The field of heart transplantation continues to evolve, with major changes in allocation systems and the increasing use of extended-criteria donor hearts, including hearts from donation after circulatory death donors, hearts supported with ex-vivo perfusion, and hearts from hepatitis C viremic donors. The use of such non-traditional donor hearts has made transplantation available to a larger number of recipients, but the demand continues to outpace the supply. Given this persistent donor heart shortage, much attention has been paid to the topic of donor-recipient size matching. Prior studies have challenged traditional criteria for size matching,1–3 while others have attempted to identify the optimal metric for matching donor and recipient heart size by comparing criteria, such as height, weight, body mass index (BMI), body surface area (BSA), and predicted heart mass (PHM).4–7 This 36th annual adult heart transplant report is based on data submitted to the International Society for Heart and Lung Transplantation (ISHLT) Thoracic Organ Transplant Registry on 146,975 heart transplants in recipients of all ages (including 131,249 adult heart transplants) through June 30, 2018. The focus theme of the 2019 annual report is donor-recipient size match.
Statistical methods
Data collection, conventions and statistical methods
Since the Registry’s inception in 1982, a total of 481 heart transplant centers have submitted data. We estimate that the data submitted to the Transplant Registry represent approximately 80% of the worldwide transplant activity.
An overview of donor and recipient characteristics and outcomes are presented throughout the report. These data are supplemented with additional and extended analyses presented in the online slide sets (3 separate slide sets, named ”Introduction/General Statistics”, ”Overall Heart Transplantation Statistics”, and ”Adult Heart Transplantation Statistics” at https://ishltregistries.org/registries/slides.asp). The slide sets for previous annual reports are also available on this site. The report refers to specific online e-slides when particular data are discussed but not shown because of space limitations; eSlide H (overall) numbers refer to the online overall heart transplant slides and eSlide H(a) numbers refer to the online adult heart transplant slides.
The Thoracic Organ Transplant Registry website (http://ishlt.org/registries/ttx-registry) provides detailed spreadsheets of data elements collected in the Registry, as well as a list of transplant centers and collectives currently submitting data. The Registry requires the mandatory submission of many donor, recipient, and transplant procedure variables at baseline and annual follow-up; these variables therefore have low rates of missingness. The rates of missingness may be significantly higher for Registry variables that depend on voluntary reporting. Since data quality is affected by the completeness and accuracy of reporting, the Registry uses various quality control measures to ensure acceptable data quality and completeness before including data for analyses.
Analytic Conventions
Unless otherwise specified, combined heart-lung transplants are not included in the analyses of heart transplants or lung transplants. Retransplants include those with a previously reported transplant of the same organ type, same organ type in combination, or with a retransplant diagnosis. Because the identification of all transplants for an individual may not be complete, the number of retransplant events may be slightly underestimated. The Registry does not capture the exact date for most secondary outcomes (e.g., renal dysfunction), but it does capture the window of occurrence (i.e., the event occurred between the first and the second annual follow-up visits). For the Registry reports, the midpoint between annual patient follow-ups is used as a surrogate for the event date. To reduce the possibility of underestimating event rates or other outcomes, some analyses are limited to surviving recipients. For time-to-event rates and cumulative morbidity rates, follow-up of recipients not experiencing the event of interest was censored at the last time the recipient was reported not to have had the event, either at the most recent annual follow-up or at the time of retransplantation. Time-to-event graphs (survival graphs) are truncated when the number of individuals still at risk is <10. Additional information regarding the general statistical methods used for analyses and data interpretation is included in the Supplementary Material available online (www.jhltonline.org).
Focus Theme Methods: Donor-Recipient Size Match
The Registry Steering Committee selected donor-recipient size match as the theme topic for the 2019 annual report given the recent interest in identifying the optimal metric for matching donor and recipient heart size and in studying the short- and longterm clinical consequences of size mismatch. Body weight has been the traditional metric for matching donor and recipient size2,3 as suggested by the ISHLT guidelines on the care of heart transplant recipients, which state that “As a general rule, the use of hearts from donors whose body weight is no greater than 30% below that of the recipient is uniformly safe. Use of a female donor whose weight is more than 20% lower than that of a male recipient should be viewed with caution.”8 However, some transplant centers prefer height to match donor and recipient size,9 while BMI and BSA have also been suggested as appropriate measures. Based on recent studies using data derived from cardiac magnetic resonance imaging, PHM, an estimate of heart size that incorporates height, weight, age, and sex, has been proposed as the optimal metric for size matching in adult heart transplantation. Prior publications have shown that when donors are undersized based on differences in the donor-recipient PHM, this is associated with the development of primary graft dysfunction10 and reduced one-year survival after heart transplantation,4,5 and that PHM is a better predictor than weight, height, BMI, or BSA mismatch.4 Therefore, we used PHM mismatch as the primary size match variable for our focus theme analyses and calculated PHM by combining right and left ventricular mass as follows11,12:
PHM = left ventricular mass + right ventricular mass
Left ventricular mass = a × Height 0.54 (m) × Weight 0.61 (kg), where a = 6.82 for women; a = 8.25 for men
Right ventricular mass = a × Age −0.32 (years) × Height 1.135 (m) × Weight 0.315 (kg), where a = 10.59 for women; a = 11.25 for men
PHM mismatch is calculated as [(donor PHM—recipient PHM) / donor PHM] × 100. A calculator to compute PHM mismatch can be found at: www.TBD.
Adult heart transplant statistics
Transplant volumes
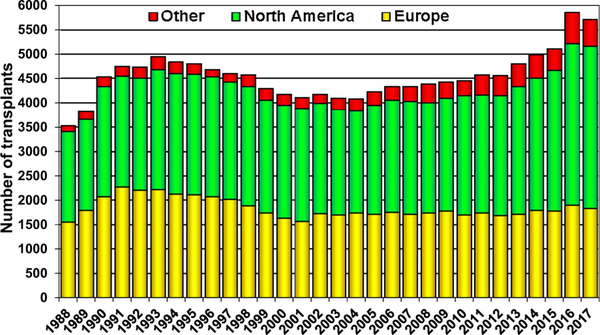
The number of heart transplants reported to the ISHLT Transplant Registry is significantly higher than a decade ago (Figure 1), driven mainly by higher heart transplant volumes, particularly in the most recent years in North America and Other (non-North American, non-European) countries. This sustained increase in transplant volume may reflect increased donor availability with the rising number of deaths owing to drug overdoses in the United States, increased reporting from some countries, and increasing use of “higher-risk” donor hearts. In addition, nascent heart transplant programs in developing countries have started contributing data to the ISHLT Registry. The majority of adult heart transplant programs perform 10 to 19 transplants per year, and only 12 centers perform 50 or more transplants per year, on average (eSlides H(overall) 3).
Figure 1.
Number of heart transplants (adult and pediatric) by year (transplants: 1988–2017) and geographic region.
