Abstract
Background
We report detailed results of The Physical Function Trial (PFT), one of seven Testosterone Trials (TTrials), which determined testosterone’s effects on mobility, self-reported physical function, falls, and patient global impression-of-change (PGIC) in older men with self-reported mobility limitation and walking speed<1.2 m/sec. We determined if testosterone’s effects on mobility differed according to baseline walking speed, mobility-limitation, or other participant-level factors.
Methods
The participants were 788 men≥65 years, with total testosterone<275 ng/dL, of which 390 men with mobility limitation and walking speed<1.2 m/sec enrolled in the PFT. Participants were assigned double-blind to 1% testosterone gel or placebo gel daily for 12-months. Primary outcome was Increase in 6-minute walk distance (6MWD) of ≥50 m; secondary outcomes included absolute increase in 6MWD, physical component of Short Form-36 (PF10), and exploratory outcomes PGIC and falls.
Findings
Intervention groups were similar at baseline. 6MWD improved significantly more in testosterone than in placebo group among all men in TTrials, and separately in men who were not enrolled in the PFT (treatment effect 8.9, 95% CI (2.2,15.6) p=0.01), but not in those who were enrolled in the PFT (treatment effect 4.1, 95% CI (−3.0,11.2) p=0.25). PF10 improved more in testosterone than in placebo group in all men in TTrials (treatment effect 3.1, 95% CI (1.2, 4.9), p=0.002) and separately in both men enrolled and not enrolled in the PFT (treatment effect 2.8, 95% CI (0.41, 5.2), p=0.02; and treatment effect 4.0, 95% CI (1.5, 6.5), p=0.002, respectively). Testosterone-treated men with baseline walking speed ≥1.2 m/sec experienced significantly greater improvements in 6MWD and in PF10 than placebo-treated men. Men reporting mobility limitation showed significantly more improvement in 6MWD and in PF10 than placebo-treated men. Fall frequency was similar in the two groups. Changes in 6MWD were significantly associated with changes in testosterone, free testosterone, DHT, and hemoglobin levels.
Interpretation
Testosterone consistently improved self-reported walking ability, modestly improved 6MWD in all men participating in the Testosterone Trials, but did not affect falls. Testosterone’s effect on mobility measures in older men with low testosterone were related to baseline gait speed and self-reported mobility limitation, and changes in testosterone and hemoglobin levels.
Trial Registration
ClinicalTrials.gov number,
Keywords: Testosterone, older men, mobility, testosterone’s effects on mobility, late onset hypogonadism, physical function, falls
INTRODUCTION
The observation that testosterone administration increases skeletal muscle mass and maximal voluntary muscle strength (1–9) has led to considerable pharmaceutical interest in applying testosterone as an anabolic therapy to improve physical function and to reduce the burden of disability in older men with mobility limitation. However, randomized trials of testosterone have not demonstrated consistent improvements in performance-based measures of physical function in older men with functional limitations (1–16). Some earlier trials were limited by their small size and suboptimal statistical power; inclusion of healthy older men without functional limitations; heterogeneity of testosterone doses, on-treatment levels, and outcomes ascertainment; and relatively short intervention durations of 3 to 6 months. An Institute of Medicine panel concluded that there was insufficient evidence of a beneficial effect of testosterone replacement on physical function and mobility in older men with functional limitations (16).
The Testosterone Trials (The TTrials) were a set of seven coordinated placebo-controlled trials, designed to determine the efficacy of testosterone in improving sexual function, physical function, vitality, and other outcomes in older men with unequivocally low testosterone levels and low libido, mobility limitation and/or low vitality (17–19). The main findings of the TTtrials have been published (19). The Physical Function Trial (PFT), one of the seven TTrials, enrolled men based on gait speed < 1.2 m/sec in the 6-minute walk test and mobility limitation defined as self-reported difficulty in walking or climbing stairs.
We report here detailed results of The Physical Function Trial (PFT), which determined testosterone’s effects on mobility, self-reported physical function, and patient global impression-of-change (PGIC) in older men with self-reported mobility limitation and walking speed<1.2 m/sec (19); additionally, we determined the effects of testosterone on falls in all TTrials participants. We determined if testosterone’s effects on mobility differed according to baseline walking speed, mobility-limitation, hormone levels, or other participant-level factors.
The primary analyses revealed that among men enrolled in the PFT, the 6MWD did not improve by at least 50 meters significantly more frequently in the testosterone group than in the placebo group (19); however, pre-specified analyses considering all men in TTrials revealed a statistically significant difference between arms in the proportion of men improving with a benefit among men treated with testosterone. These findings led us to investigate results in the men who were not enrolled in the Physical Function Trial, and to assess whether the baseline characteristics defining eligibility for the PFT were related to the treatment response. Accordingly, in the current report, we compared the changes in 6MWD and PF10 in men enrolled in any of the TTrials whose baseline gait speed was <1.2 m/sec vs those with baseline gait speed ≥1.2 m/sec, and in men who reported mobility limitation vs. those who did not report mobility limitation.
