) 135) does include these variables. Many of the variables appearing in this model are similar to previous reports. This model suggests that the more recent transplant era, bilateral lung transplantation, and CF or lymphangioleiomyomatosis as the indication for transplantation are favorably associated with survival. Cytomegalovirus mismatch (D+/R−) status, a history of hypertension or diabetes mellitus in the donor, retransplantation, and poor pre-transplant functional status are all independently associated with lower survival (Figure 5, eSlide L(a) 77). Of interest, a history of transplantation from a donation after circulatory death (DCD) donor (non–heart beating donor is the term used in the Registry) has appeared as independently associated with higher 10-year survival (hazard ratio for mortality of 0.64 [95% confidence interval: 0.47–0.86], p < 0.01, n = 110; eSlide L(a) 77, Figure 5). This is the second year that a history of DCD donation has been found to be associated with higher survival; however, the association is stronger this year than in the 35th Report.1 Although this is of interest and is independent of potential explanatory variables included in the model, it should be noted that these associations do not necessarily imply causation and that the models include only variables collected in the Registry. Potentially important explanatory variables may therefore have been omitted from these analyses. Nevertheless, this is an important finding.
Table 2.
Known Causes of Death for Adult Lung Transplant Recipients (Deaths: January 1995–June 2018)
| Cause of death | 0–30 days (n = 3,361) |
31 days–1 year (n = 6,489) |
>1 year–3 years (n = 6,775) |
>3 years–5 years (n = 4,177) |
>5 years–10 years (n = 5,404) |
>10 years (n = 2,364) |
|---|---|---|---|---|---|---|
| OB/BOS | 5 (0.1%) | 278 (4.3%) | 1,728 (25.5%) | 1,224 (29.3%) | 1,321 (24.4%) | 497 (21.0%) |
| Acute rejection | 92 (2.7%) | 121 (1.9%) | 107 (1.6%) | 31 (0.7%) | 27 (0.5%) | 5 (0.2%) |
| Lymphoma | 1 (0.0%) | 129 (2.0%) | 110 (1.6%) | 60 (1.4%) | 100 (1.9%) | 69 (2.9%) |
| Malignancy, other | 6 (0.2%) | 198 (3.1%) | 595 (8.8%) | 535 (12.8%) | 831 (15.4%) | 354 (15.0%) |
| CMV | 0 | 122 (1.9%) | 57 (0.8%) | 9 (0.2%) | 7 (0.1%) | 1 (0.0%) |
| Infection, non-CMV | 579 (17.2%) | 2,149 (33.1%) | 1,390 (20.5%) | 718 (17.2%) | 888 (16.4%) | 382 (16.2%) |
| Graft failure | 738 (22.0%) | 1,046 (16.1%) | 1,306 (19.3%) | 750 (18.0%) | 869 (16.1%) | 363 (15.4%) |
| Cardiovascular | 440 (13.1%) | 379 (5.8%) | 302 (4.5%) | 197 (4.7%) | 334 (6.2%) | 161 (6.8%) |
| Technical | 394 (11.7%) | 206 (3.2%) | 58 (0.9%) | 22 (0.5%) | 40 (0.7%) | 15 (0.6%) |
| Multiple organ failure | 488 (14.5%) | 864 (13.3%) | 358 (5.3%) | 175 (4.2%) | 249 (4.6%) | 120 (5.1%) |
| Other | 618 (18.4%) | 997 (15.4%) | 764 (11.3%) | 456 (10.9%) | 738 (13.7%) | 397 (16.8%) |
Abbreviations: BOS, bronchiolitis obliterans syndrome; CMV, cytomegalovirus; OB, obliterative bronchiolitis
Figure 5.
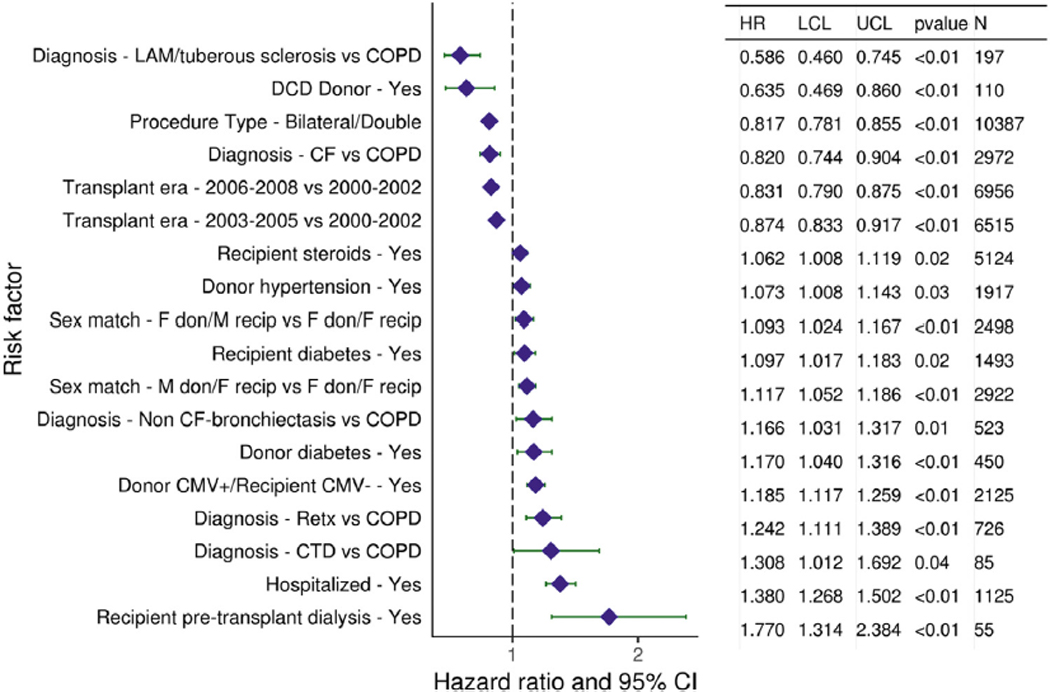
Statistically significant categorical risk factors for 10-year mortality for adult lung transplant recipients with 95% CIs (transplants: 2000–June 2008, N = 18,673). CF, cystic fibrosis; CI, confidence interval; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disorder; CTD, connective tissue disease; DCD, donation after circulatory death; F, female; HR, hazard ratio; LAM, lymphangioleiomyomatosis; LCL, lower control limit; M, male; Retx, retransplantation; UCL, upper control limit.
Continuous variables independently associated with 10-year mortality include recipient and donor age (Figure 6, eSlide L(a) 79), recipient serum creatinine (eSlide L(a) 80), recipient supplemental oxygen requirement (eSlide L(a) 81), and transplant center volume within the prior 3 years (eSlide L(a) 84).
Figure 6.