Donor and recipient demographics and characteristics
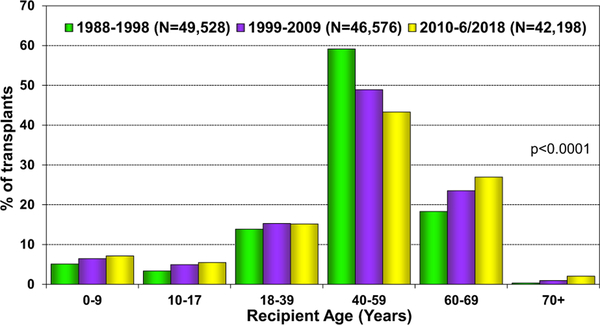
The distribution of adult donor and recipient characteristics for the most recent era (2010–June, 2018) are presented in Table 1, and for previous eras in eSlides H(a) 8 to 12. As shown in last year’s Report 13, the median recipient age for adult transplants is 55 years. Over the past 3 decades, donor age, donor weight, donor BMI, recipient weight, and recipient BMI have all increased significantly. The median donor age continues to rise in Europe (median 45 years) and remains relatively stable in North America (28 years) and Other countries (31 years) (eSlides H(overall), 7). The proportion of recipients over age 60 years continues to be higher than in prior eras (1988−2009), while the proportion of recipients aged 40 to 59 years has declined (Figure 2).
Table 1.
Donor and Recipient Characteristics for Adult Heart Transplants Performed January 1, 2010 – June 30, 2018
| Donor and Recipient Characteristics | (N = 36,883) |
|---|---|
| Recipient age (years) | 55.0 (25.0–68.0) |
| Donor age (years) | 35.0 (17.0–58.0) |
| Donor and recipient age difference (years) | −16.0 (−43.0 to 13.0) |
| Recipient weight (kg) | 80.1 (54.0–109.8) |
| Recipient height (cm) | 174.0 (157.0–188.0) |
| Recipient body mass index | 26.5 (19.5–34.9) |
| Donor weight (kg) | 80.0 (57.0–115.2) |
| Donor height (cm) | 175.0 (157.5–188.0) |
| Donor body mass index (kg/m2) | 26.0 (19.9–37.5) |
| Donor/recipient sex (% male) | 67.9%/74.4% |
| Female donor-to-male recipient | 16.10% |
| Male donor-to- female recipient | 9.70% |
| Donor/recipient diabetes mellitus | 3.5%/26.9% |
| Recipient history of prior dialysis | 4.80% |
| Donor/recipient cigarette history | 14.5%/44.9% |
| Donor/recipient hypertension | 15.4%/51.1% |
| Recipient prior cardiac surgery | 50.30% |
| Recipient previous malignancy | 8.60% |
| Ischemic time (hours) | 3.2 (1.5–5.0) |
| Panel reactive antibody > 10%a | 22.0% |
| Panel reactive antibody (%)a | 0.0 (0.0–71.0) |
| Creatinine at time of transplant (mg/dl) | 1.2 (0.7–2.3) |
| Pulmonary vascular resistance (Wood units) | 2.0 (0.0–5.3) |
| HLA mismatches | |
| 0–2 | 3.9% |
| 3–4 | 38.2% |
| 5–6 | 57.9% |
| Diagnosis | |
| Congenital heart disease | 3.1% |
| Hypertrophic cardiomyopathy | 3.4% |
| Ischemic cardiomyopathy | 32.4% |
| Non-ischemic cardiomyopathy | 50.8% |
| Restrictive cardiomyopathy | 3.5% |
| Retransplant | 2.8% |
| Valvular cardiomyopathy | 2.5% |
| Other | 1.5% |
| Donor cause of death | |
| Head trauma | 40.5% |
| Stroke | 19.9% |
| Anoxia | 21.5% |
| Central nervous system tumor | 0.5% |
| Other | 17.7% |
| Pre-operative support (multiple items may be reported) | |
| Hospitalized at time of transplant | 44.5% |
| On intravenous inotropes | 39.1% |
| Ventilator | 1.7% |
| Intra-aortic balloon pump | 7.3% |
| Mechanical circulatory support | 45.1% |
| Left ventricular assist device | 42.9% |
| Right ventricular assist device | 2.9% |
| Total artificial heart | 1.3% |
| Extracorporeal membrane oxygenation | 1.2% |
Continuous Factors Are Reported as Median (50th–95th Percentiles).
Panel reactive antibody includes Panel reactive antibody or Calculated panel reactive antibody values as reported by the centers. If Class I and Class II Panel reactive antibody values were reported separately, the higher of the 2 values was used.
Figure 2.
Recipient age distribution (adult and pediatric) by era.
The main indications for transplant (primary diagnoses) in adult recipients are non-ischemic dilated cardiomyopathy (51%) and ischemic cardiomyopathy (32%) in the current era (January, 2010–June, 2018). As shown in eSlides H(a) 4–6, the primary diagnosis varies somewhat by region and age group with a greater proportion of ischemic cardiomyopathy (35%) among North American centers and a higher proportion of non-ischemic cardiomyopathy among Other countries (59%). The overall number of retransplants remains relatively steady at 118 per year, representing 2% to 3% of all transplants in the past decade (eSlides H(a) 7).
As seen previously, the distribution of recipient comorbidities varies by location. More recipients are obese (BMI >30 kg/m2) and have diabetes mellitus in North America, compared with Europe and Other regions, while cigarette smoking is most common in European recipients (eSlides H (a) 18–20). Longer ischemic times (>4 hours) tend to be more common in Europe and Other countries compared with North America (eSlides H(a) 21), but these data should be interpreted with caution given the incomplete reporting from some international regions. Additional analyses focused on ischemic time can be found in the 2017 Adult Heart Transplant Registry Report.13
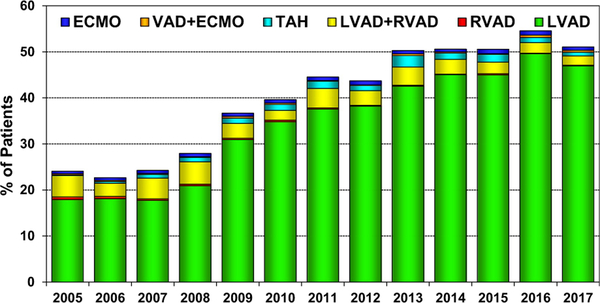
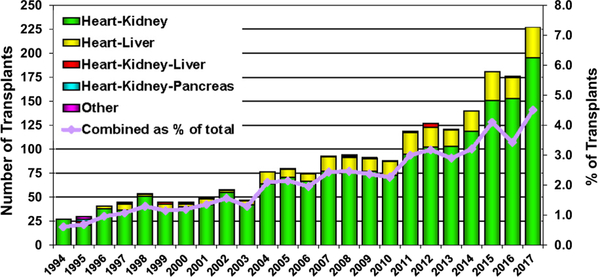
The proportion of transplant recipients bridged with mechanical circulatory support (MCS) rose rapidly from 2007 to 2013 and has since remained relatively constant at approximately 50% (Figure 3), mainly reflecting the use of left ventricular assist devices. In contrast, the number of multiorgan transplants performed worldwide increased from 176 to 227 from 2016 to 2017, mainly owing to an increase in the number of combined heart-kidney and heart-liver transplants performed in North America (Figure 4).14
Figure 3.
