ABSTRACT
Human IgG antibodies containing terminal alpha 2,6-linked sialic acid on their Fc N-glycans have been shown to reduce antibody-dependent cell-mediated cytotoxicity and possess anti-inflammatory properties. Although terminal sialylation on complex N-glycans can happen via either an alpha 2,3-linkage or an alpha 2,6-linkage, sialic acids on human serum IgG Fc are almost exclusively alpha 2,6-linked. Recombinant IgGs expressed in Chinese hamster ovary (CHO) cells, however, have sialic acids through alpha 2,3-linkages because of the lack of the alpha 2,6-sialyltransferase gene. The impact of different sialylation linkages to the structure of IgG has not been determined. In this work, we investigated the impact of different types of sialylation to the conformational stability of IgG through hydrogen/deuterium exchange (HDX) and limited proteolysis experiments. When human-derived and CHO-expressed IgG1 were analyzed by HDX, sialic acid-containing glycans were found to destabilize the CH2 domain in CHO-expressed IgG, but not human-derived IgG. When structural isomers of sialylated glycans were chromatographically resolved and identified in the limited proteolysis experiment, we found that only alpha 2,3-linked sialic acid on the 6-arm (the major sialylated glycans in CHO-expressed IgG1) destabilizes the CH2 domain, presumably because of the steric effect that decreases the glycan-CH2 domain interaction. The alpha 2,6-linked sialic acid on the 3-arm (the major sialylated glycan in human-derived IgG), and the alpha 2,3-linked sialic acid on the 3-arm, do not have this destabilizing effect.
KEYWORDS: Antibody, IgG1, IgG2, N-glycan, sialic acid, conformation, hydrogen/deuterium exchange, limited proteolysis, mass spectrometry
Introduction
Monoclonal antibodies (mAbs), especially immunoglobulin gamma (IgG) antibodies are used as therapeutic agents for their high target specificity, long serum half-life, and the capability to be produced routinely with consistency. Human IgG antibodies have a conserved N-glycosylation site at their Fc CH2 domain (Asn-297 according to the Eu numbering system1). As many studies have demonstrated, Fc N-glycosylation plays important roles in pharmacokinetics,2,3 antibody stability, and effector functions.4–11
N-linked glycans have a basic common trimannosyl core structure, with the two mannose residues attached to the 3- and 6-carbon positions of the core mannose, forming the 3-arm and 6-arm antennas. N-linked glycans are classified into oligomannose (or high-mannose), hybrid, and complex types.12 As glycoproteins pass through the endoplasmic reticulum, the non-reducing ends of the oligosaccharides are trimmed by multiple enzymes to form oligomannose type Man9GlcNAc2 (referred to as Man9 or M9) or Man8GlcNAc2 (referred to as Man8 or M8), which are further processed in the Golgi apparatus for the removal of mannose residues and the addition of N-acetylglucosamine (GlcNAc), fucose, galactose, and sialic acid residues to form hybrid and complex glycans. Examples of N-glycans of each type are shown in Table S1.
Most therapeutic recombinant antibodies are expressed in mammalian cells, with Chinese hamster ovary (CHO) cells being the most common host. For mAbs expressed in CHO cells, major Fc N-glycans are asialo biantennary complex type with zero to two galactose residues, plus small amounts of oligomannose type, hybrid type, and sialylated glycans. In general, recombinant IgGs expressed in CHO cells contain similar types of glycans compared to those present in natural human IgGs.
It has been reported that Fc-glycans lacking the 6-arm GlcNAc (e.g., unglycosylated, oligomannose type and some hybrid type glycans) destabilize the CH2-domain conformation compared to other complex glycans because of the lack of interaction between the 6-arm antenna GlcNAc to the Phe-243 side chain.5,10 Sialylation was also found to destabilize the CH2 domain in CHO-expressed antibodies.5,13
Human IgG antibodies containing terminal α2,6-linked sialic acid on their Fc N-glycans have been shown to reduce antibody-dependent cell-meditated cytotoxicity14 and possess anti-inflammatory properties,15,16 although some evidences also suggest otherwise.17 Terminal sialylation on complex N-glycans can happen via either an α2,3-linkage or an α2,6-linkage. Sialic acids in natural human IgG Fc are almost exclusively α2,6-linked.16 Due to the lack of α2,6-sialyltransferase gene, recombinant IgGs expressed in CHO cells have sialic acids solely through α2,3-linkages.18 Additionally, sialic acid on monosialylated glycans can reside on either the 6-arm or the 3-arm in CHO-expressed IgGs. For human IgGs, sialylation is mostly on the 3-arm.19 While the level of terminal monosialylated glycans in human-derived IgG is at about 10%,20 sialylated glycans are usually present in trace amount in CHO-expressed IgGs.
To understand the impact of sialylation with different linkages and locations on IgG structure, we used mass spectrometry (MS)-based tools21 to compare the CH2-domain conformational differences between human-derived IgGs and CHO-expressed IgGs with different glycoforms. Specifically, we examined the conformational stability of the CH2-domain residues near the glycosylation site by hydrogen/deuterium exchange-mass spectrometry (HDX-MS),5,22,23 as well as limited proteolysis under a native-like condition.5,13 Monitoring the glycopeptides containing different glycoforms in both HDX and proteolysis experiments by MS allows direct assessment of the impact of different glycoforms to CH2-domain stability without the need of purified glycoforms. Understanding the effect of different sialylation isomers to IgG structure facilitates, for example, better understanding of how Fc-glycans participate in effector functions and the design of better antibody therapeutics through glycan-engineering.24,25
Results
Glycan nomenclature
Nomenclature of glycans follows that described by Zhang and Shah.26 Specifically, the name of a complex glycan starts with the number of antenna, followed by the number of each terminating residue. For example, A2S1G1F represents a core-fucosylated glycan with two antennas, one antenna terminating with a sialic acid, and the other antenna terminating with a galactose. For hybrid type with more than three mannose residues, the number of mannose residue is also given. Oligomannose type glycans are named by the number of mannose residues. Detailed structures and nomenclature of each glycoform described in this report are listed in Table S1.
HDX of human-derived and CHO-expressed IgG1
In earlier works on CHO-expressed antibodies, we and others have found that the CH2 domain containing sialylated glycans is less stable than asialylated complex glycans.5,13 This observation contradicts previous observations made by both NMR and X-ray crystallography that sialylation does not alter the interaction between the glycan and the CH2 domain for human-derived antibodies,27–29 although other evidence suggests that sialylated Fc-glycans do slightly alter the CH2 domain conformation.30 It was suspected that the discrepancy was the result of different location and linkage of sialic acid residues between human-derived and CHO-expressed antibodies.5,13 To confirm this hypothesis, we performed HDX-MS experiments on a human myeloma IgG1 and a CHO-expressed recombinant IgG1, and compared the deuterium incorporation curves for the proteolytic glycopeptides containing different glycoforms (Figure 1). Indeed, sialylated glycans were found to destabilize the CH2 domain in CHO-expressed IgG1 but not human-derived IgG1, confirming the hypothesis. The HDX-MS experiment on the IgG2 materials, however, did not generate good-quality results due to the low levels of sialylated glycans in the IgG2 materials.
Figure 1.
