ABSTRACT
Kyasanur Forest Disease (KFD) is a tick-borne hemorrhagic fever of human, caused by Kyasanur forest disease virus (KFDV) in India. The tick, Haemaphysalis spinigera, has been incriminated as the vector of KFDV. In human, KFD clinically presents with high fever, frontal headache, and severe myalgia, followed by bleeding from the nasal cavity, throat, gingivae, and in some cases, gastrointestinal tract. The mortality rate in KFDV infected cases is estimated to be 3–10%. Monkeys infected with the virus also develop the disease and die. Though the incidence of KFD was found to be confined only to the sylvatic area of Shimoga district in Karnataka state in India during 1967, recent reports indicate its expanding potential to the neighboring states such as Kerala, Tamil Nadu, and Goa. The administration of an indigenous, inactivated tissue culture vaccine was found to drastically decrease the percentage of incidence; however, the recurrence of KFD in vaccinated subjects warrants innovative strategies for effective control of the infection. The present communication proposes and discusses innovative intervention strategies for the effective prevention and control of KFD in India.
KEYWORDS: Kyasanur Forest Disease, Monkey fever, Tick borne virus infection, Haemaphysalis spinigera, hemorrhagic fever
Kyasanur Forest Disease (KFD), a tick-borne hemorrhagic fever of man, is caused by KFD virus (KFDV) belonging to the family Flaviviridae. Haemaphysalis spinigera, a highly anthropophagic tick, is the vector of KFDV. KFD in humans presents with high fever, frontal headache, and severe myalgia, followed by bleeding from the nasal cavity, throat, gingivae, and in some cases, gastrointestinal tract. Humans are dead-end hosts of the virus. Earlier, the incidence of KFD was limited only to Shimoga forest, Karnataka state but now, new cases have recently been detected in the neighboring districts of the Western Ghats, namely Chamarajanagar district in Karnataka, Nilgiri district in Tamil Nadu, Wayanad and Malappuram districts in Kerala, and Pali village in Goa (Figure 1).1 The widespread circulation of KFDV through serological evidence has already been documented in many Indian provinces (Gujarat, Rajasthan, Maharashtra, West Bengal, Tamil Nadu, and Andaman and Nicobar Islands).2 In the enzootic cycle, KFDV circulates through small mammals (rodents, shrews, ground birds, etc.) and ticks. KFDV was also isolated from the insectivorous bat, Rhinolophus rouxi. Monkeys, predominantly, the black-faced langur (Presbytis entellus) and the red-faced bonnet monkey (Macaca radiata) are also susceptible to KFDV, similar to humans. After the death of the infected monkeys, ticks droop down and feed on carrion and form a potential hotspot for further transmission to human. A man encounters KFDV infection by entering the hotspot area or while clearing the moribund monkeys in the forest.3 The mortality rate in KFD infected cases is about 3–10%. The expanding potential of KFDV indicates the possibility of becoming a potential public health threat in India as well as the neighboring countries.
Figure 1.