Percent of patients bridged with mechanical circulatory support by year and device type (adult heart transplants: 2005–2017); ECMO: extracorporeal membrane oxygenation; LVAD: left ventricular assist device; RVAD: right ventricular assist device; TAH: total artificial heart; VAD: ventricular assist device.
Figure 4.
Number and percent of multiorgan transplants reported by year and type of transplant (adult heart transplants; 1994–2017).
Survival
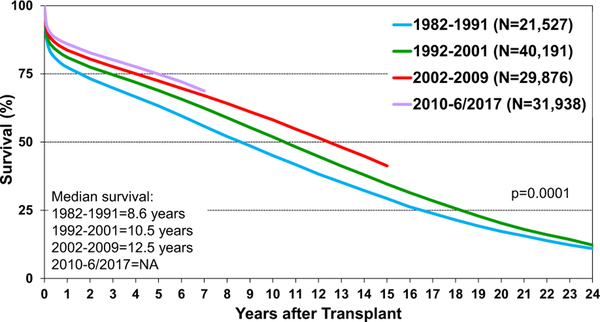
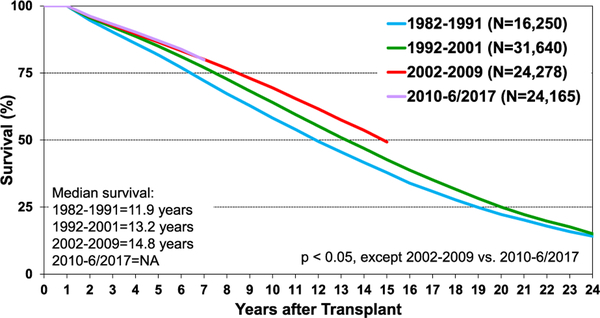
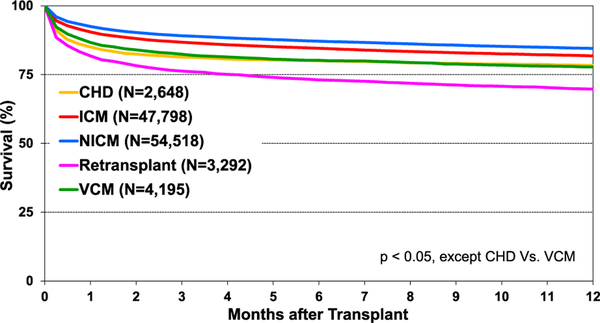
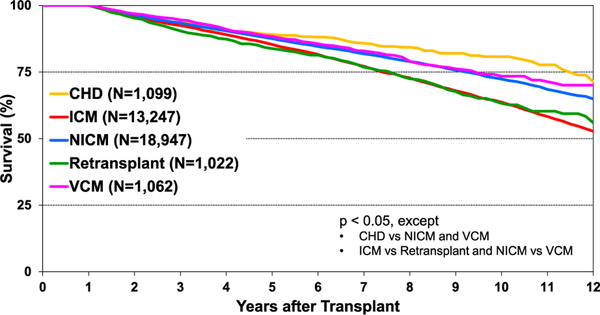
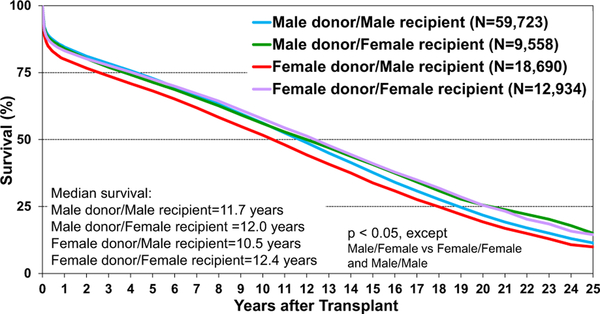
Post-transplant survival has improved over time. The median survival after adult heart transplants performed between 2002 and 2009 is 12.5 years (Figure 5) extending to 14.8 years among 1-year survivors (Figure 6). Survival within the first year post-transplant and overall survival vary by primary diagnosis. For instance, patients transplanted for non-ischemic and ischemic cardiomyopathy have the highest 1-year survival, while retransplants have the lowest survival (Figure 7). Congenital heart disease has the highest longterm survival conditional on survival to 1 year, while ischemic and retransplants have the lowest survival (Figure 8). As one may expect, higher recipient age is associated with reduced long-term survival (Figure 9), while higher donor age is also associated with higher early post-transplant mortality (eSlide H(a) 42). Consistent with prior years, female recipients have significantly higher post-transplant survival than men (median survival 12.2 years in women, 11.4 years in men; eSlides H(a) 43–44), even after accounting for donor or recipient sex combinations (Figure 10, eSlide H(a) 45–46). This observation may be confounded by differences in comorbidities and indication for transplant, and should be studied further in separate multivariable analyses. Long-term survival by recipient primary diagnosis categories and medical comorbidities is shown in eSlides H(a) 47 to 55.
Figure 5.
Kaplan-Meier survival by era (adult heart transplants: January 1982–June 2017). NA, not available.
Figure 6.
Kaplan-Meier survival by era, conditional on survival to 1 year after transplant (adult heart transplants: January 1982–June 2017). NA, not available.
Figure 7.
Kaplan-Meier survival within 1 year of transplant by diagnosis (adult heart transplants: January 1985–June 2017); CHD: congenital heart disease; ICM: ischemic cardiomyopathy; NICM: non-ischemic cardiomyopathy; VCM: valvular cardiomyopathy.
Figure 8.
Kaplan-Meier survival by diagnosis, conditional on survival to 1 year after transplant (adult heart transplants: January 2005–June 2017); CHD: congenital heart disease; ICM: ischemic cardiomyopathy; NICM: non-ischemic cardiomyopathy; VCM: valvular cardiomyopathy.
Figure 9.
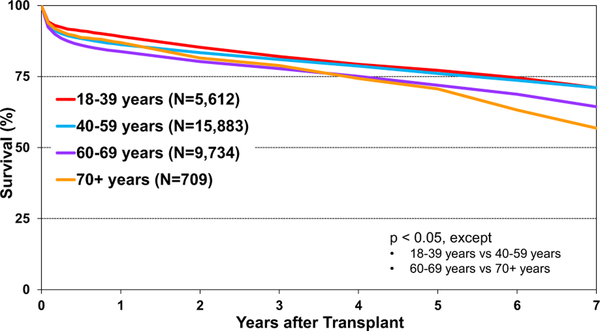
Kaplan-Meier survival by recipient age group (years) (adult heart transplants: January 2010–June 2017).
Figure 10.
Kaplan-Meier survival by donor/recipient sex (adult heart transplants: January 1992–June 2017).
