ABSTRACT
Jixuepaidu Tang-1 is obtained from the decoction of the Chinese traditional medicinal plants including Centella asiatica, Astragalus membranaceus, and Sanguis draconis. Transforming growth factor-β1 (TGF-β1)/serum- and glucocorticoid-inducible kinase-1 (SGK1)-induced epithelial-mesenchymal transition (EMT) plays a pivotal role in the pathogenesis of diabetic nephropathy (DN). In addition, long non-coding RNAs (lnRNAs) participate in the development of DN, but the role of lncRNA LOC498759 in DN is still unclear. This study aims to investigate the role of Jixuepaidu Tang-1 in regulating podocyte injury and renal damage in DN and to validate whether the mechanisms involve TGF-β1/SGK1 signaling and LOC498759. The drug treatment was initiated 2 weeks after the DN modeling. The MTT method and TUNEL staining were used to measure cell viability and apoptosis, respectively. Immunofluorescence staining was used to detect the expression of nephrin and desmin in podocytes. Sera from the Jixuepaidu Tang-1-treated mice reversed the high glucose (HG)-induced podocyte injury and EMT in mouse podocytes. Further in vivo assay revealed that Jixuepaidu Tang-1 not only reduced the ratio of the kidney to body weight, 24 h-urine total protein, and blood glucose, but alleviated glomerular mesangial extracellular matrix deposition and glomerular cell apoptosis in the streptozotocin-induced DN mice. Mechanically, the mechanisms of Jixuepaidu Tang-1 may involve the suppression of EMT by inhibiting the TGF-β1/SGK1-induced LOC498759 expression. Collectively, Jixuepaidu Tang-1 attenuates podocyte injury and renal damage in DN, and inhibits EMT through suppressing TGF-β1/SGK1-LOC498759 signaling.
KEYWORDS: Diabetic nephropathy, Jixuepaidu Tang-1, epithelial-mesenchymal transition, LOC498759, transforming growth factor, β1/serum- and glucocorticoid- inducible kinase-1
Introduction
Diabetic nephropathy (DN) is one of the most common complications of diabetes and is the leading cause of end-stage renal disease (ESRD). Podocytes are a key component of the kidney filtration barrier, and podocyte injury leads to the disruption of the filtration barrier and to protein leakage in DN [1]. In addition, podocyte injury has been indicated as a major contributor to the development and progression of DN [2]. Increasing evidence also suggested that the epithelial-mesenchymal transition (EMT) is a possible cause of podocyte injury in DN [3,4]. EMT is a process in which epithelial cells are transformed into mesenchymal cells in response to injurious stimuli [3]. EMT plays a significant role in the damage of the glomerular filtration barrier, proteinuria, and glomerular sclerosis, and thus has been considered as a new target for preventing DN [3,5,6].
Jixuepaidu Tang-1 is obtained from the decoction of the Chinese traditional medicinal plants including Centella asiatica (Jixuecao), Astragalus membranaceus (Huangqi), and Sanguis draconis (Xuejie). Several studies have shown that Centella asiatica and Astragalus membranaceus exert renal-protective effects on DN [7–10]. Centella asiatica was found to reduce podocyte damage induced by doxorubicin [11] and exhibit anti-oxidant and anti-inflammatory activities [12]. In addition, asiatic acid, an essential ingredient of Centella asiatica, can not only reduce renal function and attenuate abnormal pathological changes of podocytes in kidney tissue of diabetic rats, but increased expression of nephrin (an epithelial marker) and decreased that of desmin (a mesenchymal marker) [7]. Astragalus membranaceus has been widely used as an immune stimulant and antioxidant and has been recently shown to protect against podocyte injury in streptozotocin-induced diabetic rats [13]. Furthermore, administration with Sanguis draconis ethanol extract can prevent STZ-induced diabetogenic effects [14]. These above-mentioned findings implied that Jixuepaidu Tang-1 which is composed of these three herbs may also alleviate renal damage in DN.
Long non-coding RNA (lnRNAs) play an important role in the development of various diseases, including DN [15,16]. Studies have highlighted the potential role of lncRNAs in the fibrogenesis and EMT in DN [5,6]. LncRNA LOC498759 is highly expressed in podocytes isolated from STZ-induced DN rats and in high glucose (HG)-stimulated podocytes, suggesting that LOC498759 may be involved in the regulation of podocyte function in DN [1]. However, the role of LOC498759 in the pathogenesis of DN and podocyte EMT is unclear.
Transforming growth factor-β1 (TGF-β1)/serum- and glucocorticoid-inducible kinase-1 (SGK1) signaling pathway is the main signaling pathway regulating EMT and plays a pivotal role in the pathogenesis of DN [17–20]. However, the regulation of TGF-β1/SGK1 signaling by Jixuepaidu Tang-1 remains unclear. In this current study, we explored the role of Jixuepaidu Tang-1 in regulating the HG-induced podocyte injury, streptozotocin (STZ)-induced mouse renal damage, and EMT progression. Furthermore, we investigated whether the mechanisms underlying the Jixuepaidu Tang-1-mediated effect involved the regulation of TGF-β1/SGK1 signaling and LOC498759. Moreover, we clarified the regulation of LOC498759 by TGF-β1/SGK1 signaling.
Materials and methods
Experimental animals
Specific pathogen-free (SPF) C57BL/6J male mice (8 weeks old) were purchased from the Shanghai laboratory animal center of Chinese academy of science. All experiments were performed in compliance with the guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health.
Model establishment and grouping
After 11 days of adaptive feeding, the C57BL/6J mice were randomly divided into 5 groups: normal control group (Control), DN model group (DN), high dose of Jixuepaidu Tang-1 group (HD), high dose of Jixuepaidu Tang-1 + empty lentivirus group (HD + LV-vector) group, and high dose of Jixuepaidu Tang-1 + LOC498759 lentivirus group (HD + LV-LOC498759).
With the exception of the normal control group, the mice in the other groups were fasted for 16 h and then given a single intraperitoneal injection of streptozotocin (STZ; 150 mg/kg; Sigma) which was dissolved in a sterile 10 mmol/L sodium citrate buffer (pH4.5) to induce DN. After the STZ injection, all the mice were fed with a normal diet. The random blood glucose was measured using a reflectance meter (Accu-Chek, Roche Diagnostics GmbH, Mannheim, Germany) 72 hours after STZ injection. The DN model was considered successful if the random blood glucose exceeded 16.7 mmol/L for 3 consecutive times.
