Abstract
Medication treatment (MT) is one of the few evidence-based strategies proposed to combat the current opioid epidemic. We examined national trends and correlates of offering MT in substance use treatment facilities in the United States. According to data from national surveys, the proportion of these facilities that offered any MT increased from 20.0 percent in 2007 to 36.1 percent in 2016—mainly the result of increases in offering buprenorphine and extended-release naltrexone. Only 6.1 percent of facilities offered all three MT medications in 2016. Facilities in states with higher opioid overdose death rates, facilities that accepted health insurance overall (and, more specifically, those that accepted Medicaid in states that opted to expand eligibility for Medicaid), and facilities in states with more comprehensive coverage of MT under their Medicaid plans had higher odds of offering MT. The findings highlight the persistent unmet need for MT nationally and the role of expansion of health insurance in the dissemination of these treatments.
The United States is in the midst of an opioid epidemic.1–3 The number of adults who misuse prescription opioids or use heroin has grown rapidly in the past decade, along with fatal and nonfatal opioid overdoses.4,5 Opioid overdose claimed more than 45,000 lives in 20176—more deaths than traffic accidents or HIV infection.7,8
Among evidence-based strategies to reverse these trends, increased access to outpatient medication treatment (MT) features prominently.9–12 There is strong evidence supporting the efficacy, effectiveness, and cost-effectiveness of treatment with methadone, buprenorphine, and extended-release naltrexone, the three medications approved by the Food and Drug Administration (FDA) for long-term management of opioid use disorder.13–20 Yet only a minority of people with opioid use disorders receive any substance use treatment (for example, counseling or inpatient treatment), and an even smaller minority receive MT.21–23 In a 2014–15 national study, only 50 percent of those with past-year heroin use disorder and 23 percent with prescription opioid use disorder received any substance use treatment.24 In a study of commercially insured youth, only 27.5 percent of those with opioid use disorder received buprenorphine or naltrexone treatment within six months of diagnosis.22
The low prevalence of MT use is partly due to its limited availability in many communities. In a nationally representative survey of 345 private-sector specialty addiction treatment facilities, only 20.9 percent offered buprenorphine and 7.8 percent offered methadone. Even among programs that offered these medication treatments, fewer than half of eligible patients received them.25
The use of methadone for opioid use disorders is strictly regulated: It can be dispensed only by opioid treatment programs that are certified by the Substance Abuse and Mental Health Services Administration (SAMHSA). In contrast, buprenorphine can be prescribed for opioid use disorder by any physician after brief training and certification by the Drug Enforcement Administration, and extended-release naltrexone can be prescribed by any licensed health care provider.
The less restrictive laws governing the prescription of buprenorphine and extended-release naltrexone may have contributed to the expansion of MT in recent years. A 2017 report indicated increasing use of MT in substance use treatment facilities, especially in programs that did not house a federally approved opioid treatment program.26
Little is known about the factors that distinguish between substance use treatment facilities that do and do not offer MT. In particular, the impact of the rise in opioid overdose mortality on the adoption of MT by specialty treatment facilities remains unexplored. Even before a national state of emergency was announced in October 2017, several states had declared emergencies in response to the increases in opioid overdose deaths in their jurisdictions.27 Furthermore, some of the hardest-hit states have mounted multipronged initiatives that include dispensing naloxone for the prevention of overdose deaths, expanding treatment services, and enhancing access to MT at substance use treatment facilities.28–30 An improved understanding of the national growth and distribution of MT availability within the facilities could help policy makers and planners identify and close critical gaps in the opioid treatment infrastructure.
In this study we used national data on substance use treatment services for 2007–16 from the National Survey of Substance Abuse Treatment Services to examine temporal trends in MT use in the US and data from 2016 survey to examine associations of facility- and state-level characteristics with offering MT for opioid use disorders. Our results highlight state environments and facility characteristics associated with the availability of MT at substance use treatment facilities. This work seeks to inform efforts to broaden access to these potentially lifesaving treatments.
Study Data And Methods
DATA
Completed by or under the direction of program directors, the National Survey of Substance Abuse Treatment Services is an annual census of all substance abuse treatment facilities known to SAMHSA, both public and private. These facilities include programs operated by federal agencies (primarily the Department of Veterans Affairs, Department of Defense, and Indian Health Service); state and local, county, community, or tribal governments; private nonprofit and for-profit organizations; opioid treatment programs certified by SAMHSA; hospital-based programs; and small group practices. The facilities do not include solo practitioners (unless their inclusion is specifically requested by the state) or jails and prisons.31
Response rates to the survey were over 90 percent for the period 2007–16 (the numbers of responding facilities ranged from 13,339 to 14,399 per year). We restricted the sample to facilities with outpatient services whose patients were not limited to people convicted of driving while intoxicated (DWI) or driving under the influence (DUI). Approximately 11,000 facilities (range per year: 10,650–11,753) met these inclusion criteria in the study period. Analyses for facility- and state-level correlates of MT in 2016 were further limited to 11,693 facilities with outpatient services in the fifty states or the District of Columbia.
KEY VARIABLES
The National Survey of Substance Abuse Treatment Services collected information about types of medications offered at each facility. Certified methadone-dispensing opioid treatment programs and programs offering buprenorphine with or without naloxone or offering extended-release naltrexone were categorized as MT-offering facilities. In 2011 the survey started recording extended-release naltrexone, which was approved by the FDA in October 2010.
