The 22nd International Society for Heart and Lung Transplantation (ISHLT) Transplant Registry Report summarizes data from pediatric lung and combined heart-lung transplant recipients and their donors for transplants that occurred through June 30, 2018. This year’s report focuses on an overall theme of donor and recipient size match. In addition to reporting key data for pediatric lung and heart-lung transplant recipients, we report transplant types, historical trends, geographic associations, indications, donor and recipient characteristics, and transplant outcomes including rejection burden, bronchiolitis obliterans syndrome (BOS), and survival for transplant recipients associated with donor and recipient size match. The full Registry slide set available online (https://ishltregistries.org/registries/slides.asp) provides more detail, additional analyses, and other information not included in this printed report.
Statistical methods
Data collection, conventions, and statistical methods
National and multinational organ and data exchange organizations and individual centers submit data to the ISHLT International Thoracic Organ Transplant Registry. Since the Registry’s inception, 481 heart transplant centers, 260 lung transplant centers, and 184 heart-lung transplant centers have reported data to the Registry. In our estimations, data submitted to the Registry represent approximately 80% of worldwide transplant activity.
An overview of donor and recipient characteristics and outcomes is presented throughout the report. The data are supplemented with additional and extended analyses presented in the online slide sets (5 separate slide sets, named “Introduction/General Statistics”, “Overall Lung Transplantation Statistics”, “Pediatric Lung Transplantation Statistics”, “Overall Heart-Lung Transplantation Statistics”, and “Pediatric Heart-Lung Transplantation Statistics”; https://ishltregistries.org/registries/slides.asp). Slide sets for previous annual reports are also available on this site. The report refers to specific online e-slides when particular data are discussed but not shown because of space limitations; eSlide L(p) refers to the online pediatric lung transplant slides, and eSlide HL(p) refers to the online pediatric heart-lung transplant slides.
The International Thoracic Organ Transplant Registry website (http://ishlt.org/registries/ttx-registry) provides detailed spreadsheets of data elements collected in the Registry. The Registry requires submission of core donor, recipient, and transplant procedure variables at baseline and annual follow-up, and these variables therefore have low rates of missingness. Nevertheless, data quality depends on the accuracy and completeness of reporting. Rates of missingness may significantly increase for Registry variables that depend on voluntary reporting. The Registry uses various quality control measures to ensure acceptable data quality and completeness before including data for analyses.
Analytical conventions
Unless otherwise specified, combined heart-lung transplants are not included in analyses of heart transplants or lung transplants. Retransplant includes those with a previously reported transplant of the same organ type, same organ type in combination, or with a retransplant diagnosis. Because identification of all transplants for an individual may not be complete, the number of retransplant events could be underestimated slightly. The Registry does not capture the exact occurrence date for most secondary outcomes (e.g., renal dysfunction), but it does capture the window of occurrence (i.e., the event occurred between the first and the second year annual follow-up visits). For the annual report, the midpoint between annual follow-ups is used as a surrogate for the event date. There is some bias in reporting secondary outcomes and other information on the follow-up where a death is reported. To reduce the possibility of underestimating event rates or other outcomes, some analyses are limited to surviving patients. For time-to-event rates and cumulative morbidity rates, follow-up of recipients not experiencing the event of interest was censored at the last time the recipient was reported not to have had the event, either the most recent annual follow-up or the time of retransplantation. Time-to-event graphs (survival graphs) are truncated when the number of individuals still at risk was <10. Additional information regarding the general statistical methods used for analyses and data interpretation is included in the Supplementary Material available online (www.jhltonline.org).
Focus theme methods: Donor and recipient size match
The Registry Steering Committee selected donor and recipient size match as the theme topic for the 2019 report, given the need to identify the optimal metric for matching donor and recipient. Recently, a study investigating size mismatch with donor to recipient height, weight, and predicted total lung capacity calculations in pediatric lung recipients reported that oversized allografts were associated with worse outcomes in a cohort of younger children.1 These findings are in direct opposition to adult studies where oversized lung allografts are not a risk for worse outcomes or are associated with improved outcomes.2–7
Although widely accepted as critical to decision making at the time of receipt of an organ donor offer, there is surprisingly little literature outlining current practice and the impact of size (mis) matching on outcomes. Donor and recipient weight matching is not commonly used in the decision-making process for donor lung offers, so we included weight in our analysis. Therefore, this year’s Registry report focuses on an overall theme of donor and recipient size matching in pediatric lung and pediatric heart-lung transplant recipients.
Pediatric lung transplantation
Transplant center and indications
A total of 2,514 pediatric lung and 733 pediatric heart-lung transplants performed through June 30, 2018 have been reported to the Transplant Registry. The number of pediatric lung transplants reported to the Registry annually (eSlide L(p) 6) has remained relatively stable, whereas the number of reported pediatric heart-lung transplants (eSlide HL(p) 7) is decreasing. Over the past decade, the number of annual pediatric lung transplants reported to the Registry has ranged between 97 and 136 (eSlide L(p) 6).