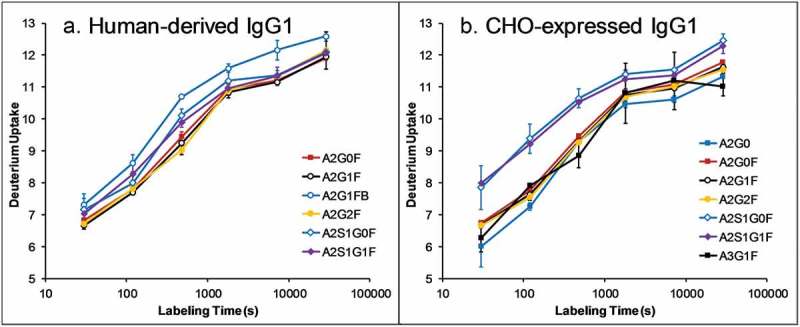
Deuterium uptake curves of glycopeptides (YVDGVEVHNAKTKPREEQYN*STYRVVSVL) containing different glycoforms in human-derived (a) and CHO-expressed (b) IgG1. Data is not corrected for deuterium back exchange. Sialylated glycopeptides are found to have faster H/D exchange rate than other complex glycans in CHO-expressed IgG1, but not human-derived IgG1, indicating that sialylated glycans destabilize the CH2 domain in CHO-expressed IgG1, but not human-derived IgG1.
Characterization of N-glycans released from human-derived and CHO-expressed IgGs by HILIC
Glycans released from human myeloma and CHO-expressed IgG1 and IgG2 were labeled with 2AB and, together with the α2,3- and α2,6-sialylated glycan libraries, analyzed by hydrophilic interaction liquid chromatography (HILIC)-MS in negative-ion mode. Figure 2 shows the selected-ion chromatograms (SIC) of each sialylated glycan species. The glycan structure for each peak is identified (described below) and labeled in Figure 2.
Figure 2.
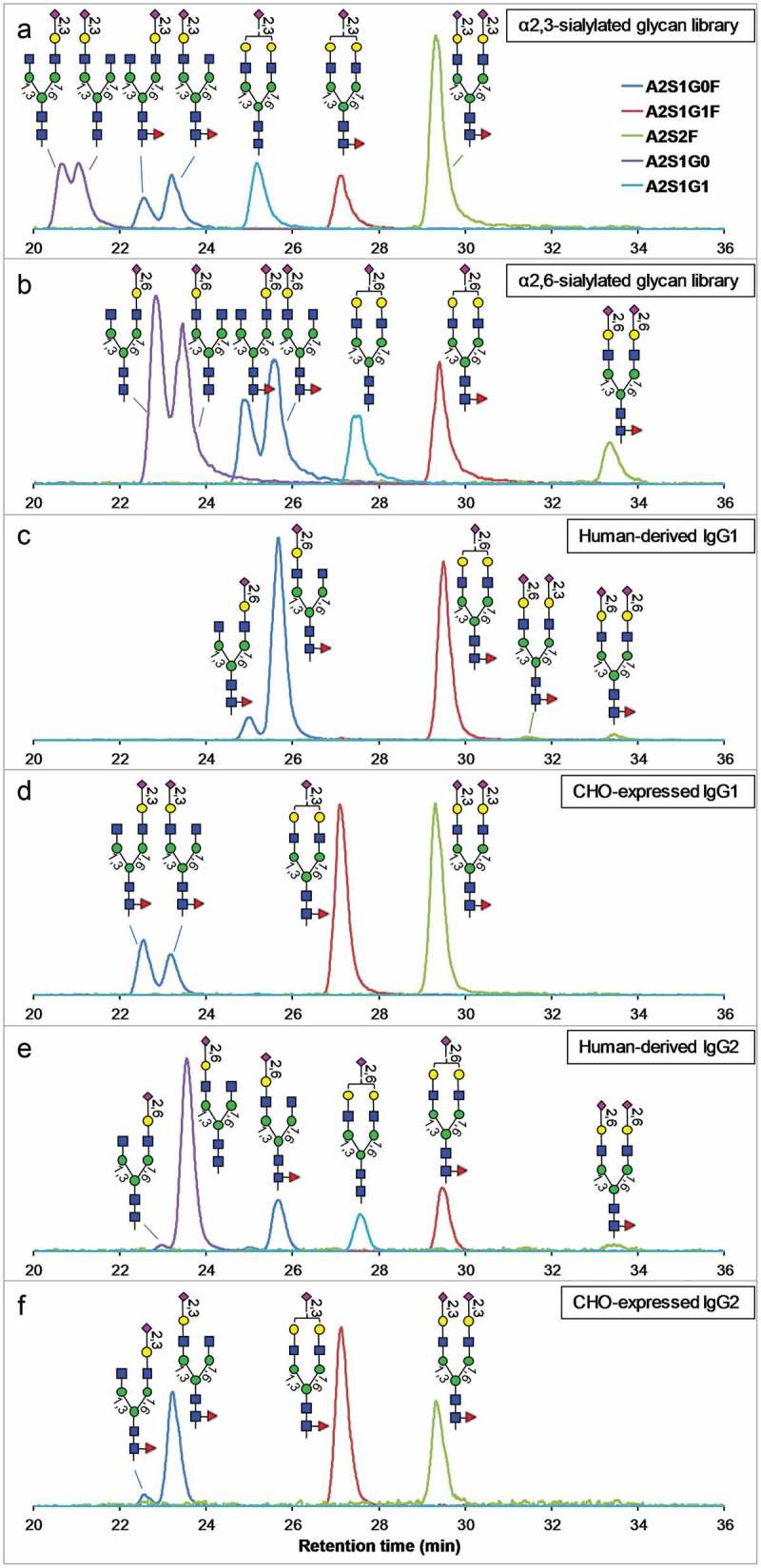
HILIC profiles (SIC of each glycoform) of sialylated N-glycans released from human myeloma and CHO-expressed IgG1 and IgG2, compared to the profiles of α2,3- and α2,6-sialylated glycan libraries. The vertical axes represent the relative signal intensities of each SIC. Panels a and b are profiles of α2,3- and α2,6-sialylated glycan libraries, respectively, panels c and d are profiles of human-derived and CHO-expressed IgG1, respectively, and panels e and f are profiles of human-derived and CHO-expressed IgG2, respectively.
First, the structures of sialylated glycans in the α2,3- and α2,6-sialylated glycan libraries (Figure 2(a,b)) were identified based on their relative retention time in HILIC: (1) α2,3-sialylated N-glycans elute earlier in HILIC than isomeric α2,6-sialylated N-glycans;31 (2) sialylated N-glycans with the sialic acid on the 6-arm elute earlier than isomeric sialylated N-glycans with the sialic acid on the 3-arm.32 The identity of glycans released from human-derived and CHO-expressed IgGs were determined by comparing the retention time of each isomeric species to those in the sialylated glycan libraries as shown in Figure 2.
To further confirm the location of each sialic acid in the monosialylated species, MS/MS of each glycan isomer was examined for the D-ions, which are indicative of the location of the sialic acid in monosialylated species.32,33 As shown in Figures S1 to S6, sialic acid locations of virtually all monosialylated A2S1G0 and A2S1G0F isomers were confirmed by the presence of diagnostic D-ions. Isomers of A2S1G1 and A2S1G1F were not resolved by the chromatography under the conditions used in this study (Figure 2a-e).
