ABSTRACT
Paradoxically, most if not all previously known appetite-stimulatory hormones are downregulated in human obesity, reflecting failing homeostatic circuitries. Recently, we discovered that acyl-coenzyme-A binding protein/diazepam-binding inhibitor (ACBP/DBI) acts as a lipogenic and appetite stimulator, when systemically injected into mice. ACBP/DBI plasma levels are also elevated in obese subjects, supporting the notion that it may represent the elusive “hunger protein” that explains overeating in human obesity.
KEYWORDS: Aging, anorexia, metabolism, obesity
Acyl-coenzyme A binding protein (ACBP) is a phylogenetically conserved protein that is found in some eubacteria, unicellular fungi, plants and animals. In humans, this protein is also known as diazepam-binding inhibitor (DBI), because it displaces the benzodiazepine diazepam from its two receptors (i) the so-called central benzodiazepine receptor (now called γ-aminobutyric acid (GABA) receptor A) that is actually expressed by most nucleated cell types in the body, and (ii) the so-called peripheral benzodiazepine receptor (now called translocator protein, TSPO) that is an ubiquitous outer mitochondrial membrane protein).
We became interested in ACBP/DBI for three major reasons. First, in the past we involved in elucidating the role of TSPO in the regulation of mitochondrial membrane permeabilization in the context of cell death, advocating the use of pharmacological TSPO ligands in cancer therapy.1,2 Second, we developed a strong interest in acetyl-coenzyme A, which is the shortest natural acyl-coenzyme A, discovering that nutrient deprivation triggers a drop in intracellular acetyl-coenzyme A concentrations, which then stimulates autophagy via the reduction of cytoplasmic protein acetylation.3–5 Third, we became intrigued by the observation that autophagy is associated to the cellular release of ACBP/DBI through a phylogenetically conserved pathway, from yeast to human astrocytes.6 In Dictyostelium discoideum, the ACBP/DBI orthologue (AcbA) is released during starvation and acts as a precursor of the 34 amino acid peptide SDF-2, which potently stimulates terminal differentiation and sporulation of the cells.7 Hence ACBP/DBI exemplifies a factor that links a cell-autonomous stress response to a systemic response.8 However, the role of ACBP/DBI in animal physiology was largely unknown, apart from the fact that ACBP/DBI is the precursor of the octadecaneuropeptide that has potent anorexigenic (appetite-inhibitory) effects when injected into the brain of rodents.9
Intrigued by these premises, we decided to generate a series of tools for the exploration of the physiological function of ACBP/DBI in mammalian cell biology and physiology, namely, recombinant ACBP/DBI protein, ACBP/DBI-encoding vectors for hepatic transgenesis, ACBP/DBI-specific monoclonal antibodies, mice in which ACBP/DBI can be knocked out in a conditional fashion by injection of tamoxifen (that activates the recombinase CRE to excise the second exon of the Dbi gene), as well as a protocol for breaking self-tolerance to ACBP/DBI and hence to induce autoantibodies against this factor. We then performed in vitro cell biology experiments, as well as in vivo experiments, to elucidate the potential role of ACBP/DBI in autophagy and metabolism in mice.10 In the course of these efforts, which started in 2012, we made a series of observations that, in our opinion, reveal a major role for ACBP/DBI in feedback inhibition of autophagy and in metabolic control.
ACBP/DBI is indeed released from murine and human cells upon starvation in an autophagy-dependent fashion, through a non-conventional protein secretion pathway.10 Moreover, in mice, the plasma concentration of ACBP/DBI increases after one day of starvation. This effect can be blocked by knockout of the pro-autophagic gene Atg4b. Hence, ACBP/DBI is a potential endocrine factor that raises when cells initiate autophagy. At difference with true hormones, however there is no specific cell type that would produce this factor. Rather, many different cells (e.g. adipocytes, hepatocytes, leukocytes …) can release ACBP/DBI in vivo (Figure 1(a)).
Figure 1.