The drug treatment was initiated 2 weeks after the DN modeling. The mice in the HD, HD + LV-vector, and HD + LV-LOC498759 groups were administered 12.6 g/(kg.d) of Jixuepaidu Tang-1 via gastric perfusion once a day for 4 weeks. The mice in the normal control and DN groups were treated with the same volume of 0.9% physiological saline for 4 weeks. The mice in the HD + LV-vector and HD + LV+LOC498759 groups were also injected respectively with lentivirus containing LOC498759 and control vectors via the tail vein.
Sample collection and metabolic indices detection
Before the mice were sacrificed, they were collected in the metabolic cages for 1 day, and the 24-hour urine samples were collected. The 24-hour urine total protein (24UTP) was detected using an automated biochemical analyzer (DADE Xpand, USA). The random blood glucose (BG) was measured using a reflectance meter (Accu-Chek, Roche Diagnostics GmbH, Mannheim, Germany). The mouse serum creatinine (SCr) was measured using a Mouse Creatinine ELISA Kit (YAJI Biological, Shanghai, China). Within 5 min after the mice were sacrificed, the body weight (BW) and kidney weight (KW) of the mice were weighed, and the ratio of KW (mg) to BW (g) was calculated. A portion of kidneys were cut into sections and fixed in 4% buffered paraformaldehyde for Periodic acid‐Schiff (PAS) staining and TUNEL staining. The remaining kidneys were stored at −80°C before being used for western blot and qRT-PCR.
Histological examination
The kidney tissues were fixed in 4% buffered paraformaldehyde, embedded in paraffin, and then cut into 4-µm tissue sections. The glomerular mesangial extracellular matrix (ECM) deposition was assessed using PAS staining. At least 50 intact glomeruli were observed in each section and photographed. All sections were evaluated using a light microscope (Olympus BH-2; Olympus Corporation, Japan). The percentage of glomerular mesangial ECM/glomerular area was calculated. The apoptosis of glomerular cells in renal tissues was examined using TUNEL Apoptosis Assay Kit (Beyotime, Shanghai, China) following the manufacturer’s instructions. The number of TUNEL positive cells (brown) was counted.
Preparation of mouse sera containing Jixuepaidu Tang-1
Following DN modeling, C57BL/6J mice (8 weeks old) were randomly divided into four groups (n = 8 in each group) according to dosage of Jixuepaidu Tang-1: the normal control (NC) group, low dose (LD) group, mid dose (MD) group, and high dose (HD) group. The mice in the LD, MD, and HD groups were administered 3.15 g/(kg.d), 6.3 g/(kg.d), and 12.6 g/(kg.d) of Jixuepaidu Tang-1 respectively via gastric perfusion once a day for 3 days, whereas the NC group was treated with the same volume of 0.9% physiological saline. One hour after the final administration, blood was taken from the abdominal aortas of the mice, and the sera were separated by centrifugation of the blood at 720 × g for 15 min. Following 2 rounds of filtration using a 0.22 µm cellulose acetate membrane, the sera were sealed in vials, heated in a water bath at 56°C for 30 min, and then preserved at −80°C for future use.
Cell culture
Differentiated, immortalized murine podocytes at passage 12 were kindly provided by Dr. Peter Mundel at Division of Nephrology, Harvard Medical School (Boston, MA). Podocytes were cultured in RMPI 1640 medium containing5% fetal bovine serum (FBS; Gibco) and 20 U/mL interferon-γ (IFN-γ) under a permissive condition at 33°C in a humidified air with 5% CO2. The cells were then transferred to 5% FBS containing RMPI 1640 medium without IFN-γ, and cultured at 37°C (nonpermissive condition) for differentiation for 10–14 days. When the cells grew to 70–80% confluence, the cells were synchronized by starvation for 24 h in serum-free medium. Then cells were randomly grouped according to different experimental purposes.
Plasmid construction and transfection
To overexpress LOC498759, LOC498759 cDNA of full length was subcloned into the pcDNA3.1 expression vector (Invitrogen), generating pcDNA3.1- LOC498759. An empty pcDNA3.1 vector was used as negative control. To knock down LOC498759 and SGK1, LOC498759 siRNA (si-LOC498759) and SGK1 siRNA (si-SGK1) were designed and synthesized by GenePharma (Shanghai, China). A scramble siRNA was used as negative control (si-Ctrl). Cells were transfected with these plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocols. Following transfection for 48 h, podocytes were harvested for qRT-PCR analysis to examine the knockdown or overexpression efficiency.
Cell viability assay
The cell viability was determined using the MTT method. Cells were seeded in 96-well plates at a density of 5 × 103 cells/well. The cell viability was assessed using an MTT assay kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. The absorbance at 490 nm was read on a microplate reader.
Cell apoptosis assay
The cell apoptosis was determined using the TUNEL Apoptosis Assay Kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions. Apoptosis index (AI) was calculated as the percentage of positive nuclei (brown) in sections (×400) stained by TUNEL.
Determination of nephrin and desmin expression by immunofluorescence staining
The immunofluorescence staining was used to detect the expression of nephrin and desmin in podocytes. Briefly, podocytes were cultured in six-well plates containing glass slides, washed in PBS, and fixed in 4% paraformaldehyde for 20 min. After permeabilization in 0.2% Triton X-100 for 5 min, cells were washed in PBS, and then blocked with 3% bovine serum albumin (BSA) at room temperature to eliminate the nonspecific fluorescence. Immunofluorescence staining was performed by incubating the cells with primary antibodies against nephrin (1:200; Abcam) and desmin (1:200; Abcam) overnight at 4°C. After the cell preparations were washed three times in PBS, they were incubated with DyLight 488 (Green)/594 (Red)-labeled secondary antibodies (Sigma-Aldrich) for 1 h at room temperature. After that, the cells were washed in PBS and then incubated with DAPI (Sigma-Aldrich) for 1 min at room temperature. Finally, the sections were sealed and images were photographed under an inverted fluorescence microscope.
Determination of LOC498759 expression by quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from podocytes or mouse kidney tissues using TRIzol reagent (Invitrogen) and was reverse transcribed into cDNAs using an ABI High Capacity cDNA RT Kit (Life Technologies, USA). After that, the cDNA was synthesized through qRT-PCR using SYBR Premix Dimmer Eraser kit (TaKaRa) by the ABI7900 system (Applied Biosystem). The relative LOC498759 expression was calculated by the 2−ΔΔCt method and normalized to the internal control GAPDH.