Information on the organization operating each facility (federal agency as listed above; state, local, county, community, or tribal government; or private for-profit or nonprofit organization), whether the facility was located in or operated by a hospital, whether the facility had inpatient or residential services, the number of patients seen in March 2016, and the types of funding received or accepted by the facility (federal, state, county, or local funds; Medicaid; Medicare; a state-financed health insurance plan other than Medicaid; private insurance; or cash or self-payment) and whether it offered free treatment for all patients were also recorded.
The National Survey of Substance Abuse Treatment Services provided binned data on the number of patients seen in each facility in March of each year. The numbers in the bins differed for outpatient facilities that offered an opioid treatment program and those that offered DWI/DUI services and other outpatient facilities. For the 2016 data we defined facilities with outpatient opioid treatment services that were provided to more than 315 patients, outpatient DWI/DUI services provided to more than 70 patients, or other outpatient services provided to more than 51 patients as large facilities, and we defined all other facilities as being small to medium-size.
State-level variables included the 2015–16 aggregated prevalence of past-year heroin use in adults based on data from the National Survey on Drug Use and Health32 and averaged 2015–16 opioid overdose death rates based on data from the Centers for Disease Control and Prevention online data source (CDC Wonder)33 extracted by the authors. As a negative control, we also computed averaged 2015–16 overall age-adjusted death rates for analyses of geographical distribution of mortality data. States’ ACA Medicaid expansion status by 2016 and the number of MT medications covered under states’ Medicaid programs were obtained from data collected by the Kaiser Family Foundation.34,35 (For states’ expansion status and MT coverage, see online appendix exhibit A.)36
States were classified based on whether their Medicaid programs covered one, two, or all three of the MT medications. (For the number of MT medications covered by each state, see appendix exhibit A.)36 In addition, we used data on state Medicaid coverage of substance use treatment according the American Society of Addiction Medicine’s classification of levels of coverage,37 assessed in the 2013 National Drug Abuse Treatment System Survey.38 The society’s levels of care include outpatient, intensive outpatient, residential, and detoxification services. State Medicaid programs were categorized as either covering all four or fewer levels of care. (For states’ levels of coverage, see appendix exhibit A.)36
ANALYSES
Analyses were conducted in four stages. First, percentages of outpatient facilities that offered MT were examined for the period 2007–16, using data from the National Survey of Substance Abuse Treatment Services.
Second, the geographical distribution of facilities that offered MT across states in 2016 and the prevalence of opioid overdose deaths, overall deaths, and heroin use in those states in 2015–16 were examined. We also computed Pearson correlation coefficients for these associations. Two-year periods of death rate data were averaged to obtain more stable estimates.
Third, logistic regression analyses were conducted to assess the facility-level correlates of offering MT. Variables included type of operating agency, relationship with a hospital, other services provided by the facility, size of the program, and funding sources. Unadjusted and adjusted logistic regression analyses were conducted.
Data on facility size were missing for 17.7 percent of facilities, and data on the sources of facility funding were missing for 1.3–6.2 percent of facilities. We used multiple imputation with five imputed data sets to replace the missing values on these variables (all variables were dichotomous).
Fourth, the associations of state-level variables with offering MT at each facility were examined using logistic regression analyses. The models included state overdose death rates and prevalence of heroin use, both averaged for 2015–16; Medicaid expansion status; the number of MT medications covered by the state’s Medicaid program; and the American Society of Addiction Medicine’s levels of care that were covered under the state’s Medicaid program. Overdose deaths and heroin use were dichotomized based on median split. Two sets of models were fitted: a set of unadjusted models and a set of models that adjusted for the facility-level variables described above. Because Medicaid policies are most likely to affect facilities that accept this type of health insurance, analyses for Medicaid expansion, Medicaid coverage of MT medications, and Medicaid’s coverage of the American Society of Addiction Medicine’s levels of care were repeated after we limited the sample to facilities that accepted Medicaid.
The state was entered as a random intercept in all logistic regression models to adjust for the nesting of facilities within states. All analyses were conducted using Stata, version 15.1. The mi impute routine of Stata with a logistic link was used for the multiple imputations.
LIMITATIONS
This analysis had some limitations. First, the National Survey of Substance Abuse Treatment Services collected information only about the availability of MT and not about the proportion of eligible patients who received these treatments. Past research has found that only a minority of eligible patients with opioid use disorder in facilities that offer MT receive it.21
Second, several important correlates of MT provision were not measured, such as the availability of providers with prescriptive authority.
Third, the survey does not capture medical offices, where a significant proportion of buprenorphine and extended-release naltrexone are prescribed.39,40 Therefore, these data underrepresent MT medications dispensed nationwide. Nevertheless, other data sources corroborate that there is substantial unmet need for MT across the country.23
Fourth, data on the American Society of Addiction Medicine’s levels of care covered under state Medicaid programs were based on the 2013 survey,38 the source of the most recent data publicly available.
Finally, the associations of state-level mortality and heroin use with MT availability are ecological in nature and prone to ecological fallacy.