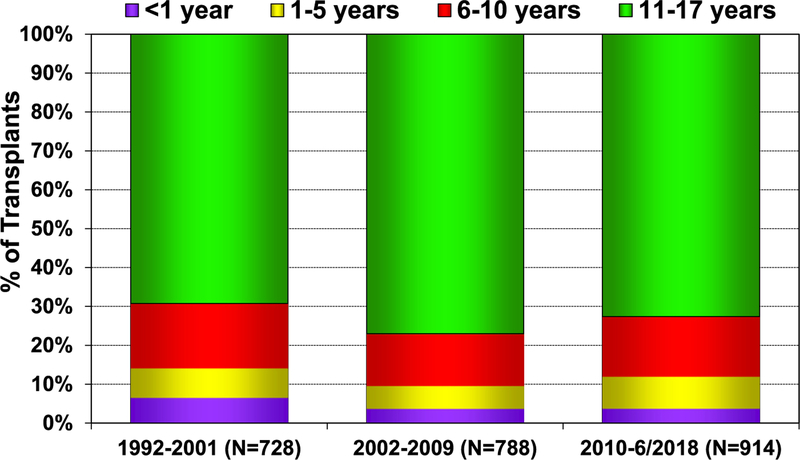
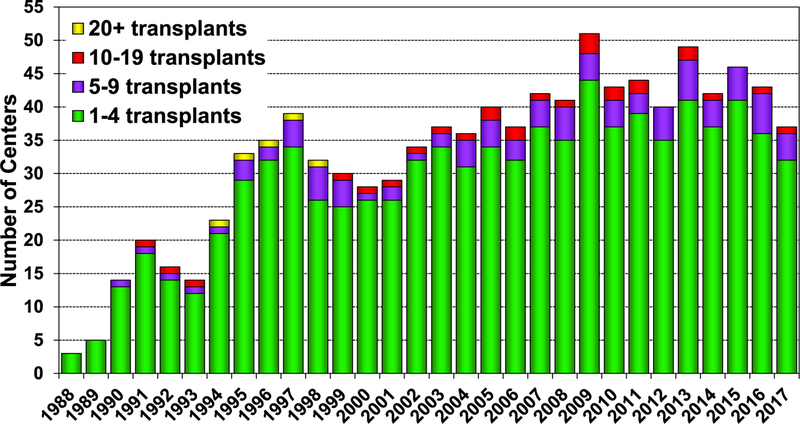
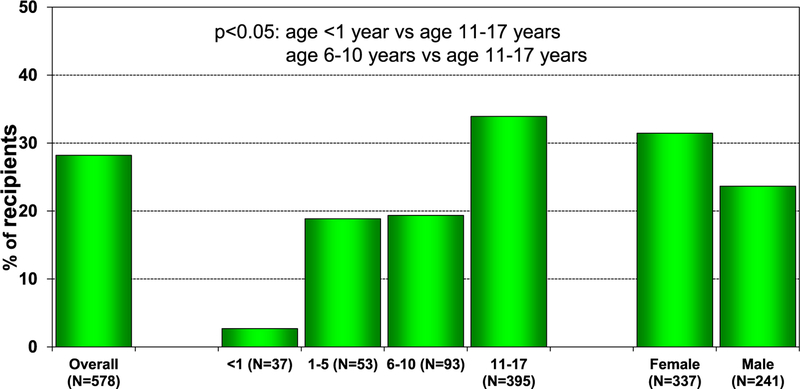
In 2017, 77% of pediatric lung transplants performed were in older children (11–17 years) (78/101), whereas only 3 lung transplants were performed in children <1 year of age (eSlide L(p) 6). Over the past 3 eras (1992–2001, 2002–2009, and 2010–June 2018), the age distribution of pediatric lung recipients has significantly changed (Figure 1). For 2017, the number of centers reporting the performance of a pediatric lung transplant decreased to 37, compared with 46 centers in 2015 and 43 centers in 2016. Of those centers performing pediatric lung transplants in 2017, 86% (32/37) performed <5 transplants (Figure 2). Although a single pediatric center performed between 10 and 19 lung transplants, the majority of pediatric lung transplants are performed at centers performing between 1 and 4 procedures annually (eSlide L(p) 9).
Figure 1.
Recipient age distribution for pediatric lung transplants (transplants: January 1992–June 2018).
Figure 2.
Number of centers reporting pediatric lung transplants by transplant year and pediatric center volume (transplants: 1988–2017).
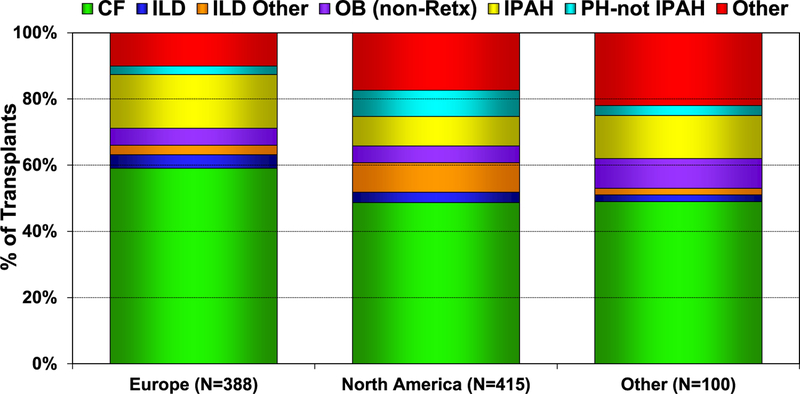
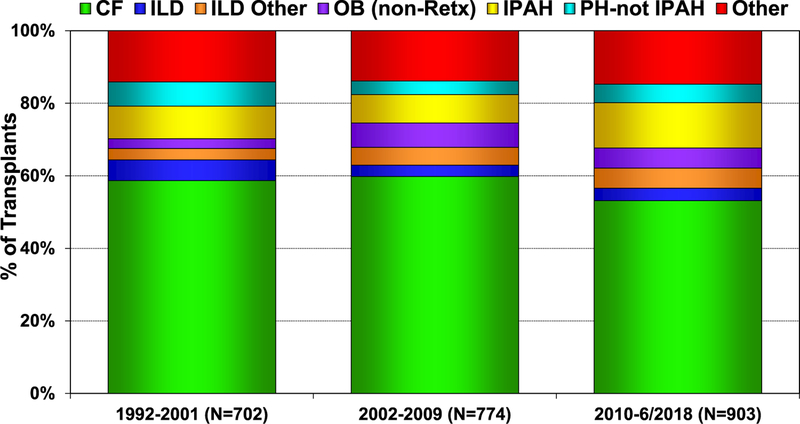
From January 2010 to June 2018, there were slight differences in the indications for pediatric lung transplants based on location (Europe, North America, and other Regions). More children with cystic fibrosis (CF) and idiopathic pulmonary arterial hypertension (IPAH) undergo lung transplant in Europe, whereas more children with interstitial lung disease undergo lung transplant in North America (Figure 3). Since January 2010, IPAH has been an increasing indication for pediatric lung transplantation (Figure 4). Comparing 2002–2009 to 2010–June 2018, subtle trends across the various indications for pediatric lung transplant are evident, with patients transplanted for IPAH being younger in the modern era, whereas children with pulmonary hypertension (PH, not IPAH) are older (eSlide L(p) 16). There has been no significant change in the age of pediatric patients transplanted for CF, but both interstitial lung disease groups are older, and the obliterative bronchiolitis (not retransplant) group is younger in the modern era (eSlide L(p) 16).
Figure 3.
Diagnosis distribution for pediatric lung transplants by location (transplants: January 2010–June 2018). CF, cystic fibrosis; ILD, interstitial lung disease; IPAH, idiopathic pulmonary arterial hypertension; OB, obliterative bronchiolitis; PH-not IPAH, pulmonary hypertension-not idiopathic pulmonary arterial hypertension; Retx, retransplantation.
Figure 4.
Diagnosis distribution by era for pediatric lung transplants (transplants: January 1992–June 2018). CF, cystic fibrosis; ILD, interstitial lung disease; IPAH, idiopathic pulmonary arterial hypertension; OB, obliterative bronchiolitis; PH-not IPAH, pulmonary hypertension-not idiopathic pulmonary arterial hypertension; Retx, retransplant.