Determination of protein levels by western blot
Total protein was extracted from podocytes or mouse kidney tissues in RIPA lysis buffer. The protein concentrations were determined by BCA assay, after which equal protein from cell lysates was separated by 10% SDS-PAGE gels and electro-transferred onto PVDF membranes (Millipore Corp., Billerica, MA, USA). After blocked with 5% fat-free milk, the membranes were probed with primary antibodies against nephrin (1:1000; Abcam), P-cadherin (1:1000; Santa Cruz Biotechnology), desmin (1:1000; Abcam), vimentin (1:1000; Cell Signaling Technology), TGF-β1 (1:1000; Santa Cruz Biotechnology), and SGK1 (1:1000; Cell Signaling Technology), overnight at 4°C, followed by further 1 h of incubation with secondary antibody horseradish peroxidase-conjugated goat anti-rabbit IgG. The protein levels were quantified using enhanced chemiluminescence (Thermo Scientific). β-actin was used as the loading control.
Statistical analysis
All statistical analyzes were performed using SPSS 16.0. The data are presented as the mean ± standard deviation (SD) from three independent experiments. The unpaired Student’s t-test was used to analyze differences between two groups. One-way analysis of variance (ANOVA) was used to analyze differences among multiple groups. p < 0.05 was considered to indicate a statistically significant difference.
Results
Jixuepaidu Tang-1 sera reversed the HG-induced podocyte injury and EMT
As expected, HG stimulation significantly inhibited podocyte viability (Figure 1(a)) and promoted podocyte apoptosis (Figure 1(b)). Importantly, treatment with sera from the Jixuepaidu Tang-1-treated mice (Jixuepaidu Tang-1 sera) notably reversed the HG-induced podocyte injury in a dose-dependent manner, as revealed by a significant increase in podocyte viability (Figure 1(a)) and decrease in podocyte apoptosis (Figure 1(b)).
Figure 1.
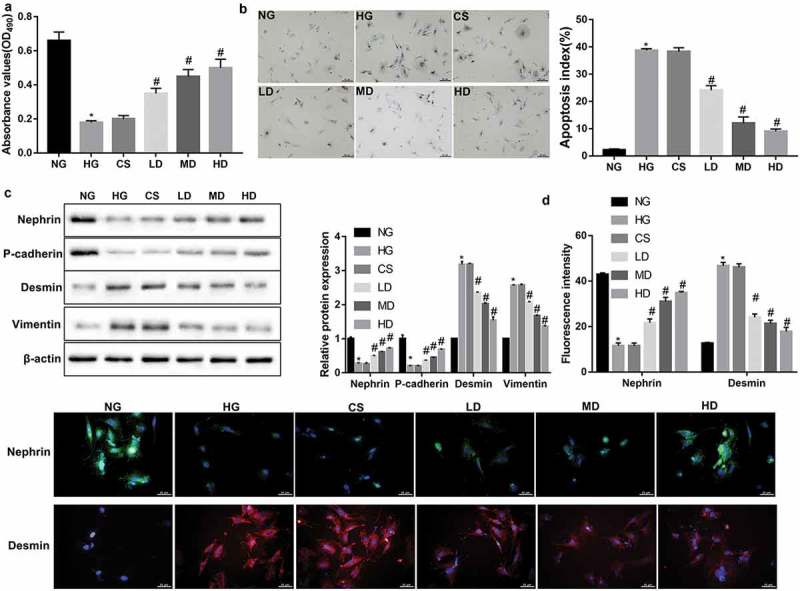
Effect of sera from the Jixuepaidu Tang-1-treated mice on the viability, apoptosis, and EMT process in HG-treated podocytes.
The podocytes were cultured with media containing normal glucose (NG; 5.6 mmol/L D-glucose), high glucose (HG; 30 mmol/L D-glucose), sera from the control physiological saline-treated mice (CS), and sera from the low/mid/high dose of Jixuepaidu Tang-1-treated mice (LD/MD/HD). With the exception of the NG group, the podocytes in the other groups were stimulated with HG. Following 24 h of culture, (a) the cell viability was determined using the MTT method. (b) The cell apoptosis was determined using the TUNEL staining. The apoptosis index (AI) was evaluated as the percentage of TUNEL-positive nuclei (brown) in sections. (c) Representative western blot analysis of nephrin, P-cadherin, desmin, and vimentin expression in podocytes. The graph represents the densitometric analysis normalized to β-actin. (d) Representative immunofluorescence images of the slit diaphragm protein nephrin (green) and podocyte injury marker desmin (red). Cell nucleus was labeled in blue by DAPI. Scale bar: 25 μm. Data are presented as mean ± SD; n = 3; *p < 0.05 vs. NG; #p < 0.05 vs. CS.
EMT has been identified as a possible cause of podocyte injury, an important early pathologic marker of DN. Therefore, we next explored the effect of Jixuepaidu Tang-1 sera on the EMT process in podocytes. Western blot analyses showed that Jixuepaidu Tang-1 sera dose-dependently reversed the HG-mediated decrease in protein levels of nephrin and P-cadherin (epithelial markers) and increase in protein levels of desmin and vimentin (mesenchymal markers) (Figure 1(c)). Immunofluorescence analyses further confirmed the Jixuepaidu Tang-1 sera-mediated increase in nephrin and decrease in desmin under HG stimulation (Figure 1(d)). These results suggested that Jixuepaidu Tang-1 sera reversed the HG-induced EMT.