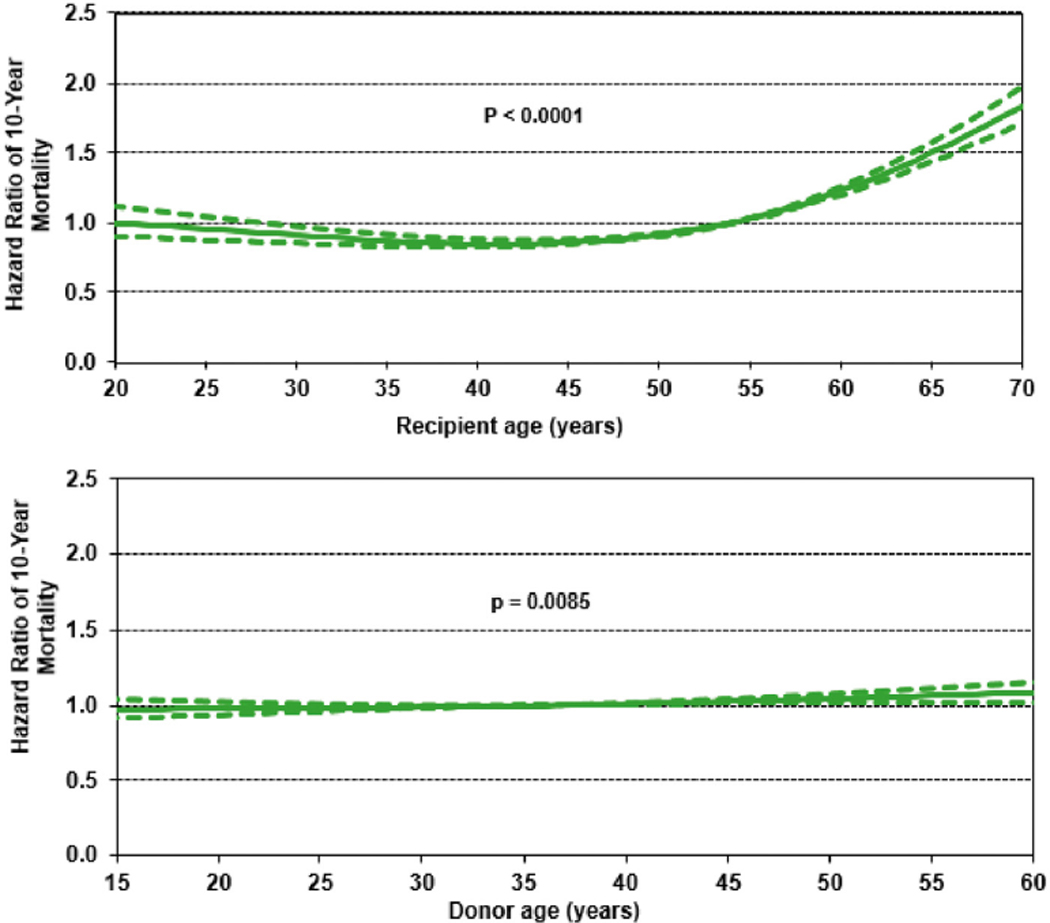
Hazard ratio for 10-year mortality for adult lung transplant recipients by recipient and donor age (transplants: 2000–June 2008, N = 18,673). This model excludes size match. The reference for recipient age is 54 years and for donor age is 36 years.
Lung transplantation donor and recipient size match
Donor-recipient height difference by transplant type, indication, and sex match
Data from 36,628 adult lung transplants performed between January 2005 and June 2018 and reported to the Registry were included in this analysis. Bilateral lung transplants accounted for 75%. Differences between donor and recipient height (expressed as donor-recipient height in cm), stratified by transplant type and diagnosis category, and listed by year of transplant are presented in Table 3. On average, patients transplanted for COPD and alpha1-antitypsin deficiency–related emphysema (A1ATD), diseases associated with gas trapping and supranormal total lung capacity, received lungs from donors who were 5.1 cm taller for bilateral transplants and 6.6 cm taller for single lung transplants. Patients with ILD received lungs from donors who were on average 1.9 cm shorter for bilateral procedures and 0.5 cm shorter for single lung procedures. Donors for patients with CF were on average 4.1 cm taller. There were no significant trends over time.
Table 3.
Mean Donor-Recipient Height Difference (cm) by Diagnosis Category and Procedure Type (Transplants: January 2005–June 2018)
| Year | A1ATD/COPD |
IIP/ILD-not IIP |
CF |
Other |
All |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bilateral | Single | Bilateral | Single | Bilateral | Single | Bilateral | Single | Bilateral | Single | |
| 2005 | 4.3 | 6.6 | −1.2 | 1.2 | 3.7 | 5.2 | 2.0 | 2.5 | 2.4 | 3.8 |
| 2006 | 5.2 | 5.7 | −0.4 | 0.3 | 3.0 | 7.1 | 1.3 | 2.0 | 2.6 | 2.9 |
| 2007 | 5.0 | 5.3 | −1.9 | −0.5 | 3.1 | 13.8 | 1.8 | 0.6 | 2.2 | 1.8 |
| 2008 | 5.2 | 7.6 | −2.8 | 0.1 | 3.8 | 5.3 | 1.1 | 1.4 | 1.9 | 3.1 |
| 2009 | 4.7 | 6.6 | −2.1 | −0.9 | 4.3 | 14.0 | 1.3 | 0.2 | 2.1 | 1.8 |
| 2010 | 5.7 | 6.1 | −2.4 | −1.4 | 4.0 | 6.5 | 0.9 | 1.5 | 2.1 | 1.3 |
| 2011 | 4.9 | 6.6 | −2.0 | −1.8 | 4.0 | 9.9 | 1.4 | 1.1 | 2.1 | 1.1 |
| 2012 | 5.1 | 7.4 | −2.4 | −1.3 | 4.4 | 6.4 | 1.0 | 0.6 | 1.8 | 1.4 |
| 2013 | 5.0 | 6.2 | −1.8 | −0.8 | 4.6 | −3.7 | 1.2 | 0.9 | 2.0 | 1.2 |
| 2014 | 4.9 | 6.7 | −2.2 | −0.9 | 4.7 | 6.3 | 1.1 | −1.0 | 1.6 | 0.8 |
| 2015 | 4.7 | 5.7 | −2.3 | −0.2 | 3.5 | −0.7 | 1.3 | 2.9 | 1.5 | 1.7 |
| 2016 | 5.3 | 5.6 | −1.5 | −0.6 | 5.2 | — | 0.7 | 1.8 | 2.0 | 1.3 |
| 2017 | 5.4 | 8.7 | −1.6 | 0.8 | 4.9 | — | 1.2 | 1.7 | 2.0 | 3.1 |
| 2018 | 5.7 | 8.0 | −2.2 | −0.3 | 4.7 | — | 2.1 | −0.2 | 1.7 | 2.0 |
| Mean height difference | 5.1 | 6.6 | −1.9 | −0.5 | 4.1 | 6.4 | 1.3 | 1.1 | 2.0 | 2.0 |
Abbreviations: A1ATD, alpha-1-antitrypsin deficiency; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; IIP, idiopathic interstitial pneumonia; ILD-not IIP, interstitial lung disease-not idiopathic interstitial pneumonia
The distribution of donor-recipient height differences by diagnostic category and procedure type are presented in Figure 7 (eSlide L(a) 90). As per the previously described convention, green coloration indicates an oversized donor, whereas darker blue coloration indicates an undersized donor. The lightest shade of blue indicates transplant procedures where there is little (−5 cm to 5 cm) difference between donor and recipient height. Somewhat surprisingly, a significant proportion of transplants for ILD (IIP or non-IIP) were performed after donation from oversized donors (21% of bilateral procedures and 27% of single procedures, Figure 7, eSlide L(a) 90); however, as previously highlighted, the Registry does not capture procedures performed to cut down oversized donor lungs. As expected, there were very few single lung transplants performed for CF (n = 39).
Figure 7.