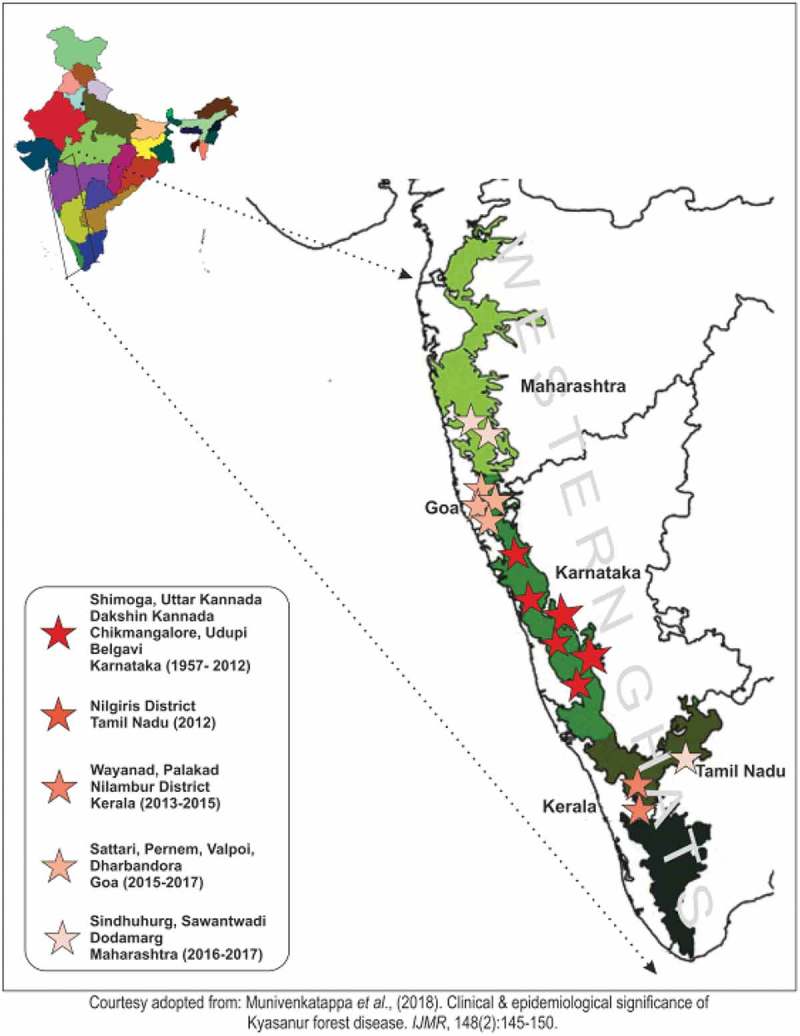
Map showing the expanding potential of Kyasanur forest disease along the Western Ghats, India.
KFDV virus is a single-stranded, positive-sense RNA virus belonging to the family Flaviviridae. The virus genome is 11 kb in size and encodes for three structural, namely, capsid, envelop, and membrane (C, E, and M) and seven non-structural proteins (NS1, NS2a, 2b, NS3, NS4a, 4b, and NS5). KFDV, though antigenically related to the tick-borne encephalitis virus sero-complex, has been shown genetically to have 97% homology with Alkhurma Hemorrhagic fever virus, recovered in Jeddah, Saudi Arabia.4 The virus can be isolated from clinical specimens, i.e., from serum of virus-infected man and monkeys or from the infected vector ticks by inoculating in infant mice or Vero E6, BHK–21 or Chick embryo cells.
KFDV is controlled by the application of pesticides, such as acaricides, which are effective against ticks and mites. In the KFD affected area, acaricides are applied around the 5-meter circle of the spot where infected and dead monkeys are found or reported to have been found. Additionally, intramuscular administration of formalin-inactivated indigenous tissue-culture KFD vaccine to the susceptible population has been found to drastically reduce the percentage of incidences. The vaccination schedule includes administration of two doses to 7–65 year age group in one month apart. As the vaccine-induced immunity is short-lived, the first booster dose is recommended in about 6–9 months after the primary vaccination; thereafter, administration of annual booster dose is recommended for five years after the last confirmed case in the area.5 However, recent data show recurrence of KFD cases even in the vaccinated subjects, which may probably be due to the sub-optimal efficacy of the current vaccine or vaccination protocol.5,6 Possible variants of the circulating virus and low acceptance of vaccine by the community, due to adverse effects, could also lead to recurrence. Additionally, the vaccine-associated side effects, such as pain, and administration of booster doses for five years are also believed to be some of the potential discouraging factors that might affect the vaccine acceptability. The current vaccination strategy covers the administration of the vaccine to the susceptible group in 5 km radius of the KFD reported area. However, the appearance of the cases at locations more than 5 km apart from the affected area indicates the need for revising the ongoing vaccination strategy. Moreover, the movement of KFDV infected monkeys to the nearby areas in the forest may spread the virus to the unvaccinated population and render them susceptible to KFDV infection. Hence, there is an urgent need for developing innovative next generation KFD control strategies.