Jixuepaidu Tang-1 sera inhibited the HG-induced podocyte injury and EMT through blocking the TGF-β1/SGK1 signaling
TGF-β1-induced EMT and fibrosis play a pivotal role in the development and progression of DN [21]. SGK1 has been indicated to mediate TGF-β1-induced EMT and fibrosis [18,22,23]. Interestingly, western blot analyses showed that Jixuepaidu Tang-1 sera diminished the HG-induced elevation in protein levels of TGF-β1 and SGK1 (Figure 2(a)). This indicated that the inhibition of podocyte injury and EMT by Jixuepaidu Tang-1 sera may be mediated through suppression of TGF-β1 and SGK1. To this end, the podocytes were either transfected with si-SGK1 or treated with recombinant TGF-β1 or recombinant SGK1, before treatment with HG and high dose of Jixuepaidu Tang-1 sera. The results showed that recombinant TGF-β1 or SGK1 impaired the Jixuepaidu Tang-1 sera-mediated promotion of cell viability (Figure 2(b)), inhibition of cell apoptosis (Figure 2(c)), increase in levels of nephrin and P-cadherin, and decrease in levels of desmin and vimentin (Figure 2(d,e)). In contrast, SGK1 silencing exerted the opposite effect (Figure 2(b–e)). Together, these results indicated that Jixuepaidu Tang-1 sera may inhibit podocyte injury and EMT through suppressing TGF-β1 and SGK1. Furthermore, treatment with recombinant TGF-β1 significantly upregulated protein levels of SGK1. However, either recombinant SGK1 or SGK1 silencing exerted no significant effect on protein levels of TGF-β1 (Figure 2(d)). These findings suggested that TGF-β1 acted as upstream of SGK1. Collectively, these results indicated that Jixuepaidu Tang-1 sera may inhibit the HG-induced podocyte injury and EMT through blocking the TGF-β1/SGK1 signaling.
Figure 2.
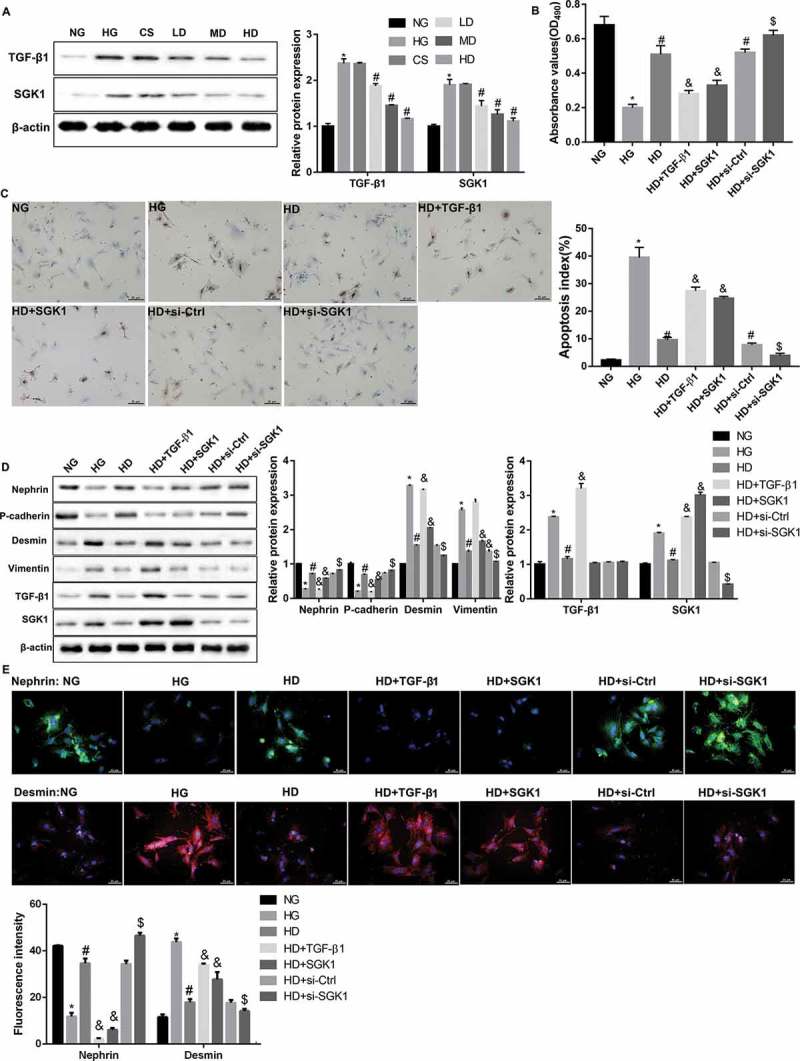
Jixuepaidu Tang-1 sera inhibited the HG-induced podocyte injury and EMT through blocking the TGF-β1/SGK1 signaling.
(a) Representative western blot analysis of TGF-β1 and SGK1 expression in podocytes in the groups treated the same as in Figure 1. The graph represents the densitometric analysis normalized to β-actin. *p < 0.05 vs. NG; #p < 0.05 vs. CS. (b–e) The podocytes were cultured with media containing normal glucose (NG; 5.6 mmol/L D-glucose), high glucose (HG; 30 mmol/L D-glucose), sera from the high dose of Jixuepaidu Tang-1-treated mice (HD), TGF-β1 recombinant protein (TGF-β1), or SGK1 recombinant protein (SGK1) (Thermo Scientific). The podocytes in the si-Ctrl and si-SGK1 groups were transfected with si-Ctrl and si-SGK1 respectively before treatment with HG and HD. With the exception of the NG group, the podocytes in the other groups were stimulated with HG. Following 24 h of culture, (b) the cell viability was determined using the MTT method. (c) The cell apoptosis was determined using the TUNEL staining. The apoptosis index (AI) was evaluated as the percentage of TUNEL-positive nuclei (brown) in sections. (d) Representative western blot analysis of nephrin, P-cadherin, desmin, vimentin, TGF-β1, and SGK1 expression in podocytes. Graph represents the densitometric analysis normalized to β-actin. (e) Representative immunofluorescence images of nephrin (green) and desmin (red). Cell nucleus was labeled in blue by DAPI. Scale bar: 25 μm. Data are presented as mean ± SD; n = 3; (B-E) *p < 0.05 vs. NG; #p < 0.05 vs. HG; &p < 0.05 vs. HD; $p < 0.05 vs. HD+si-Ctrl.
Jixuepaidu Tang-1 sera may inhibit the HG-induced podocyte injury and EMT through blocking the TGF-β1/SGK1-LOC498759 signaling
Treatment with Jixuepaidu Tang-1 sera significantly diminished the HG-induced increase in LOC498759 expression (Figure 3(a)). Furthermore, treatment with recombinant TGF-β1 and SGK1 significantly increased LOC498759 expression in the presence or absence of HG and Jixuepaidu Tang-1 sera (Figure 3(a,b)). This indicated that LOC498759 acted as downstream of TGF-β1/SGK1 signaling under Jixuepaidu Tang-1 condition.
Figure 3.