The leading indication for pediatric lung transplantation depends on the age group. CF is the most frequent indication for children aged ≥11 years (65%), compared with 48% in the 6- to 10-year-old patient group (Table 1). In infants, pulmonary vascular disorders are the predominant indication for lung transplant, with the next largest group being surfactant disorders. Regional differences persist with regards to indications for a lung transplant in children, with CF being more common in Europe than in North America and other Regions (eSlide L(p) 13).
Table 1.
Indications for Pediatric Lung Transplants by Age Group (Transplants: January 2002–June 2018)
| Indication | <1 year | 1–5 years | 6–10 years | 11–17 years | ||||
|---|---|---|---|---|---|---|---|---|
| CF | 0 | — | 4 | 3.4% | 116 | 48.1 | 823 | 65.4% |
| Non-CF bronchiectasis | 0 | — | 0 | — | 2 | 0.8% | 25 | 2.0% |
| ILD | 5 | 8.1% | 6 | 5.2% | 5 | 2.1% | 39 | 3.1% |
| ILD other specified cause | 6 | 9.7 | 10 | 8.6% | 21 | 8.7% | 51 | 4.1% |
| IPAH | 7 | 11.3% | 31 | 26.7% | 24 | 10.0% | 112 | 8.9% |
| PH-not IPAH | 16 | 25.8% | 25 | 21.6% | 7 | 2.9% | 27 | 2.1% |
| Obliterative bronchiolitis (non-retransplant) | 0 | — | 10 | 8.6% | 32 | 13.3% | 60 | 4.8% |
| ABCA3 transporter mutation | 6 | 9.7% | 6 | 5.2% | 2 | 0.8% | 1 | 0.1% |
| Bronchopulmonary dysplasia | 3 | 4.8% | 4 | 3.4% | 3 | 1.2% | 3 | 0.2% |
| Surfactant protein B deficiency | 14 | 22.6% | 4 | 3.4% | 1 | 0.4% | 0 | — |
| Surfactant protein C deficiency | 0 | — | 1 | 0.9% | 0 | — | 2 | 0.2% |
| Retransplant (obliterative bronchiolitis) | 0 | — | 4 | 3.4% | 7 | 2.9% | 35 | 2.8% |
| Retransplant (not obliterative bronchiolitis) | 0 | — | 5 | 4.3% | 7 | 2.9% | 43 | 3.4% |
| COPD, with or without A1ATD | 2 | 3.2% | 1 | 0.9% | 3 | 1.2% | 11 | 0.9% |
| Other | 3 | 4.8% | 5 | 4.3% | 11 | 4.6% | 26 | 2.1% |
Abbreviations: A1ATD, alpha-1-antitrypsin deficiency; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; IPAH, idiopathic pulmonary arterial hypertension; PH-not IPAH, pulmonary hypertension-not idiopathic pulmonary arterial hypertension.
Immunosuppressive therapy
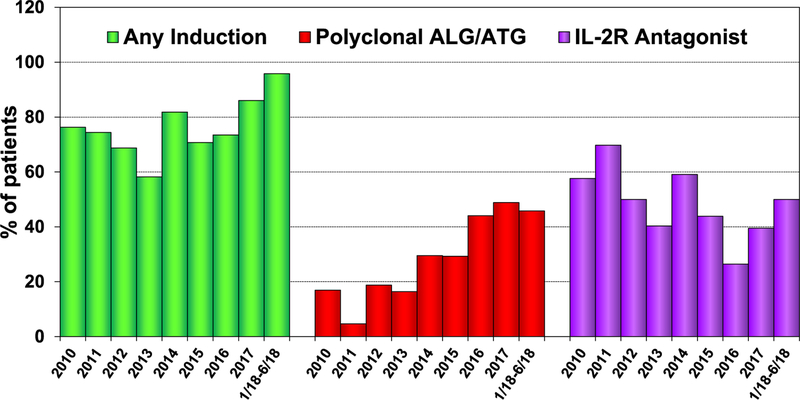
With rates of induction immunosuppression steadily rising in the modern era, 96% of pediatric lung transplant recipients received some form of induction immunosuppression in the first 6 months of 2018 (Figure 5). Looking at the agents used for induction, there was an acute drop in the use of interleukin-2 receptor (IL-2R) antagonist therapy in 2016 that has since reversed, whereas polyclonal anti-lymphocyte or anti-thymocyte globulin use remains stable (Figure 5). Induction therapy with either IL-2R antagonist or polyclonal antibody compared with no induction was not associated with the likelihood of being treated for rejection (eSlide L(p) 52) between discharge and the first year after transplant. Moreover, the univariate analyses did not demonstrate a survival difference in pediatric lung transplant recipients receiving or not receiving induction therapy (eSlide L(p) 48).
Figure 5.
Induction immunosuppression use for pediatric lung transplants (transplants: January 2010–June 2018). ALG, anti-lymphocyte globulin; ATG, anti-thymocyte globulin; IL-2R, interleukin-2 receptor.
Since 2010, the combination of tacrolimus and mycophenolate mofetil or mycophenolic acid has been the maintenance immunosuppressive regimen of choice, with 82% of patients receiving that combination at 1-year post-transplant follow-up (eSlide L(p) 51). The next most common combinations included tacrolimus and azathioprine (7%) and single-agent therapy with tacrolimus (5%).
Survival
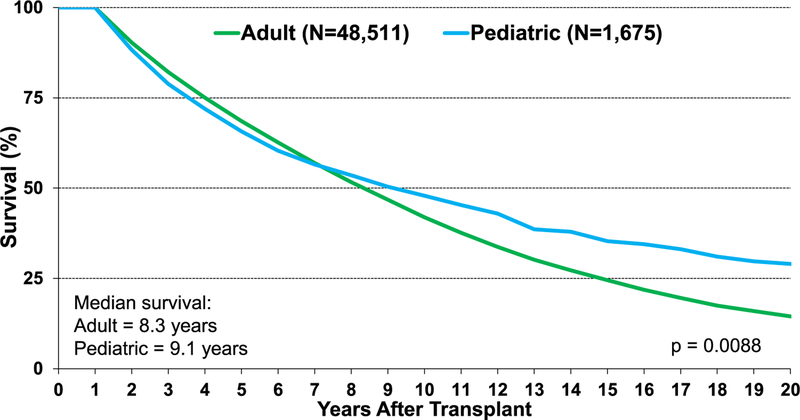
Median survival in children following lung transplantation is 5.7 years for patients transplanted between January 1992 and June 2017, compared with adults who have a median survival of 6.2 years (eSlide L(p) 18). As suggested by the Kaplan-Meier survival curve, there appears to be separation of the survival curve slope suggesting superior survival in pediatric recipients beyond the 8-year mark. For patients surviving 1 year post-lung transplant, the median survival for pediatric recipients is 9.1 years, compared with 8.3 years for adults (Figure 6). Further analysis comparing survival after pediatric lung transplantation to adult single and bilateral lung transplantation shows differences between groups, with adult bilateral lung transplants having longer unadjusted survival and adult recipients of single lung transplants having the shortest unadjusted survival (eSlide L(p) 20). Single lung transplant is infrequently performed in children and is associated with shorter unadjusted survival than bilateral transplant (eSlide L(p) 26).