As testosterone’s anabolic effects on skeletal muscle are related to testosterone dose and concentrations (1–2), we also assessed whether the changes in 6MWD and physical function component (PF10) of the Medical Outcomes Study Short Form 36 (MOS SF36) were related to changes in total and free testosterone, dihydrotestosterone (DHT), or estradiol levels.
STUDY DESIGN
The TTrials were a set of seven placebo-controlled, double-blind, parallel group trials conducted at 12 academic sites in the United States. The study design and the main findings of the TTrials have been published (17–19). Briefly, the participants had to meet eligibility requirements for one or more of the three main trials (Sexual Function, Physical Function, and Vitality). If the participants qualified for any of the three main trials, they could participate in one or more of the other trials.
The study protocol was approved by the institutional review boards of the University of Pennsylvania and each of the 12 trial sites. A Data and Safety Monitoring Board reviewed the study’s progress every 6 months, with additional quarterly safety reviews.
Participants
The participants were community-dwelling men, 65 years or older, with an average of two morning fasting testosterone concentrations 9.5 nmol/L (<275 ng/dL) (first value 9.5 nmol/L (<275 ng/dL), second 10.4 nmol/L (<300 ng/dL) and the average of two values <9.5 nmol/L (275 ng/dL)), measured using liquid chromatography tandem mass spectrometry (LC-MS/MS) at the Quest Diagnostics Laboratory, San Juan Capistrano, CA. To qualify for the PFT, the participants had to have mobility limitation, defined by self-reported difficulty walking one-quarter mile and/or walking up one flight of stairs, and a 6MWS <1.2 m/sec (17). Walking speeds <1.2 m/sec have been associated with increased mortality (20). Our expectation was that men who walked more slowly and perceived mobility problems would be more likely to benefit from testosterone treatment than men who were functioning at a higher level.
The exclusion criteria have been published (17); briefly, they included conditions that could potentially be worsened by testosterone treatment or would preclude assessment of primary or secondary outcomes.
Intervention
The participants initially applied either 5-g 1% testosterone gel (AndroGel 1%, AbbVie Pharmaceuticals, North Chicago, IL) containing 50-mg testosterone or an equivalent amount of placebo gel daily on the skin. Serum testosterone concentration was measured at months 1, 2, 3, 6 and 9, and dose was adjusted after each measurement, as necessary, to maintain testosterone concentration between 500–800 ng/dL (17–19).
Participant Allocation
The participants were assigned to receive testosterone or placebo gel for one year using the method of minimization (21). The balancing factors included in the minimization procedure were participation in the three main trials, trial site, screening testosterone less than or greater than 200 ng/dL, age less than or greater than 75 years, antidepressant use, and PDE5 inhibitor use. An automated computer algorithm assigned the treatment providing optimal balance on the above factors with 80% probability to maintain some randomness to the assignment.
Blinding
The participants and the study staff were unaware of the intervention allocation, which was known only to Data Coordinating Center and the Central Pharmacy. The testosterone and placebo preparations were similar in look, smell and feel. When the testosterone dose was adjusted in a man in the testosterone arm, a participant in the placebo group was also asked to change his dose to maintain blinding.
Outcomes
The primary outcome of the PFT was the proportion of men whose 6MWD increased by ≥50 m from baseline. The 6MWD, a widely used measure of mobility, was selected as the primary outcome because walking is essential for most activities of daily living; walking speed and distance predict clinical outcomes including disability and mortality (22–24); and estimates of the minimum clinically important difference (MCID) (50 meters) were available (25–27). The 6MWD was measured at baseline and at months 3, 6, 9 and 12.
Secondary outcomes included change in 6MWD as a continuous variable, change in 6MWD in all men enrolled in the TTrials, and self-reported physical function, assessed using the physical function component (PF10) of the Medical Outcomes Study Short Form-36 (MOS SF36) (28). PF10 was selected as the self-reported measure of mobility because this instrument includes a number of questions about difficulty in walking short as well as long distances. The MCID for PF10 is 8 points. Prespecified exploratory endpoints included falls and patient global impression of change (PGIC). The falls were ascertained every 3 months using a structured questionnaire which asked subjects whether they had encountered a fall in the interval period, and if so, whether they had sought medical attention, and whether they had suffered a fracture. The PGIC was ascertained every 3 months using a standardized question which asked if the subjects felt their walking ability had improved since the beginning of the intervention using a Likert scale of 1 to 7.
At the completion of the trial, serum testosterone, dihydrotestosterone, and estradiol levels were measured using LC-MS/MS and free testosterone using equilibrium dialysis in the Brigham Research Assay Core Laboratory, as described (19).