Study Results
TEMPORAL TRENDS IN FACILITIES OFFERING MEDICATION TREATMENT
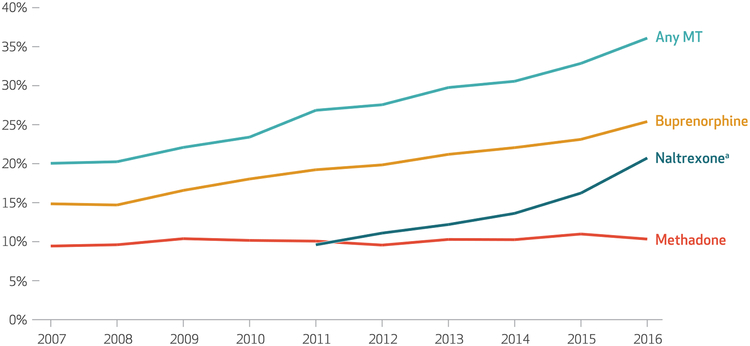
The percentage of substance use treatment facilities with outpatient services offering any MT increased from 20.0 percent in 2007 to 36.1 percent in 2016 (exhibit 1). This was mainly due to increases in the percentages of facilities offering buprenorphine (from 14.9 percent to 25.4 percent) and extended-release naltrexone (from 9.6 percent to 20.7 percent), rather than to the increase in the percentage of facilities offering methadone through an opioid treatment program (from 9.4 percent to 10.3 percent). The survey data showed that in 2016, 2,968 (70.4 percent) of the 4,218 facilities that offered any MT offered buprenorphine, and 2,429 (57.6 percent) offered extended-release naltrexone. Only 1,208 (28.7 percent) offered methadone, and only 256 (6.1 percent) offered all three medications.
Exhibit 1. Trends in the proportion of outpatient substance use treatment facilities that offered medication treatment (MT), 2007–16.
source Authors’ analysis of data for 2007–16 from the National Survey of Substance Abuse Treatment Services. note All changes from the first year to the last year were statistically significant (p < 0:001). aExtended release.
GEOGRAPHICAL DISTRIBUTION
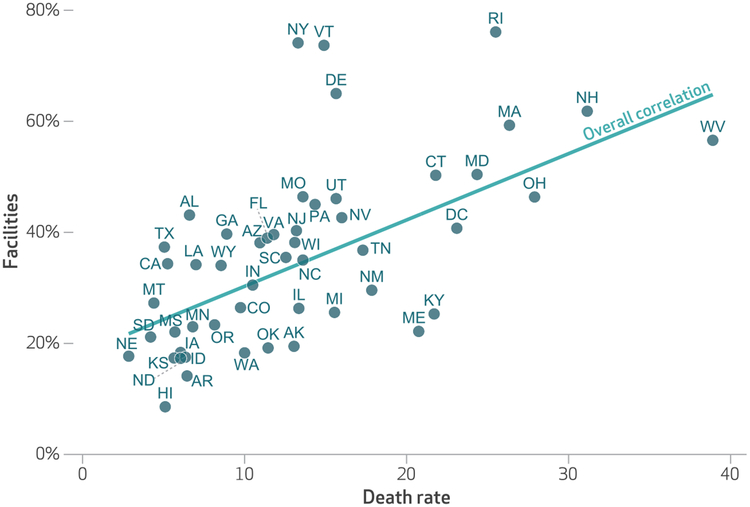
The geographical distribution of facilities offering MT in 2016 and the distributions of heroin use, opioid overdose deaths, and overall deaths in 2015–16 are presented in appendix exhibits B1–B4.36 There was a strong correlation between past-year prevalence of heroin use and facilities offering MT in each state (correlation coefficient [r] 0:46; p < 0:001). (For the association of states’ prevalence of past-year heroin use and offering MT, see appendix exhibit C.)36 A similarly strong association was noted between opioid overdose deaths and MT use (r = 0:61; p < 0:001) (exhibit 2). However, overall deaths were not associated with MT use (r = − 0:09; p > 0:1). (For the association of states’ prevalence of overall deaths with MT use, see appendix exhibit D.)36
Exhibit 2. Association between the proportion of outpatient substance use treatment facilities in each state that offered medication treatment (MT) in 2016 andstates’ opioid overdose death rates per 100,000 population in 2015–16.
source Authors’ analysis of data for 2016 from the National Survey of Substance Abuse Treatment Services and for 2015–16 based on data from the Centers for Disease Control and Prevention drawn by the authors from the CDC Wonder database. notes The exhibit shows death rates for the District of Columbia as well as the fifty states. The correlation between the share of facilities offering MT and death rates was significant (p < 0:001), with a correlation coefficient of 0.61.
Facilities in Rhode Island (76.1 percent), New York (73.7 percent) and Vermont (73.7 percent) were more likely than facilities in other states to offer MT, while facilities in Hawaii (8.6 percent), Arkansas (14.1 percent), and Idaho (16.8 percent) were less likely to offer MT (exhibit 2). A comparison of relative state rankings in the percentage of facilities offering MT and in opioid overdose deaths identified Maine, Kentucky, and New Mexico as states with lower prevalence of facilities offering MT (22.3 percent, 25.2 percent, and 29.1 percent, respectively) and relatively higher opioid overdose death rates (21, 22, and 18 per 100,000, respectively).
FACILITY-LEVEL CORRELATES
In unadjusted logistic regression analyses, we found that offering MT was more common in Department of Veterans Affairs facilities, compared to private for-profit facilities; facilities located or operated by a hospital, compared to those not related to a hospital; and facilities with inpatient and residential services, compared to those without these services (exhibit 3). Offering MT was also more common in large facilities than in those of small to medium size; and in facilities accepting Medicare, state-sponsored insurance, private insurance, and cash or self-payment than in facilities not accepting these forms of payment. In contrast, offering MT was less common in private nonprofit facilities; state, local, county, community, or tribal government facilities; and Department of Defense facilities, compared to private for-profit facilities. Facilities that received federal, state, county, or local funds (which included block grants) also had lower odds of offering MT, as did facilities that provided free treatment to all of their patients. With few exceptions, these associations persisted in adjusted logistic regression analysis, although the odds ratios were attenuated following adjustment. Furthermore, the association with state-financed health insurance changed direction: Facilities that accepted this type of insurance had 13 percent lower odds of offering MT in adjusted analysis.