Human-derived IgG1 contains two A2S2F isomers (Figure 2(c)). One of them (~33.5 min) eluted at the same time as the A2S2F in the α2,6-sialylated glycan library. The other one (~31.4 min) eluted between the A2S2F peaks in the α2,3- and α2,6-sialylated glycan libraries, indicating that it has α2,6-linked sialic acid on one arm and α2,3-linked sialic acid on the other arm. As will be demonstrated below, this isomer has α2,6-linked sialic acid on the 3-arm and α2,3-linked sialic acid on the 6-arm.
From analysis of release glycans, we concluded that, in the human-derived IgG1 and IgG2, the sialic acids are primarily α2,6-linked on the 3-arm. This conclusion is consistent with previously reported observations.16,19 In the CHO-expressed IgG1 and IgG2, the sialic acids are all α2,3-linked as expected, and are located on either the 3-arm or the 6-arm. No antigen-binding fragment (Fab) N-glycans are present in the CHO-expressed IgG1 and IgG2 based on the Fab amino acid sequences. Based on the low level of disialylated species observed in the human-derived IgG1 and IgG2, it is clear that the human myeloma IgG1 and IgG2, being mAbs, do not contain Fab glycans either, because Fab N-glycans often contain large amount of disialylated complex glycans.34–36 This conclusion is also supported by intact mass analysis of the human myeloma IgG1, which showed a typical mAb Fc glycosylation heterogeneity from different number of galactose residues37 (Figure S7). Therefore, the glycan profile determined from released glycans largely represents the Fc-glycans. As discussed below, the human myeloma IgG2 material may be contaminated with a small amount of other IgGs, but this does not affect the conclusion described here.
Identification of glycopeptides generated from limited proteolysis
Stability of the CH2 domain containing different glycans can be conveniently monitored by their susceptibility to proteolytic digestion under a native-like condition.5,13 To determine the impact of different linkage and positional isomers of sialylated glycans on the CH2-domain conformation, the structure of each glycan isomer on the proteolytic glycopeptides that are resolved in the chromatogram must be identified.
Figure 3 shows the SIC of sialylated glycopeptides, after tryptic digestion of the four IgG antibodies for 24 h under a native-like condition. It has been shown previously that after 24 h of digestion the CH2-domain glycopeptides are nearly fully released, as the glycoform profile is similar to that from a full digestion.5,13 As described below, glycoforms were identified based on a combination of evidences, including the glycan profiles determined from HILIC-MS/MS analysis of released glycans, exoglycosidase digestion, as well as the order of elution.
Figure 3.
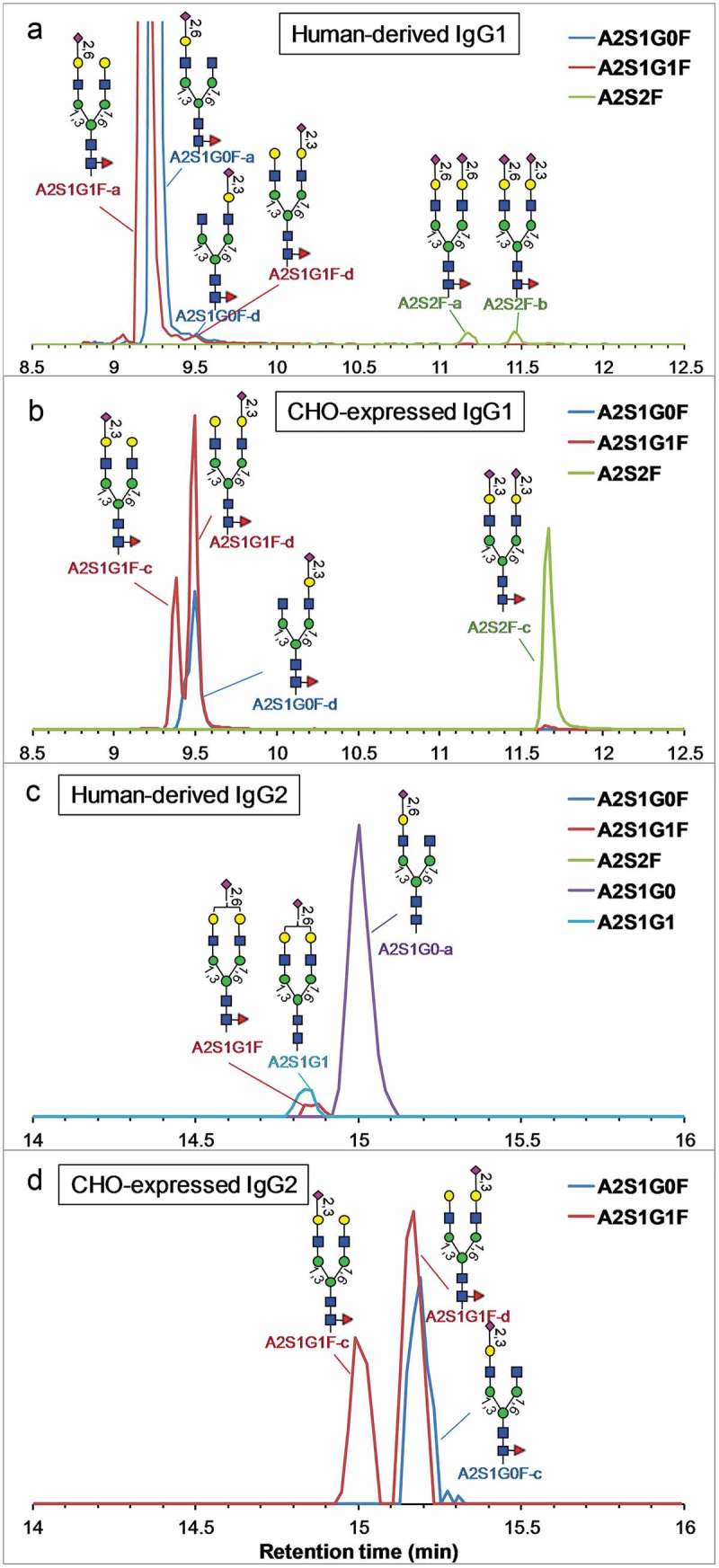
Reversed-phase profile (SIC of each glycoform) of sialylated glycopeptides released after 24-h tryptic digestion of the human-derived and CHO-expressed IgG1 and IgG2 under a native-like condition. The vertical axes represent the relative signal intensities of each SIC.
First, in HILIC of 2AB-labeled N-glycans, glycans containing an α2,3-linked sialic acid elute much earlier than glycans containing an α2,6-linked sialic acid (Figure 2). Additionally, glycans with a sialic acid on the 6-arm elute slightly earlier than glycans with a sialic acid on the 3-arm. Because reversed-phase chromatography uses opposite molecular property for elution, it is rational to believe that glycopeptides containing monosialylated glycan isomers elute with opposite order of retention time, i.e., α2,6-linked sialic acid on 3-arm (a) < α2,6-linked sialic acid on 6-arm (b) < α2,3-linked sialic acid on 3-arm (c) < α2,3-linked sialic acid on 6-arm (d); they are labeled here with suffix of – a, -b, -c, and – d, respectively.
For the human-derived IgG1 (Figure 3(a)), it has been determined from HILIC analysis of released glycans (Figure 2(c)), as well as reported in the literature,16,19 that the sialic acid is primarily α2,6-linked on the 3-arm, and therefore the major monosialylated N-glycans are identified as such (labeled as A2S1G1F-a and A2S1G0F-a).