Statistical Analyses and Sample Size
The sample size estimate for the PFT was based on the MCID of 50 meters, the assumption that 15% of men in the placebo group would increase their walk distance by at least this amount, and the goal of detecting a difference if ≥30% of men in the testosterone group showed such an increase, with 90% power using a two-sided 0.05 significance level and a repeated measures analysis including all post-baseline assessments. We inflated the sample size by 5% to compensate for the small number of men expected to have no post-baseline values. The sample size target was 175 per treatment arm.
The primary analysis was performed using random effects models for longitudinal data, which included visit time as a categorical variable and a single main effect for treatment, and included balancing factors and baseline value of the 6-minute walking distance as fixed effect covariates. For linear models of continuous outcomes, the treatment effect denoted the average difference in response by treatment arm across all visits. For logistic models of binary outcomes, the treatment effect was the log odds ratio of a positive versus negative outcome for testosterone versus placebo participants, averaged over all visits. The association of PGIC with treatment was assessed in a random effects proportional odds model, adjusted for balancing factors. The extreme responses at each end of the 7-point scale were collapsed to make a 5-point rather than a 7-point scale.
As the anabolic effects of testosterone on the skeletal muscle are related to increase in testosterone concentrations (12), we also evaluated the relation of changes in hormone levels with the changes in 6MWD and PF-10 in all men participating in the TTrials. These analyses were performed using marginal models with parameters estimated using generalized estimating equations (GEE), including balancing variables and change from baseline of hormone levels at each measured time point as time-varying covariates and baseline value of the hormones and the 6MWD. In these models, effects denote the average change in outcome associated with a unit change in hormone. We also evaluated the effect of changes in hemoglobin on changes in 6MWD, accounting for change in testosterone, using GEE regression with change in hemoglobin and change in testosterone as time-varying covariates. The association of PGIC with other outcomes was assessed in a mixed effects model for longitudinal data, considering PGIC as a time-varying covariate and including treatment arm, balancing factors and baseline value of the outcome in the model. Tests for treatment interaction with other covariates were performed by adding a term for the interaction to the model.
We investigated whether the baseline characteristics defining eligibility for the PFT were related to the treatment response. Accordingly, we compared the changes in 6MWD and PF10 in men whose baseline gait speed was <1.2 m/sec vs those with baseline gait speed ≥1.2 m/sec, and in men who reported mobility limitation vs. those who did not report mobility limitation.
All randomized participants with any follow-up data were analyzed in the group to which they were allocated regardless of their compliance (intention-to-treat). We did not adjust the analyses for multiple comparisons as we anticipated that these outcomes would be highly correlated with each other.
The Role of the Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author (SB) and the Chief Biostatistician (SE) had full access to all of the data and assume the final responsibility to submit for publication.
RESULTS
The Flow of Participants Through the Trial
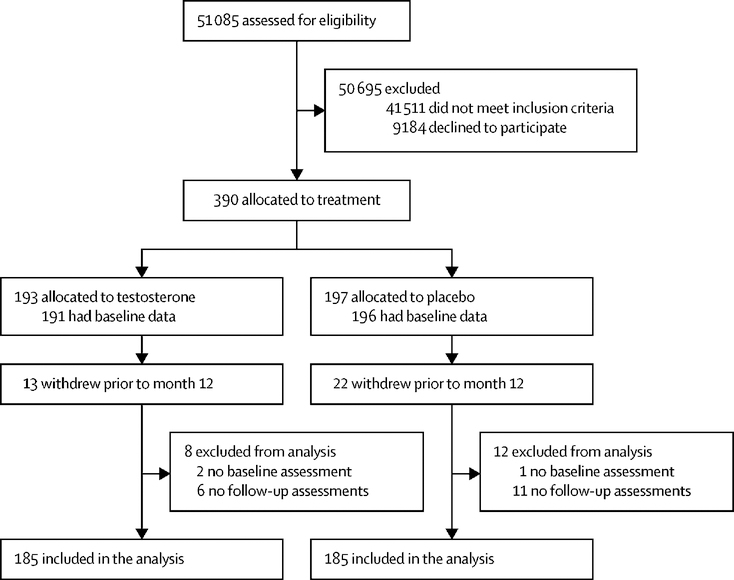
As described (19), among 790 men who were enrolled in the TTrials, 390 were enrolled in the PFT; 193 men were allocated to the testosterone arm and 197 to the placebo arm. Among the 390 men who were enrolled in the PFT, 35 withdrew prior to month 12, 13 in the testosterone group and 22 in the placebo group (CONSORT diagram; Figure 1). The modified intention-to-treat analytical sample included all men who were enrolled and had at least one post-baseline assessment. The actual number included in the analyses are shown in the tables and figures.
Figure 1.
CONSORT Diagram. The figure shows the flow of subjects through the various phases of the trial.