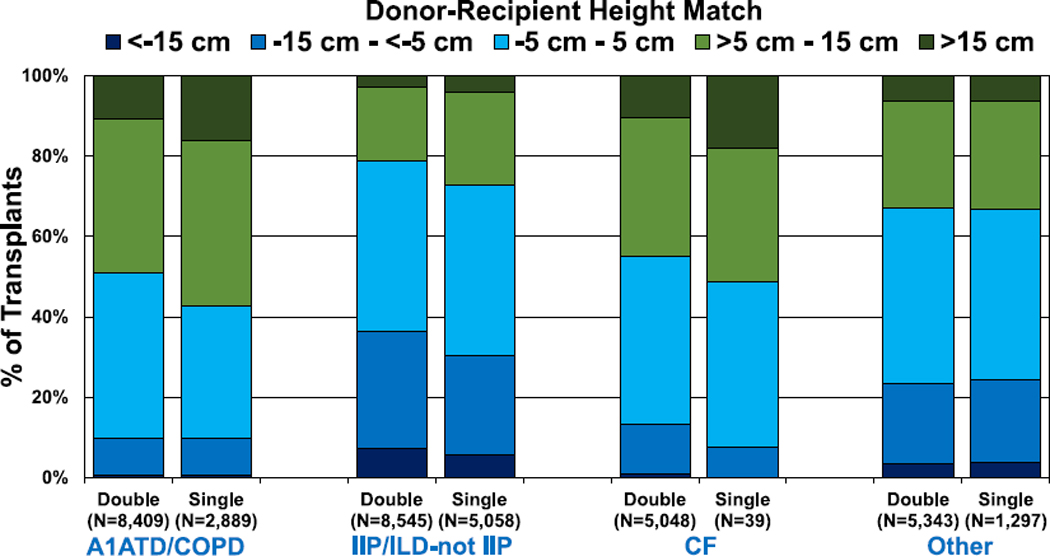
Distribution of donor-recipient height difference for adult lung transplant recipients by procedure type and recipient diagnosis (transplants: January 2005–June 2018). A1ATD, alpha-1-antitrypsin deficiency; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; IIP, idiopathic interstitial pneumonia; ILD-not IIP, interstitial lung disease-not idiopathic interstitial pneumonia.
Differences in donor and recipient height by diagnostic category, procedure type, and sex (mis)match are presented in eSlides L(a) 91–94. As would be expected, male-to-female transplants were generally oversized, whereas female-to-male transplants were generally undersized. Non–sex-mismatched transplants were generally well matched for size. There were no obvious associations between donor and recipient height difference and ischemic time or donor age (eSlides L(a) 95–102). North American recipients were more likely to receive organs from oversized donors compared with Europe and other regions (eSlide L(a) 103).
Survival
Transplants performed between January 1995 and June 2017 were included in the 1-year outcomes analyses and from January 1995 to June 2013 for the 5-year outcomes analyses. For these analyses, weight difference (donor-recipient, in kg) was calculated and categorized (<−15 kg; −15 kg to −5 kg; 5 kg to −5 kg; 5 kg−15 kg; and >15 kg) with green coloration again indicating an oversized donor and blue an undersized donor. Unadjusted survival curves for adult lung transplant recipients by weight difference are displayed in Figure 8 (1-year survival, eSlide L(a) 106) and Figure 9 (5-year survival, eSlide L(a) 115). One-year and 5-year survival were lower for recipients of undersized (by weight) donors. However, these Kaplan-Meier survival analyses are unadjusted for potentially important explanatory variables (e.g., single vs bilateral lung transplantation).
Figure 8.
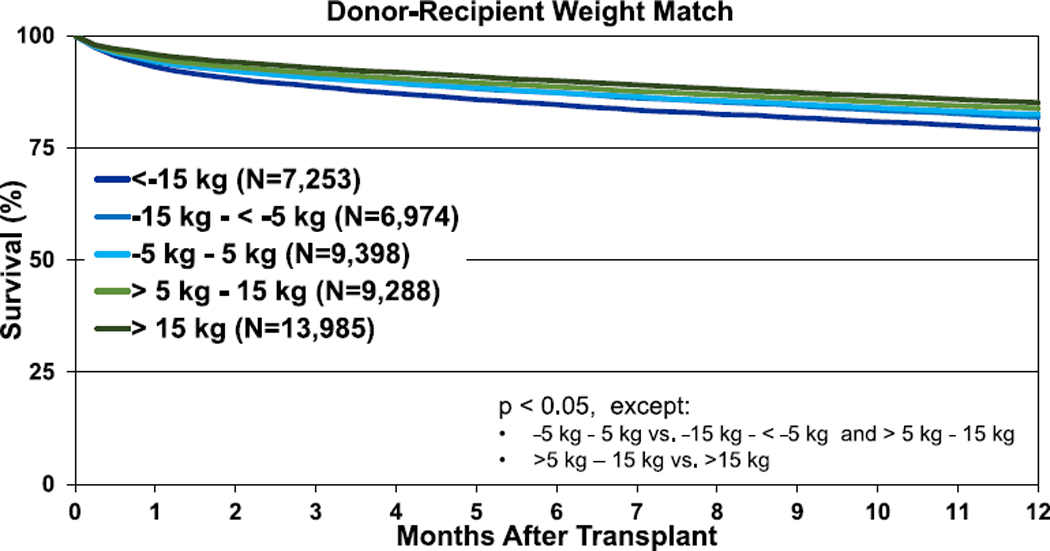
Kaplan-Meier survival within 1 year for adult lung transplant recipients by donor-recipient weight difference (transplants: January 1995–June 2017).
Figure 9.
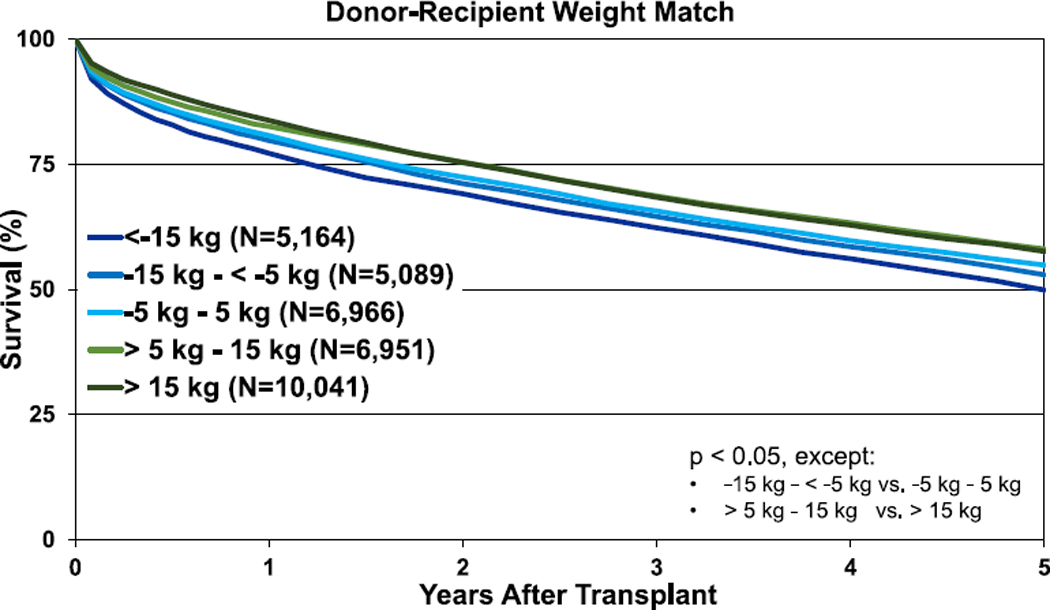
Kaplan-Meier survival within 5 years for adult lung transplant recipients by donor-recipient weight difference (transplants: January 1995–June 2013).