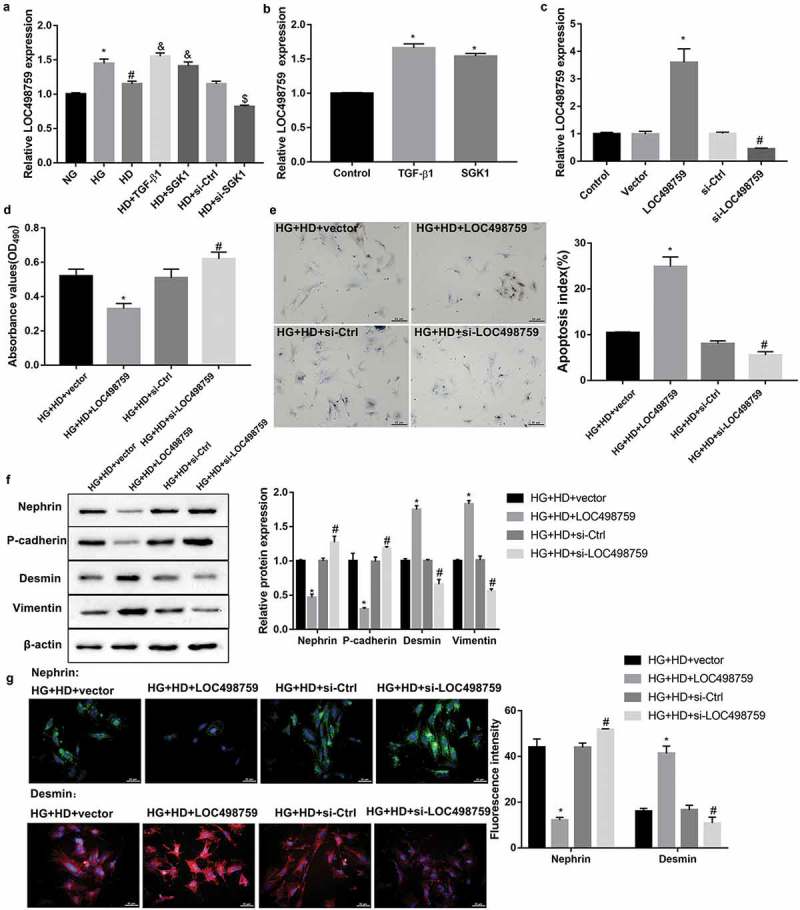
LOC498759 was involved in the Jixuepaidu Tang-1 sera-mediated inhibition of the HG-induced podocyte injury and EMT.
qRT-PCR was performed to detect relative LOC498759 expression in podocytes in the groups treated the same as in Figure 2(b–e). *p < 0.05 vs. NG; #p < 0.05 vs. HG; &p < 0.05 vs. HD; $p < 0.05 vs. HD+si-Ctrl. (b) Treatment with TGF-β1 and SGK1 recombinant proteins significantly increased LOC498759 expression. *p < 0.05 vs. Control. (c–g) The podocytes were transfected with LOC498759 overexpression vector and control vector, or si-LOC498759 and scramble si-Ctrl, followed by treatment with high glucose (HG; 30 mmol/L D-glucose) and sera from the high dose of Jixuepaidu Tang-1-treated mice (HD). (c) qRT-PCR was performed to examine the knockdown or overexpression efficiency. Following 24 h of culture, (d) the cell viability was determined using the MTT method. (e) The cell apoptosis was determined using the TUNEL staining. The apoptosis index (AI) was evaluated as the percentage of TUNEL-positive nuclei (brown) in sections. (f) Representative western blot analysis of nephrin, P-cadherin, desmin, and vimentin, expression in podocytes. The graph represents the densitometric analysis normalized to β-actin. (G) Representative immunofluorescence images of nephrin (green) and desmin (red). Cell nucleus was labeled in blue by DAPI. Scale bar: 25 μm. Data are presented as mean ± SD; n = 3; (C-G) *p < 0.05 vs. HG+HD+vector; #p < 0.05 vs. HG+HD+si-Ctrl.
To further elucidate whether LOC498759 was involved in the Jixuepaidu Tang-1 sera-mediated inhibition of podocyte injury and EMT, we overexpressed or silenced LOC498759 in podocytes before treatment with HG and Jixuepaidu Tang-1 sera. The knockdown or overexpression efficiency was confirmed by qRT-PCR (Figure 3(c)). Subsequently, cell viability, cell apoptosis, and EMT in podocytes were evaluated. Of note, data revealed that LOC498759 overexpression notably suppressed cell viability (Figure 3(d)), facilitated cell apoptosis (Figure 3(e)), decreased levels of nephrin and P-cadherin, and increased levels of desmin and vimentin (Figure 3(f,g)) under treatment with HG and Jixuepaidu Tang-1 sera. In contrast, LOC498759 silencing exerted the opposite effect (Figure 3(d–g)). These results suggested that LOC498759 overexpression diminished; whereas LOC498759 knockdown enhanced Jixuepaidu Tang-1 sera-mediated inhibition of the HG-induced podocyte injury and EMT.
According to the above-mentioned results, it was proposed that Jixuepaidu Tang-1 sera may inhibit the HG-induced podocyte injury and EMT through blocking the TGF-β1/SGK1-LOC498759 signaling.
LOC498759 was involved in the Jixuepaidu Tang-1-mediated renal protection in DN mice
As expected, compared with the control group, the mice in the DN group showed an increased ratio of the kidney to body weight, 24 h-urine total protein, and blood glucose (Table 1). Furthermore, the kidney tissues from the DN model mice exhibited increased glomerular mesangial extracellular matrix deposition (Figure 4(a)) and apoptosis of glomerular cells (Figure 4(b)), accompanied by decreased protein levels of nephrin and P-cadherin, plus increased levels of desmin, vimentin, TGF-β1, and SGK1 (Figure 4(c)). Importantly, these indices were notably improved in the high dose of Jixuepaidu Tang-1 (HD) group when compared with the DN group (Table 1, Figure 4(a–c)). These results indicated that Jixuepaidu Tang-1 not only reduced the ratio of the kidney to body weight, 24 h-urine total protein, and blood glucose, but alleviated glomerular mesangial extracellular matrix deposition and apoptosis of glomerular cells in DN mice. The mechanisms may involve the inhibition of renal EMT and TGF-β1/SGK1 signaling.
Table 1.