Exhibit 3.
Outpatient substance use treatment facilities that did and did not offer medication treatment (MT) in 2016, by facility characteristics
| Facility characteristic | Facilities offering MT | Odds ratios, comparison of facilities offering and not offering MT | ||
|---|---|---|---|---|
| Numbera | Percent | Unadjusted | Adjustedb | |
| Operated by: | ||||
| Private for-profit organization (n = 4,549) | 1,774 | 39.0 | 1.00 | 1.00 |
| Private nonprofit organization (n = 5,751) | 1,929 | 33.5 | 0.68**** | 0.52**** |
| State government (n = 205) | 68 | 33.2 | 0.65*** | 0.45**** |
| Local, county, or community government (n = 631) | 182 | 28.8 | 0.51**** | 0.41**** |
| Tribal government (n = 242) | 56 | 23.1 | 0.64*** | 0.82 |
| Department of Veterans Affairs (n = 201) | 177 | 88.1 | 12.36**** | 5.51**** |
| Department of Defense (n = 86) | 23 | 26.7 | 0.53** | 0.92 |
| IHS or other federal agency (n = 28) | 9 | 32.1 | 0.99 | 1.21 |
| Located in or operated by a hospital | ||||
| No (n = 10,659) | 3,518 | 33.0 | 1.00 | 1.00 |
| Yes (n = 1,033) | 699 | 67.7 | 3.90**** | 3.24**** |
| Has inpatient services | ||||
| No (n = 11,286) | 3,872 | 34.3 | 1.00 | —c |
| Yes (n = 407) | 346 | 85.0 | 11.96**** | —c |
| Has nonhospital residential services | ||||
| No (n = 10,517) | 3,588 | 34.1 | 1.00 | 1.00 |
| Yes (n = 1,176) | 630 | 53.6 | 2.63**** | 3.20**** |
| Size | ||||
| Small to medium (n = 6,140) | 1,708 | 27.8 | 1.00 | 1.00 |
| Large (n = 3,484) | 1,700 | 48.8 | 1.80**** | 2.01**** |
| Receives any federal, state, county, or local funds | ||||
| No (n = 5,357) | 2,092 | 39.1 | 1.00 | —d |
| Yes (n = 5,854) | 1,926 | 32.9 | 0.74**** | —d |
| Accepts Medicaid | ||||
| No (n = 3,776) | 1,239 | 32.8 | 1.00 | 1.00 |
| Yes (n = 7,661) | 2,878 | 37.6 | 0.99 | 1.20*** |
| Accepts Medicare | ||||
| No (n = 7,039) | 2,362 | 33.6 | 1.00 | 1.00 |
| Yes (n = 4,321) | 1,744 | 40.4 | 1.22**** | 1.14** |
| Accepts state-financed health insurance other than Medicaid | ||||
| No (n = 5,380) | 1,893 | 35.2 | 1.00 | 1.00 |
| Yes (n = 5,583) | 2,018 | 36.1 | 1.52**** | 0.87** |
| Accepts private insurance | ||||
| No (n = 3,098) | 898 | 29.0 | 1.00 | 1.00 |
| Yes (n = 8,360) | 3,246 | 38.8 | 1.52**** | 1.17** |
| Accepts cash or self-payment | ||||
| No (n = 982) | 237 | 24.1 | 1.00 | 1.00 |
| Yes (n = 10,549) | 3,909 | 37.4 | 1.69**** | 1.62**** |
| Provides free treatment for all clients | ||||
| No (n = 11,279) | 4,153 | 36.8 | 1.00 | 1.00 |
| Yes (n = 272) | 22 | 8.1 | 0.18**** | 0.38**** |
source Authors’ analysis of data for 2016 from the National Survey of Substance Abuse Treatment Services.
notes Facilities that treated only patients convicted of driving while intoxicated or driving under the influence were excluded. Of the 11,693 facilities in the study, 4,218 (36.1 percent) offered MT (defined as offering methadone, buprenorphine, or extended-release naltrexone). Small-to-medium and large facilities are defined in the text. IHS is Indian Health Service.
Actual number of facilities, not imputed data.
Adjusted for type of operating organization, whether in or operated by a hospital, whether residential services were available in the facility, size of facility, and types of insurance accepted.
Not included in the multivariable model due to overlap with the variable of being located in or operated by a hospital.
Not included in the multivariable model due to overlap with the variable of being operated by a government agency.
p < 0:05
p < 0:01
p < 0:001
STATE-LEVEL CORRELATES
In unadjusted logistic regression analyses of state-level correlates, offering MT was associated with higher twelve-month prevalence of heroin use and opioid overdose death rates in 2015–16 (exhibit 4). Offering MT was also associated with a state’s having expanded Medicaid in analyses of all facilities and of facilities accepting Medicaid. Facility location in a Medicaid expansion state was associated with 21 percent overall higher odds of offering MT and 89 percent higher odds among facilities accepting Medicaid. Furthermore, offering MT in each facility was associated with the number of MT medications covered under the state’s Medicaid program in analyses of all facilities and of facilities accepting Medicaid. Each additional medication covered was associated with 44 percent higher odds of offering MT overall and 52 percent higher odds among facilities accepting Medicaid. Most of these associations persisted in analyses that adjusted for facility-level characteristics, except for the association of Medicaid expansion with all facilities. Facility location in a Medicaid expansion state remained a significant predictor of offering MT in adjusted analyses limited to facilities accepting Medicaid.
Exhibit 4.