To reduce complexity, single and double lung transplants were analyzed separately for the donor-recipient height analyses. Unadjusted 1-year survival curves for adult lung transplant recipients by height difference are displayed in Figure 10 (pooled data, eSlide L(a) 107) and Figure 11 (by diagnostic category, eSlides L (a) 111–114). Unadjusted 1-year survival was lower for recipients of undersized donor organs (Figure 10). When individual diagnostic categories were considered, undersizing by height was associated with lower unadjusted 1-year survival for bilateral adult lung recipients with COPD or A1ATD and CF (eSlides L(a) 111 and 113). Undersizing was not associated with lower 1-year survival in bilateral adult lung recipients transplanted for ILD (eSlide L(a) 112). Similar trends were observed in the 5-year survival analyses for bilateral lung transplants (Figure 11, eSlides L(a) 120–123). Unadjusted 5-year survival was lower for adult lung transplant recipients of undersized donor organs (eSlide L(a) 116). When individual diagnostic groups were analyzed, unadjusted 5year survival was lower for bilateral adult lung transplant recipients who received undersized allografts and who had a diagnosis of COPD or A1ATD and CF, but not ILD (Figure 11). Unadjusted 5-year freedom from BOS was not different among the donor-recipient height groups (eSlide L(a) 124).
Figure 10.
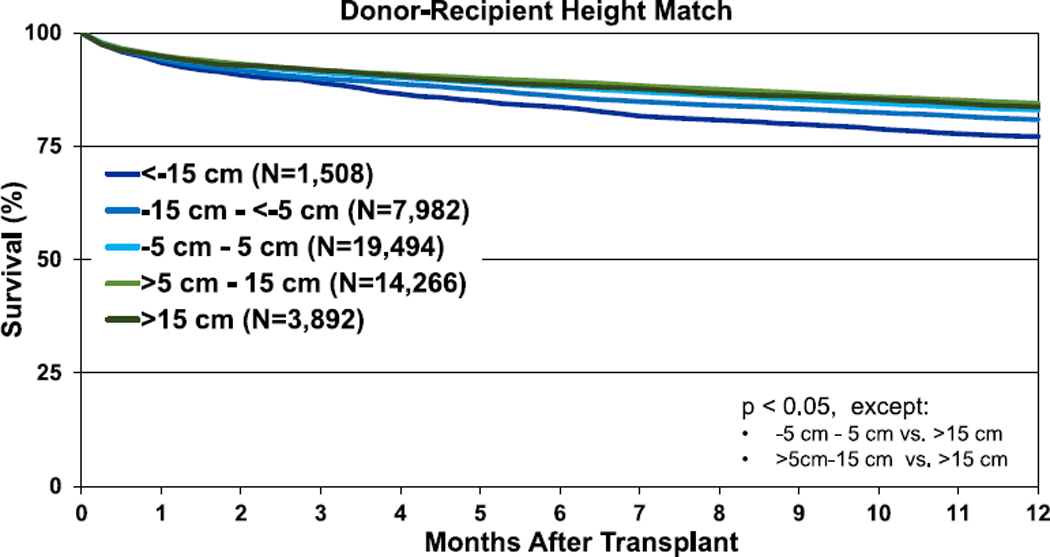
Kaplan-Meier survival within 1 year for adult lung transplant recipients by donor-recipient height difference (transplants: January 1995–June 2017).
Figure 11.
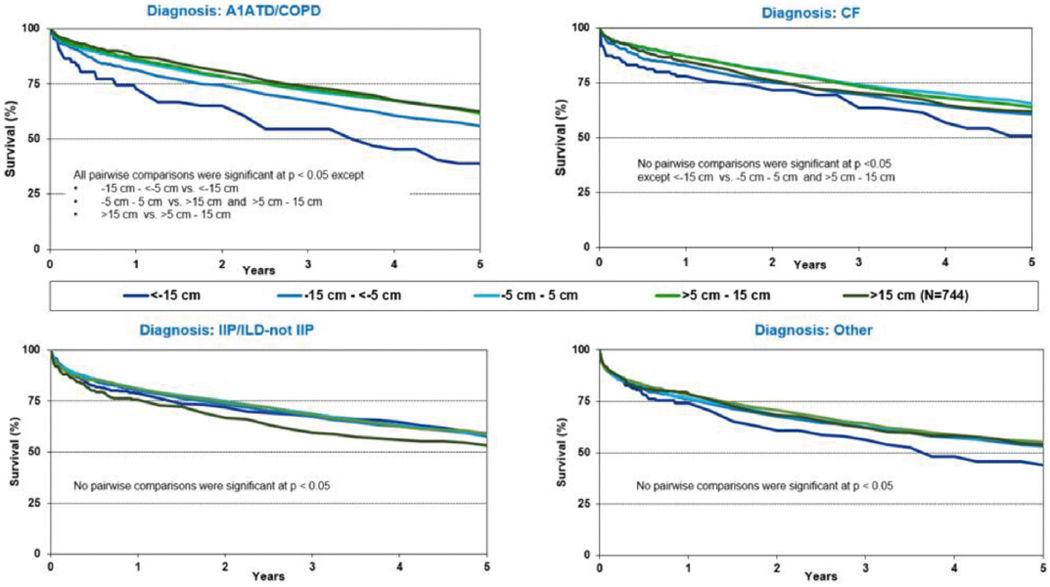
Kaplan-Meier survival within 5 years for adult lung transplant recipients by donor-recipient height difference for (a) A1ATD/COPD, (b) CF, (c) IIP/ILD-not IIP, and (d) other diagnoses (double lung transplants: January 1995–June 2013). A1ATD, alpha-1-antitrypsin deficiency; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; IIP, idiopathic interstitial pneumonia; ILD-not IIP, interstitial lung disease-not idiopathic interstitial pneumonia.
The results of multivariable analyses for statistically significant categorical risk factors for 5-year mortality for adult lung transplant recipients with 95% confidence limits (transplants: 2005–June 2013) are presented in Figure 12 (eSlide L(a) 139). This model includes both donor-recipient height difference and weight difference. Independent risk factors for mortality are similar to previous reports. Continuous factors which were retained in the model were (eSlides L(a) 140–151): recipient age (years); donor age (years); recipient creatinine (mg/dl); recipient oxygen requirement (L/min); recipient forced vital capacity % predicted; transplant center volume within the prior 3 years; recipient bilirubin (mg/dl); recipient panel reactive antibody (%); weight difference (kg); and height difference (cm). The hazard ratio of 5-year mortality for donor-recipient weight difference (relative to a weight difference of 5 kg) is shown in Figure 13 (eSlide L(a) 149), for donor-recipient height difference (relative to a height difference of 2.4 cm) in Figure 14 (eSlide L(a) 150), and for height difference and diagnosis interaction in Figure 15 (eSlide L(a) 151). Of note, the shape of the interaction curves is different between COPD or A1ATD and CF vs ILD, suggesting that the 5-year mortality hazard is higher for ILD recipients who receive oversized allografts and higher for COPD or A1ATD recipients who receive undersized allografts.
Figure 12.