Post-transplant survival does not appear to be adversely affected by the pre-transplant use of left ventricular assist devices, alone or in combination with right ventricular support (eSlides H(a) 56) in our univariate analyses. However, it is important to recognize that selection bias may affect outcomes, since sicker patients on mechanical support may not survive through transplantation. Survival is notably lower in patients bridged to transplant with extracorporeal membrane oxygenation (ECMO), particularly in the early post-transplant period. This observation is of interest given the recent change to the heart transplant allocation policy in the United States that prioritizes candidates supported with ECMO and other forms of acute MCS.15,16 Additional information regarding the association of MCS and outcomes is presented in the multivariable analyses section of this report.
Causes of death
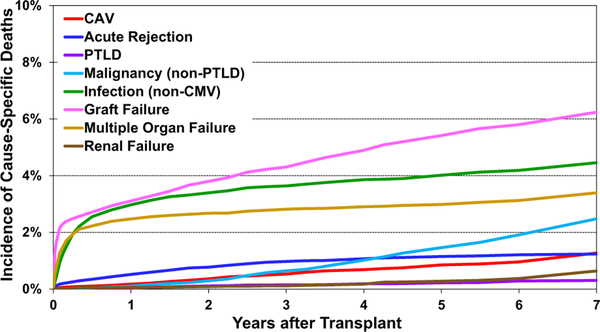
The leading causes of long-term mortality have not changed in recent years and include graft failure, non-cytomegalovirus infection and multiple organ failure (Figure 11, eSlides H(a) 58–59). Death owing to acute graft failure is highest in the first 30 days; death because of infectious complications is highest in the first year, and malignancy, acute rejection, cardiac allograft vasculopathy (CAV), and renal failure death increase with the time since transplant. Most cases of chronic graft failure are likely because of CAV.
Figure 11.
Cumulative incidence of leading causes of death (adult heart transplants: January 2010–June 2017); CAV: cardiac allograft vasculopathy; CMV: cytomegalovirus; PTLD: posttransplant lymphoproliferative disorder.
Immunosuppression
Induction immunosuppression
Just over 50% of heart transplant recipients received induction therapy at the time of transplantation in the most recent era (2010–June, 2018) (eSlide H(a) 23). The use of induction therapy is more common in North America than Europe, particularly for anti-lymphocyte and anti-thymocyte globulins (eSlide H(a) 24). Overall, there is no significant difference in post-transplant survival based on the use or type of induction immunosuppression (eSlide H(a) 25). More comprehensive analyses of the effects of induction therapy that adjust for relevant covariates have recently been published.17–19
Maintenance immunosuppression
Tacrolimus and mycophenolate mofetil and/or mycophenolic acid are the leading maintenance immunosuppressants at 1 year after transplant (eSlide H(a) 26–27) with 80% of the patients remaining on corticosteroids (information on corticosteroid dose is not available in the Registry). The tacrolimus-mycophenolate mofetil and/or mycophenolic acid combination was used in 78% of recipients during the first year post-transplant (eSlide H(a) 28). A total of 8.5% of the patients are started on a proliferation signal inhibitor (sirolimus or everolimus) within the first year after transplant, most commonly in combination with a calcineurin inhibitor or mycophenolate mofetil and/or mycophenolic acid.
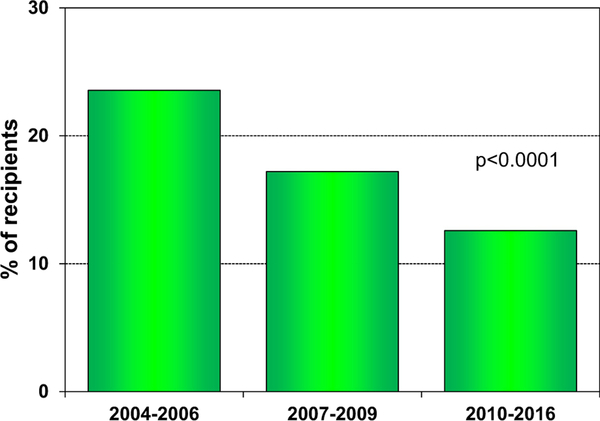
The incidence of treated rejection after transplant has continued to decrease; of patients transplanted between 2010 and 2016, 12.6% were treated for rejection between discharge and 1 year after transplant (Figure 12). The rates of treated rejection between discharge and 1 year after transplant were lowest in the absence of induction (eSlides H(a) 30, possibly due to lack of induction therapy for patients felt to be at low risk for acute rejection) and in recipients treated with tacrolimus rather than cyclosporine in combination with a cell-cycle inhibitor (eSlides H(a) 31). Overall, hospitalization for rejection has also continued to decline in consecutive transplant eras (eSlides H(a) 32).
Figure 12.
Percent of recipients experiencing treated rejection between transplant discharge and 1-year follow-up, by transplant era (adult heart transplants: 2004–2016).
Post-transplant morbidity
As noted in last year’s Report,13 the post-transplant length of hospitalization appears to have increased in the most recent era (2015–June, 2018), particularly in Europe, with a higher proportion of patients with lengths of stay ≥15 days, and particularly ≥29 days (eSlides H(a) 62–63). This may reflect transplantation of higher risk recipients and use of hearts from higher risk donors. Fortunately, 60% of the surviving recipients do not require rehospitalization within the first year, and approximately 75% are not rehospitalized between 2 and 5 years post-transplant (eSlides H(a) 66).
Severe renal dysfunction (defined as serum creatinine >2.5 mg/dl, chronic dialysis, or renal transplant), diabetes, and development of CAV are other important post-transplant morbidities (Table 2). One-third of the surviving recipients have diabetes by 5 years post-transplant (the Registry no longer collects data on the incidence of diabetes at 10 years). Almost a quarter of the recipients develop severe renal dysfunction, and almost half have CAV by 10 years post-transplant. However, it is important to note that the definition of CAV may vary between reporting centers.
Table 2.
Cumulative Morbidity Rates in Survivors within 1, 5 and 10 Years Post-Transplant (Adult Heart Transplants Performed from January 1, 1995 to June 30, 2017)
| Outcome | Within 1 year | Total N with known response | Within 5 years | Total N with known response | Within 10 years | Total N with known response |
|---|---|---|---|---|---|---|
| Severe renal dysfunctiona | 6.7% | (N = 39,544) | 15.7% | (N = 22,462) | 22.3% | (N = 9,195) |
| Creatinine > 2.5 mg/dl | 5.1% | 12.2% | 14.3% | |||
| Chronic dialysis | 1.5% | 2.9% | 6.0% | |||
| Renal transplant | 0.1% | 0.6% | 2.0% | |||
| Diabetesb | 20.0% | (N = 39,834) | 33.8% | (N = 22,720) | - | |
| Cardiac allograft vasculopathy | 7.7% | (N = 36,774) | 29.0% | (N = 17,392) | 46.8% | (N = 5,962) |
Severe renal dysfunction = creatinine >2.5 mg/dl (221 μmol/liter), dialysis, or renal transplant
Data are not available 10 years post-transplant
CAV continues to be associated with lower survival (eSlides H(a) 68). Female recipients are less likely to develop CAV than males, perhaps because of fewer comorbidities, and survival is reduced in recipients who develop CAV within 3 years of transplantation (eSlides H(a) 70–71). Fortunately, survival in patients with CAV has improved in the most recent era, perhaps owing to the availability of therapies that may prevent disease progression (eSlides H(a) 72).