Outpatient substance use treatment facilities that did and did not offer medication treatment (MT) in 2016, by state characteristics
| State characteristic | Facilities offering MT | Odds ratios, comparison of facilities offering and not offering MT | ||
|---|---|---|---|---|
| Numbera | Percent | Unadjusted | Adjustedb | |
| 12-month prevalence of heroin use, 2015–16c | ||||
| Low (<0.37%; n = 5,666) | 1,790 | 31.6 | 1.00 | 1.00 |
| High (≥0.37%; n = 6,027) | 2,428 | 40.3 | 1.46**** | 1.67*** |
| Opioid overdose death rate per 100,000 in 2015–16d | ||||
| Low (<13.1; n = 5,729) | 1,716 | 30.0 | 1.00 | 1.00 |
| High (≥13.1; n = 5,964) | 2,502 | 42.0 | 1.69**** | 2.25**** |
| Expanded eligibility for Medicaid (all facilities) | ||||
| No (n = 4,118) | 1,371 | 33.3 | 1.00 | 1.00 |
| Yes (n = 7,575) | 2,847 | 37.6 | 1.21**** | 1.31 |
| Expanded eligibility for Medicaid (facilities accepting Medicaid) | ||||
| No (n = 2,608) | 713 | 27.3 | 1.00 | 1.00 |
| Yes (n = 5,053) | 2,165 | 42.8 | 1.89*** | 1.81*** |
| Number of MT medications covered by state’s Medicaid programe (all facilities) | ||||
| One (n = 703) | 174 | 24.8 | 1.00 | 1.00 |
| Two (n = 1,818) | 559 | 30.7 | 1.35*** | 1.68 |
| All three (n = 9,172) | 3,485 | 38.0 | 1.86**** | 2.32* |
| Test of trendf | —g | —g | 1.44** | 1.44** |
| Number of MT medications covered by state’s Medicaid programe (facilities accepting Medicaid) | ||||
| One (n = 342) | 102 | 29.8 | 1.00 | 1.00 |
| Two (n = 1,232) | 323 | 26.2 | 1.34 | 1.31 |
| All three (n = 6,087) | 2,453 | 40.3 | 2.11 | 2.16 |
| Test of trendf | —g | —g | 1.52** | 1.57** |
| All ASAM levels of care covered under state’s Medicaid program (all facilities)?h | ||||
| No (n = 4,587) | 1,543 | 33.6 | 1.00 | 1.00 |
| Yes (n = 7,106) | 2,675 | 37.6 | 1.19 | 1.22 |
| All ASAM levels of care covered under state’s Medicaid program (facilities accepting Medicaid)?h | ||||
| No (n = 2,950) | 958 | 32.5 | 1.00 | 1.00 |
| Yes (n = 4,711) | 1,920 | 40.8 | 1.33 | 1.33 |
source Authors’ analysis of data for 2016 from the National Survey of Substance Abuse Treatment Services and other sources listed below.
notes Facilities that treated only patients convicted of driving while intoxicated or driving under the influence were excluded. Of the 11,693 facilities in the study, 4,218 (36.1 percent) offered MT (defined as offering methadone, buprenorphine, or extended-release naltrexone). ASAM is American Society of Addiction Medicine.
Actual number of facilities, not imputed data.
Adjusted as explained in the notes to exhibit 1.
Based on data from Substance Abuse and Mental Health Services Administration. 2015–2016 National Survey on Drug Use and Health (see note 32 in text).
Based on data from Centers for Disease Control and Prevention drawn by authors from the CDC Wonder database (see note 33 in text).
Based on data from Henry J. Kaiser Family Foundation. Medicaid’s role in addressing the opioid epidemic (see note 35 in text).
Trend was tested by entering the continuous variable of the number of medications covered under Medicaid (one, two, or all three) into the logistic regression models. The odds ratios associated with this variable represent changes in the odds of outcome for each additional medication covered.
Not applicable.
Based on data for 2013 from the National Drug Abuse Treatment System Survey reported in Grogan CM, et al. Survey highlights differences (see note 36 in text).
p < 0:1
p < 0:05
p < 0:01
p < 0:001
Discussion
In the past decade there has been an increase in the proportion of substance use treatment facilities that offer medication treatment for opioid use disorders. The increase has been mainly due to the availability of buprenorphine and extended-release naltrexone—two medications that, unlike methadone, can be prescribed outside of a federally certified opioid treatment program. The share of facilities that provided methadone treatment increased minimally over the study period.
Despite growth in the proportion of facilities offering MT, almost two-thirds of facilities did not provide any MT in 2016. The low availability of these services in substance use treatment facilities is likely one of the reasons for the large unmet need for MT across the country.21,22 As the US grapples with the opioid epidemic, there is a pressing need to expand access to the only evidence-based form of treatment available for managing opioid use disorder. The experiences of states in which most treatment facilities offer MT—Rhode Island, New York, and Vermont—may provide guidance to other states that are facing high or increasing opioid overdose death rates.28,41–44 The experience of the Department of Veterans Affairs also provides useful guidance for organizationwide initiatives.45 Curbing the opioid epidemic will likely require a multipronged approach that involves both prevention and treatment initiatives.
In 2016 only 6.1 percent of substance use treatment facilities offered all three MT treatments. Although the two opioid agonists—methadone and buprenorphine—are similarly efficacious, there is some evidence that methadone may be more effective in retaining patients in treatment and may be especially beneficial for patients with prior treatment failure.13,46 Furthermore, some patients may benefit from the structured treatment and accountability offered by methadone maintenance programs, while others may perceive treatment at a methadone clinic as stigmatizing or find the required daily travel overly burdensome.47 Other patients might not be able or willing to complete the induction period required for extended-release naltrexone treatment.48 Thus, the limited availability of all three medications in most treatment settings poses a barrier to the optimal use of MT.