The two intervention groups were similar in their baseline characteristics among men enrolled and not enrolled in the PFT (Table 1), among men whose baseline gait speed was <1.2 m/sec or ≥1.2 m/sec, and among men with and without self-reported mobility limitation (Supplementary tables 1 and 2). The men enrolled in the PFT were on average older, had higher BMI, were more likely to have comorbid conditions, and, as expected, had slower gait speed and lower PF10 score than those not enrolled in this trial. Among men not enrolled in the PFT, 175 had baseline gait speed less than 1.2 m/sec, and 57 reported mobility limitation.
Table 1.
Baseline Characteristics of Men in the Physical Function Trial and Not in the Physical Function Trial
| Treatment Group | Men IN the Physical Function Trial | Men NOT in the Physical Function Trial | ||
|---|---|---|---|---|
| Placebo |
Testosterone |
Placebo |
Testosterone |
|
| N | 197 | 193 | 197 | 201 |
| Demographics | ||||
| Age (yr) | 73.2 (5.9) | 73.4 (6.4) | 71.4 (5.4) | 70.8 (4.6) |
| Race | ||||
| Caucasian (%) | 168 (85.3%) | 172 (89.1%) | 182 (92.4%) | 176 (87.6%) |
| African-American (%) | 13 (6.6%) | 10 (5.2%) | 7 (3.6%) | 11 (5.5%) |
| Other (%) | 16 (8.1%) | 11 (5.7%) | 8 (4.1%) | 14 (7.0%) |
| Ethnicity | ||||
| Hispanic (%) | 8 (4.1%) | 8 (4.1%) | 2 (1.0%) | 10 (5.0%) |
| Non-Hispanic (%) | 189 (95.9%) | 184 (95.3%) | 195 (99.0%) | 191 (95.0%) |
| Concomitant conditions | ||||
| BMI (kg/m2) | 31.7 +/− 3.4 | 31.5 +/− 3.5 | 30.3 +/− 3.6 | 30.5 +/− 3.6 |
| BMI >30 (%) | 135 (68.5%) | 135 (69.9%) | 110 (55.8%) | 116 (57.7%) |
| Alcohol Use (no. drinks/week) | 3.5 +/− 5.3 | 2.9 +/− 4.1 | 3.4 +/− 4.8 | 3.1 +/− 4.4 |
| Smoking | ||||
| Current smoker (%) | 20 (10.2%) | 19 (9.8%) | 14 (7.1%) | 11 (5.5%) |
| Ever smoker (%) | 136 (69.0%) | 133 (68.9%) | 132 (67.0%) | 123 (61.2%) |
| Diabetes (%) | 85 (43.1%) | 81 (42.0%) | 59 (29.9%) | 67 (33.3%) |
| Hypertension (%) | 145 (73.6%) | 143 (74.1%) | 134 (68.0%) | 143 (71.1%) |
| History of myocardial infarction (%) | 35 (17.8%) | 28 (14.5%) | 28 (14.2%) | 25 (12.4%) |
| History of stroke (%) | 11 (5.6%) | 11 (5.7%) | 6 (3.0%) | 5 (2.5%) |
| Sleep apnea | 34 (17.3%) | 43 (22.3%) | 42 (21.3%) | 34 (16.9%) |
| Sex Hormones | ||||
| Testosterone (ng/dL) | 233.4 +/− 64.0 | 230.5 +/− 64.3 | 238.8 +/− 69.3 | 233.0 +/− 62.1 |
| Free testosterone (pg/mL) | 63.9 +/− 23.0 | 60.5 +/− 21.9 | 66.0 +/− 23.8 | 63.4 +/− 21.0 |
| Dihydrotestosterone (ng/dL) | 20.9 +/− 14.0 | 21.6 +/− 12.8 | 20.8 +/− 11.9 | 20.9 +/− 10.3 |
| Estradiol (pg/mL) | 21.4 +/− 6.5 | 20.2 +/− 6.6 | 19.5 +/− 6.1 | 20.3 +/− 6.8 |
| Sex hormone binding globulin (nM) | 29.3 +/− 14.0 | 32.3 +/− 16.1 | 29.8 +/− 15.5 | 30.4 +/− 14.3 |
| Physical Performance | ||||
| Gait speed (m/s) | 1.0 +/− 0.2 | 1.0 +/− 0.2 | 1.2 +/− 0.2 | 1.2 +/− 0.2 |
| PF10 | 64.8 ± 21.3 | 65.4 ± 20.0 | 76.9 ± 18.9 | 79.8 ± 17.4 |
Legend: Data are mean ± SD. BNI, body mass index; PF10, physical component domain of the Medical Outcomes Study Short Form-36 questionnaire
Adherence to assigned treatment in men enrolled in the PFT, assessed by weighing the returned bottles and comparing it to the expected weight based on the prescribed dose, was high in both the testosterone and placebo groups (means 97% and 92%), respectively, with fewer than 5% of men with compliance<60% and fewer than 5% with compliance >135%). As reported earlier, the rates of prostate, cardiovascular and serious adverse events did not differ significantly between groups (19).