Malignancy continues to be an important cause of longterm morbidity (eSlides H(a) 75–78), with an incidence of 28% (mostly skin cancers) at 10 years post-transplant. Survival after the development of post-transplant lymphomas remains poor, despite recent advances in diagnosis and treatment (eSlides H(a) 78).20 Fortunately, the development of post-transplant lymphomas remains uncommon (1%–2% by 5–10 years post-transplant) in adult recipients.
Other comorbidities that are known to be prevalent after heart transplant include hypertension and hyperlipidemia.21 However, these comorbidities are no longer reported to the Registry.
Functional status
Compared to living with advanced heart failure, functional status after heart transplantation remains very good, with over 70% of recipients reporting that they are able to perform normal activities of their daily life with minimal to no symptoms (90%–100% on the Karnofsky Performance Scale [eSlide H(a) 64]). The extent of employment in heart transplant recipients appears disproportionately low (<40% at 3–5 years post-transplant, eSlides H(a) 65), which may in part be due to medical insurance considerations, particularly in the United States.
Multivariable Analyses
Unadjusted mortality and morbidity rates are presented earlier. Next, we performed multivariable proportional hazards regression analyses for recent transplant eras to identify independent risk markers for and potential risk factors associated with subsequent mortality and morbidity. These analyses establish independent associations between risk factors and outcomes but cannot establish causality.
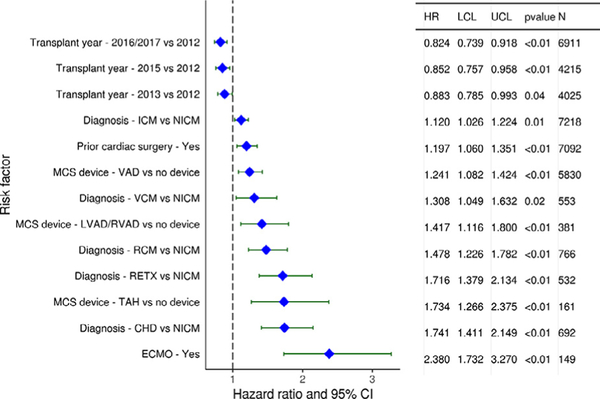
Categorical variables associated with increased 1-year mortality after adult heart transplantation (for transplants performed between 2012 and June, 2017) include indication for transplant, with valvular and restrictive cardiomyopathy, congenital heart disease, and retransplantation each conferring a higher risk for death than non-ischemic cardiomyopathy. Prior cardiac surgery and use of MCS devices (ventricular assist device, total artificial heart, ECMO) were also associated with increased 1-year mortality in multivariable analyses, as shown in Figure 13. Continuous variables that were statistically significant risk factors and/or markers for 1-year mortality include older donor and recipient age, higher recipient BMI, longer ischemic time, worse recipient kidney and liver function, lower transplant center volume, and size match by PHM (see focus theme analyses) (eSlides H(a) 128–136).
Figure 13.
Categorical risk factors for 1-year mortality (adult heart transplants: 2012−June 2017). CHD: congenital heart disease; ECMO: extracorporeal membrane oxygenation; HR, hazard ratio; ICM: ischemic cardiomyopathy; LCL, lower confidence limit; LVAD: left ventricular assist device; MCS: mechanical circulatory support; NICM: non-ischemic cardiomyopathy; RCM: restrictive cardiomyopathy; RETX: retransplant; RVAD: right ventricular assist device; TAH: total artificial heart; UCL, upper confidence limit; VAD: ventricular assist device; VCM: valvular cardiomyopathy.
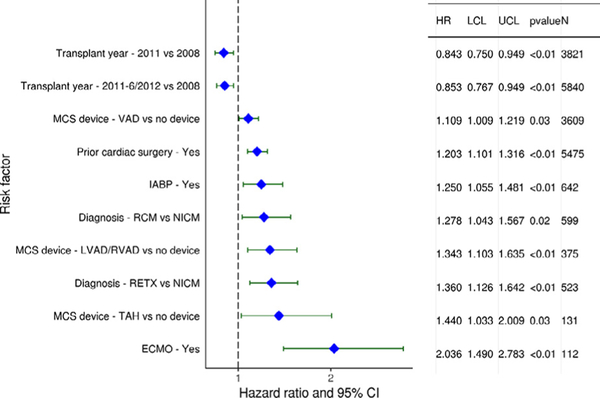
Recipient categorical variables associated with increased post-transplant mortality at 5 years (Figure 14) included several variables that were also predictive of 1-year mortality (prior cardiac surgery, ventricular assist device, total artificial heart), as well as retransplantation when compared to non-ischemic cardiomyopathy. Recipient pre-transplant support with ECMO was a strong risk factor for 5-year mortality, with a hazard ratio of 2.04 (p < 0.01). Continuous variables associated with 5-year mortality once again included older donor and recipient age, higher recipient creatinine and bilirubin, higher recipient BMI, longer ischemic time, and donor-recipient size match by PHM (eSlides H(a) 138−146). Risk factors for 5-year mortality conditional on survival to 1 year are displayed in eSlides H(a) 80 to86.
Figure 14.
Statistically significant categorical risk factors for 5-year mortality (adult heart transplants: 2008–June 2013). CI, confidence interval; ECMO: extracorporeal membrane oxygenation; HR, hazard ratio; IABP: intra-aortic balloon pump; LCL, lower confidence limit; LVAD: left ventricular assist device; MCS: mechanical circulatory support; NICM: non-ischemic cardiomyopathy; RCM: restrictive cardiomyopathy; RETX: retransplant; RVAD: right ventricular assist device; TAH: total artificial heart; UCL, upper confidence limit; VAD: ventricular assist device.
Multivariable analyses for mortality 10 years after heart transplantation are displayed in eSlides H(a) 87 to 95. In addition to several risk factors that were associated with 5-year mortality, several other significant predictors were identified, including male donor to female recipient combination, recipient diabetes mellitus, recipient panel-reactive antibodies, recipient mechanical ventilation at transplant, and recipient dialysis use prior to transplant. Allograft ischemic time and average transplant center volume remained significant risk factors for 10-year mortality.
Finally, the results of multivariable analyses for the development of CAV and severe renal dysfunction within 5 years (conditional on survival to discharge) are shown in eSlides H(a) 96 to 104. Significant risk factors for CAV development include transplant because of ischemic cardiomyopathy, retransplantation, recipient pre-transplant dialysis, older donor age, and higher transplant center volume. Predictors of severe renal dysfunction (in patients without severe renal dysfunction at the time of transplant) include recipient pre-transplant creatinine, recipient bilirubin and recipient dialysis prior to discharge. The non-use of calcineurin inhibitors was also associated with severe renal dysfunction, likely because this class of medication was discontinued in patients with worsening renal function.