For CHO-expressed IgG1 (Figure 3(b)), only α2,3-linked sialic acids are present (Figure 2(d)). Because the glycopeptide with a sialic acid on the 3-arm should elute earlier than on the 6-arm, the two peaks labeled A2S1G1F-c and A2S1G1F-d are therefore identified as α2,3-linked sialic acid on the 3-arm and 6-arm, respectively. These assignments are supported by the observation that a sialic acid on the 6-arm has about twice the abundance as a sialic acid on the 3-arm in both HILIC analysis of released glycans (Figure 2(d)) and reversed-phase analysis of the glycopeptides (Figure 3(b)). The minor A2S1G1F peak coeluting with A2S2F (Figure 3(b)) is from in-source fragmentation of A2S2F. The two positional isomers for A2S1G0F are not well resolved, but we believe the major form is A2S1G0F-d (α2,3-linked sialic acid on the 6-arm) based on its peak shape (the minor form appears to elute earlier) and the fact that A2S1G0F-d is more abundant than A2S1G0F-c (Figure 2(d)). The two very small coeluting peaks of A2S1G0F and A2S1G1F at 9.5 min in human IgG1 (Figure 3(a)) elute at the same retention time as A2S1G0F-d and A2S1G1F-d in CHO IgG1 (Figure 3(b)), suggesting that they are the same species. The minor unresolved peak between A2S1G1F-a and A2S1G1F-d in the human-derived IgG1 (Figure 3(a)) is likely A2S1G1F-b (α2,6-linked sialic acid on the 6-arm).
To further confirm the structure of the monosialylated glycans, we digested human-derived and CHO-expressed IgG1 with trypsin under the native-like condition for 3 h, followed by sialidase α-(2–3) treatment. The sialidase α-(2–3) treatment should remove all α2,3-linked sialic acid residues from each glycan. Figure 4 shows the reversed-phase SIC of each glycoform of interest before and after the sialidase α-(2–3) treatment of human myeloma IgG1. While the two major monosialylated forms (A2S1G1F-a and A2S1G0F-a) remained constant, the two minor peaks at 9.9 min (corresponding to the 9.5-min peaks in (Figure 3(a)) because of retention time shift from use of a different column of the same type) clearly disappeared, confirming the sialic acids are α2,3-linked in both forms (A2S1G1F-d and A2S1G0F-d). The disialylated species at 11.5 min also disappeared, indicating it also contains α2,3-linked sialic acid. The same experiment is also performed on CHO-expressed IgG1, in which all sialylated glycopeptides disappeared (Figure S8), confirming that all sialic acids in the CHO-expressed IgG are α2,3-linked.
Figure 4.
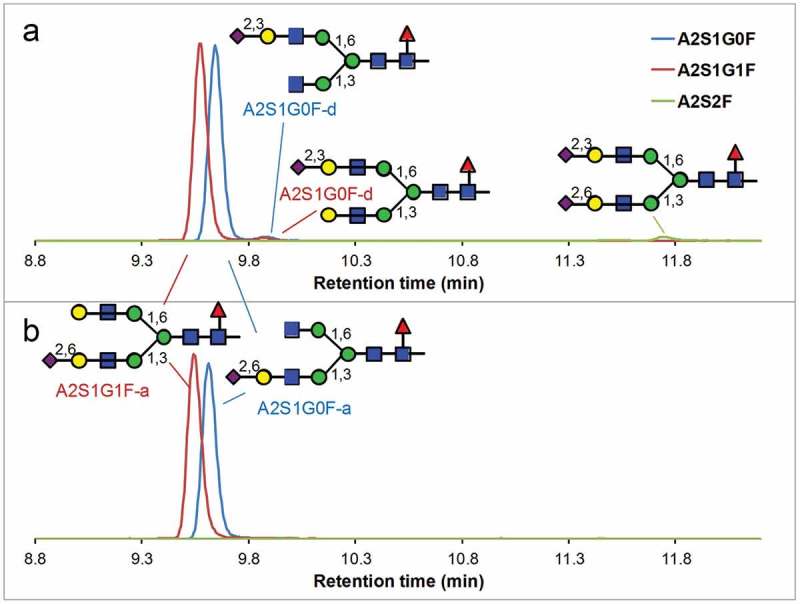
Reversed-phase profile of sialylated glycopeptides after a 3-h tryptic digestion of human-derived IgG1under a native-like condition (a) and followed by sialidase α-(2–3) treatment (b). Disappearance of the minor peaks (A2S1G0F-d, A2S1G1F-d and A2S2F) indicates that all of these minor peaks contain α2,3-linked sialic acid. The vertical axes represent the relative signal intensities of each SIC.
To determine the structure of the two disialylated glycoforms (A2S2F) in human-derived IgG1 (Figure 3(a)), human myeloma IgG1 was digested under the native-like condition for 24 h, followed by reversed-phase separation. The fractions covering the retention time range of the two disialylated peaks were collected, followed by sialidase α-(2–3) treatment and chromatographic analysis (Figure 5). After sialidase treatment, the later-eluting peak clearly diminished, with increased abundance of A2S1G1F-a (α2,6-linked sialic acid on 3-arm), indicating the late-eluting disialylated glycans have an α2,3-linked sialic acid on the 6-arm and an α2,6-linked sialic acid on the 3-arm. The earlier-eluting disialylated glycopeptide has α2,6-linked sialic acids on both arms. It is interesting to note that, although the 3-arm strongly favors the α2,6-linked sialic acid in human-derived IgG1, the 6-arm favors the α2,3-linked sialic acid. This observation also supports the A2S1G1F-d assignment (α2,3-linked sialic acid on the 6-arm) of the minor peak at 9.5-min in (Figure 3(a)), and thus the A2S1G1F-d assignment of the major peak at the same retention time in CHO-expressed IgG1 (Figure 3(b)).
Figure 5.
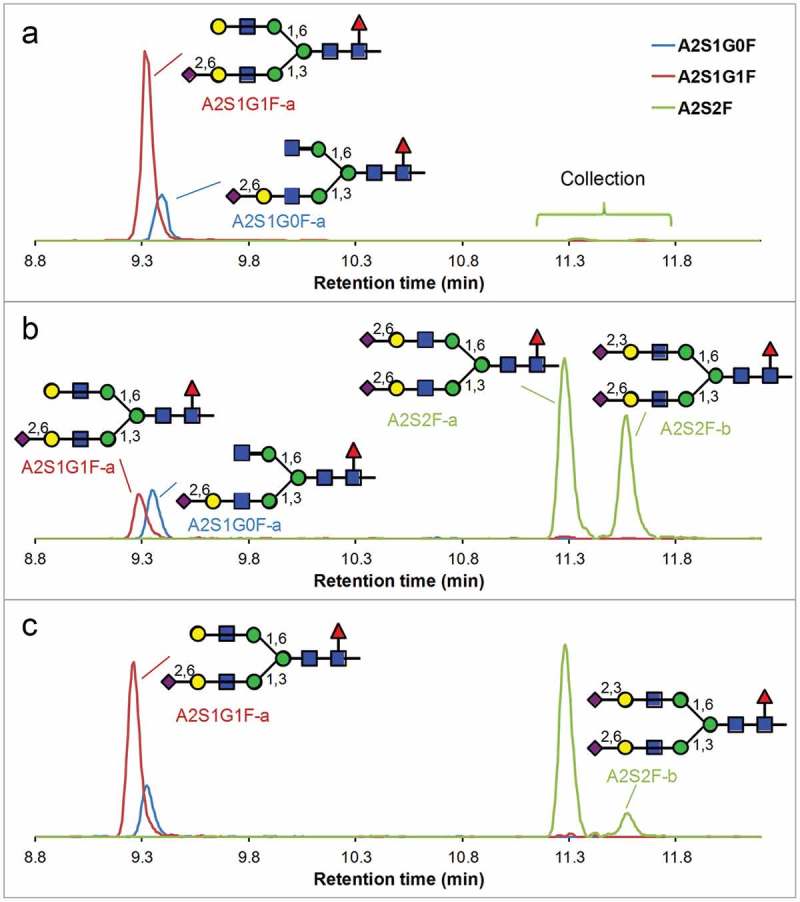
Reversed-phase profiles of sialylated glycopeptides after a 24-h tryptic digestion of human myeloma IgG1under a native-like condition (a), followed by collection of fractions containing the disialylated glycopeptides (b), then followed by sialidase α-(2–3) treatment (c). Disappearance of A2S2F-b and appearance of A2S1G1F-a indicates that A2S2F-b has α2,3-sialic acid on the 6-arm and α2,6-sialic acid on the 3-arm. The vertical axes represent the relative signal intensities of each SIC.