Metabolic data of mice at the end of the experiment (mean ± SD).
| BW(g) | KW(mg) | KW/BW (mg/g) | BG(mmol/L) | SCr(mg/dL) | 24UTP(mg) | |
|---|---|---|---|---|---|---|
| Control(n = 8) | 23.98 ± 2.05 | 320.1 ± 55.1 | 13.35 ± 2.3 | 6.12 ± 1.06 | 0.52 ± 0.04 | 0.60 ± 0.20 |
| DN(n = 8) | 15.55 ± 0.56* | 345.2 ± 45.5 | 22.20 ± 2.93* | 25.61 ± 3.12* | 0.56 ± 0.04 | 4.51 ± 0.45* |
| HD(n = 8) | 19.52 ± 1.65# | 322.7 ± 43.6 | 16.53 ± 2.23# | 20.45 ± 3.56# | 0.57 ± 0.03 | 1.56 ± 0.52# |
| LV-vector(n = 8) | 19.98 ± 1.55 | 325.8 ± 41.1 | 16.31 ± 2.06 | 21.12 ± 3.41 | 0.53 ± 0.04 | 1.54 ± 0.41 |
| LV-LOC498759 (n = 8) | 17.12 ± 1.14& | 335.7 ± 43.9 | 19.61 ± 2.56& | 22.23 ± 3.15& | 0.55 ± 0.03 | 2.54 ± 0.52& |
BW, body weight; KW, kidney weight; KW/BW, kidney weight-to-body weight ratio; BG, blood glucose; SCr, serum creatinine; 24UTP, 24 h-urine total protein; *p < 0.05 vs. control; #p < 0.05 vs. DN; &p < 0.05 vs. HD+LV-vector.
Figure 4.
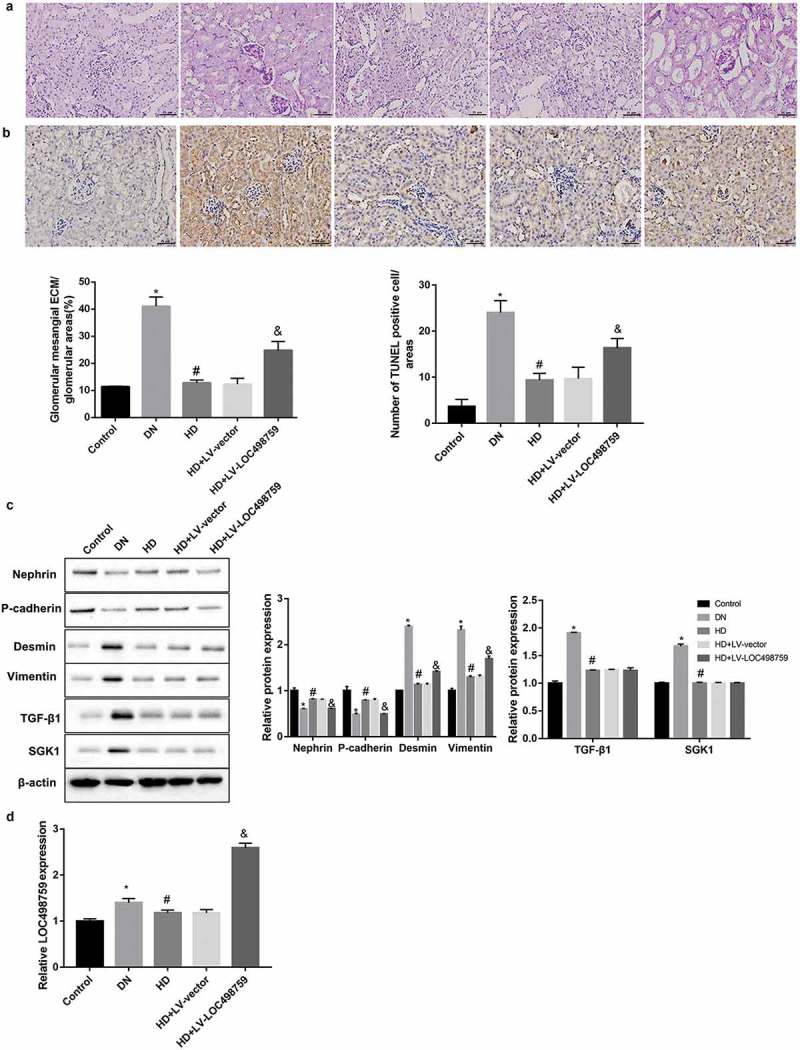
LOC498759 was involved in the Jixuepaidu Tang-1-mediated renal protection in DN mice.
C57BL/6J mice were randomly divided into 5 groups: normal control group (Control), DN model group (DN), high dose of Jixuepaidu Tang-1 group (HD), high dose of Jixuepaidu Tang-1 + empty lentivirus group (HD + LV-vector) group, and high dose of Jixuepaidu Tang-1 + LOC498759 lentivirus group (HD + LV-LOC498759). At the end of the experiment, the mice were sacrificed and the kidneys were separated for the following analysis. (a) The glomerular mesangial extracellular matrix (ECM) deposition in mouse kidney tissues was assessed using PAS staining. The percentage of glomerular mesangial ECM/glomerular area was calculated. Scale bar: 25 μm. (b) The apoptosis of glomerular cells in mouse kidney tissues was examined using TUNEL staining. The number of TUNEL positive cells (brown) was counted. Scale bar: 25 μm. (c) Representative western blot analysis of nephrin, P-cadherin, desmin, vimentin, TGF-β1, and SGK1 expression in mouse kidney tissues. The graph represents the densitometric analysis normalized to β-actin. (d) qRT-PCR was performed to detect relative LOC498759 expression in mouse kidney tissues. *p < 0.05 vs. control; #P < 0.05 vs. DN; &p < 0.05 vs. LV-vector.
Moreover, as shown in Figure 4(d), LOC498759 expression in the kidney tissues in the DN group was significantly higher than that in the control group. Jixuepaidu Tang-1 significantly downregulated LOC498759 expression compared with the DN group. To further verify the role of LOC498759 in the Jixuepaidu Tang-1-mediated renal protection, we injected the Jixuepaidu Tang-1-treated DN mice with LV-LOC498759 lentivirus. Renal LOC498759 expression in the LV-LOC498759 group was notably higher than that in the HD+LV-vector group (Figure 4(d)), confirming that LOC498759 was successfully overexpressed. Furthermore, data showed that LOC498759 overexpression diminished the Jixuepaidu Tang-1-mediated reduction in the ratio of the kidney to body weight, 24 h-urine total protein, and blood glucose (Table 1), and alleviation of glomerular mesangial extracellular matrix deposition (Figure 4(a)) and apoptosis of glomerular cells (Figure 4(b)). Furthermore, LOC498759 overexpression attenuated the Jixuepaidu Tang-1-mediated inhibition of EMT, but had no significant effect on TGF-β1/SGK1 signaling (Figure 4(c)).