Figure 6.
Kaplan-Meier survival conditional on survival to 1 year for pediatric and adult lung transplants (transplants: January 1992–June 2017).
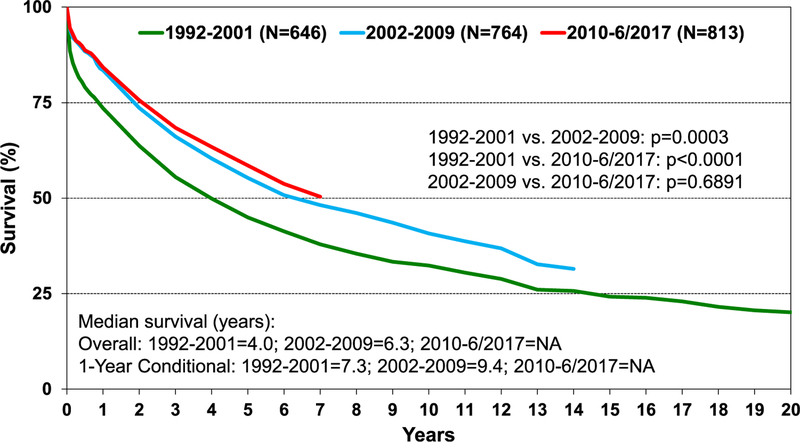
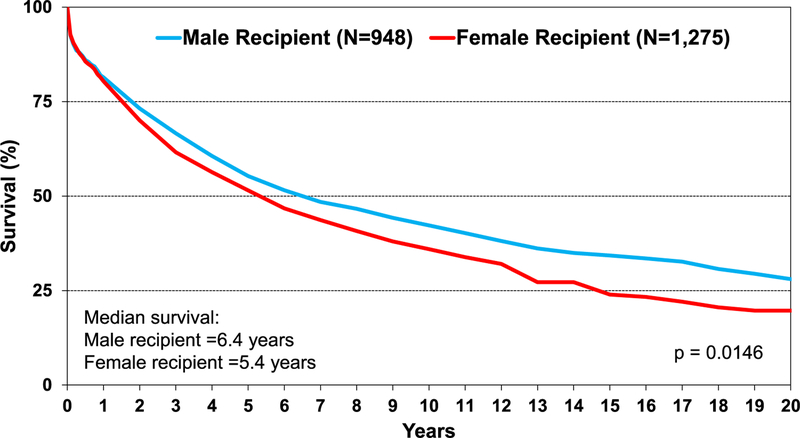
Survival was similar across age groups in pediatric lung transplant recipients (eSlide L(p) 29). In addition, there is no difference in survival, conditional to 1 year, by recipient age group (eSlide L(p) 30) and by diagnosis (eSlide L(p) 28). Across eras, there has been improvement in unadjusted survival for pediatric lung transplant recipients in the most recent eras (2002–2009 and 2010–June 2017) compared with the previous era (1992–2001) (Figure 7). Survival analysis stratified by sex reveals that male recipients have higher unadjusted survival than female recipients (median survival of 6.4 vs 5.4 years, respectively, p = 0.015) (Figure 8). This survival difference is even more pronounced when limiting the cohort to conditional 1-year survival (median survival for males was 10.5 years vs 8.2 years for female recipients, p < 0.01) (eSlide L(p) 32). The effect of donor age on survival was explored in children aged 11 to 17 years undergoing lung transplants. Donor age did not appear to be associated with survival in pediatric recipients aged 11 to 17 years. However, the Kaplan-Meier survival curve suggests donors over 50 years of age may lead to unfavorable outcomes (eSlide L(p) 35). In addition, donor and recipient cytomegalovirus status at time of lung transplant in children did not appear to impact survival (eSlide L(p) 44).
Figure 7.
Kaplan-Meier survival for pediatric lung transplants by era (transplants: January 1992–June 2017). NA, not applicable.
Figure 8.
Kaplan-Meier survival for pediatric lung transplants by sex (transplants: January 1992–June 2017).
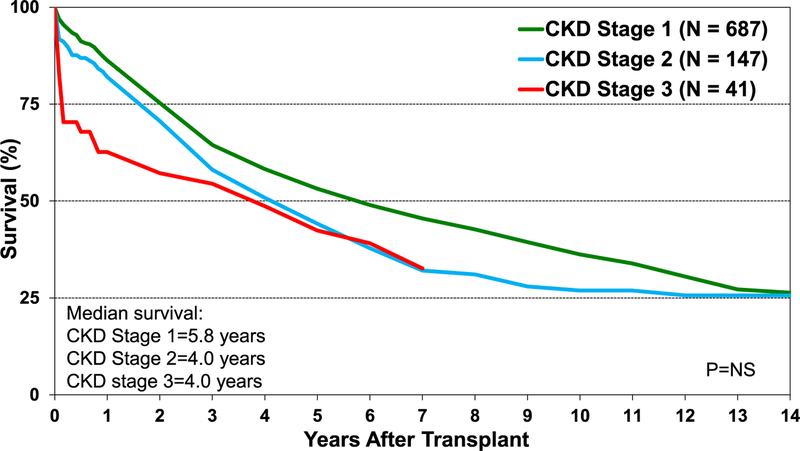
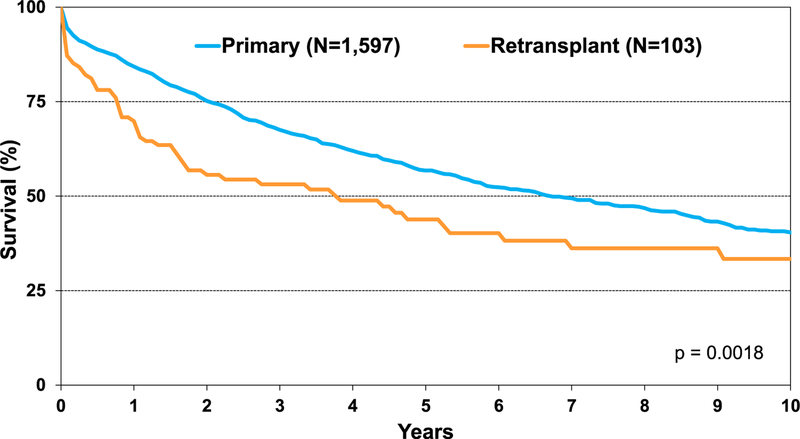
With renal dysfunction being a potential comorbidity in children requiring lung transplant, we explored the effect of chronic kidney disease (CKD) using estimated glomerular filtration rate calculated with the Schwartz formula. Although the differences did not reach statistical significance, survival of recipients with CKD Stage 2 and Stage 3 was numerically lower than recipients with Stage 1 CKD (Figure 9). Finally, retransplant in pediatric recipients continues to be associated with lower survival than primary lung transplant recipients (Figure 10).