Hormone Levels
In men enrolled in the PFT, serum total testosterone levels increased from an average of 8.0 nmol/L (230.5 ng/dL) at baseline to an average of 17.9 nmol/L (516.4 ng/dL) at month 12 in the testosterone group men, but remained unchanged in placebo-treated men (mean 8.1 nmol/L (233.4 ng/ml) at baseline vs 8.0 nmol/L (230.3 ng/ml) at month 12). Serum free testosterone, DHT and estradiol concentrations also increased in the testosterone group, but did not change in the placebo group.
Walking Speed
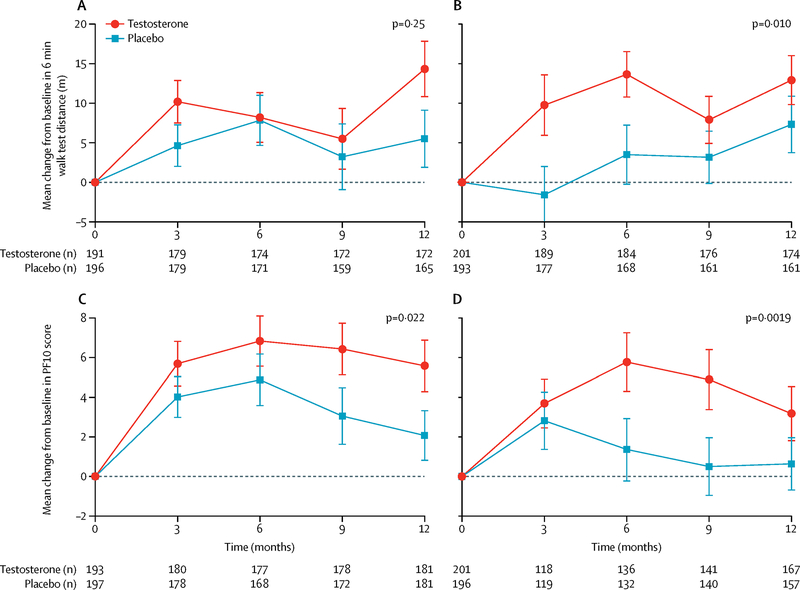
As reported previously (19), neither the proportion of men increasing their 6MWD by more than 50 m [35 (20.4%) in the testosterone arm and 20 (12.1%) in the placebo arm], nor the absolute change from baseline in 6MWD differed significantly between the two intervention arms in men enrolled in the PFT. Among men not enrolled in the PFT, those assigned to testosterone arm improved their 6MWD more than those assigned to the placebo arm (Figures 2A and 2B, treatment effect 8.9 m, 95% CI (2.2, 15.6) P=0.01). However, a test for statistical interaction between treatment and enrollment in the PFT with respect to the absolute change in walk distance did not show a significant effect (p= 0.33).
Figure 2.
Comparison of change in 6-minute walking distance and PF10 scores in men enrolled in the Physical Function Trial and men not enrolled in the physical function trial.
The point estimates are means and the error bars represent 95% confidence intervals.
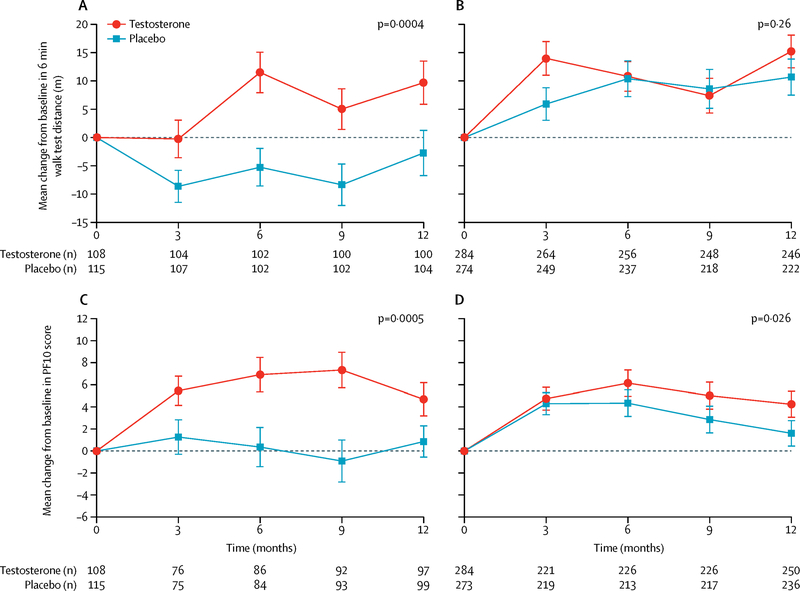
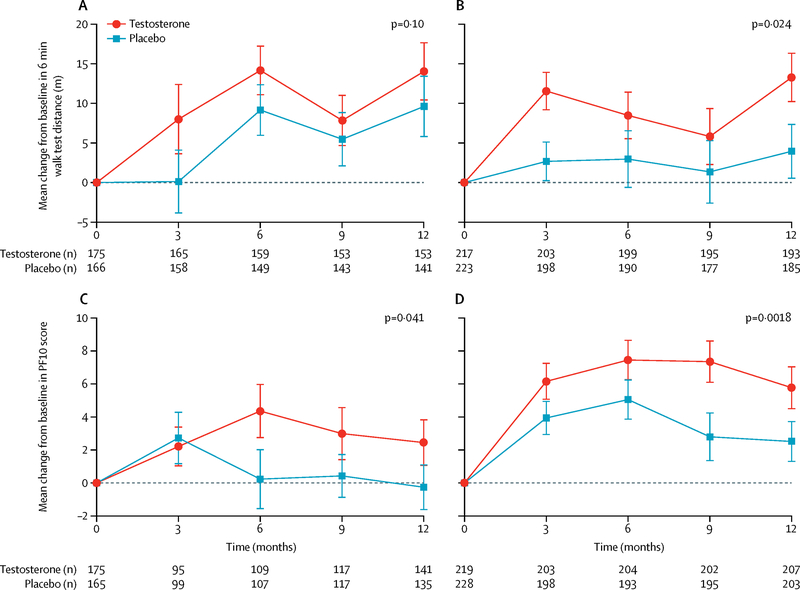
Because enrollment in the PFT required men to have baseline 6MWS <1.2 m/sec plus self-reported mobility difficulty, we evaluated whether the treatment response differed depending on baseline 6MWS or mobility limitation. We also performed tests of interaction of treatment with baseline 6MWS. Among all men enrolled in the TTrials, the men treated with testosterone whose baseline 6MWS was ≥1.2 m/sec improved their 6MWD significantly more than men treated with placebo (treatment effect 14.2 m, 95% CI (6.5, 21.9) P<0.001), while men with baseline 6MWS <1.2 m/sec showed no significant benefit of testosterone (treatment effect 3.5 m, 95% CI (−2.6, 9.7) P=0.26) (Figure 3). The interaction between treatment arm and baseline 6MWS was significant (p = 0.02). Testosterone treatment had a statistically significant effect on 6MWD in men with self-reported mobility limitation at baseline (treatment effect 7.6 m, 95% CI (1.0, 14.1) P=0.02) but the effect did not quite reach statistical significance in men who did not report mobility limitation (treatment effect 5.9 m, 95% CI (−1.2, 13.1) P=0.10) (Figure 4). The interaction between treatment arm and baseline self-reported mobility limitation was not statistically significant (p = 0.77).