Under the genera Haemaphysalis, a total of 167 species have been recorded around the world and about 40 of them exist in India. Though Haemaphysalis turturis and H. spinigera both are the main vectors of KFDV, around 95% of virus isolations have been done from H. spinigera ticks. The vector H. spinigera is distributed in India, Sri Lanka, and Vietnam. The adult stages of the vector H. spinigera is multi-host and usually feed on large animals like cattle, monkeys, birds, etc., and are not involved in virus transmission to humans due to low viremia. Human gets an infection mostly by the bite of virus-infected nymphs. Small mammals such as rodents and shrews have been considered as the reservoir of the virus since they are also susceptible to KFDV infection and capable of developing low-level viremia and transmitting the pathogen to bloodsucking ticks. Though KFDV had been in circulation through “enzootic cycle” among ticks and small mammals, infection in monkeys and their subsequent death started before the appearance of human cases. Monkeys were found to show a high titer of KFDV which facilitated further transmission to man. The studies undertaken in the past have clearly shown the significant role played by the nymphal stage of the tick in the transmission of the virus to humans, as epidemics of KFD and high nymphal activity have been reported to occur simultaneously. The high nymphal activity of the vector is recorded during January–May. Human cases of KFD appear during the post-monsoon season in the affected areas and such incidences can be correlated with the death of infected monkeys and the appearance of human KFD cases. This also explains the essential role of monkeys in triggering KFD in humans in the affected areas. Among the KFDV infected clinical cases, 80% of them recover with no consequences and around 20% develop biphasic symptoms. In some cases, few patients also suffer from hemorrhagic manifestations or neurological sequelae.7 Among the recovered patients, long term or permanent sequelae are rare. The incidence of confirmed cases of KFD in humans, the death of infected monkeys, and virus activity in the vector are reported every year in Karnataka and are also being reported recently from the adjoining States of Kerala and Tamil Nadu.1 The attack rate from different outbreaks has been found to range from 2–20% and estimated 400–500 cases of KFD occur every year.8 The case fatality rate is estimated to be 3–10%.9 Hence, it is appropriate to design prevention and control strategies by targeting the monkey-tick contact that usually precedes human infections.
The available options for the intervention of KFD are shown in Figure 2.
Figure 2.
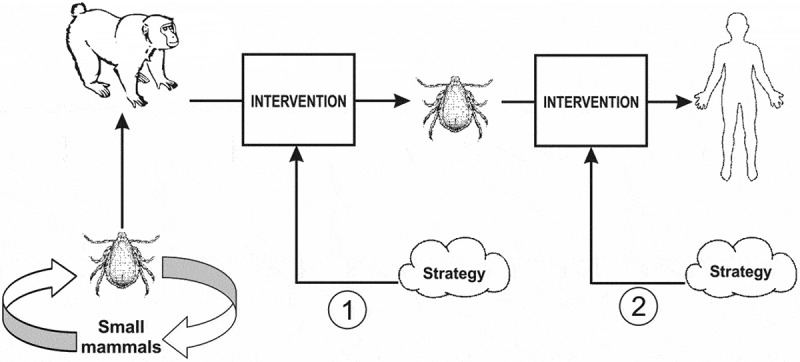
Schematic description of KFD control targets for the proposed strategies.
Following are the possible strategies:
Strategy 1: to intervene at the monkey-vector contact.
Strategy 2: to intervene at the man-vector contacts.
or
A common prevention strategy targeting both man and monkey to curtail KFDV transmission.
By careful analysis of the epidemiology, it is obvious that the option available for prevention and control of KFDV is to ultimately interrupt the man-vector contact. Recent reports also indicate the requirement of a highly effective anti-KFD vaccine for human use.