Figure 9.
Kaplan-Meier survival for pediatric lung transplants by stages of chronic kidney disease at transplant (transplants: January 1992–June 2017). eGFR was calculated using the Schwartz formula. The stages of CKD are as follows: 1: eGFR ≥90; 2: eGFR = 60–89; 3: eGFR = 30–59. CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; NS, not significant.
Figure 10.
Kaplan-Meier survival for primary pediatric lung transplants and pediatric lung retransplants (transplants: January 2000–June 2017).
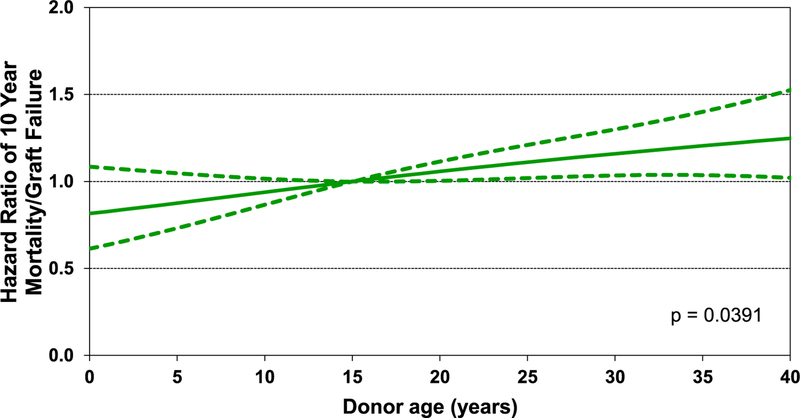
Although a number of causes are responsible for mortality after pediatric lung transplants, BOS and graft failure emerge as the key causes of mortality beyond the first year after transplant (eSlide L(p) 68). In multivariable analysis, donor age (Figure 11), recipient estimated glomerular filtration rate (eSlide L(p) 74), and female donor-female recipient (vs male donor-male recipient) combination were identified as predictors of 10-year mortality after transplant.
Figure 11.
Hazard ratio of 10-year mortality/graft failure for pediatric lung transplants by donor age (transplants: 2000–June 2008).
Functional status and rehospitalization
More than 80% of surviving children with follow-up between January 2010 and June 2018 have a Lansky score of ≥80%, indicating that most pediatric lung transplant recipients are fully active or have only minor restrictions in strenuous physical activity through 3 years post-transplant (eSlide L(p) 39). Additionally, rehospitalization rates decrease over the first 3 years after lung transplant, with 47% of recipients not requiring rehospitalization in the first year post-transplant, and 57% remaining hospitalization-free in years 4 and 5 post-transplant (eSlide L(p) 40).
Post-transplant morbidities
Table 2 outlines the cumulative incidence of commonly encountered post-transplant morbidities in children, including severe renal dysfunction, diabetes mellitus, and BOS, at 1, 5, and 7 years after lung transplantation. The use of induction immunosuppression at the time of lung transplant in children was not associated with the rates of these frequently encountered morbidities at 1 year post-transplant (Table 3). The risk for severe renal dysfunction in children progressively increases after lung transplant (eSlide L(p) 61), but the incidence has decreased in the modern era (2005–June 2017) compared with the previous era (1995–2004) (eSlide L(p) 62). At 5 years and 7 years post–lung transplant, >90% of surviving pediatric recipients have no malignancies (eSlide L(p) 63). Lymphoma represented nearly all of the malignancies that did occur in pediatric lung recipients.
Table 2.
Cumulative Morbidity Rates in Survivors within 1, 5, and 7 Years Post-Transplant (Pediatric Lung Transplants: January 1, 1995–June 30, 2017)
| Outcome | Within 1 year | Total N with known response |
Within 5 years | Total N with known response |
Within 7 years | Total N with known response |
|---|---|---|---|---|---|---|
| Severe renal dysfunction | 2.0% | 867 | 5.8% | 343 | 7.4% | 215 |
| Creatinine > 2.5 mg/dl | 1.5% | — | 3.8% | — | 5.6% | — |
| Chronic dialysis | 0.3% | — | 1.5% | — | 0.9% | — |
| Renal transplant | 0.1% | — | 0.6% | — | 0.9% | — |
| Diabetes | 18.2% | 872 | 27.1% | 354 | — | — |
| Bronchiolitis obliterans syndrome | 9.2% | 815 | 36.5% | 277 | 46.3% | 162 |
Severe renal dysfunction is defined as creatinine > 2.5 mg/dl (221 μmol/liter), dialysis, or renal transplant. Data are not available for diabetes mellitus 7 years post-transplant.
Table 3.
Cumulative Morbidity Rates in Survivors within 1 Year Post-Transplant by Induction Use (Pediatric Lung Transplants: January 1, 2000–June 30, 2017)
| Induction |
No induction |
|||
|---|---|---|---|---|
| Outcome | Within 1 year | Total N with known response | Within 1 year | Total N with known response |
| Severe renal dysfunction | 2.0% | 446 | 0.9% | 227 |
| Creatinine > 2.5 mg/dl | 1.8% | 0.4% | ||
| Chronic dialysis | 0.2% | 0.0% | ||
| Renal transplant | 0.0% | 0.4% | ||
| Diabetes | 16.3% | 447 | 21.2% | 226 |
| Bronchiolitis obliterans syndrome | 9.2% | 425 | 8.0% | 213 |
Severe renal dysfunction is defined as creatinine > 2.5 mg/dl (221 μmol/liter), dialysis, or renal transplant.