The distribution of MT across the country parallels the trend in heroin use and opioid overdose deaths. Another study also found an association between opioid overdose deaths and the availability of buprenorphine-prescribing physicians.49 Contextual factors, such as publicity associated with heroin use and opioid overdose deaths, may have contributed to policy initiatives that in turn led to greater access to buprenorphine.28–30
Consistent with some past research and early predictions, Medicaid expansion was associated with offering MT, especially in facilities that accepted Medicaid.50–54 This result could be related to the more robust coverage of MT under Medicaid programs in expansion states.55 Nevertheless, a large proportion of people, especially those in poorer states, do not have ready access to treatment facilities that accept Medicaid.56
The shift in financing away from state and local government general revenues and toward Medicaid and private insurance may have promoted the integration of mental health treatment into general medical settings and more widespread use of prescription medications for the treatment of mental disorders.57,58 In contrast to mental health care, the sources of funding for substance use treatment services did not markedly change during the study period. By 2014 over 40 percent of spending for substance use disorder services came from state, local, and federal sources, including block grants.57 In comparison, only 20 percent of spending for mental health care came from these sources.57 With the implementation of the ACA, this financing landscape may be gradually changing, as the proportion of people with health insurance among patients receiving treatment in substance use disorder services has increased.24 There are some indications that treatment with buprenorphine in general medical settings may have also increased in tandem with these funding changes.51,53
Conclusion
Despite recent growth in the offering of medication treatment in substance use treatment facilities, a large unmet need for this treatment remains. A majority of facilities offer no MT, and very few offer all three of the relevant medications. Changes in policy, financing of care, and insurance coverage typically have a slow pace and might not be extensive enough to meet the present urgent need for the expansion of MT. In the meantime, state and federal governments may be able to leverage block grants and other local funding mechanisms to promote more expeditious implementation of MT in substance use treatment facilities.55
Acknowledgments
All of the authors were supported in this work by the National Institute on Drug Abuse (Grant No. R01DA039137).
Contributor Information
Ramin Mojtabai, Department of Mental Health at the Johns Hopkins Bloomberg School of Public Health, in Baltimore, Maryland..
Christine Mauro, Mailman School of Public Health, Columbia University, in New York City..
Melanie M. Wall, Department of Psychiatry, College of Physicians and Surgeons, Columbia University..
Colleen L. Barry, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health..
Mark Olfson, Department of Psychiatry, College of Physicians and Surgeons, Columbia University, and a research psychiatrist at the New York State Psychiatric Institute, in New York City..
NOTES
- 1.Gostin LO, Hodge JG Jr, Noe SA. Reframing the opioid epidemic as a national emergency. JAMA. 2017; 318(16):1539–40. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy M US declares opioid epidemic a “national emergency.” BMJ. 2017;358:j3881. [DOI] [PubMed] [Google Scholar]
- 3.Bonnie RJ, Kesselheim AS, Clark DJ. Both urgency and balance needed in addressing opioid epidemic: a report from the National Academies of Sciences, Engineering, and Medicine. JAMA. 2017;318(5):423–4. [DOI] [PubMed] [Google Scholar]
- 4.Vivolo-Kantor AM, Seth P, Gladden RM, Mattson CL, Baldwin GT, Kite-Powell A, et al. Vital signs: trends in emergency department visits for suspected opioid overdoses—United States, July 2016–September 2017. MMWR Morb Mortal Wkly Rep. 2018;67(9):279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones CM. Trends and key correlates of prescription opioid injection misuse in the United States. Addict Behav. 2018;78:145–52. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad FB, Rossen LM, Spencer MR, Warner M, Sutton P. Provisional drug overdose death counts [Internet]. Hyattsville (MD): National Center for Health Statistics; [page last updated 2018. November 14; cited 2018 Nov 27]. Available from: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm [Google Scholar]
- 7.National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. 2018 annual surveillance report of drug-related risks and outcomes—United States [Internet]. Atlanta (GA): CDC; [last revised 2018. August 31; cited 2018 Nov 28]. (Surveillance Special Report). Available from: https://www.cdc.gov/drugoverdose/pdf/pubs/2018-cdc-drug-surveillance-report.pdf [Google Scholar]
- 8.Centers for Disease Control and Prevention. Deaths: final data for 2016. Natl Vital Stat Rep. 2018; 67(5):1–76. [PubMed] [Google Scholar]
- 9.Barry CL. Fentanyl and the evolving opioid epidemic: what strategies should policy makers consider? Psychiatr Serv. 2018;69(1):100–3. [DOI] [PubMed] [Google Scholar]
- 10.Tetrault JM, Fiellin DA. More beds or more chairs? Using a science-based approach to address the opioid epidemic. Ann Intern Med. 2018; 168(1):73–4. [DOI] [PubMed] [Google Scholar]
- 11.Vestal C In fighting an opioid epidemic, medication-assisted treatment is effective but underused. Health Aff (Millwood). 2016;35(6): 1052–7. [DOI] [PubMed] [Google Scholar]
- 12.Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063–6. [DOI] [PubMed] [Google Scholar]