Figure 3.
Comparison of change in 6-minute walking distance and PF10 scores between testosterone and placebo-treated men among those whose baseline gait speed was < 1.2 m/sec vs ≥ 1.2 m/sec. Upper left panel: The change in 6-minute walking distance in testosterone and placebo-treated men among those with baseline gait speed ≥ 1.2 m/sec. Upper right panel: The change in 6-minute walking distance in testosterone and placebo-treated men among those with baseline gait speed ≤ 1.2 m/sec. Lower left panel: Change in PF10 score in testosterone and placebo-treated men among those with baseline gait speed ≥ 1.2 m/sec. Lower right panel: The change in PF10 score in testosterone and placebo-treated men among those with baseline gait speed < 1.2 m/sec.
The point estimates are means and the error bars represent 95% confidence intervals.
Figure 4.
Comparison of change in 6-minute walking distance and PF10 scores between testosterone and placebo-treated men among those who had mobility limitation vs those who did not have mobility limitation at baseline. The point estimates are means and the error bars represent 95% confidence intervals.
Upper left panel: The change in 6-minute walking distance in testosterone and placebo-treated men among those who did not have mobility limitation at baseline; Upper right panel: The change in 6-minute walking distance in testosterone and placebo-treated men among those who had mobility limitation at baseline.
Lower left Panel: Change in PF10 score in testosterone and placebo-treated men among those without mobility limitation at baseline; Lower right panel: Change in PF10 score in testosterone and placebo-treated men among those with mobility limitation at baseline.
Among the baseline factors (age, body mass index, 6MWS and PF10 scores, testosterone levels) that were included as covariates in the primary analysis of men enrolled in the PFT, age, the baseline 6MWS and baseline PF10 scores were significantly associated with the change in 6MWD; body mass index, baseline testosterone levels, and the use of anti-depressants were not associated with change from baseline in these outcomes.
The changes in total and free testosterone, and DHT, but not estradiol levels were significantly associated with changes in 6MWD in all men enrolled in the TTrials (effect size for 3.5 nmol/L (100 ng/dL) change in total testosterone 1.0 m 95% CI (0.4, 1.8) P=0.002; for 69.3 pmol/L (20 pg/mL) change in free testosterone 0.22 m 95% CI (0.11, 0.37) P=0.009; for 0.34 nmol/L (10 ng/dL) change in DHT 0.52 m 95% CI (0.14, 0.9) P=0.008). The changes in hormone levels were not significantly associated with changes in PF10.