Allograft rejection
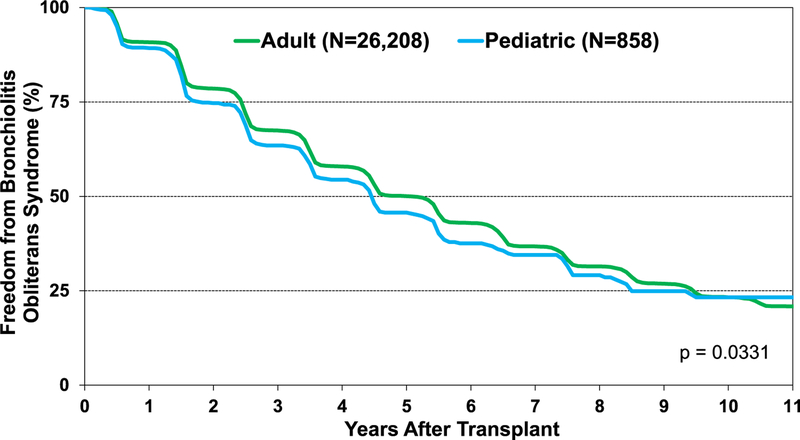
From January 2005 to June 2018, 26% of pediatric lung transplant recipients required treatment for acute rejection between discharge and 1 year after transplant (eSlide L(p) 42). The percentage of pediatric lung transplant recipients that developed any acute rejection varies according to age, with infants having the lowest rate (3%) and children 11 to 17 years of age having the highest rate of rejection (34%) in this time period (Figure 12). Chronic rejection, which is now termed chronic lung allograft rejection, remains the primary limiting factor for long-term survival in pediatric lung transplants, with BOS being the most common type. In the modern era, there has been a rise in freedom from BOS in pediatric lung transplant recipients (eSlide L(p) 59). However, freedom from BOS appears to be lower in pediatric than adult lung transplant recipients (Figure 13).
Figure 12.
Percentage of pediatric lung recipients experiencing any rejection between discharge and 1-year follow-up (follow-ups: January 2005–June 2018).
Figure 13.
Freedom from bronchiolitis obliterans syndrome for lung transplants by age group (pediatric vs adult) (transplants: January 1995–June 2017).
Focus theme: Donor and recipient size match
The objective of the 2019 focus theme was to report pretransplant donor and recipient size match data and to examine associations of donor and recipient size match with outcomes. To perform the analysis, we categorized donor-recipient height differences as <−15 cm, −15 cm to <−5 cm, −5 cm to 5 cm, >5 cm to 15 cm, and >15 cm and donor-recipient weight differences as <−15 kg, −15 kg to <−5 kg, 5 kg to 5 kg, >5 kg to 15 kg, and >15 kg.
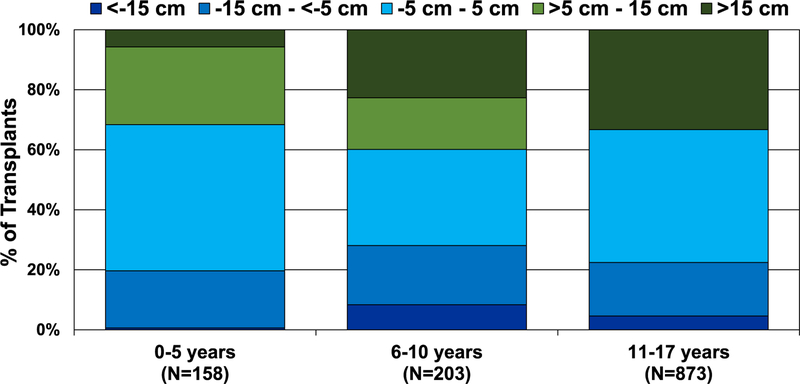
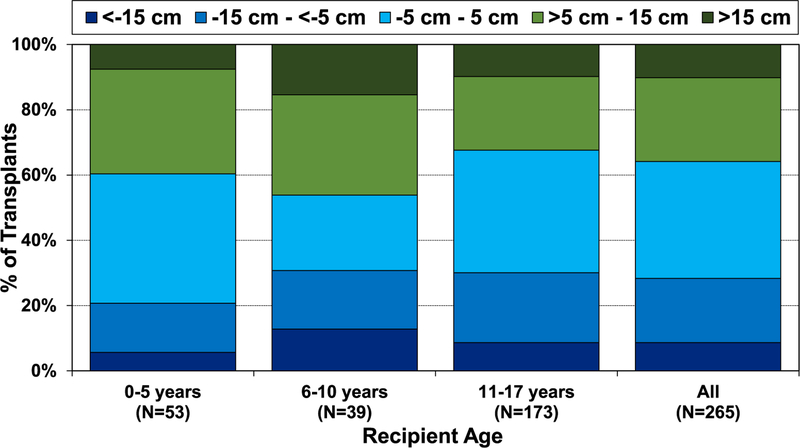
In the modern era, especially from 2015 to June 2018, there has been a trend of greater donor-recipient height differences in pediatric lung transplantation (eSlide L(p) 77). From January 2000 to June 2018, the pediatric lung transplant age group in which the most substantial donor-recipient height differences occurred was among 6- to 10-year-olds (Figure 14). This finding is likely due to limited donor organ availability for candidates in this age range leading to willingness to accept greater height differences. Donor-recipient sex match was associated with small differences in donor-recipient size match with male-to-female and male-to-male groups having the 2 highest donor-recipient height differences (>5 cm−15 cm and >15 cm) (eSlide L(p) 79). Notably, we found that pediatric lung transplants with higher donor-recipient height differences have the shortest ischemic times (eSlide L(p) 81). One possible explanation is the willingness of transplant centers to accept a larger donor-recipient height discrepancy when they are geographically close.
Figure 14.
Distribution of donor-recipient height difference for pediatric lung transplants by recipient age (transplants: January 2000–June 2018).