It is important to recognize that categorical and continuous risk factors for post-transplant morbidity and mortality will differ by time since transplant, and long-term data reflect patients transplanted in earlier eras, such that the findings may not be applicable to current conditions. Therefore, specific associations observed among these data should be interpreted with caution and are better explored in more detailed analyses of Registry data.
Focus theme: donor and recipient size match
The continued interest in safely expanding the use of available donor hearts has generated great interest in the acceptable boundaries for donor-recipient size match. Size mismatch has been reported to be a risk factor for primary graft failure22 and for increased long-term mortality after heart transplantation,23 particularly in cases of an undersized donor. Several recent reports using the United Network for Organ Sharing Heart Transplant Registry have compared different metrics to assess size mismatch, including weight, height, BMI, BSA, and PHM4,5 and have concluded that PHM is the optimal donor-recipient size metric for the prediction of 1-year mortality after heart transplant. Therefore, we selected donor-recipient size match, based on PHM, for further exploration as part of this year’s Registry report.
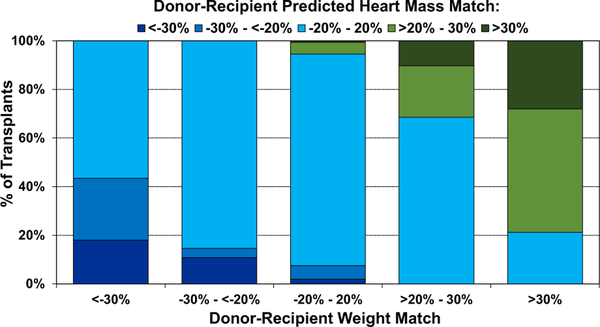
Size matching for adult heart transplantation has traditionally been weight-based.8,24 Therefore, we started by comparing the distribution of donor-recipient PHM mismatch by donor-recipient weight mismatch, as shown in Figure 15, where weight match was calculated as [(donor weight-recipient weight)/donor weight] × 100. As shown in the far-left column, the majority of donor-recipient pairs with donor-recipient weight match <−30%, which is often considered a contraindication to donor heart acceptance, had an acceptable PHM match of −20% to 20%. Thus, the use of PHM as the metric for assessing donor-recipient size match may lead to an increase in the use of available donor hearts. The middle (third) column shows that 87% of the donor-recipient pairs that are matched by weight are also matched by PHM, while the far-right column shows that oversized donor hearts by weight (donor-recipient weight match >30%) are also usually oversized >20% by PHM. The Pearson correlation coefficient (R) for weight mismatch compared to PHM mismatch is moderate-strong at 0.74 (eSlides H(a) 108).
Figure 15.
Distribution of donor-recipient predicted heart mass (PHM) match by donor-recipient weight match (adult heart transplant: January 2010–June 2018).
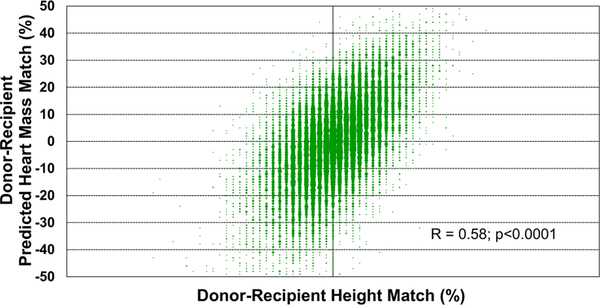
Some heart transplant centers choose to match donors and recipients primarily based on height, rather than weight. Height match, however, appears to be weakly-moderately correlated with PHM match, with R = 0.58 (Figure 16). Similarly, we found no correlation between recipient age and donor-recipient PHM match (R = −0.025, eSlides H(a) 110 and 113), which suggests that similar conventions are followed for donor-recipient size matching, regardless of recipient age, in adult heart transplantation.
Figure 16.
Donor-recipient predicted heart mass match by donor-recipient height match (adult heart transplant: January 2010–June 2018).
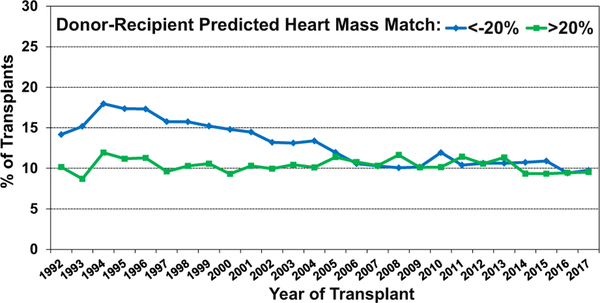
The analysis of size-matched heart transplants over time (donor-recipient PHM match <−20% and >20% from 1992 to 2017) showed that the use of undersized donors has decreased over time, while the use of oversized donors has been relatively stable (Figure 17). This observation suggests increased avoidance of donor undersizing in the current era.
Figure 17.
Transplants with donor-recipient predicted heart mass match of <−20% and >20% by transplant year (adult heart transplants: January 1992–December 2017).
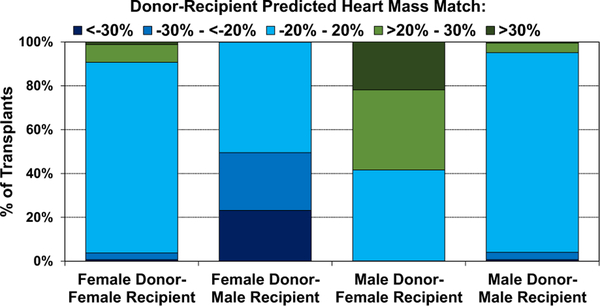
Next, we examined donor-recipient PHM match according to other relevant donor heart acceptance considerations, including sex match, ischemic time, and recipient pulmonary vascular resistance (PVR). As shown in Figure 18, same-sex donor-recipient pairs tend to be well matched for size, while female donor-male recipient combinations tend to be undersized (50% have PHM match exceeding −20%), and male donor-female recipient combinations tend to be oversized (58% have PHM match > 20%). Differences in size matching may account, in part, for mortality differences seen in different sex-mismatch combinations after adult heart transplantation.5,25
Figure 18.
Distribution of donor-recipient predicted heart mass match by donor-recipient sex combinations (adult heart transplants: January 2010–June 2018).