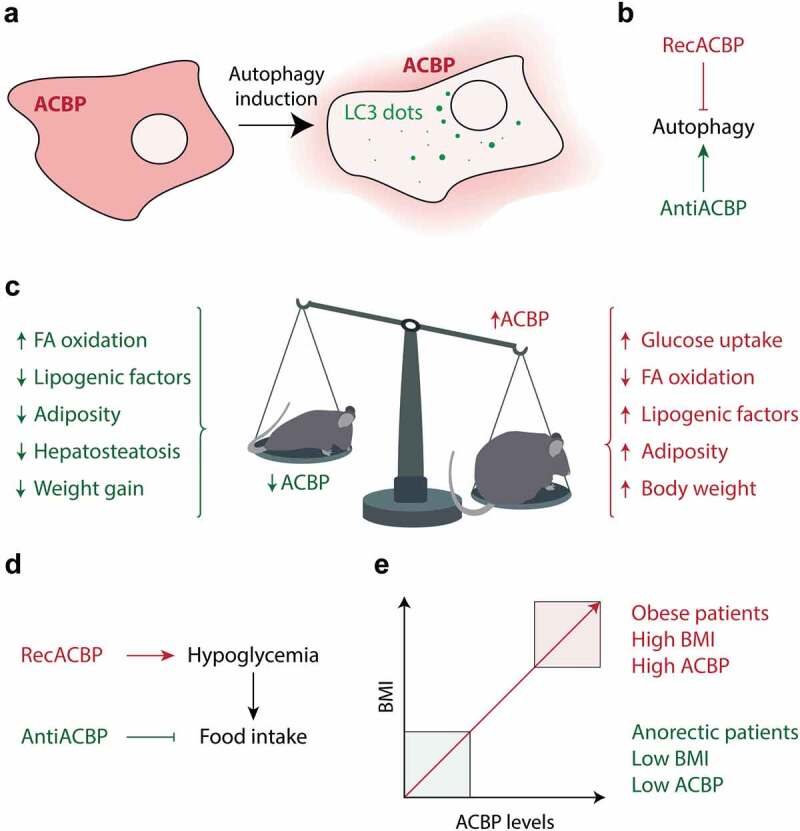
Schematic representation of ACBP/DBI effects on autophagy and metabolism. (a) Autophagy-dependent release of ACBP/DBI from cells. (b) Involvement of ACBP/DBI in feedback inhibition of autophagy. (c) Obesogenic effects of ACBP/DBI in mice. (d) Orexigenic effects of ACBP/DBI in mice. (e) Deviations in circulating ACBP/DBI levels in human appetite control disorders.
In vitro, addition of recombinant ACBP/DBI protein to cell cultures inhibits starvation-induced autophagy, while addition of a neutralizing antibody specific for ACBP/DBI stimulates autophagy. Similar results are obtained in mice when ACBP/DBI protein or the neutralizing antibody are injected into the peritoneal cavity.10 Thus, it appears that ACBP/DBI is engaged in a feedback loop that controls autophagy. When ACBP/DBI is released from starved cells into the extracellular space, it then inhibits autophagy, perhaps as a homeostatic control mechanism (Figure 1(b)).
Transgenic overexpression of ACBP/DBI in the liver or intravenous injection of the recombinant protein has obesogenic effects: increased glucose uptake by liver cells and adipocytes, inhibition of fatty acid oxidation, upregulation of lipogenic transcription factors and enzymes, enhanced adiposity, and increased body weight. In contrast, neutralization of ACBP/DBI by three different methods, namely, (i) injection of neutralizing monoclonal antibodies, (ii) induction of autoantibodies specific for ACBP/DBI, or (iii) tamoxifen-inducible whole-body knockout had marked anorexigenic effects: induction of fatty acid oxidation; downregulation of lipogenic transcription factors and enzymes; browning of fat; reduced adiposity, less hepatosteatosis, no diabetes and attenuated weight gain in the context of a high-fat diet, as well as a major weight less when mice were switched from a high-fat to a normal diet (Figure 1(c)).
Beyond the aforementioned effects, injection of ACBP/DBI stimulated increased food uptake, commensurate with the activation of orexigenic neurons in the hypothalamus, while neutralization of ACBP/DBI caused a reduction of food intake after transient starvation, accompanied by the inhibition of orexigenic and the activation of anorexigenic neurons. Thus, peripherally administered ACBP/DBI has the opposite effect than ACBP/DBI that is provided by by intrathecal (intracerebroventricular) or intrahypothalamic injection.9 This orexigenic ACBP/DBI effect is most likely mediated by a modulation of systemic metabolism. Thus, intravenous injection of ACBP/DBI causes a reduction in circulating glucose concentrations, and a glucose clamp (that maintains glucose levels stable) prevents the activation of orexigenic neurons and the hyperphagia induced by ACBP/DBI in this context (Figure 1(d)).