As tick vector gets sufficient KFDV load from monkeys, the intervention of monkey-tick contact could be a potential point for targeting and implementing prevention and control strategies. Therefore, new interventions could be planned to prevent ticks from receiving high KFDV titer from monkeys for further transmission to human. At the same time, to be on the safer side humans should also be vaccinated against KFDV as they stride through the forest. However, the development of two sets of vaccines would be an expensive process.
The present communication proposes two strategies for the prevention and control of ticks and tick-borne infections. The first strategy proposes the development of a common and safe, preferably an edible KFDV vaccine for vaccinating both man and the non-human primates. The aim of proposing administration of an edible vaccine to monkeys through appropriate baits in the hot spot areas before the onset of nymphal activity (August to October) is to decrease the transmission of the pathogen from the infected and vaccinated monkeys to humans.
The concept of immunizing wild animals such as red fox, raccoons, grey foxes, and coyotesetc through baited rabies vaccine is already in practice in the Americas and Europe.10 The success story documents dropping of chicken head baited with rabies vaccine in the areas covering about 2.36 million km2 in nine countries in Western and Central Europe for the rabies elimination in wild animals.11
Therefore, targeting the area of the Western Ghats of about 60,000 km2 (62,000 sq mi) for dropping oral KFD vaccine is practically possible.12 However, the areas for dropping the vaccine will need to be precisely determined. The administration of the vaccine to monkeys once a year would be sufficient. At the same time, mopping up exercise may also be carried out as and when required in the areas where KFD cases are detected. The proposal of edible vaccine development for KFD control seems to be a practical long term strategy. Though the development of such a vaccine will probably take more than a decade, if possible. The development of an effective rabies vaccine from an injectable to an edible form took long term inputs of R&D activities. Meanwhile, the efforts on the development of an effective vaccine using other feasible technologies may be encouraged. Strategy 1 of dual vaccination is shown in Figure 3a.
Figure 3.
a. Schematic illustration showing KFD control Statergy 1.
A combined approach for vaccinating human and monkeys with an edible anti-KFD vaccine could be expected to protect them against KFD infection. The vaccine can protect monkeys from KFDV infection and curtail its further transmission to human through ticks. At the same time, an edible vaccine can also be used to immunize humans against KFDV.
On the other hand, Strategy 2 of developing an edible chimeric vaccine (anti KFDV and anti-tick multi antigenic epitope peptides) for monkeys only could be a better option. This, in addition, will also reduce ticks as well as tick-borne infections. However, the chimeric vaccine with anti-tick antigenic peptide will not be suitable for the human purpose and a separate potential KFDV vaccine will need to be developed. Strategy 2 is illustrated in Figure 3b. Similar to the development of an edible KFD vaccine, developing a successful anti-tick vaccine against KFDV vector will be a challenging and time-consuming venture. Since discovering the anti-tick antigens is difficult and needs a long time. The successful anti-tick vaccine could also be advantageous in controlling other potential tick-borne rickettsial and viral infections. As the application of multiple vaccines has already been proved to be effective for several infectious diseases, the exploitation of the proposed strategy cannot be overruled.
Figure 3.
b. Schematic illustration showing KFD control Strategy 2.
The advantages of immunizing monkeys and man with a common edible vaccine are as follows:
The wild population of susceptible monkeys will be protected from the transmission of KFDV through tick bites,
KFD transmission to human from virus infected monkeys via ticks will be curtailed.
Vaccinated susceptible humans will acquire immunity against KFDV by the common vaccine as a personal protection measure.
As an edible vaccine, its acceptance and compliance are expected to be higher.
There is an argument that how to vaccinate monkeys which are freely living in the forest and are habitually moving from one place to other frequently in the forest. Vaccinating monkeys with an edible vaccine through bait is expected to be advantageous in the long run as the average life span of monkeys (Presbytis entellus and Macaca radiata) is above 30 years, which is an advantage for vaccination as the immunity of the vaccinated herd would sustain for a considerable long period. Along with this, a well-designed model of KFD spread in the Western Ghats needs to be developed.