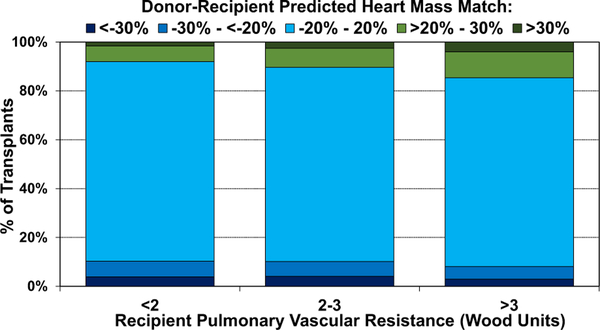
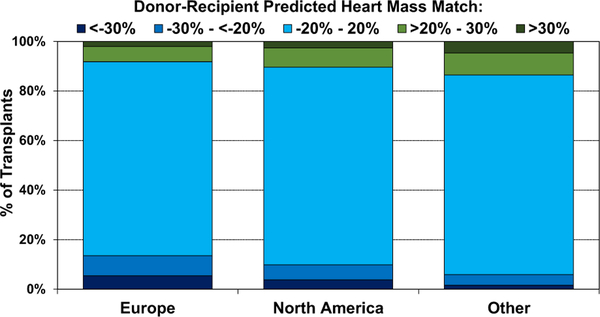
We also examined donor-recipient PHM match by ischemic time, donor age, recipient PVR, and geographic location. We observed a higher proportion of undersized donors with longer ischemic time (eSlides H(a) 114) and older donor age (eSlides H(a) 115). This suggests that transplant centers may have been willing to accept hearts from donors with a combination of high-risk features for critically ill recipients, or conversely for stable recipients who otherwise may not have received a donor heart. As one may expect, donor oversizing (PHM >20%) was more common in recipients with pulmonary hypertension (PVR > 3 Wood Units, Figure 19), as several prior studies have reported worse survival in recipients with elevated PVR who received undersized donor hearts.5,9 We finally examined donor-recipient size mismatch by geographic location (Figure 20). Undersizing was more common in Europe, compared to North America and Other locations, likely owing to the severe donor shortage in this region.
Figure 19.
Distribution of donor-recipient predicted heart mass match by recipient pulmonary vascular resistance (adult heart transplants: January 2010–June 2018).
Figure 20.
Distribution of donor-recipient predicted heart mass match by geographic location (adult heart transplants: January 2010–June 2018).
We then studied post-transplant outcomes by donorrecipient PHM match. Interestingly, we found a significant decrease in the incidence of treated acute rejection between transplant discharge and 1-year with increasing donor-recipient PHM match (less rejection in oversized donors, eSlides H(a) 119). The reason for this observation is not readily apparent, but it is possible that early graft dysfunction in undersized donor hearts may be treated empirically as acute rejection.
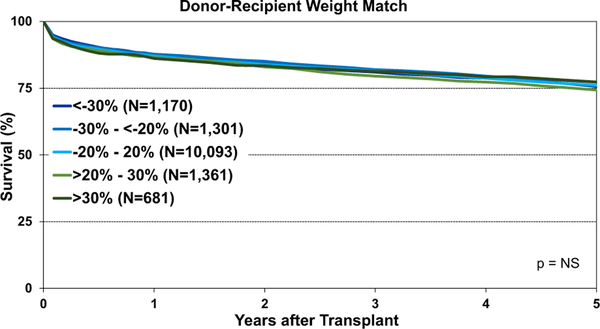
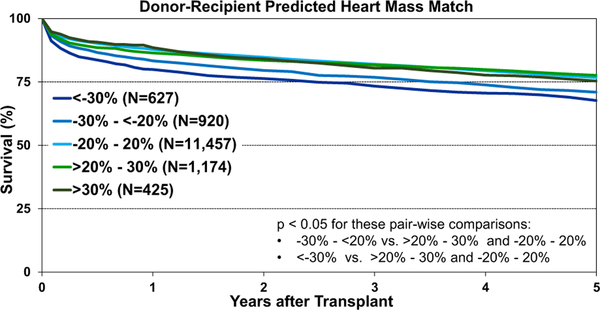
Post-transplant survival was examined according to different metrics for donor-recipient size match. We observed significantly lower 1-year survival after transplantation using oversized donors by height (donor-recipient height difference >10% versus <−10%, eSlides H(a) 120). Longer-term survival was also significantly lower after the transplantation of hearts from oversized donors by height (eSlides H(a) 121). However, no survival differences were observed according to donor-recipient weight match groups (Figure 21), which suggests that weight is not a reliable indicator of size mismatch. We finally examined post-transplant survival by PHM match and found that donor hearts that were undersized by PHM (donor-recipient PHM match < −20%) had significantly reduced survival compared with other size match categories (Figure 22). These results support prior work suggesting that PHM is the superior metric for assessing donor-recipient size mismatch.4,5
Figure 21.
Kaplan-Meier survival by donor-recipient weight match (adult heart transplants: January 2008–June 2013). NS, not significant.
Figure 22.
Kaplan-Meier survival by donor-recipient predicted heart mass match (adult heart transplants: January 2008–June 2013).
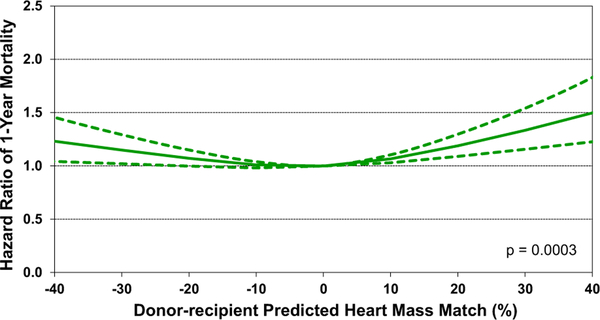
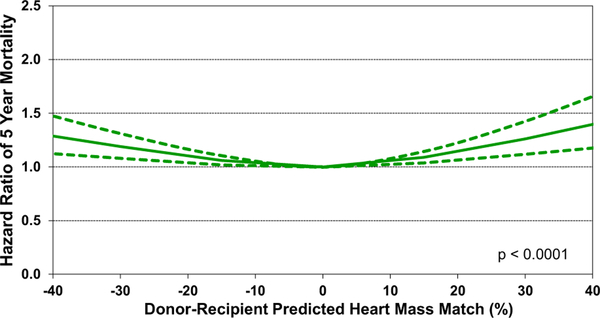
As mentioned earlier, both univariable and multivariable analyses identified donor-recipient size match by PHM as a significant predictor of both 1-year and 5-year mortality after heart transplant (univariable: eSlide H(a) 127 and eSlide H(a) 137, multivariable eSlide H(a) 129, eSlide H(a) 139). The recipients of undersized donors have increased mortality relative to size-matched donor-recipient pairs (PHM mismatch −10% to 10%). After multivariable adjustment, we also identified an increased risk of mortality in the recipients of oversized donors (Figure 23—multivariable hazard ratio plot of 1-year mortality by PHM match; Figure 24—multivariable hazard ratio plot of 5-year mortality by PHM match). What factors are responsible for this elevated risk remains unclear. One possibility is that oversized donor hearts are being accepted for sicker transplant candidates who subsequently have higher post-transplant mortality. Not all recipient covariates that convey this increased risk may be fully accounted for in the multivariable analysis, which may result in residual confounding.
Figure 23.
Multivariable hazard ratio plot for 1-year mortality by donor-recipient predicted heart mass match compared to no mismatch (adult heart transplants: 2012–June 2017).
Figure 24.
Multivariable hazard ratio plot for 5-year mortality, by donor-recipient predicted heart mass match, compared to no mismatch (adult heart transplants: 2008–June 2013).