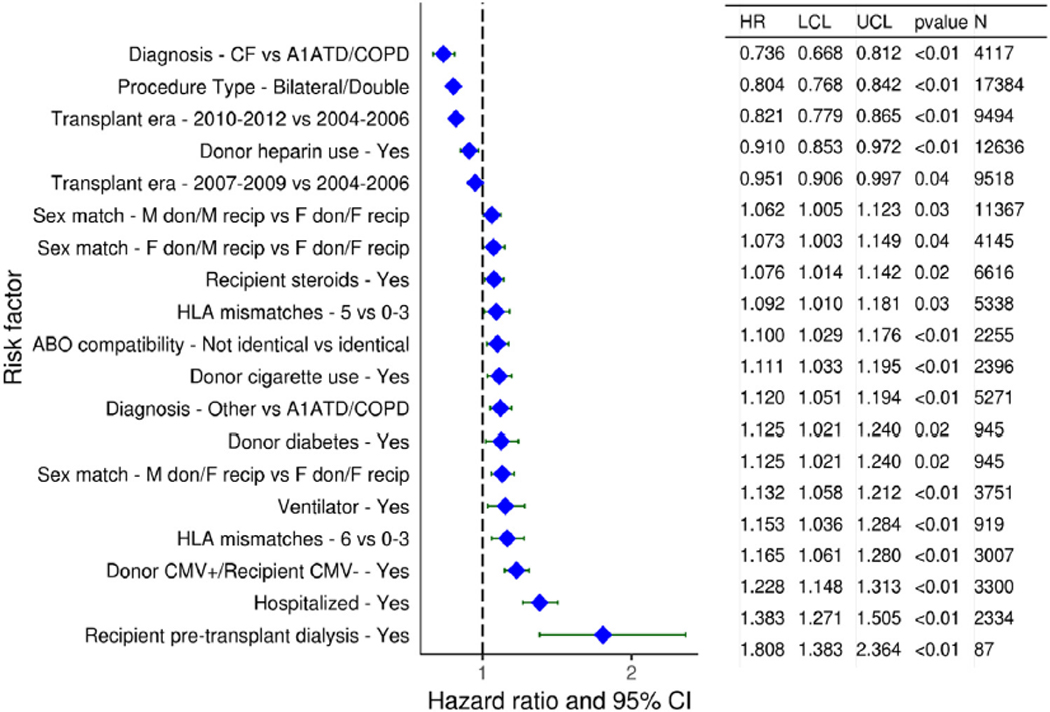
Statistically significant categorical risk factors for 5-year mortality for adult lung transplant recipients with 95% CIs (transplants: 2005–June 2013, N = 27,023). This model includes size match. A1ATD, alpha-1-antitrypsin deficiency; CF, cystic fibrosis; CI, confidence interval; CMV, cytomegalovirus; COPD, chronic obstructive pulmonary disease; F, female; HLA, human leukocyte antigen; HR, hazard ratio; IIP, idiopathic interstitial pneumonia; ILD-not IIP, interstitial lung disease-not idiopathic interstitial pneumonia; LCL, lower control limit; M, male; UCL, upper control limit.
Figure 13.
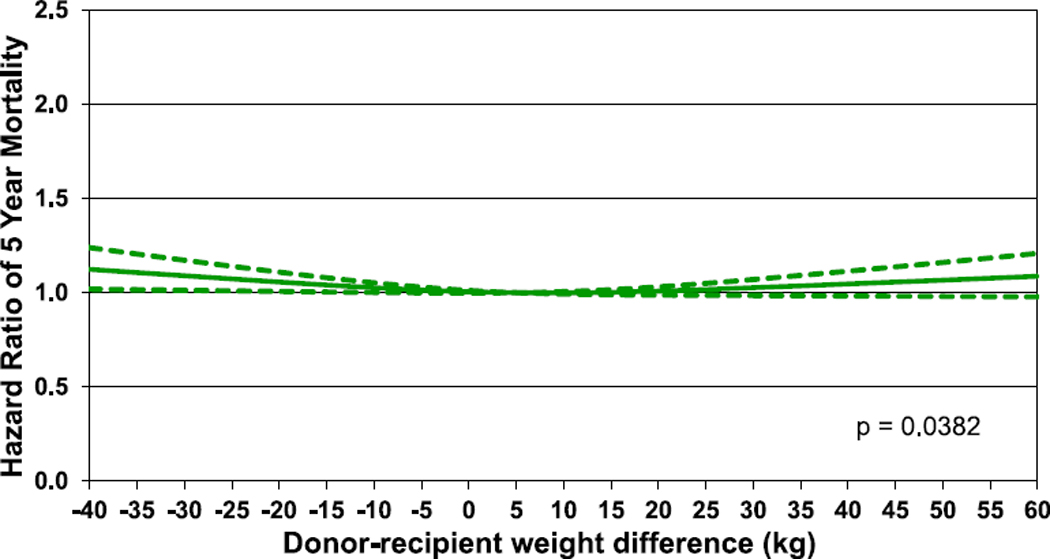
Hazard ratio of 5-year mortality for adult lung transplant recipients by donor-recipient weight difference (transplants: 2005–June 2013, N = 27,023). The reference value for weight difference is 5 kg.
Figure 14.
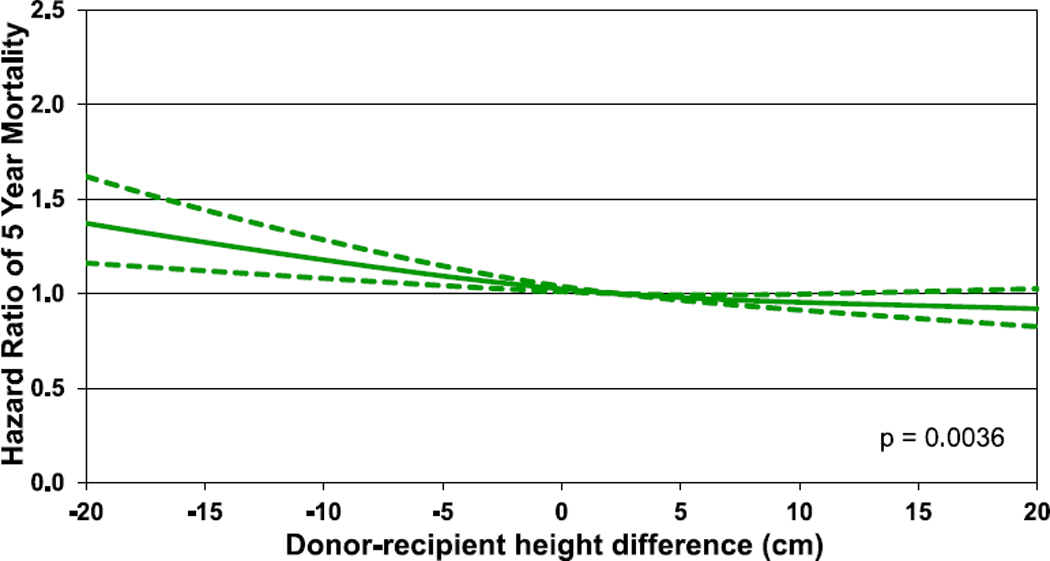
Hazard ratio of 5-year mortality for adult lung transplant recipients by donor-recipient height difference (transplants: 2005–June 2013, N = 27,203). The reference value for height difference is 2.4 cm.
Figure 15.