Alkhurma hemorrhagic fever virus causes clinical symptoms in humans that include gastroenteric as well as CNS manifestations with an estimated case fatality rate of less than 0.5%.13 On the other hand, no clinical severity or case fatality due to Nanjianyin virus, a variant of KFDV, has been documented, except the single isolation from a patient with fever in Yunnan Province, China, in 1989. Moreover, its distribution was demonstrated by the serological evidence in residents of the Hengduan Mountain region of Yunnan Province, China.14 Though the problems of closely related variants such as Alkhurma and Nanjianyin virus have been documented in Saudi Arabia and China, respectively, KFD is largely restricted to India. Hence, a KFD consortium on “MISSION MODE” needs to be urgently constituted in order to conduct research for developing an intervention strategy for KFD in India.
The broad priority research areas are as follows (Figure 4);
Figure 4.
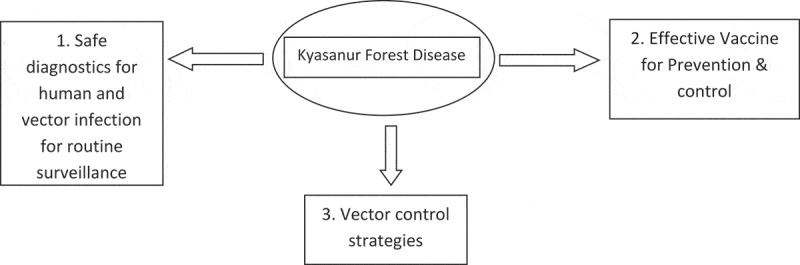
The broad priority research areas to control KFD.
1. Development of safer diagnostics enabling detection of the infected human subjects and vectors for routine surveillance
Innovative approaches such as exploitation of synthetic multi antigenic peptides for virus-based diagnostics should be attempted for developing a safer and rapid point of care assay. Such a safer assay may be helpful in routine surveillance of KFD in vectors as well as in humans in India at the hotspots of the disease. The availability of diagnostic assays will be helpful in mapping the extent of pathogen distribution, diversity if any, by conducting a large scale screening in the country.
2. Development of an effective vaccine for the prevention and control of KFD
As KFDV vaccinated subjects have been observed to experience the occurrence of the disease, the effectiveness of existing vaccine needs to be re-looked. This may be due to the circulation of virus variants or low coverage or less vaccine acceptance by the community due to the adverse effects such as severe pain. Hence, efforts should be undertaken for developing better vaccines and the innovative approaches, such as using recombinant subunit or chimeric strategy, may be attempted as suggested by Pattnaik (2006).15 However, the most promising approaches could be the cell culture-based live attenuated virus similar to the successful 17 D Yellow fever vaccine. The successful flavivirus vaccines currently available on the market are summarized in Table 1. Many of the flavivirus vaccines using novel strategies are in pipeline.16 Hence, efforts for developing an effective vaccine for a human with novel strategies should be encouraged.
Table 1.
List of successful flavivirus vaccines commercially available on the market.