Allograft rejection and survival
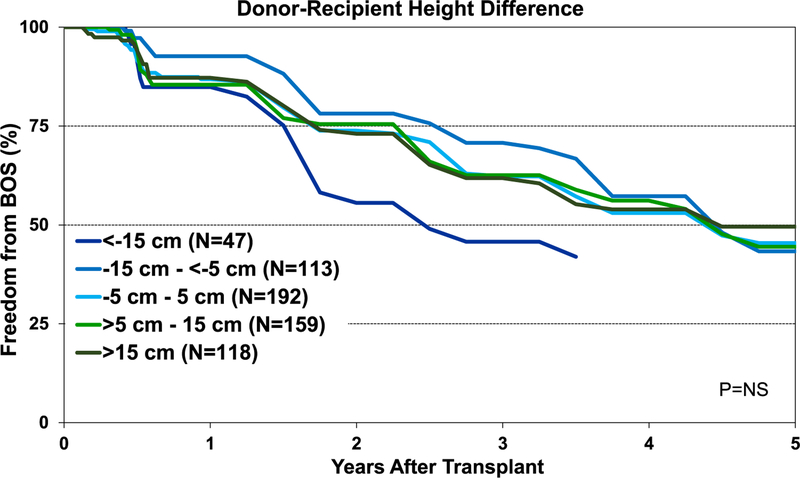
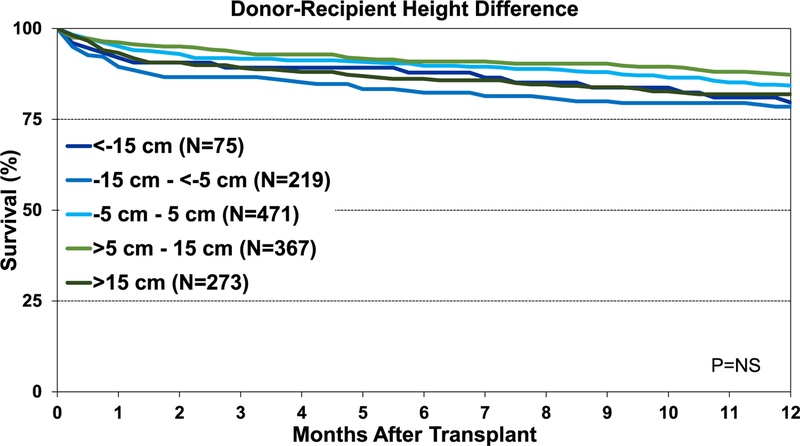
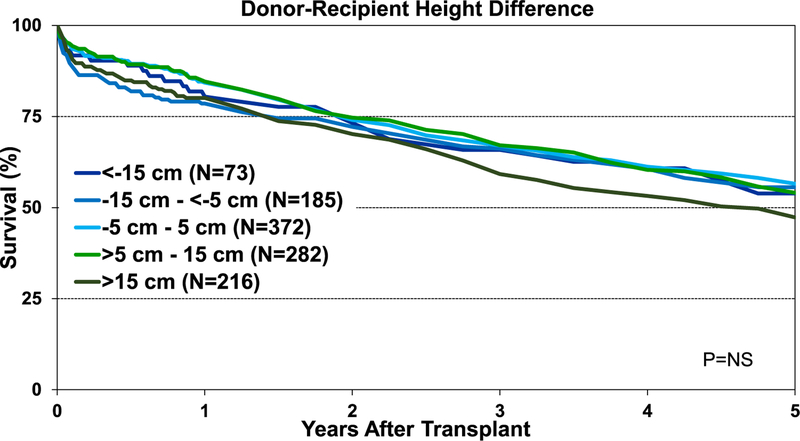
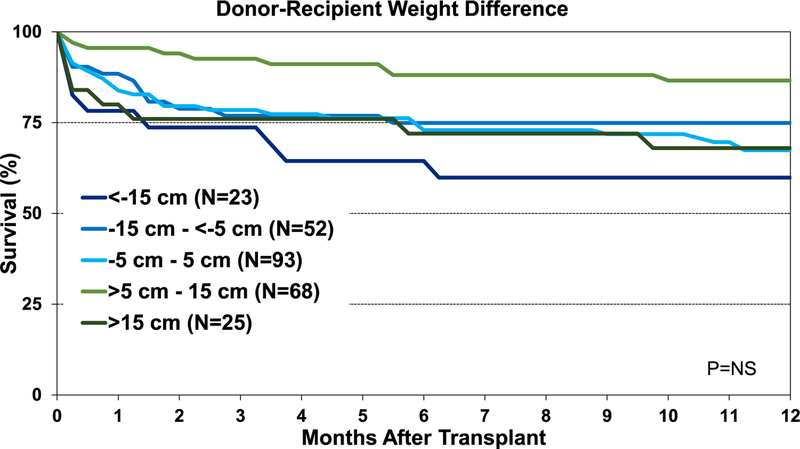
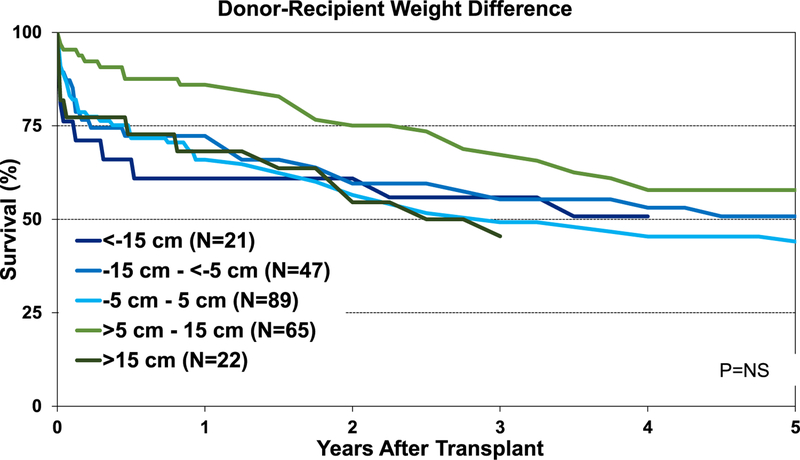
Although the percentage of pediatric lung transplant recipients requiring treatment for allograft rejection within 1 year was higher in the <−15-cm donor-recipient height difference group than in the other groups, this difference was not statistically significant (eSlide L(p) 84). Additionally, our analysis did not identify an association between donor-recipient height differences and freedom from BOS within 5 years after pediatric lung transplant (Figure 15). Unlike adult lung transplant recipients, where donor-recipient height difference was significantly associated with survival,8 differences in height between the donor and recipient were not associated with 1-year (Figure 16) and 5-year survival (Figure 17) for pediatric recipients. Similarly, donor-recipient weight differences were not associated with 1-year (eSlide L(p) 89) and 5-year survival (eSlide L(p) 94). This was also true for multivariable analysis. It must be noted, however, that the number of patients with significant over- and undersizing was relatively low in this pediatric lung transplant cohort.
Figure 15.
Kaplan-Meier freedom from BOS within 5 years for pediatric lung transplants by donor-recipient height difference (transplants: January 1995-June 2013). BOS, bronchiolitis obliterans syndrome; NS, not significant.
Figure 16.
Kaplan-Meier survival within 1 year for pediatric lung transplants by donor-recipient height difference (transplants: January 1995–June 2017). NS, not significant.
Figure 17.
Kaplan-Meier survival within 5 years for pediatric lung transplants by donor-recipient height difference (transplants: January 1995–June 2013). NS, not significant.