The change in hemoglobin was highly significantly associated with the change in 6MWD, even after accounting for the effect of change in total testosterone (effect size 3.8, 95% CI (1.7, 6.0), p<0.001); for each 1.0 g/dL increase in hemoglobin, the 6MWD improved by an average 3.8 meters. The change in hemoglobin was not significantly associated with change in PF10 (effect size 0.41, 95% CI (−0.51, 1.3), p=0.38).
Self-reported Physical Function
Self-reported mobility assessed using the PF-10 improved significantly more in the testosterone group than in the placebo group both in men enrolled and not enrolled in the PFT (treatment effect 2.8, 95% CI (0.41, 5.2) P=0.02, and 4.0, 95% CI (1.5, 6.5) P=0.002, respectively, Figures 2C and 2D). The time X treatment interaction was not statistically significant; thus the apparent fluctuations in PF10 scores over time may be a chance finding. The change in PF10 from baseline in men treated with testosterone was not significantly related to the change in total and free testosterone, DHT and estradiol level (data not shown).
The PF-10 scores improved significantly more in men treated with testosterone than in men treated with placebo among men whose baseline 6MWS was ≥1.2 m/sec (treatment effect 4.9, 95% CI (2.2, 7.7) P<0.001) as well as in those with baseline 6MWS <1.2 m/sec (treatment effect 2.5, 95% CI 0.29, 4.6), P=0.03 (Figure 3). Among men who reported mobility limitation at baseline, those treated with testosterone improved significantly more than men treated with placebo (treatment effect 3.6, 95% CI (1.3, 5.9) P=0.002). Testosterone treatment also significantly improved PF-10 scores in men who did not report mobility limitation at baseline (treatment effect 2.7, 95% CI (0.11, 5.3), P=0.04), but to a lesser extent (Figure 4).
We asked men at each visit whether they perceived any changes in their walking ability since the start of the trial using a 7-point scale ranging from “much worse” to “much better” (PGIC). Men in the testosterone arm were significantly more likely to perceive improvement in their walking ability than men in the placebo arm, both for men enrolled and not enrolled in the PFT (p=0.002 and p=0.001, respectively). The PGIC in walking ability was positively associated with changes in 6MWD as well as in PF10 score (Figures 5A and 5B, P < 0.001).
Falls
Among all men enrolled in the TTrials, the number of men with one or more falls (103 versus 103), the number of men who reported seeking medical attention for fall-related injury (25 versus 26), and the number of men with one or more fractures (6 versus 6) was nearly identical between intervention arms during the intervention period (Table 2).
Table 2.
Reported Falls, by Treatment Arm and Year: All Men in TTrials
| Arm | ||||
|---|---|---|---|---|
| Testosterone | Placebo | |||
| YEAR 1 | N | n=380 | n=380 | |
| At least one fall recorded | Yes | 103 (27%) | 103 (27%) | |
| No | 277 (73%) | 277 (73%) | ||
| Number of falls recorded | 0 | 277 (73%) | 277 (73%) | |
| 1 | 73 (19%) | 58 (15%) | ||
| >1 | 30 (8%) | 45 (12%) | ||
| Sum of all falls | 184 | 202 | ||
| YEAR 2 | N | N=351 | N=337 | |
| At least one fall recorded | Yes | 78 (22%) | 58 (17%) | |
| No | 273 (78%) | 279 (83%) | ||
| Number of falls recorded | 0 | 273 (78%) | 279 (83%) | |
| 1 | 46 (13%) | 34 (10%) | ||
| >1 | 32 (9%) | 24 (7%) | ||
| Sum of all falls | 161 | 112 | ||
Legend: The number (percent) of falls by categories is shown.
DISCUSSION
Testosterone consistently improved self-reported measures of physical function. in older men with mobility limitation. Testosterone also likely improved 6MWD but the treatment effect was modest and appeared to be related to baseline gait speed, the self-reported mobility limitation, and changes in testosterone and hemoglobin levels. Testosterone did not reduce fall frequency. Taken together, these findings suggest a likely small benefit of testosterone on mobility in older men with low testosterone levels. The improvement in self-reported mobility and function, measured by the PF-10 and the PGIC, was observed in all men treated with testosterone, regardless of the baseline walk speed, although the specific effect on 6MWD was greater in men with higher gait speed.