Discussion
Jixuepaidu Tang-1 is obtained from the decoction of the three medicinal plants including Centella asiatica, Astragalus membranaceus, and Sanguis draconis. Centella asiatica has been used for the treatment of diabetes and diabetic complications [8]. Asiatic acid, an essential ingredient of Centella asiatica, can reduce renal function and attenuate abnormal pathological findings of podocytes in kidney tissue of diabetic rats [7]. Besides, Astragalus membranaceus also shows therapeutic effects on different stages of DN [9]. Furthermore, administration with Sanguis draconis ethanol extract can prevent STZ-induced diabetogenic effects [14]. Thus, Jixuepaidu Tang-1 may have potential therapeutic effects on DN. In line with our speculation, our results in the present study showed that sera from the Jixuepaidu Tang-1-treated mice reversed the HG-induced podocyte injury (decrease in cell viability and increase in cell apoptosis) in mouse podocytes in vitro. Further in vivo assay revealed that Jixuepaidu Tang-1 not only reduced the ratio of the kidney to body weight, 24 h-urine total protein, and blood glucose, but alleviated glomerular mesangial extracellular matrix deposition and apoptosis of glomerular cells in the streptozotocin-induced DN mice. Collectively, these findings indicated the important potential of Jixuepaidu Tang-1 in treating DN.
EMT plays an important role in the damage of glomerular filtration barrier, proteinuria, tubulointerstitial fibrosis and glomerular sclerosis, and thus has been considered as a new target for preventing DN [3,5,6,24]. EMT in podocytes is characterized by increased expression of epithelial markers (e.g. nephrin and P-cadherin) and decreased expression of mesenchymal markers (e.g. desmin and vimentin) [3]. The results in this study showed that Jixuepaidu Tang-1 sera dose-dependently reversed the EMT under HG stimulation (upregulation of nephrin and P-cadherin, and downregulation of desmin and vimentin). This indicates that the inhibition of EMT may be one of the mechanisms by which Jixuepaidu Tang-1 alleviates podocyte injury and kidney damage.
In view of the important role of EMT activation in the development of DN, identifying the mechanisms of EMT activation could be meaningful. TGF-β1 is currently recognized as one of the most potent pro-fibrogenic factors and is a central mediator involved in the increase of extracellular matrix, fibroblast activation and phenotypic transformation [25]. Numerous studies have shown that sustained expression of TGF-β1 under high glucose stimulation ultimately leads to glomerular sclerosis and renal interstitial fibrosis. TGF-β1 could induce EMT in various diseases [26,27], including DN [21]. Accumulating studies have indicated that TGF-β1-induced EMT and renal fibrosis plays an important role in the development and progression of DN [21,28]. SGKl, a serine/threonine protein kinase, covers a wide range of physiological functions and participates in fibrosis stimulation [29,30]. Studies have demonstrated that in a variety of fibrosing diseases such as DN, the expression of SGK1 was significantly higher than that of normal tissues. It is speculated that SGK1 may be a new transcriptional target of TGF-β1 and mediates TGF-β1-induced EMT and fibrosis [18,22,23]. Our results in this study further confirmed that TGF-β1 acted as upstream of SGK1 and suggested that inhibition of TGF-β1/SGK1 signaling may be one of the mechanisms by which Jixuepaidu Tang-1 suppressed EMT.
LncRNAs are transcripts of more than 200 nucleotides in length without coding potential, which participates in regulating multiple cellular processes [5,6]. LncRNA LOC498759 is highly expressed in both podocytes isolated from STZ-induced DN rats and in HG-stimulated podocytes, suggesting that LOC498759 may be involved in the pathogenesis of DN [1]. Interestingly, our results showed that LOC498759 expression was decreased by Jixuepaidu Tang-1 both in vitro and in vivo. Furthermore, LOC498759 overexpression attenuated the Jixuepaidu Tang-1-mediated inhibition of podocyte injury, renal damage, and EMT. This indicates that inhibition of LOC498759 may be one of the mechanisms by which Jixuepaidu Tang-1 inhibits podocyte injury, renal damage, and EMT.
Our results also showed that treatment with recombinant TGF-β1 and SGK1 significantly increased LOC498759 expression in podocytes in vitro. Furthermore, LOC498759 overexpression had no significant effect on TGF-β1/SGK1 signaling in DN mice. These findings indicated that LOC498759 acted as downstream of TGF-β1/SGK1 signaling under Jixuepaidu Tang-1 condition.
In conclusion, our findings in this study supported the notion that Jixuepaidu Tang-1 sera inhibited podocyte injury, renal damage, and EMT through the block of the TGF-β1/SGK1-LOC498759 signaling. These findings indicated the important potential of Jixuepaidu Tang-1 in treating DN, providing promising options for DN treatment. Furthermore, LOC498759 might serve as a novel target for reducing podocyte injury and renal fibrosis in DN.
Funding Statement
This work was supported by the Scientific Research Foundation of Chinese Ministry of Education for the Returned Overseas Chinese Scholars [2015,311]; Nature and Science Foundation of Hubei Province in China [2014CFB408].