Conclusions
In this 2019 Report, we observed a record high number of heart transplants reported to the Registry as compared to several years ago. Donor age continues to increase (particularly in Europe), and more recipients over the age of 60 years were transplanted compared to prior eras. Despite these observations, post-transplant survival remains robust, with a current median survival of 12.5 years overall and 14.8 years for patients who survived the first year (for transplants performed between 2002 and 2009). The use of MCS as a bridge to transplant remained stable through 2017, with 50% of patients on left ventricular assist device support and <1% on ECMO. It would be of interest to explore whether recent changes in allocation policies will have regional effects on pre-transplant MCS use and on post-transplant survival.
The 2019 focus theme presents more details on donor and recipient size match, mainly quantified as PHM match. Our analysis shows that donor-recipient PHM match is modestly associated with weight match and poorly associated with height match. We observed differences in the use of donor hearts with PHM match by geographic region and recipient PVR, as described previously. Finally, we identified donor-recipient PHM match as a significant predictor of 1-year and 5-year mortality after heart transplantation, with the best survival seen in donor-recipient pairs where the PHM match was within 10%. Our focus theme analysis supports donor-recipient size matching by PHM in the current era.
Supplementary Material
Acknowledgments
E.H is supported by NIH/ NHLBI award R01HL141892 to study disparities in survival among heart transplant candidates and recipients. D.C. received research funding from Astellas and Boeringher-Ingelheim.
Footnotes
Disclosure statement
K.K.K. serves as a scientific advisor and speaker for CareDx, Inc; L.P. serves as a speaker for Thermofisher, Sandoz, One Lambda and Novartis, and is an advisory board member for Qiagen and Novartis; J.S. serves as a consultant for Medtronic and Abbott; J.R. serves as a consultant for Bayer, Novartis, Amgen, and CSL Behring, and A.Z. serves on the speakers bureau of Paragonix, Novartis, Mallincrodt, Sanofi-Genzyme, Franz K€ohler Chemie and on the advisory board for Chiesi. M.H., D.H., T.S., B.M., W.C., A.R. and A.S. have no conflicts to disclose.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2019.08.004.
References
- 1.Blackbourne LH, Tribble CG, Langenburg SE, et al. Successful use of undersized donors for orthotopic heart transplantation—with a caveat. Ann Thorac Surg 1994;57:1472–6. [DOI] [PubMed] [Google Scholar]
- 2.Sethi GK, Lanauze P, Rosado LJ, et al. Clinical significance of weight difference between donor and recipient in heart transplantation. J Thorac Cardiovasc Surg 1993;106:444–8. [PubMed] [Google Scholar]
- 3.Taghavi S, Wilson LM, Brann SH, Gaughan J, Mangi AA. Cardiac transplantation can be safely performed with low donor-to-recipient body weight ratios. J Card Fail 2012;18:688–93. [DOI] [PubMed] [Google Scholar]
- 4.Kransdorf EP, Kittleson MM, Benck LR, et al. Predicted heart mass is the optimal metric for size match in heart transplantation. J Heart Lung Transplant 2019;38:156–65. [DOI] [PubMed] [Google Scholar]
- 5.Reed RM, Netzer G, Hunsicker L, et al. Cardiac size and sex-matching in heart transplantation: size matters in matters of sex and the heart. JACC Heart Fail 2014;2:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo MJ, Hong KN, Davies RR, et al. The effect of body mass index on survival following heart transplantation: do outcomes support consensus guidelines? Ann Surg 2010;251:144–52. [DOI] [PubMed] [Google Scholar]
- 7.Patel ND, Weiss ES, Nwakanma LU, et al. Impact of donor-to-recipient weight ratio on survival after heart transplantation: analysis of the United Network for Organ Sharing Database. Circulation 2008;118:S83–8. [DOI] [PubMed] [Google Scholar]
- 8.Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010;29:914–56. [DOI] [PubMed] [Google Scholar]
- 9.Kobashigawa J, Khush K, Colvin M, et al. Report From the American Society of Transplantation conference on donor heart selection in adult cardiac transplantation in the United States. Am J Transplant 2017;17:2559–66. [DOI] [PubMed] [Google Scholar]
- 10.Gong TA, Joseph SM, Lima B, et al. Donor predicted heart mass as predictor of primary graft dysfunction. J Heart Lung Transplant 2018;37:826–35. [DOI] [PubMed] [Google Scholar]
- 11.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol 2008;52:2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawut SM, Lima JA, Barr RG, et al. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation 2011;123:2542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lund LH, Khush KK, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1037–46. [DOI] [PubMed] [Google Scholar]
- 14.Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult heart transplantation Report-2018; focus theme: Multiorgan transplantation. J Heart Lung Transplant 2018;37:1155–68. [DOI] [PubMed] [Google Scholar]
- 15.Davies RR, Farr M, Silvestry S, et al. The new United States heart allocation policy: progress through collaborative revision. J Heart Lung Transplant 2017;36:595–6. [DOI] [PubMed] [Google Scholar]
- 16.Meyer DM, Rogers JG, Edwards LB, et al. The future direction of the adult heart allocation system in the United States. Am J Transplant 2015;15:44–54. [DOI] [PubMed] [Google Scholar]
- 17.Ansari D, Lund LH, Stehlik J, et al. Induction with anti-thymocyte globulin in heart transplantation is associated with better long-term survival compared with basiliximab. J Heart Lung Transplant 2015;34:1283–91. [DOI] [PubMed] [Google Scholar]
- 18.Briasoulis A, Inampudi C, Pala M, et al. Induction immunosuppressive therapy in cardiac transplantation: a systematic review and meta-analysis. Heart Fail Rev 2018;23:641–9. [DOI] [PubMed] [Google Scholar]
- 19.Whitson BA, Kilic A, Lehman A, et al. Impact of induction immunosuppression on survival in heart transplant recipients: a contemporary analysis of agents. Clin Transpl 2015;29:9–17. [DOI] [PubMed] [Google Scholar]
- 20.Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med 2018;378:549–62. [DOI] [PubMed] [Google Scholar]
- 21.Lund LH, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtythird adult heart transplantation Report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016;35:1158–69. [DOI] [PubMed] [Google Scholar]
- 22.Iyer A, Kumarasinghe G, Hicks M, et al. Primary graft failure after heart transplantation. J Transplant 2011;2011:175768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergenfeldt H, Stehlik J, H€oglund P, Andersson B, Nilsson J. Donorrecipient size matching and mortality in heart transplantation: influence of body mass index and gender. J Heart Lung Transplant 2017;36:940–7. [DOI] [PubMed] [Google Scholar]
- 24.Reichart B. Size matching in heart transplantation. J Heart Lung Transplant 1992;11:S199–202. [PubMed] [Google Scholar]
- 25.Khush KK, Kubo JT, Desai M. Influence of donor and recipient sex mismatch on heart transplant outcomes: analysis of the International Society for Heart and Lung Transplantation Registry. J Heart Lung Transplant 2012;31:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.