- 13.Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014; (2):CD002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009; (3):CD002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsch LA. The efficacy of methadone maintenance interventions in reducing illicit opiate use, HIV risk behavior, and criminality: a meta-analysis. Addiction. 1998;93(4): 515–32. [DOI] [PubMed] [Google Scholar]
- 16.Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JD, Nunes EV Jr, Novo P, Bachrach K, Bailey GL, Bhatt S, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018; 391(10118):309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanum L, Solli KK, Latif ZE, Benth JS, Opheim A, Sharma-Haase K, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74(12):1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krebs E, Enns B, Evans E, Urada D, Anglin MD, Rawson RA, et al. Cost-effectiveness of publicly funded treatment of opioid use disorder in California. Ann Intern Med. 2018; 168(1):10–9. [DOI] [PubMed] [Google Scholar]
- 20.Krebs E, Urada D, Evans E, Huang D, Hser YI, Nosyk B. The costs of crime during and after publicly funded treatment for opioid use disorders: a population-level study for the state of California. Addiction. 2017; 112(5):838–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nosyk B, Li L, Evans E, Urada D, Huang D, Wood E, et al. Utilization and outcomes of detoxification and maintenance treatment for opioid dependence in publicly-funded facilities in California, USA: 1991–2012. Drug Alcohol Depend. 2014;143:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, Larochelle MR. Trends in receipt of buprenorphine and naltrexone for opioid use disorder among adolescents and young adults, 2001–2014. JAMA Pediatr. 2017;171(8):747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015; 105(8):e55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feder KA, Mojtabai R, Krawczyk N, Young AS, Kealhofer M, Tormohlen KN, et al. Trends in insurance coverage and treatment among persons with opioid use disorders following the Affordable Care Act. Drug Alcohol Depend. 2017;179:271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knudsen HK, Abraham AJ, Roman PM. Adoption and implementation of medications in addiction treatment programs. J Addict Med. 2011; 5(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alderks CE. Trends in the use of methadone, buprenorphine, and extended-release naltrexone at substance abuse treatment facilities: 2003–2015 (update). CBHSQ Report [serial on the Internet]. 2017. August 22 [cited 2018 Nov 27]. Available from: https://www.samhsa.gov/data/sites/default/files/report_3192/ShortReport-3192.pdf [Google Scholar]
- 27.Gomez AM. This is how 6 states declared an emergency over the opioid crisis, with no national precedent. ThinkProgress [serial on the Internet]. [Updated 2017. October 26; cited 2018 Nov 27]. Available from: https://thinkprogress.org/this-is-how-6-states-declared-an-emergency-over-the-opioid-crisis-with-no-national-precedent-0a5537d19e51/ [Google Scholar]
- 28.Raimondo GM. Rhode Island Overdose Prevention and Intervention Task Force action plan [Internet]. Providence (RI): Office of the Governor; 2016. [cited 2018 Nov 27]. Available from: http://www.governor.ri.gov/documents/press/051116.pdf [Google Scholar]
- 29.Heroin Inter-Agency and Opioid Coordinating Council. Mid-year report to the governor: executive order 01.01.2015.13 [Internet]. Annapolis (MD): Department of Health and Mental Hygiene; 2016. August [cited 2018 Nov 27]. Available from: https://governor.maryland.gov/ltgovernor/wp-content/uploads/sites/2/2016/09/Interagency-Heroin-and-Opioid-Coordinating-Council-Mid-Year-Report-to-the-Governor-August-2016.pdf [Google Scholar]
- 30.Opioid Task Force. Recommended strategies to address opioid related harm in New Hampshire: 2017–2020 [Internet]. Concord (NH): Opioid Task Force; [cited 2018 Nov 27]. Available from: http://1viuw040k2mx3a7mwz1lwva5.wpengine.netdna-cdn.com/wp-content/uploads/2018/02/NHGovComm_AOD-OpioidTF_Priorities_2017-20.pdf [Google Scholar]
- 31.Substance Abuse and Mental Health Services Administration. National Survey of Substance Abuse Treatment Services (N-SSATS): 2016 [Internet]. Rockville (MD): SAMHSA; [cited 2018 Nov 26]. Available from: https://wwwdasis.samhsa.gov/dasis2/nssats/2016_nssats_rpt.pdf [Google Scholar]
- 32.Substance Abuse and Mental Health Services Administration. 2015–2016 National Survey on Drug Use and Health: model-based prevalence estimates (50 states and the District of Columbia) [Internet]. Rockville (MD): SAMHSA; 2017. [cited 2018 Nov 13]. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUHsaePercents2016/NSDUHsaePercents2016.pdf [Google Scholar]
- 33.Centers for Disease Control and Prevention. CDC Wonder [home page on the Internet]. Atlanta (GA): CDC; [last revised 2018. November 5; cited 2018 Nov 26]. Available from: https://wonder.cdc.gov/ [Google Scholar]
- 34.Henry J. Kaiser Family Foundation. Status of state action on the Medicaid expansion decision [Internet]. San Francisco (CA): KFF; 2018. November 26 [cited 2018 Nov 27]. Available from: https://www.kff.org/health-reform/state-indicator/state-activity-around-expanding-medicaid-under-the-affordable-care-act/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D [Google Scholar]
- 35.Henry J. Kaiser Family Foundation. Medicaid’s role in addressing the opioid epidemic [Internet]. San Francisco (CA): KFF; 2018. February 27 [cited 2018 Nov 26]. Available from: https://www.kff.org/infographic/medicaids-role-in-addressing-opioid-epidemic/ [Google Scholar]
- 36. To access the appendix, click on the Details tab of the article online.