For human-derived IgG2 (Figure 3(c)), the disialylated glycans are not detected. The major glycoform in the human-derived IgG2 was identified based on the major isomeric component determined by the HILIC analysis of the released glycans (Figure 2(e)). It is interesting that, while A2S1G0F and A2S2F are observed in the released glycans of human-derived IgG2 (Figure 2(e)), they are not observed in the glycopeptides (Figure 3(c)). This is likely because the human myeloma IgG2 material used here is contaminated with a small amount of other IgGs. For CHO-expressed IgG2 (Figure 3(d)), the A2S1G0F isomer was identified based on the major isomeric component determined by HILIC (Figure 2(f)), and the A2S1G1F isomers were identified based on the retention order (not resolved in HILIC).
Glycopeptides identified in the four IgG samples, together with their retention times, determined masses and theoretical masses are shown in Tables S2 to S5.
Impact of sialylation on CH2 stability by limited proteolysis
Decreased conformational stability of the CH2 domain can be monitored via tryptic digestion of the antibody under a native-like condition and examination of the release of the glycopeptides containing different glycoforms,5,13 because a less stable domain is more susceptible to enzymatic degradation. The human-derived and CHO-expressed IgG1 and IgG2 were subjected to trypsin digestion under a native-like condition (pH 7, 37°C). The glycan profiles (abundance of each glycoform as a percentage of total glycan abundance) of released glycopeptides (EEQYN*STYR for IgG1 and EEQFN*STFR for IgG2) containing several representative glycoforms are plotted against digestion time in Figure 6. When structural isomers are resolved chromatographically, they are plotted separately with appropriate suffix, e.g., – a, -b, based on elution order. If the abundance of a glycoform starts high and then decreases with the digestion time (show as red line in Figure 6), it means that the glycoform destabilizes the CH2 domain and therefore makes it more susceptible to enzymatic degradation. Note that a much larger number of glycoforms are reported in Figure 6 than in the HDX-MS experiment (Figure 1) because of the better sensitivity of the LC-MS peptide mapping experiment described here.
Figure 6.
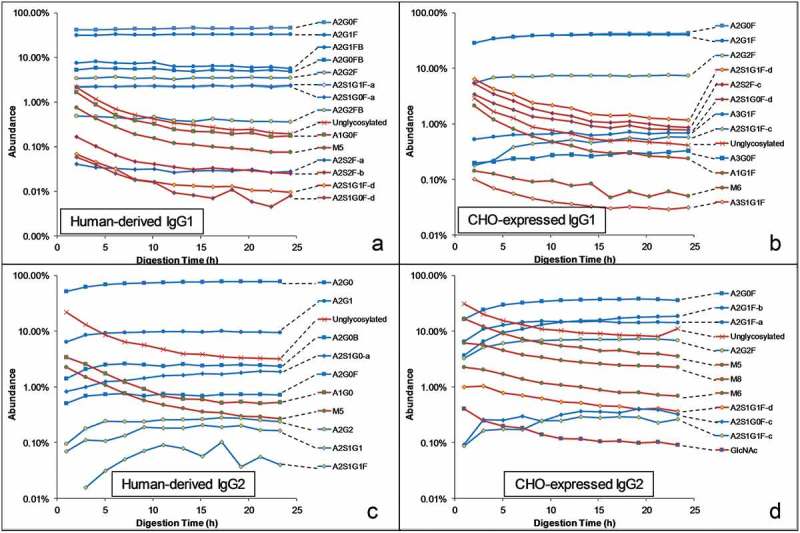
Glycan profiles of released glycopeptides (EEQYNSTYR for IgG1 and EEQFNSTFR for IgG2) after tryptic digestion of human-derived and CHO-expressed IgGs for varying lengths of time under a native-like condition. Glycoforms that increase or do not change with time are shown in blue lines and glycoforms that decrease with time are shown in red lines.
As expected from our previous study,5 the unglycosylated peptide and the glycopeptides lacking the 6-arm GlcNAc (including oligomannose type and some hybrid type glycans) are released early, indicating the CH2 domain containing these glycoforms are less stable and more susceptible to enzymatic degradation. Among the monosialylated glycans, only the (d) isomers (A2S1G1F-d and A2S1G0F-d) exhibit this destabilizing behavior, while the (a) and (c) isomers exhibit stabilizing effects as other asialo complex glycans. The (b) isomers are not detected because of their low abundance in all four IgGs.
The (d) isomers of monosialylated glycans have an α2,3-linked sialic acid on the 6-arm, indicating an α2,3-linked sialic acid on the 6-arm as a necessary condition for the destabilizing effect. Among the disialylated glycans, A2S2F-b and A2S2F-c have the destabilizing effect, while A2S2F-a does not. Both A2S2F-b and A2S2F-c contains an α2,3-sialic acid on the 6-arm, supporting the same conclusion. The A2S2F-a isomer, on the other hand, has α2,6-linked sialic acid on both arms, indicating α2,6-linked sialic acid does not have the destabilizing effect regardless of its location.
The limited proteolysis experiment was applied to additional CHO-expressed IgG molecules (Sigma-Aldrich or Amgen). The results support the same conclusion (Figure S9).
Discussion
Results from the limited proteolysis experiment demonstrated that an α2,3-linked sialic acid on the 6-arm of the complex N-glycan weakens the interaction between the Fc glycan and the peptide backbone and destabilizes the CH2 domain. For a mechanistic understanding of this phenomenon, the crystal structure of the CH2-domain containing α2,6-disialylated glycan28 is shown in Figure 7. The IgG1 antibody containing α2,6-disialylated glycans were prepared by sequential treatment of serum IgG with β-1,4-galactosyltransferase and α2,6-sialyltransferase.38 The 3-arm is entirely solvent accessible, and as a result, the sialic acid residues on the 3-arm are too flexible to be defined by crystallography. Sialylation on the 3-arm, therefore, does not affect the CH2-domain stability. The GlcNAc and Gal residues on the 6-arm, on the other hand, closely interact with the protein backbone to stabilize the CH2 domain. Addition of sialic acid on the 6-arm may introduce steric hindrance that weakens the glycan–protein interaction. Closer examination of the orientation of the galactose residue on the 6-arm reveals that while the 6-position of the galactose points away from the protein backbone, the 3-position points towards the backbone. As a result, sialylation on the 3-position potentially causes steric effects that weaken the glycan–protein interaction, resulting in a less stable CH2-domain conformation. Interestingly, Raju and Scallon also observed that when terminal sialic acids were added to Fc-glycan in vitro using α2,3-sialyltransferase, the antibody became less resistant to papain digestion.39 The model described above predicts that the effect will not be observed, or become less prominent, when α2,6-sialyltransferase is used.