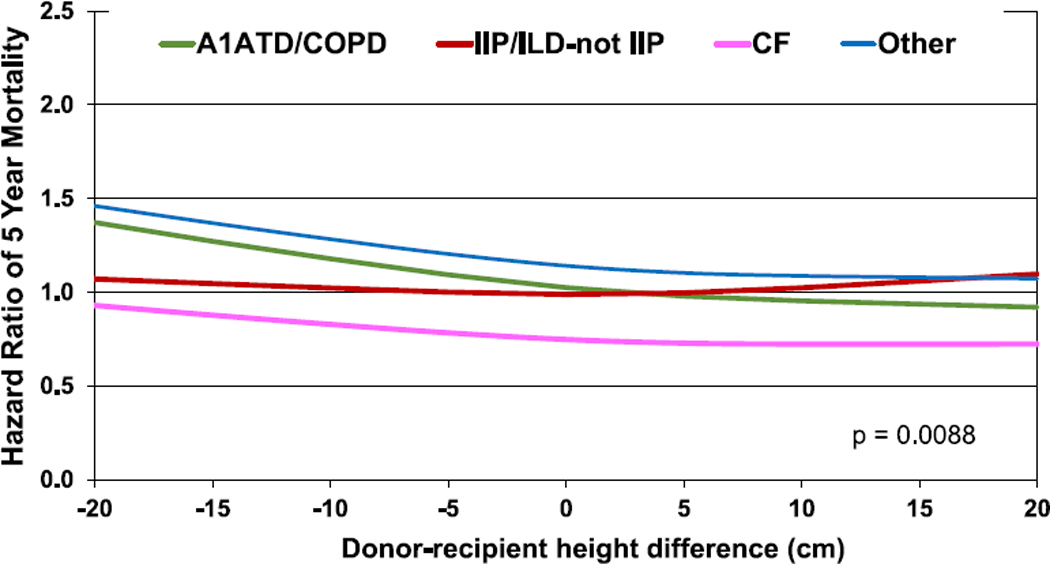
Hazard ratio of 5-year mortality for adult lung transplant recipients by donor-recipient height difference and diagnosis interaction (transplants: 2005–June 2013, N = 27,023). A1ATD, alpha-1-antitrypsin deficiency; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; IIP, idiopathic interstitial pneumonia; ILD-not IIP, interstitial lung disease-not idiopathic interstitial pneumonia.
Heart-lung transplantation
Centers, transplant activity, and recipient characteristics
The annual reported number of heart-lung transplants remains static with 59 procedures reported in 2017 (Figure 16, eSlide HL (a) 4). At least 1 heart-lung transplant was reported performed at 87 centers (33.7% of the total number of reporting centers) between 2010 and June 2018. The majority of these centers reported an average of 1 procedure per year (eSlide HL(a) 5).
Figure 16.
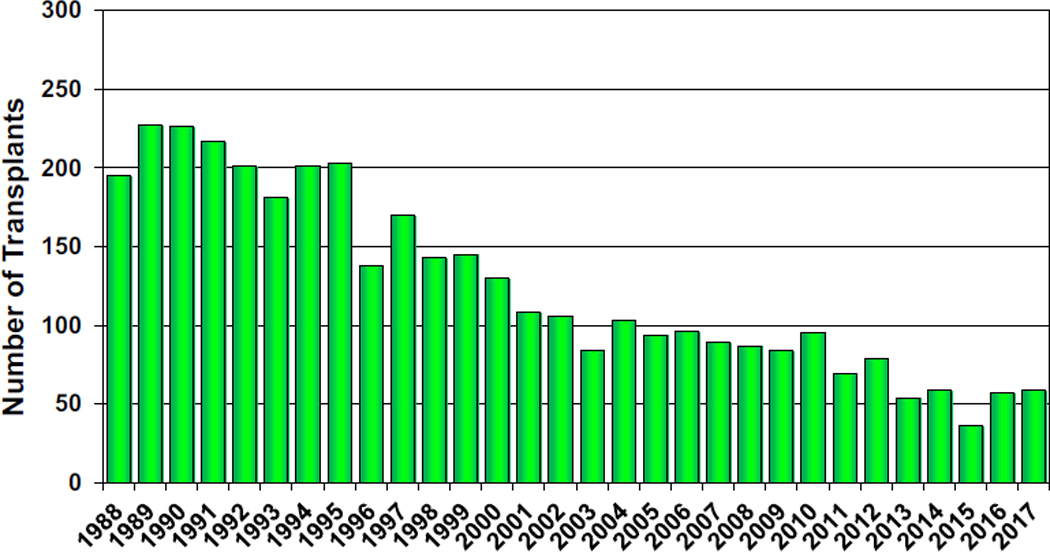
Number of adult heart-lung transplants by transplant year.
There were no significant changes in indication for heart-lung transplantation, with pulmonary hypertension accounting for the majority of procedures (eSlides HL(a) 8–9). The trend for a small but increasing number of heart-lung transplants performed for IIP and other diagnoses continued unabated in the past year (eSlides HL(a) 8–9). This increased activity has occurred at the expense of heart-lung transplantation for CF, which has become a rare indication compared with the 1990s. As has been the case for the past few years, an increasing proportion of heart-lung transplant recipients are older than 50 years at the time of transplant. The trend for older, non-CF recipients is particularly strong in North America, where 34% of recipients are now 50 years or older, and 8% 60 years or older (eSlide HL(a) 10).
Immunosuppression, morbidity, and BOS
A smaller proportion of heart-lung transplant recipients than that of lung transplant recipients receive induction therapy, although the proportion varies significantly from year to year (eSlide HL(a) 23). Immunosuppression regimens, however, are similar (eSlide HL(a) 24). Renal dysfunction, diabetes mellitus, malignancy, and chronic allograft rejection (allograft vasculopathy and BOS) are unfortunately common complications of heart-lung transplantation (eSlides HL(a) 26–30).
Survival
The median survival for heart-lung transplant recipients has increased over the past few decades to 6.5 years in the most recent era; much of this mortality occurs early after transplantation, with median survival, conditional on survival to 1 year after transplant, almost double that at 12.8 years (Figure 17, eSlide HL(a) 15). Recipients transplanted for IIP have particularly poor outcomes, with median survival of only 1.9 years, significantly lower than median survival for CF recipients (Figure 18, eSlide HL(a) 16). The results of multivariable analysis of 1-year mortality for adult heart-lung transplant recipients are presented in eSlides L(a) 48–49. This model includes donor-recipient height and weight difference. Independent risk factors for mortality were earlier transplant era, cytomegalovirus mismatch, recipient ventilator dependence, and older donor age.
Figure 17.
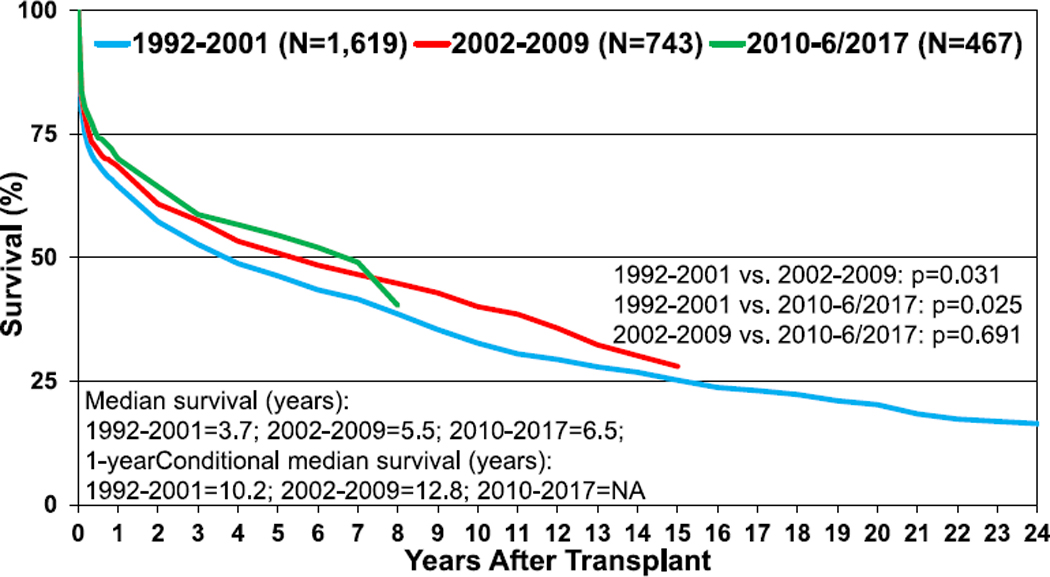
Kaplan-Meier survival for adult heart-lung transplant recipients by transplant era (transplants: January 1992–June 2017). NA, not applicable.