Pediatric heart-lung transplantation
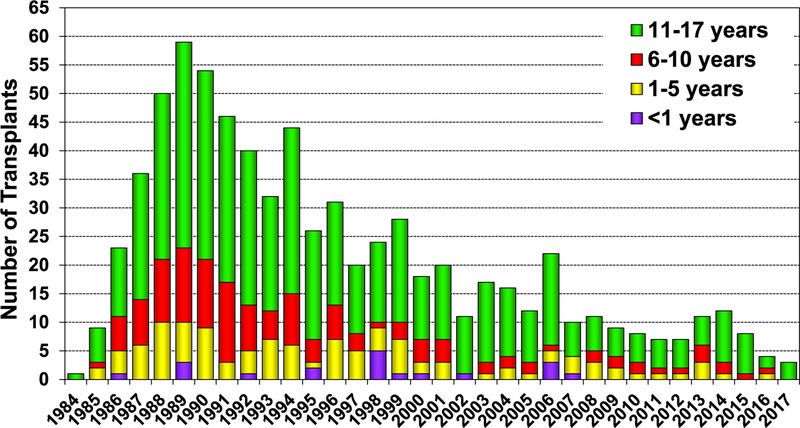
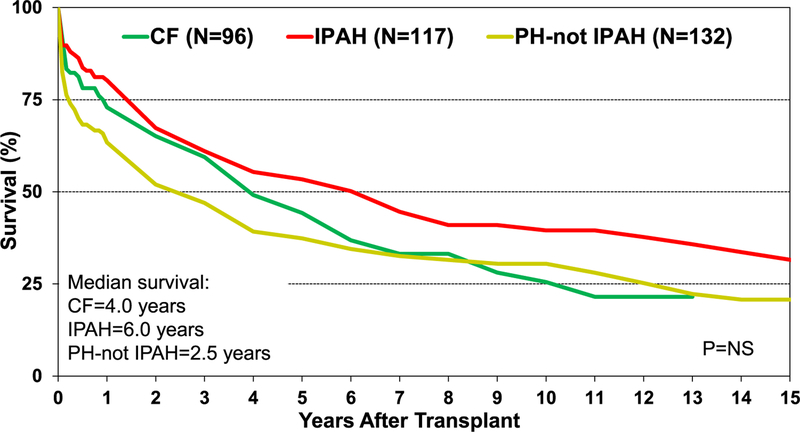
Pediatric heart-lung transplantation is a rare procedure for children with cardiopulmonary failure, with most transplantations performed in the 11- to 17-year age group (Figure 18). Across eras, there has been a steady decline in the number of pediatric heart-lung transplants performed and reported to the Registry (eSlide HL(p) 7). From January 2010 to June 2018, significant differences were seen with respect to indication for pediatric heart-lung transplantation between Europe, North America, and other Regions. In Europe, CF is a more common indication, whereas IPAH and PH-not IPAH are more common in North America and other Regions (eSlide HL(p) 12). Unadjusted Kaplan-Meier survival in pediatric heart-lung recipients across the 3 major indications (CF, IPAH, and PH-not IPAH) was not statistically different; however, numerical differences in survival are present (Figure 19).
Figure 18.
Volume of transplantation and recipient age distribution for pediatric heart-lung transplants (transplants: 1984–2017).
Figure 19.
Kaplan-Meier survival for pediatric heart-lung transplants by major diagnosis (transplants: January 1992–June 2017). CF, cystic fibrosis; IPAH, idiopathic pulmonary arterial hypertension; NS, not significant; PH-not IPAH, pulmonary hypertension-not idiopathic pulmonary arterial hypertension.
In our analysis evaluating donor-recipient size match on outcomes after pediatric heart-lung transplants, the distribution of donor-recipient height matching was more consistent across recipient age groups (Figure 20) when compared with pediatric lung transplants (Figure 14). Similar to pediatric lung transplants, survival differences across donor-recipient height categories were not statistically significant, which may be related to small sample sizes. Numerical differences between the groups were present, with higher survival seen especially in the >5 cm to 15 cm group, both at 1 and 5 years after transplant (Figures 21 and 22). There were no statistically significant differences in survival based on donor-recipient weight differences (eSlides HL(p) 30 and 32).
Figure 20.
Distribution of donor-recipient height difference for pediatric heart-lung transplants by recipient age (transplants: January 1992–June 2018).
Figure 21.
Kaplan-Meier survival within 1 year for pediatric heart-lung transplants by donor-recipient height difference (transplants: January 1992–June 2017). NS, not significant.
Figure 22.
Kaplan-Meier survival within 5 years for pediatric heart-lung transplants by donor-recipient height difference (transplants: January 1992–June 2013). NS, not significant.
Conclusions
The 2019 ISHLT Registry Report on Pediatric Lung and Heart-Lung Transplantation demonstrates continued improvements in survival of children after lung and heart-lung transplantation. These findings support pediatric lung and heart-lung transplantation as an essential therapy for certain pediatric lung diseases or disorders resulting in cardiopulmonary failure. Our analyses provide additional insights into the role of donor-recipient height or weight differences in children after lung and heart-lung transplantation.
Supplementary Material
Acknowledgments
K.K serves as scientific adviser and speaker for CareDx, Inc; L.P serves as a speaker for Thermofisher, Sandoz, One Lambda, and Novartis, and is advisory board member for Qiagen and Novartis; J.S serves as consultant for Medtronic and Abbott; J.R serves as consultant for Bayer, Novartis, Amgen, and CSL Behring; A.Z serves on the speakers bureau of Paragonix, Novartis, Mallincrodt, Sanofi-Genzyme, and Franz Köhler Chemie and on the advisory board for Chiesi. D.C received research funding from Astellas and Boeringher-Ingelheim.
Footnotes
Disclosure statement
M.H, D.H, T.S, E.H, B.M, W.C, A.R, and A.S have no conflicts to report.
References
- 1.Fraser CD 3rd, Zhou X, Grimm JC, et al. Size mismatching increases mortality following lung transplantation in preadolescent patients. Ann Thorac Surg 2019;108:130–7. [DOI] [PubMed] [Google Scholar]
- 2.Eberlein M, Permutt S, Chahla MF, et al. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest 2012;141: 451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberlein M, Arnaoutakis GJ, Yarmus L, et al. The effect of lung size mismatch on complications and resource utilization after bilateral lung transplantation. J Heart Lung Transplant 2012;31:492500. [DOI] [PubMed] [Google Scholar]
- 4.Eberlein M, Reed RM, Maidaa M, et al. Donor-recipient size matching and survival after lung transplantation. A cohort study. Ann Am Thorac Soc 2013;10:418–25. [DOI] [PubMed] [Google Scholar]
- 5.Eberlein M, Reed RM, Bolukbas S, et al. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant 2015;34:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberlein M, Diehl E, Bolukbas S, Merlo CA, Reed RM. An oversized allograft is associated with improved survival after lung transplantation for idiopathic pulmonary arterial hypertension. J Heart Lung Transplant 2013;32:1172–8. [DOI] [PubMed] [Google Scholar]
- 7.Delom F, Danner-Boucher I, Dromer C, et al. Impact of donor-to-recipient weight ratio on survival after bilateral lung transplantation. Transplant Proc 2014;46:1517–22. [DOI] [PubMed] [Google Scholar]
- 8.Chambers DC, Cherikh WS, Harhay MO, et al. Thirty-sixth adult lung and heart–lung transplantation Report—2019; focus theme: Donor and recipient size match. J Heart Lung Transplant 2019;38: 1052–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.