| Disease | Commercial Name of Vaccine | Vaccine Type | Manufacturer | Countries available |
|---|---|---|---|---|
| Yellow Fever | 17DD | Live attenuated | Bio-Manguinhos, Brazil | Brazil |
| 17D–204 (Stamaril® in Senegal) | Sanofi Pasteur France | France | ||
| Chumakov Institute of Poliomyelitis and Viral Encephalitides (IPVE), Moscow | Russia | |||
| 17D–204 YF-VAX® |
Sanofi Pasteur, USA | USA | ||
| 17D–204 Yellow Fever Vaccine (live) |
Beijing Tiantan Biological Products, China | China | ||
| Japanese encephalitis | IXIARO® (SA14–14–2) |
Inactivated | Valneva SE (France), Biological E (India) | Europe, USA, Canada, Hong Kong, Singapore, and Israel, Norway, Liechtenstein, and Iceland. |
| JESPECT® | Inactivated | Australia and New Zealand | ||
| CD. JEVAXTM (SA14–14–2) | Live attenuated | Chengdu Institute of Biological Products (China) | China, Cambodia, North Korea, India, Laos, Myanmar, Nepal, South Korea, Sri Lanka, and Thailand. | |
| IMOJEV® | Live attenuated Chimeric vaccine (prM/E genes replaced YFV–17D genes) |
Sanofi Pasteur (France) | Australia | |
| THAIJEV® | Thailand | |||
| Tick-borne encephalitis | FSME-IMMUNE | Inactivated | Pfizer | Vienna and Austria |
| Encepur | GlaxoSmithKline | Germany | ||
| TBE-MOSCOW | Chumakov Institute of Poliomyelitis and Viral Encephalitides (IPVE),Moscow | Russia | ||
| EnceVir | Microgen, Tomsk, Russia | Russia | ||
| CIBP | Changchun Institute of Biological Products, China | China |
3. Vector control strategies
The emerging symbiotic organism based biocontrol system for ticks could also be explored. Multi tick peptide-based vaccines could be a potential strategy for controlling ticks by immunizing monkeys and cattle, which will help in reducing the tick infestation of monkeys and cattle thereby reducing the risk of transporting and transmission of zoonotic pathogen infected ticks. The reverse vaccinological approaches may be attempted by exploring the valuable genomic information available for ticks in order to identify highly immunogenic multiple antigenic peptides, as the promising efforts are already underway.17
The proposed strategy of developing a common edible KFD vaccine with a dual-purpose, i.e., immunizing non-human primates and humans would be advantageous over the conventional strategy of a vaccine targeting human only in the view of the cost, and time required. The combined effect of vaccinating non-human primates would also help in decreasing the death of monkeys as well as curtailing further KFDV transmission to humans. The proposed strategy would complement the ongoing prevention and control measures against KFD in the country, as integrated control approach would always be required along with the proposed strategy for the effective prevention and control of KFD in India.
Acknowlegdement
The author thanks Dr. P. Jambulingam, Director, ICMR-VCRC, Pondicherry, for his valuable suggestions to improve the manuscript and Dr. A. Venkatesh for his assistance in illustrations. The author also thanks Dr. J. Muthukrishnan, Professor of Environmental Biology (Retired), Madurai Kamaraj University, Madurai for reviewing the manuscript and his valuable remarks.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
References
- 1.Murhekar MV, Kasabi GS, Mehendale SM, Mourya DT, Yadav PD, Tandale BV.. On the transmission pattern of Kyasanur Forest disease (KFD) in India. Infect Dis Poverty. 2015;19(4):37. doi: 10.1186/s40249-015-0066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padbidri VS, Wairagkar NS, Joshi GD, Umarani UB, Risbud AR, Gaikwad DL, Bedekar SS, Divekar AD, Rodrigues FM. A serological survey of arboviral diseases among the human population of the Andaman and Nicobar Islands, India. Southeast Asian J Trop Med Public Health. 2002;33(4):794–800. PMID:12757228 [PubMed] [Google Scholar]
- 3.Work TH. Russian spring-summer encephalitis virus in India. Kyasanur Forest disease. Prog Med Virol. 1958;1:248–79. PMID:13579010. [PubMed] [Google Scholar]