Acknowledgments
The present study was supported by the Nature and Science Foundation of Hubei Province in China (2014CFB408) and the Scientific Research Foundation of Chinese Ministry of Education for the Returned Overseas Chinese Scholars (2015,311).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Ling L, Tan Z, Zhang C, et al. Long noncoding RNA ENSRNOG00000037522 is involved in the podocyte epithelial‑mesenchymal transition in diabetic rats. Int J Mol Med. 2018;41:2704–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dai H, Liu Q, Liu B.. Research progress on mechanism of podocyte depletion in diabetic nephropathy. J Diabetes Res. 2017;2017:2615286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Loeffler I, Wolf G.. Epithelial-to-mesenchymal transition in diabetic nephropathy: fact or fiction? Cells. 2015;4:631–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yamaguchi Y, Iwano M, Suzuki D, et al. Epithelial-mesenchymal transition as a potential explanation for podocyte depletion in diabetic nephropathy. Am J Kidney Dis. 2009;54:653–664. [DOI] [PubMed] [Google Scholar]
- [5].Wang X, Xu Y, Zhu YC, et al. LncRNA NEAT1 promotes extracellular matrix accumulation and epithelial-to-mesenchymal transition by targeting miR-27b-3p and ZEB1 in diabetic nephropathy. J Cell Physiol. 2019;234:12926–12933. [DOI] [PubMed] [Google Scholar]
- [6].Gao J, Wang W, Wang F, et al. LncRNA-NR_033515 promotes proliferation, fibrogenesis and epithelial-to-mesenchymal transition by targeting miR-743b-5p in diabetic nephropathy. Biomed Pharmacothe. 2018;106:543–552. [DOI] [PubMed] [Google Scholar]
- [7].Chen YN, Wu CG, Shi BM, et al. The protective effect of asiatic acid on podocytes in the kidney of diabetic rats. Am J Transl Res. 2018;10:3733–3741. [PMC free article] [PubMed] [Google Scholar]
- [8].Alqahtani A, Hamid K, Kam A, et al. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr Med Chem. 2013;20:908–931. [PubMed] [Google Scholar]
- [9].Liao H, Hu L, Cheng X, et al. Are the therapeutic effects of huangqi (Astragalus membranaceus) on diabetic nephropathy correlated with its regulation of macrophage iNOS activity? J Immunol Res. 2017;2017:3780572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim J, Moon E, Kwon S. Effect of Astragalus membranaceus extract on diabetic nephropathy. Endocrinol Diabetes Metab Case Rep. 2014;2014:140063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang Z, Sun WS, Liu JT. Impacts of asiaticoside on cytoskeleton and p38 pathway in podocytes (in Chinese). World J Integr Tradit West Med. 2015;10:1456–1459. [Google Scholar]
- [12].Ramesh BN, Girish TK, Raghavendra RH, et al. Comparative study on anti-oxidant and anti-inflammatory activities of Caesalpinia crista and Centella asiatica leaf extracts. J Pharm Bioallied Sci. 2014;6:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhai R, Jian G, Chen T, et al. Astragalus membranaceus and Panax notoginseng, the novel renoprotective compound, synergistically protect against podocyte injury in streptozotocin-induced diabetic rats. J Diabetes Res. 2019;2019:1602892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hu CM, Li JS, Cheah KP, et al. Effect of Sanguis draconis (a dragon’s blood resin) on streptozotocin- and cytokine-induced beta-cell damage, in vitro and in vivo. Diabetes Res Clin Pract. 2011;94:417–425. [DOI] [PubMed] [Google Scholar]
- [15].Li Y, Ren D, Xu G. Long noncoding RNA MALAT1 mediates high glucose-induced glomerular endothelial cell injury by epigenetically inhibiting klotho via methyltransferase G9a. IUBMB Life. 2019;71:873–881. [DOI] [PubMed] [Google Scholar]
- [16].Shen H, Ming Y, Xu C, et al. Deregulation of long noncoding RNA (TUG1) contributes to excessive podocytes apoptosis by activating endoplasmic reticulum stress in the development of diabetic nephropathy. J Cell Physiol. 2019;234:15123–15133. [DOI] [PubMed] [Google Scholar]
- [17].Lu H, Chen B, Hong W, et al. Transforming growth factor-beta1 stimulates hedgehog signaling to promote epithelial-mesenchymal transition after kidney injury. Febs J. 2016;283:3771–3790. [DOI] [PubMed] [Google Scholar]
- [18].Lu X, Li M, Zhou L, et al. Urinary serum- and glucocorticoid-inducible kinase SGK1 reflects renal injury in patients with immunoglobulin A nephropathy. Nephrology (Carlton, Vic). 2014;19:307–317. [DOI] [PubMed] [Google Scholar]
- [19].Liu W, Wang X, Wang Y, et al. SGK1 inhibition-induced autophagy impairs prostate cancer metastasis by reversing EMT. J Exp Clin Cancer Res. 2018;37:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hills CE, Squires PE. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am J Nephrol. 2010;31:68–74. [DOI] [PubMed] [Google Scholar]
- [21].Sun Z, Ma Y, Chen F, et al. miR-133b and miR-199b knockdown attenuate TGF-beta1-induced epithelial to mesenchymal transition and renal fibrosis by targeting SIRT1 in diabetic nephropathy. Eur J Pharmacol. 2018;837:96–104. [DOI] [PubMed] [Google Scholar]
- [22].Feng Y, Wang Q, Wang Y, et al. SGK1-mediated fibronectin formation in diabetic nephropathy. Cell Physiol Biochem. 2005;16:237–244. [DOI] [PubMed] [Google Scholar]
- [23].Cheng J, Truong LD, Wu X, et al. Serum- and glucocorticoid-regulated kinase 1 is upregulated following unilateral ureteral obstruction causing epithelial-mesenchymal transition. Kidney Int. 2010;78:668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhao Y, Yin Z, Li H, et al. MiR-30c protects diabetic nephropathy by suppressing epithelial-to-mesenchymal transition in db/db mice. Aging Cell. 2017;16:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yue Y, Meng K, Pu Y, et al. Transforming growth factor beta (TGF-beta) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res Clin Pract. 2017;133:124–130. [DOI] [PubMed] [Google Scholar]
- [26].Loeffler I. MKP2 suppresses TGF-beta1-induced epithelial-to-mesenchymal transition through JNK inhibition. Clin Sci. 2019;133:545–550. [DOI] [PubMed] [Google Scholar]
- [27].Kim J-H, Ham S, Lee Y, et al. TTC3 contributes to TGF-β(1)-induced epithelial-mesenchymal transition and myofibroblast differentiation, potentially through SMURF2 ubiquitylation and degradation. Cell Death Dis. 2019;10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lu Q, Wang WW, Zhang MZ, et al. ROS induces epithelial-mesenchymal transition via the TGF-beta1/PI3K/Akt/mTOR pathway in diabetic nephropathy. Exp Ther Med. 2019;17:835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lang F, Bohmer C, Palmada M, et al. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. [DOI] [PubMed] [Google Scholar]
- [30].Steinberger M, Foller M, Vogelgesang S, et al. Lack of the serum- and glucocorticoid-inducible kinase SGK1 improves muscle force characteristics and attenuates fibrosis in dystrophic mdx mouse muscle. Pflugers Arch. 2015;467:1965–1974. [DOI] [PubMed] [Google Scholar]