Finally, we determined the plasma concentration in humans with disorders in appetite control. We found a strong positive correlation (Spearman r = 0.88) between the plasma concentration of ACBP/DBI and the body mass index (BMI) across all extremes, from underweight (BMI<20), through normal weight (BMI between 20 and 25), overweight (BMI between 25 and 30) to obesity (BMI>30) and morbid obesity (BMI>35). In anorexia nervosa, ACBP/DBI levels were extremely low, while in obesity these levels were supraphysiological. Similarly, in mice obesity was coupled to supranormal levels of ACBP/DBI protein levels in the plasma, as well as enhanced Dbi mRNA levels in the liver and in white adipose tissue. Moreover, in patients, long-term variations in caloric uptake positively correlated with DBI mRNA levels in the periumbilical white adipose tissue (Figure 1(e)).
In summary, ACBP/DBI appears to act as a major obesogenic (orexigenic + lipogenic) factor that is elevated in human obesity. As such, it may be the thus far elusive “hunger protein” that mediates pathogenic hyperphagia in obese subjects, locking then in a close-to-irreversible state of metabolic deviation.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Association “Le Cancer du Sein, Parlons-en!”; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM).
References
- 1.Gonzalez-Polo RA, Carvalho G, Braun T, Decaudin D, Fabre C, Larochette N, Perfettini J-L, Djavaheri-Mergny M, Youlyouz-Marfak I, Codogno P, et al. PK11195 potently sensitizes to apoptosis induction independently from the peripheral benzodiazepin receptor. Oncogene. 2005;24(7503–7513). doi: 10.1038/sj.onc.1208907. [DOI] [PubMed] [Google Scholar]
- 2.Fulda S, Galluzzi L, Kroemer G.. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9(447–464). doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 3.Marino G, Pietrocola F, Eisenberg T, Kong Y, Malik S, Andryushkova A, Schroeder S, Pendl T, Harger A, Niso-Santano M, et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell. 2014;53(710–725). doi: 10.1016/j.molcel.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 4.Pietrocola F, Galluzzi L, Bravo-San Pedro JM, Madeo F, Kroemer G.. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 2015;21(805–821). doi: 10.1016/j.cmet.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg T, Schroeder S, Andryushkova A, Pendl T, Küttner V, Bhukel A, Mariño G, Pietrocola F, Harger A, Zimmermann A, et al. Nucleocytosolic depletion of the energy metabolite acetyl-coenzyme a stimulates autophagy and prolongs lifespan. Cell Metab. 2014;19(431–444). doi: 10.1016/j.cmet.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V. Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol. 2010;188(527–536). doi: 10.1083/jcb.200911154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manjithaya R, Subramani S. Role of autophagy in unconventional protein secretion. Autophagy. 2010;6(650–651). doi: 10.4161/auto.6.5.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galluzzi L, Yamazaki T, Kroemer G. Linking cellular stress responses to systemic homeostasis. Nat Rev Mol Cell Biol. 2018;19(731–745). doi: 10.1038/s41580-018-0068-0. [DOI] [PubMed] [Google Scholar]
- 9.de Mateos-Verchere JG, Leprince J, Tonon MC, Vaudry H, Costentin J. The octadecaneuropeptide [diazepam-binding inhibitor (33–50)] exerts potent anorexigenic effects in rodents. Eur J Pharmacol. 2001;414(225–231). doi: 10.1016/s0014-2999(01)00771-3. [DOI] [PubMed] [Google Scholar]
- 10.Bravo-San Pedro JM, Sica V, Martins I, Pol J, Loos F, Maiuri MC, Durand S, Bossut N, Aprahamian F, Anagnostopoulos G, et al. Acyl-CoA-binding protein is a lipogenic factor that triggers food intake and obesity. Cell Metab. 2019. doi: 10.1016/j.cmet.2019.07.010. [DOI] [PubMed] [Google Scholar]