- 4.Charrel RN, Zaki AM, Attoui H, Fakeeh M, Billoir F, Yousef AI, de Chesse R, De Micco P, Gould EA, de Lamballerie X. Complete coding sequence of the Alkhurma virus, a tick-borne flavivirus causing severe hemorrhagic fever in humans in Saudi Arabia. Biochem Biophys Res Commun. 2001;287:455–61. doi: 10.1006/bbrc.2001.5610. [DOI] [PubMed] [Google Scholar]
- 5.Kasabi GS, Murhekar MV, Sandhya VK, Raghunandan R, Kiran SK, Channabasappa GH, Mehendale SM. Coverage and effectiveness of Kyasanur forest disease (KFD) vaccine in Karnataka, South India, 2005–10. PLoS Negl Trop Dis. 2013;7(1):e2025. doi: 10.1371/journal.pntd.0002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiran SK, Pasi A, Kumar S, Kasabi GS, Gujjarappa P, Shrivastava A, Mehendale S, Chauhan LS, Laserson KF, Murhekar M. Kyasanur Forest disease outbreak, and vaccination strategy, Shimoga District, India, 2013–2014. Emerg Infect Dis. 2015;21(1):146–49. doi: 10.3201/eid2101.141227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munivenkatappa A, Sahay RR, Yadav PD, Viswanathan R, Mourya DT. Clinical & epidemiological significance of Kyasanur forest disease. Indian J Med Res. 2018;148(2):145–50. doi: 10.4103/ijmr.IJMR_688_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah SZ, Jabbar B, Ahmed N, Rehman A, Nasir H, Nadeem S, Jabbar I Rahman ZU, Azam S. Epidemiology, pathogenesis, and control of a tick-borne disease- Kyasanur Forest disease: current status and future directions. Front Cell Infect Microbiol. 20188(149):1–19. doi: 10.3389/fcimb.2018.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holbrook MR. Kyasanur forest disease. Antiviral Res. 2012;96:353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slate D, Algeo TP, Nelson KM, Chipman RB, Donovan D, Blanton JD, Niezgoda M, Rupprecht CE. Oral rabies vaccination in North America: opportunities, complexities, and challenges. PLoS Negl Trop Dis. 2009. 22;3(12):e549. doi: 10.1371/journal.pntd.0000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naturally Speaking reports entitled. “Outfoxing-rabies one vaccine loaded chicken head at a time” by Laurie Baker’s experiences in using lessons from fox rabies elimination in Western Europe to outfox rabies. [accessed 2018 October20] https://naturallyspeaking.blog/2016/09/28/outfoxing-rabies-one-vaccine-loaded-chicken-head-at-a-time/September 2016
- 12.Retrieved information from Wikipedia the free encyclopedia on Western Ghats. [accessed 2018 October20] https://en.wikipedia.org/wiki/Western_Ghats.
- 13.Zaki AM. Isolation of a flavivirus related to the tick-borne encephalitis complex from human cases in Saudi Arabia. Trans R Soc Trop Med Hyg. 1997;91(2):179–81. PMID: 9196762 [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Zhang H, Fu S, Wang H, Ni D, Nasci R, Tang Q, Liang G. Isolation of kyasanur forest disease virus from febrile patient, yunnan, china. Emerg Infect Dis. 2009;15(2):326–28. PMID:19193286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pattnaik P. Kyasanur forest disease: an epidemiological view in India. Rev Med Virol. 2006;16(3):151–65. doi: 10.1002/rmv.495. [DOI] [PubMed] [Google Scholar]
- 16.Scherwitzi I, Mongkolsapaja J, Screaton G. Recent advances in human flavivirus vaccines. Curr Opin Virol. 2017;23:95–101. doi: 10.1016/j.coviro.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Valle MR, Guerrero FD. Anti-tick vaccines in the omics era. Front Biosci (Elite Ed). 2018;1(10):122–36. PMID: 28930608 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Naturally Speaking reports entitled. “Outfoxing-rabies one vaccine loaded chicken head at a time” by Laurie Baker’s experiences in using lessons from fox rabies elimination in Western Europe to outfox rabies. [accessed 2018 October20] https://naturallyspeaking.blog/2016/09/28/outfoxing-rabies-one-vaccine-loaded-chicken-head-at-a-time/September 2016