Figure 7.
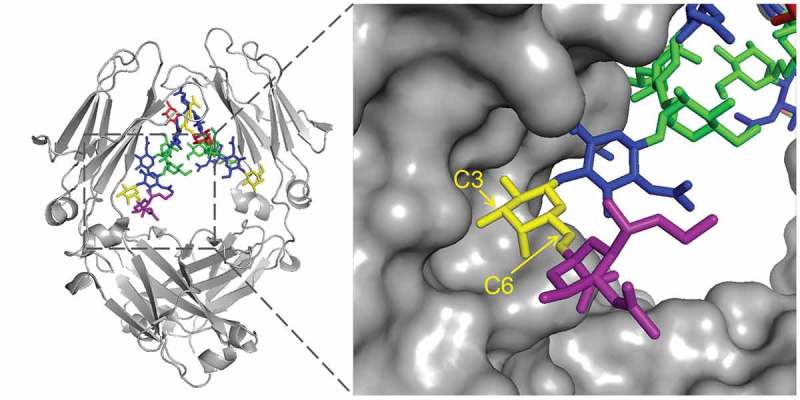
Crystal structure of the α2,6-disialylated glycan in the CH2 domain (PDB id: 4BYH). The 3-arm is pointing inward, and the 6-arm is pointing outward. Sialic acid on the 3-arm is not observable because of its flexibility. Galactose (yellow) carbon positions are labeled. While sialic acid (purple) linked to the 6-position are exposed to the solvent, linking to the 3-position will likely cause steric effect as it points toward the protein backbone.
In human-derived IgGs, sialylation is mostly on the solvent-exposed 3-arm by an α2,6-linkage, although α2,3-sialyltransferase is present. Therefore, sialylation on a human-derived IgG does not cause any changes in conformation as revealed by HDX in this work, as well as previous work by X-ray crystallography and NMR.27–29 It is also interesting to note that α2,6-linked sialic acid on the 6-arm is not favored, presumably related to the specificity of the α2,6-sialyltransferase.40
In CHO-expressed IgG1, α2,3-linked sialic acids are more favored on the 6-arm than the 3-arm, which explains the HDX result that CHO-expressed IgG1 experiences CH2 domain destabilization upon sialylation. This is not true for monogalatosylated A2S1G0F form in IgG2 (Figure 2(f)), in which the α2,3-linked sialic acid favors the 3-arm, presumably because the galactose residue favors the 3-arm in IgG2 antibodies.20
In human-derived IgG1, α2,6-linked sialic acid favors the 3-arm, while α2,3-linked sialic acid favors the 6-arm, although the 3-arm is more solvent accessible. This explains why CHO-expressed IgGs have only trace amount of Fc-sialylated glycans, because of the lack of α2,6-sialyltransferase to add sialic acid to the solvent accessible 3-arm.
In conclusion, through HDX and limited proteolysis, we discovered that α2,3-linked sialic acid on the 6-arm of the Fc-glycan (the major sialylated glycans in CHO-expressed IgG) destabilizes the CH2 domain. This discovery is important in the understanding of the impact of sialylation to the IgG structure, as how the site- and linkage-specific sialylation affects the Fc conformations and binding to respective receptors remains an open question.
Materials and methods
Materials
Recombinant IgG1 and IgG2 antibodies were expressed from CHO cells (Amgen or Sigma-Aldrich). Human-derived IgG1 and IgG2 antibodies (both with kappa light chain) were purified from the plasma of a human myeloma patient (Sigma-Aldrich part number I5154 and I5404). The 2-AB (2-aminobenzamide) labeled α2,3 and α2,6 sialylated biantennary glycan libraries and Glycoclean S cartridges were purchased from Prozyme. Trypsin (proteomics grade) was purchased from Roche. Sialidase sp α-(2–3) (part # E-S007) and the 2-AB labeling kit were purchased from QA Bio. PNGase F was purchased from New England Biolab. Extract-Clean cartridges were purchased from Alltech Associates.
HDX-MS
The detailed experimental setup for deuterium labeling and MS analysis has been described previously.23 Briefly, H/D exchange was initiated by diluting the mAb solutions (10 mg/ml) 10-fold (v/v) into a D2O buffer at 25ºC and pH 7 (87 mM phosphate, pH values were directly read from a pH meter). After a certain length of labeling time (0.5, 2, 8, 30, 120, and 480 min, each in duplicate), the reaction was quenched by mixing 20 µL of the solution with 80 µL quenching/denaturation/reduction buffer (7.25 M urea, 625 mM tris(2-carboxyethyl)phosphine, 0.45 M glycine, pH 2.7) at 1ºC. A 40 µL aliquot of the quenched solution was transferred into 120 µL of 0.4 mg/mL pepsin (Sigma-Aldrich) solution. The digestion solution was immediately injected into a sample loop at 1ºC and stayed for 6 min for digestion. The digest was separated on a Waters 1 × 50 mm CSH C18 column. The HPLC separation was performed at 1ºC with a 6-min acetonitrile gradient of 2% to 40% at a flow rate of 100 µL/min. Each mobile phase contained 0.04% trifluoroacetic acid and 0.1% formic acid. Deuterium labeling, quenching, proteolytic digestion and injection were all performed on a LEAP HD-X PAL system controlled by LEAP HDx Chronos (LEAP Technologies). A peptide mixture (containing equal molar concentration of bradykinin, angiotensin I, and leucine enkephalin) was added to the samples to track and correct back-exchange variability.
The digests were analyzed by LC-MS on an Agilent 1290 Infinity system coupled to a Thermo Scientific Orbitrap Fusion high-resolution mass spectrometer with an electrospray ionization interface (spray voltage of 4400 V). Mass spectrometric data were acquired in the Orbitrap with a resolution of 120,000 in centroid mode, a scan range of 260–2000, an AGC (automatic gain control) target of 2E5, and a maximum injection time of 50 ms. Prior to the HDX experiment, each unlabeled protein digest was analyzed three times by data-dependent LC-MS/MS for peptide identification and glycan profiling. MS/MS data was collected in the linear ion trap using collision induced dissociation (CID) (normalized collision energy of 35%), and a dynamic exclusion duration of 10 s.
All HDX-MS data were processed on MassAnalyzer41 (available from Thermo Scientific in BioPharma Finder) in a fully automated fashion. First, glycopeptides were identified by comparing the experimental MS/MS to theoretically predicted MS/MS.26 Then, using the HDX data processing function, MassAnalyzer generates the deuterium-uptake time course for each proteolytic peptide.23
Characterization of released glycans by HILIC
To release N-glycans from IgGs for HILIC analysis, 1 mg of each human-derived and CHO-expressed IgG antibodies was separately diluted into 200 µL of 50 mM phosphate buffer at pH 7.5. To each IgG, a total of 12 µL of PNGase F was added in two steps, along with incubation at 37°C for 24 h. The released glycans were purified using Extract-Clean cartridges (Alltech Associates) following the manufacturer recommended procedure. Glycans from ~0.5 mg of glycoprotein was labeled with 2-aminobenzamide using the 2-AB labeling kit. Labeled glycans were cleaned with a Glycoclean S cartridge (Prozyme) following the manufacturer recommended procedure. 2-AB labeled glycans were dissolved in 75% acetonitrile for HILIC analysis.