Figure 18.
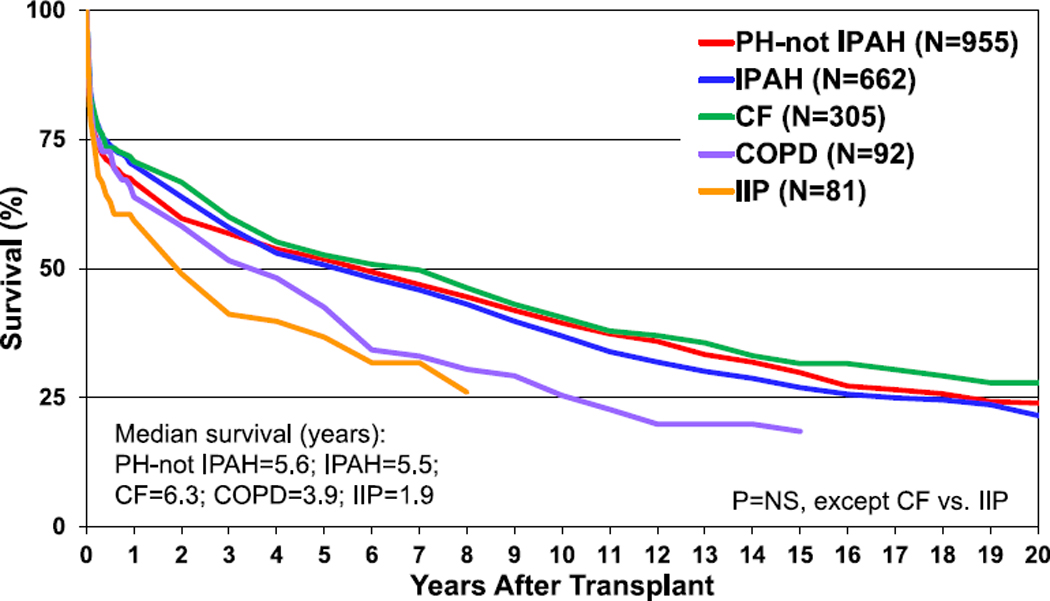
Kaplan-Meier survival for adult heart-lung transplant recipients by major diagnosis (transplants: January 1992–June 2017). CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; IIP, idiopathic interstitial pneumonia; IPAH, idiopathic pulmonary arterial hypertension; NS, not significant; PH, pulmonary hypertension.
Heart-lung transplantation donor and recipient size match
Data from 1,532 adult heart-lung transplants performed between January 1992 and June 2018 and reported to the Registry were included in this analysis. Donors and recipients were generally well matched for height with a mean difference of 1.2 cm (i.e., slightly oversized donor) and no change in height matching practice over time (eSlide HL(a) 35). There were no differences in donor-recipient height match by diagnostic category (eSlide HL (a) 36); however, female-to-male heart-lung transplants were generally undersized, whereas male-to-female transplants were generally oversized (eSlide HL(a) 37).
In contrast to lung transplantation, in heart-lung transplantation, weight difference appeared of more importance in terms of association with survival than height difference. There were no significant differences in unadjusted 1- and 5-year survival for the height difference categories (Figure 19, eSlides HL(a) 42 and 44), whereas unadjusted 1- and 5-year survival was lower in recipients of allografts from donors undersized by weight (Figure 20, eSlides 43 and 45). Nevertheless, after multivariable adjustment, donor-recipient height and weight differences were no longer independently associated with survival after heart-lung transplantation.
Figure 19.
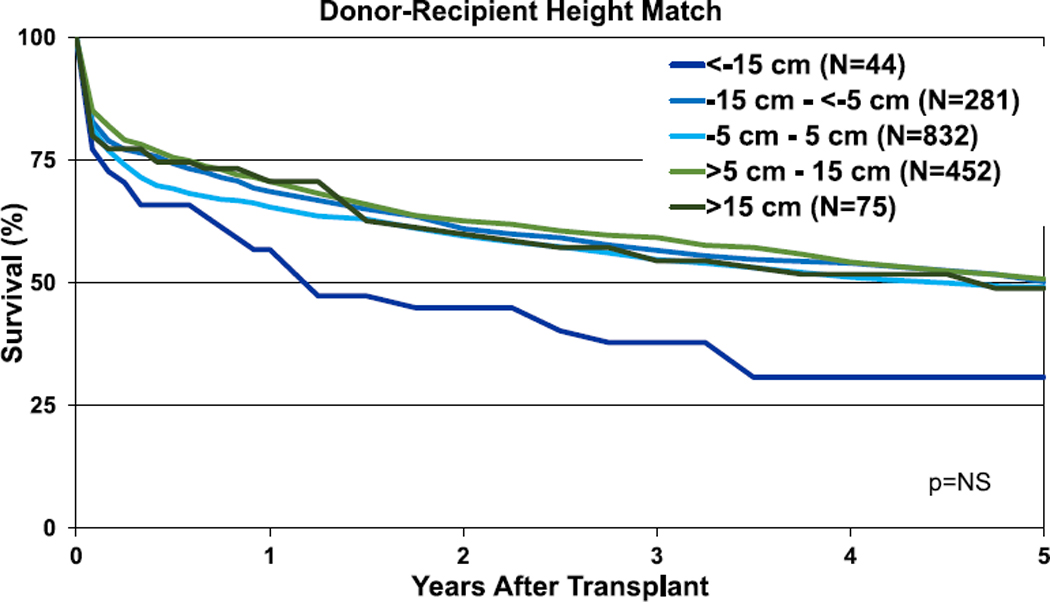
Kaplan-Meier survival for adult heart-lung transplant recipients within 5 years by donor-recipient height difference (transplants: January 1992–June 2013). NS, not significant.
Figure 20.