- 37.American Society of Addiction Medicine. What are the ASAM levels of care? [Internet]. Rockville (MD): ASAM; 2015. May 13 [cited 2018 Nov 27]. Available from: https://www.asamcontinuum.org/knowledgebase/what-are-the-asam-levels-of-care/ [Google Scholar]
- 38.Grogan CM, Andrews C, Abraham A, Humphreys K, Pollack HA, Smith BT, et al. Survey highlights differences in Medicaid coverage for substance use treatment and opioid use disorder medications. Health Aff (Millwood). 2016;35(12):2289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein BD, Pacula RL, Gordon AJ, Burns RM, Leslie DL, Sorbero MJ, et al. Where is buprenorphine dispensed to treat opioid use disorders? The role of private offices, opioid treatment programs, and substance abuse treatment facilities in urban and rural counties. Milbank Q. 2015; 93(3):561–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dick AW, Pacula RL, Gordon AJ, Sorbero M, Burns RM, Leslie D, et al. Growth in buprenorphine waivers for physicians increased potential access to opioid agonist treatment, 2002–11. Health Aff (Millwood). 2015;34(6):1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clemans-Cope L, Wishner JB, Allen EH, Lallemand N, Epstein M, Spillman BC. Experiences of three states implementing the Medicaid health home model to address opioid use disorder—case studies in Maryland, Rhode Island, and Vermont. J Subst Abuse Treat. 2017;83:27–35. [DOI] [PubMed] [Google Scholar]
- 42.Nagel FW, Kattan JA, Mantha S, Nelson LS, Kunins HV, Paone D. Promoting Health Department opioid-prescribing guidelines for New York City emergency departments: a qualitative evaluation. J Public Health Manag Pract. 2018;24(4): 306–9. [DOI] [PubMed] [Google Scholar]
- 43.Huffman A Voluntary guidelines for emergency physicians: clarifying New York City’s efforts to curb opioid misuse. Ann Emerg Med. 2013; 62(2):13A–14A. [DOI] [PubMed] [Google Scholar]
- 44.Brooklyn JR, Sigmon SC. Vermont hub-and-spoke model of care for opioid use disorder: development, implementation, and impact. J Addict Med. 2017;11(4):286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wyse JJ, Gordon AJ, Dobscha SK, Morasco BJ, Tiffany E, Drexler K, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: historical perspective, lessons learned, and next steps. Subst Abus. 2018. March 29 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burns L, Gisev N, Larney S, Dobbins T, Gibson A, Kimber J, et al. A longitudinal comparison of retention in buprenorphine and methadone treatment for opioid dependence in New South Wales, Australia. Addiction. 2015;110(4):646–55. [DOI] [PubMed] [Google Scholar]
- 47.Yarborough BJ, Stumbo SP, McCarty D, Mertens J, Weisner C, Green CA. Methadone, buprenorphine, and preferences for opioid agonist treatment: a qualitative analysis. Drug Alcohol Depend. 2016;160: 112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarvis BP, Holtyn AF, Berry MS, Subramaniam S, Umbricht A, Fingerhood M, et al. Predictors of induction onto extended-release naltrexone among unemployed heroin-dependent adults. J Subst Abuse Treat. 2018;85:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knudsen HK, Havens JR, Lofwall MR, Studts JL, Walsh SL. Buprenorphine physician supply: relationship with state-level prescription opioid mortality. Drug Alcohol Depend. 2017;173(Suppl 1):S55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharp A, Jones A, Sherwood J, Kutsa O, Honermann B, Millett G. Impact of Medicaid expansion on access to opioid analgesic medications and medication-assisted treatment. Am J Public Health. 2018;108(5):642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clemans-Cope L, Lynch V, Epstein M, Kenney G. Medicaid coverage of effective treatment for opioid use disorder: trends in state buprenorphine prescriptions and spending since 2011 [Internet]. Washington (DC): Urban Institute; [updated 2017. June; cited 2018 Nov 27]. Available from: https://www.urban.org/sites/default/files/publication/90461/2001287_medicaid_coverage_of_effective_treatment_for_opioid_use_disorder_1.pdf [Google Scholar]
- 52.Buck JA. The looming expansion and transformation of public substance abuse treatment under the Affordable Care Act. Health Aff (Millwood). 2011;30(8):1402–10. [DOI] [PubMed] [Google Scholar]
- 53.Wen H, Hockenberry JM, Borders TF, Druss BG. Impact of Medicaid expansion on Medicaid-covered utilization of buprenorphine for opioid use disorder treatment. Med Care. 2017;55(4):336–41. [DOI] [PubMed] [Google Scholar]
- 54.Saloner B, Bandara S, Bachhuber M, Barry CL. Insurance coverage and treatment use under the Affordable Care Act among adults with mental and substance use disorders. Psychiatr Serv. 2017;68(6):542–8. [DOI] [PubMed] [Google Scholar]
- 55.Saloner B, Stoller KB, Barry CL. Medicaid coverage for methadone maintenance and use of opioid agonist therapy in specialty addiction treatment. Psychiatr Serv. 2016; 67(6):676–9. [DOI] [PubMed] [Google Scholar]
- 56.Cummings JR, Wen H, Ko M, Druss BG. Race/ethnicity and geographic access to Medicaid substance use disorder treatment facilities in the United States. JAMA Psychiatry. 2014;71(2):190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mark TL, Yee T, Levit KR, Camacho-Cook J, Cutler E, Carroll CD. Insurance financing increased for mental health conditions but not for substance use disorders, 1986–2014. Health Aff (Millwood). 2016;35(6): 958–65. [DOI] [PubMed] [Google Scholar]
- 58.Frank RG, Glied SA. Better but not well: mental health policy in the United States since 1950. Baltimore (MD): Johns Hopkins University Press; 2006. [Google Scholar]