HILIC analysis of 2-AB labeled glycans were performed on an Agilent 1260 HPLC with a Waters glycan BEH amide column (2.1 × 100 mm, 1.7 µm) at 60°C. Glycans were separated with gradient elution with the following mobile phases: (A) 100 mM ammonium formate pH 4.5 and (B) acetonitrile. The elution started by maintaining at 72%B for the first 4.0 min and then decreased to 62%B in 30 min at 0.2 mL/min. Then, the flow rate decreased to 0.1 mL/min at 0%B to clean the column for 3.0 min, followed by equilibration at 72%B for 10 min at 0.2 mL/min. Glycans were detected by fluorescence (wavelengths, Ex: 330nm/Em: 420nm) as well as MS in negative-ion mode (spray voltage = −3200 V) on a Thermo Scientific Orbitrap Fusion Lumos instrument (Resolution = 60,000, AGC target = 5E5 and maximum injection time = 50 ms). Data-dependent MS/MS were collected with HCD (normalized collision energy 20%) in the orbitrap or CID (normalized collision energy of 35%) in the linear trap.
Trypsin digestion under a native-like condition
To test the proteolytic stability of IgG molecules with different glycoforms, human myeloma and CHO-expressed IgG1 and IgG2 antibodies were digested by trypsin under a native-like condition and analyzed by LC-MS/MS as described previously.5 Briefly, each antibody was diluted to 1 mg/mL into a 0.2 M tris buffer at pH 7.5, followed by adding trypsin (Roche) to a concentration of 0.05 mg/mL (20: 1 substrate to enzyme ratio). The digestion was carried out at 37°C for 1 to 24 h, followed by LC-MS/MS analysis on an Agilent 1290 HPLC connected to a Thermo Scientific Orbitrap Fusion Lumos (IgG1s) or Oribtrap Elite (IgG2s) mass spectrometer. Released peptides were separated on a Waters CSH reversed-phase column (2.1 × 100 mm) with a 40-min acetonitrile gradient (1% to 40%) containing 0.1% formic acid in the mobile phase, at a flow rate of 0.3 mL/min. The MS data were collected in the high-resolution orbitraps (resolution of 120,000 for the Fusion and 60,000 for the Elite), and data-dependent CID MS/MS data were collected in the linear ion traps at a normalized collision energy of 35%. Glycans in the released glycopeptides were identified and quantified on MassAnalyzer.41 After feature extraction, glycopeptides were identified by comparing experimental MS/MS to theoretically predicted MS/MS.26 With automated retention time alignment,42 different glycoforms in different samples were conveniently quantified and tabulated.43
Digestion with sialidase
To confirm sialic acid linkages, the human-derived and CHO-expressed IgG1 antibodies (0.3 mg) were each digested with trypsin under a native-like condition at 37°C for 3 h as described previously, then treated with 10 µL sialidase α-(2–3) at 37°C for 2 h after adjusting to pH 6.0 with 1 M phosphate buffer. Sialidase treated samples were analyzed by the same LC-MS/MS method for tryptic digested IgGs.
To confirm sialic acid linkages in disialylated biantennary glycans (A2S2F), glycopeptides from 11 to 12.5 min of peptide map were collected for human-derived and CHO-expressed IgG1 antibodies after tryptic digestion under the native-like condition for 24 h. Glycopeptides from ~ 90 µg of digested protein were collected and dried completely. Glycopeptides were redissolved in 75 µL of 50 mM phosphate buffer at pH 6.0. To 25 µL of this solution, 2 µL of sialidase α-(2–3) was added and incubated at 37°C for 2 h. Sialidase treated glycopeptides were analyzed by the same LC-MS/MS method for tryptic digestion.
Funding Statement
Work supported by Amgen Inc.
Abbreviations
- HDX,
hydrogen/deuterium exchange;
- LC,
liquid chromatography;
- MS,
mass spectrometry;
- MS/MS,
tandem mass spectrometry;
- CHO,
Chinese hamster ovary;
- IgG,
immunoglobulin gamma;
- HILIC,
hydrophilic interaction liquid chromatography;
- SIC,
selected-ion chromatogram;
- CID,
collision-induced dissociation;
- HCD,
higher-energy collision dissociation
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Edelman GM, Cunningham BA, Gall WE, Gottlieb PD, Rutishauser U, Waxdal MJ.. The covalent structure of an entire γG immunoglobulin molecule. Proc Natl Acad Sci USA. 1969;63:78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goetze AM, Liu YD, Zhang Z, Shah B, Lee E, Bondarenko PV, Flynn GC. High-mannose glycans on the Fc region of therapeutic IgG antibodies increase serum clearance in humans. Glycobiology. 2011;21:949–59. doi: 10.1093/glycob/cwr027. [DOI] [PubMed] [Google Scholar]
- 3.Alessandri L, Ouellette D, Acquah A, Rieser M, LeBlond D, Saltarelli M, Radziejewski C, Fujimori T, Correia I. Increased serum clearance of oligomannose species present on a human IgG1 molecule. MAbs. 2012;4:509–20. doi: 10.4161/mabs.20450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mimura Y, Church S, Ghirlando R, Ashton P, Dong S, Goodall M, Lund J, Jefferis R. The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: properties of a series of truncated glycoforms. Mol Immunol. 2000;37:697–706. [DOI] [PubMed] [Google Scholar]
- 5.Fang J, Richardson J, Du Z, Zhang Z. Effect of Fc-glycan structure on the conformational stability of IgG revealed by hydrogen/deuterium exchange and limited proteolysis. Biochemistry. 2016;55:860–68. doi: 10.1021/acs.biochem.5b01323. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Nowak C, Andrien B, Shao M, Ponniah G, Neill A. Impact of IgG Fc Oligosaccharides on recombinant monoclonal antibody structure, stability, safety, and efficacy. Biotechnol Progr. 2017;33:1173–81. doi: 10.1002/btpr.2498. [DOI] [PubMed] [Google Scholar]
- 7.Wada R, Matsui M, Kawasaki N. Influence of N-glycosylation on effector functions and thermal stability of glycoengineered IgG1 monoclonal antibody with homogeneous glycoforms. MAbs. 2019;11:350–72. doi: 10.1080/19420862.2018.1551044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471–78. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Jefferis R. Recombinant antibody therapeutics: the impact of glycosylation on mechanisms of action. Trends Pharmacol Sci. 2009;30:356–62. doi: 10.1016/j.tips.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Bowden TA, Baruah K, Coles CH, Harvey DJ, Yu X, Song B-D, Stuart DI, Aricescu AR, Scanlan CN, Jones EY, et al. Chemical and structural analysis of an antibody folding intermediate trapped during glycan biosynthesis. J Am Chem Soc. 2012;134:17554–63. doi: 10.1021/ja306068g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quast I, Lünemann JD. Fc glycan-modulated immunoglobulin G effector functions. J Clin Immunol. 2014;34:51–55. doi: 10.1007/s10875-014-0018-3. [DOI] [PubMed] [Google Scholar]
- 12.Stanley P, Taniguchi N, Aebi M. N-glycans. Essentials of Glycobiology. [Internet] 3rd ed. New York: Cold Spring Harbor Laboratory Press, 2017; p. 98–111. [Google Scholar]
- 13.Falck D, Jansen BC, Plomp R, Reusch D, Haberger M, Wuhrer M. Glycoforms of immunoglobulin G based biopharmaceuticals are differentially cleaved by trypsin due to the glycoform influence on higher-order structure. J Proteome Res. 2015;14:4019–28. doi: 10.1021/acs.jproteome.5b00573. [DOI] [PubMed] [Google Scholar]