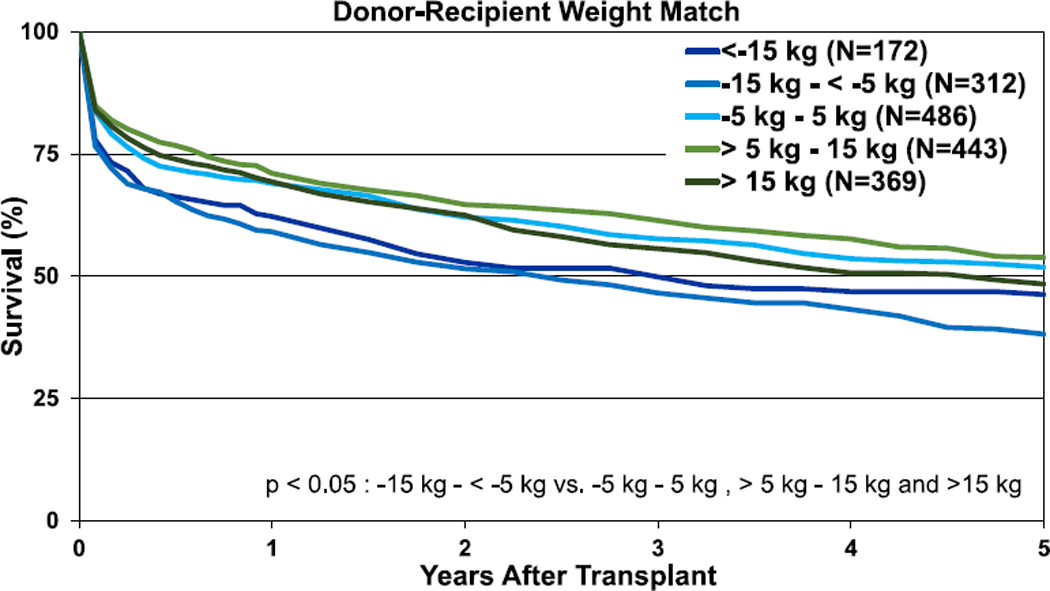
Kaplan-Meier survival for adult heart-lung transplant recipients within 5 years by donor-recipient weight difference (transplants: January 1992–June 2013).
Conclusions
The 2019 report presents important information regarding the global practice of lung and heart-lung transplantation. A history of donation after circulatory death has emerged as being associated with higher post-lung transplant survival at 10 years. Although numbers remain small, and different explanations for this finding are conceivable, the data presented here suggests that long-term survival after DCD lung transplantation is favorable. This report represents the most comprehensive description to date of size matching approaches and outcomes in lung transplantation. The consistent message across all analyses was that undersizing in terms of height and weight is associated with inferior posttransplant survival, in line with previous reports.9–12 This finding was independent of potential confounding factors recorded in the Registry dataset. The only diagnostic group where this association was not apparent was the group transplanted for ILD, where those receiving undersized allografts experienced similar survival to those receiving well-matched and even oversized grafts.
It is because of the generosity and dedication of the collectives which report to the Registry that this global resource can generate evidence to guide clinical practice in our highly specialized fields. It is the Transplant Registry Committee’s hope that the 36th Report will provide information which is useful in day-to-day practice for transplant professionals worldwide who seek to continually improve outcomes and quality of life for the patient groups they serve.
Supplementary Material
Footnotes
Disclosure statement
Kiran Khush serves as scientific adviser and speaker for CareDx, Inc; Luciano Potena serves as a speaker for Thermofisher, Sandoz, One Lambda, and Novartis, and is advisory board member for Qiagen and Novartis; Josef Stehlik serves as consultant for Medtronic and Abbott; Daniel Chambers received research funding from Astellas and Boeringher-Ingelheim; Joseph Rossano serves as consultant for Bayer, Novartis, Amgen, CSL Behring; Andreas Zuckermann serves on the speakers bureau of Paragonix, Novartis, Mallincrodt, Sanofi-Genzyme, and Franz Köhler Chemie and on the advisory board for Chiesi. The following authors have no disclosures: Michael Harhay, Don Hayes, Tajinder Singh, Eileen Hsich, Bruno Meiser, Wida Cherikh, Amanda Robinson, and Aparna Sadavarte.
Supplementary data
Supplementary data associated with this article can be found in the online version at www.jhltonline.org.
References
- 1.Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult lung and heart-lung transplant report-2018; Focus theme: multiorgan Transplantation. J Heart Lung Transplant 2018;37:1169–83. [DOI] [PubMed] [Google Scholar]
- 2.Goldfarb SB, Hayes D Jr, Levvey BJ, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation.: twenty-first pediatric lung and heart–lung transplantation report-2018; focus theme: multiorgan transplantation. J Heart Lung Transplant 2018;37:1196–206. [DOI] [PubMed] [Google Scholar]
- 3.Rossano JW, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: twenty-first pediatric heart transplantation report-2018; Focus theme: multiorgan Transplantation. J Heart Lung Transplant 2018;37:1184–95. [DOI] [PubMed] [Google Scholar]
- 4.Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult heart transplantation Report-2018; focus theme: multiorgan transplantation. J Heart Lung Transplant 2018;37:1155–68. [DOI] [PubMed] [Google Scholar]
- 5.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report—2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009–24. [DOI] [PubMed] [Google Scholar]
- 6.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-second official adult lung and heart-lung transplantation report-2015; focus theme: early graft failure. J Heart Lung Transplant 2015;34: 1264–77. [DOI] [PubMed] [Google Scholar]
- 7.Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016;35: 1170–84. [DOI] [PubMed] [Google Scholar]
- 8.Chambers DC, Yusen RD, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult lung and heart-lung transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant 2017;36:1047–59. [DOI] [PubMed] [Google Scholar]
- 9.Delom F, Danner-Boucher I, Dromer C, et al. Impact of donor-to-recipient weight ratio on survival after bilateral lung transplantation. Transplant Proc 2014;46:1517–22. [DOI] [PubMed] [Google Scholar]
- 10.Eberlein M, Permutt S, Chahla MF, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest 2012;141:451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eberlein M, Reed RM, Bolukbas S, et al. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant 2015;34:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eberlein M, Reed RM, Maidaa M, et al. Donor-recipient size matching and survival after lung transplantation. A cohort study. Ann Am Thorac Soc 2013;10:418–25. [DOI] [PubMed] [Google Scholar]
- 13.Hayden AM, Scarlett MV, Fox K. Relationship between donor/recipient lung size mismatch and functional outcome in single lung transplantation for COPD. J Transpl Coord 1996;6:155–8. [DOI] [PubMed] [Google Scholar]
- 14.Fessart D, Dromer C, Thumerel M, Jougon J, Delom F. Influence of gender donor-recipient combinations on survival after human lung transplantation. Transplant Proc 2011;43:3899–902. [DOI] [PubMed] [Google Scholar]
- 15.Iyer A, Kumarasinghe G, Hicks M, et al. Primary graft failure after heart transplantation. J Transplant 2011;2011:175768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergenfeldt H, Stehlik J, H€oglund P, Andersson B, Nilsson J .Donor-recipient size matching and mortality in heart transplantation: influence of body mass index and gender. J Heart Lung Transplant 2017;36:940–7. [DOI] [PubMed] [Google Scholar]
- 17.Khush KK, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth Adult Heart Transplantation report - 2019; Focus Theme: Donor and Recipient Size Match. J Heart Lung Transplant 2019;38:1056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilburn KH, Warshaw RH, Thornton JC, Thornton K, Miller A. Predictive equations for total lung capacity and residual volume calculated from radiographs in a random sample of the Michigan population. Thorax 1992;47:519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paoletti P, Viegi G, Carrozzi L, et al. Residual volume in a general population. Effects of body size, age, cigarette smoking, and respiratory symptoms. Chest 1992;102:1209–15. [DOI] [PubMed] [Google Scholar]
- 20.Hayes D, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-first Pediatric Lung and Heart-Lung Transplantation Report-2019; Focus Theme: Donor and Recipient Size Match. J Heart Lung Transplant 2019;37:xxxx-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