- 14.Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol. 2007;44:1524–34. doi: 10.1016/j.molimm.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–73. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 16.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–76. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell IK, Miescher S, Branch DR, Mott PJ, Lazarus AH, Han D, Maraskovsky E, Zuercher AW, Neschadim A, Leontyev D, et al. Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc portion and independent of sialylation or basophils. J Immunol. 2014;192:5031–38. doi: 10.4049/jimmunol.1301611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee E, Roth J, Paulson J. Alteration of terminal glycosylation sequences on N-linked oligosaccharides of Chinese hamster ovary cells by expression of beta-galactoside alpha 2, 6-sialyltransferase. J Biol Chem. 1989;264:13848–55. [PubMed] [Google Scholar]
- 19.Wormald MR, Rudd PM, Harvey DJ, Chang S-C, Scragg IG, Dwek RA. Variations in oligosaccharide-protein interactions in immunoglobulin G determine the site-specific glycosylation profiles and modulate the dynamic motion of the Fc oligosaccharides. Biochemistry. 1997;36:1370–80. doi: 10.1021/bi9621472. [DOI] [PubMed] [Google Scholar]
- 20.Flynn GC, Chen X, Liu YD, Shah B, Zhang Z. Naturally occurring glycan forms of human immunoglobulins G1 and G2. Mol Immunol. 2010;47:2074–82. doi: 10.1016/j.molimm.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Goswami D, Zhang J, Bondarenko PV, Zhang Z. MS-based conformation analysis of recombinant proteins in design, optimization and development of biopharmaceuticals. Methods. 2018;144:134–51. doi: 10.1016/j.ymeth.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Z, Smith DL. Determination of amide hydrogen exchange by mass spectrometry: a new tool for protein structure elucidation. Prot Sci. 1993;2:522–31. doi: 10.1002/pro.5560020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, Zhang A, Xiao G. Improved protein hydrogen/deuterium exchange mass spectrometry platform with fully automated data processing. Anal Chem. 2012;84:4942–49. doi: 10.1021/ac300535r. [DOI] [PubMed] [Google Scholar]
- 24.Le NPL, Bowden TA, Struwe WB, Crispin M. Immune recruitment or suppression by glycan engineering of endogenous and therapeutic antibodies. Biochim Biophys Acta-Gen Subj. 2016;1860:1655–68. doi: 10.1016/j.bbagen.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, Zhu Z, Chen W, Feng Y, Dimitrov DS. Crystallizable fragment glycoengineering for therapeutic antibodies development. Front Immunol. 2017;8:1554. doi: 10.3389/fimmu.2017.01554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z, Shah B. Prediction of collision-induced dissociation spectra of common N-glycopeptides for glycoform identification. Anal Chem. 2010;82:10194–202. doi: 10.1021/ac102359u. [DOI] [PubMed] [Google Scholar]
- 27.Barb AW, Meng L, Gao Z, Johnson RW, Moremen KW, Prestegard JH. NMR characterization of immunoglobulin G Fc glycan motion on enzymatic sialylation. Biochemistry. 2012;51:4618–26. doi: 10.1021/bi300319q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crispin M, Yu X, Bowden TA. Crystal structure of sialylated IgG Fc: implications for the mechanism of intravenous immunoglobulin therapy. Proc Natl Acad Sci USA. 2013;110:E3544–E6. doi: 10.1073/pnas.1310657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed AA, Giddens J, Pincetic A, Lomino JV, Ravetch JV, Wang L-X, Bjorkman PJ. Structural characterization of anti-inflammatory immunoglobulin G Fc proteins. J Mol Biol. 2014;426:3166–79. doi: 10.1016/j.jmb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. General mechanism for modulating immunoglobulin effector function. Proc Natl Acad Sci USA. 2013;110:9868–72. doi: 10.1073/pnas.1307864110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raymond C, Robotham A, Spearman M, Butler M, Kelly J, Durocher Y. Production of α2, 6-sialylated IgG1 in CHO cells. MAbs. 2015;7:571–83. doi: 10.1080/19420862.2015.1029215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao J, Li S, Li C, Wu S-L, Xu W, Chen Y, Shameem M, Richardson D, Li H. Identification of low abundant isomeric N-glycan structures in biological therapeutics by LC/MS. Anal Chem. 2016;88:7049–59. doi: 10.1021/acs.analchem.6b00636. [DOI] [PubMed] [Google Scholar]
- 33.Harvey DJ, Royle L, Radcliffe CM, Rudd PM, Dwek RA. Structural and quantitative analysis of N-linked glycans by matrix-assisted laser desorption ionization and negative ion nanospray mass spectrometry. Anal Biochem. 2008;376:44–60. doi: 10.1016/j.ab.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 34.Holland M, Yagi H, Takahashi N, Kato K, Savage C, Goodall D. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim Biophys Acta-Gen Subj. 2006;1760:669–77. doi: 10.1016/j.bbagen.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Anumula KR. Quantitative glycan profiling of normal human plasma derived immunoglobulin and its fragments Fab and Fc. J Immunol Methods. 2012;382:167–76. doi: 10.1016/j.jim.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Bondt A, Rombouts Y, Selman MH, Hensbergen PJ, Reiding KR, Hazes JM, Dolhain RJEM, Wuhrer M. Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes. Mol Cell Proteomics. 2014;13:3029–39. doi: 10.1074/mcp.M114.039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Pan H, Chen X. Mass spectrometry for structural characterization of therapeutic antibodies. Mass Spectrom Rev. 2009;28:147–76. doi: 10.1002/mas.20190. [DOI] [PubMed] [Google Scholar]
- 38.Yu X, Vasiljevic S, Mitchell DA, Crispin M, Scanlan CN. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J Mol Biol. 2013;425:1253–58. doi: 10.1016/j.jmb.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Raju TS, Scallon B. Fc glycans terminated with N‐acetylglucosamine residues increase antibody resistance to papain. Biotechnol Progr. 2007;23:964–71. doi: 10.1002/bp070118k. [DOI] [PubMed] [Google Scholar]
- 40.Barb AW, Brady EK, Prestegard JH. Branch-specific sialylation of IgG-Fc glycans by ST6Gal-I. Biochemistry. 2009;48:9705–07. doi: 10.1021/bi901430h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z. Large-scale identification and quantification of covalent modifications in therapeutic proteins. Anal Chem. 2009;81:8354–64. doi: 10.1021/ac901193n. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z. Retention time alignment of LC/MS data by a divide-and-conquer algorithm. J Am Soc Mass Spectrom. 2012;23:764–72. doi: 10.1007/s13361-011-0334-2. [DOI] [PubMed] [Google Scholar]
- 43.Shah B, Jiang XG, Chen L, Zhang Z. LC-MS/MS peptide mapping with automated data processing for routine profiling of N-glycans in immunoglobulins. J Am Soc Mass Spectrom. 2014;25:999–1011. doi: 10.1007/s13361-014-0858-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