The Physical Function Trial is one of the largest trials of testosterone’s effects on physical function and had several attributes of good trial design: concealed participant allocation and blinded intervention; inclusion of a placebo control; and subject allocation using minimization balanced on several baseline factors. TTrials is one of the largest testosterone trials to be conducted to-date which enrolled older men with unequivocally low testosterone levels, measured using liquid chromatography tandem mass spectrometry assay certified by the Center for Disease Control’s Hormone Standardization Program for Testosterone (HoST). Unlike many previous trials, which enrolled healthy older men without functional limitations, PFT enrolled men who not only had self-reported mobility limitation, but also had slow gait speed assessed objectively using the 6-minute walk test. Because patient-reported outcomes as well as laboratory-based physical performance measures each have inherent limitations, the trial included patient-reported outcomes (PF10) as well as performance-based (6MWS) measures of mobility; the combined application of both patient-reported and performance-based measures of mobility provided a more comprehensive assessment of function than either type of measure alone. Additionally, we included a patient global impression of change to corroborate whether the patients perceived their walking speed to have improved.
The trial also had some limitations. The 6MWS continued to improve throughout the intervention duration and we do not know whether a longer duration of intervention may have enabled the neuromuscular adaptations needed to translate testosterone-induced muscle mass and strength gains into clinically meaningful functional improvements. Multiple comparisons were performed and some of findings may be due to chance alone. We assumed the minimal clinically important difference in 6MWD to be 50 meters, based on the information from epidemiologic studies available at the time the trial was designed (25–27). It is possible that in the participants enrolled in this trial, the MCID for 6MWD may be lower than this estimate, as suggested by the fact that a greater proportion of men in the testosterone arm perceived their walking ability to have improved even though the mean change in 6MWD was substantially smaller than 50 meters.
Contrary to our expectations, the 6MWD improved significantly more with testosterone than with placebo administration in TTrials men who were not enrolled in the PFT (nearly half of whom had a baseline gait speed <1.2 m/sec) than those who were enrolled. We had anticipated that men with clear mobility limitations, both on objective measures and by self-report, would be more likely to show benefits of testosterone treatment on physical function measures. Our analyses show that men with higher baseline gait speed (likely reflecting better physical function at baseline) experienced significantly greater improvements in their gait speed and PF10 scores than men on placebo. The significant interaction between baseline gait speed and treatment arm suggests that the effect of baseline gait speed on response to testosterone is likely real. It is possible that men with better baseline physical function, compared to those with poor function at baseline, may engage in a higher level of physical activity or may experience greater gains in muscle mass contributing to a higher treatment effect; however, physical activity and muscle mass were not measured, which is another limitation.
The men with self-reported mobility limitation, on the other hand, showed significant effects of testosterone administration on walking speed and PF10 while those who did not report mobility limitation did not show such effects; a test of interaction, however, did not confirm an effect of self-reported mobility limitation on response to testosterone treatment. The Patient Global Impression of Change scores indicated a significantly positive impact of testosterone on participant’s perception of improvement in his walking ability overall and separately in men enrolled and not enrolled in the PFT.
The change in hemoglobin was highly significantly associated with change in 6MWD. Some of the improvements in 6MWD could be due to the testosterone-induced increase in hemoglobin, but additional direct effects of testosterone on the muscle mitochondrial function and bioenergetics, and aerobic performance could also contribute to the improvement in 6MWD.
Although lean body mass and muscle strength were not measured in this trial, testosterone administration has been shown consistently in numerous trials to increase skeletal muscle mass and maximal voluntary strength (1–11, 15–16). Therefore, it would be expected to improve those measures of physical function and mobility which are dependent upon lower extremity strength. The overall treatment effect on 6MWD was small, but not dissimilar from that of a physical activity intervention in older adults with mobility limitation (29). It is possible that the 6-minute walk test, which is more a measure of endurance than of lower extremity strength, may be less responsive to testosterone than other measures of mobility such as the stair climbing power, which is more strongly associated with lower extremity strength. Indeed, some trials have reported improvements in stair climbing power with testosterone administration (8, 14). We aimed to raise testosterone levels into the mid-range for healthy men; it is possible that higher on-treatment testosterone levels could result in greater gains in 6MWD.
The number of men reporting falls or seeking medical attention for fall-related injuries during the year on treatment was similar in each treatment group. The falls were recorded by self-report and were not adjudicated or ascertained using a structured interview; furthermore, serious fall injuries were not ascertained or adjudicated. Although it appears unlikely that testosterone treatment has any substantial effect on falls, further studies using more rigorous ascertainment methods, would be needed to determine whether testosterone might have a modest effect on falls.
In summary, testosterone administration in older men with mobility limitation consistently improved self-reported measures of physical function and likely improved mobility, but did not affect fall frequency. The treatment effect on mobility measures was small and appeared to be related to baseline gait speed and the self-reported mobility limitation. These effects may not by themselves justify use of testosterone in older men with low testosterone. Thus, testosterone should probably not be started specifically to improve physical function, but men who are treated with testosterone for other reasons may experience some improvement in physical function. It is possible that functional exercise training may augment the translation of testosterone-induced muscle mass and strength gains into functional improvements, as exercise training has been reported to augment the anabolic effects of testosterone (30). Further studies of longer duration are needed to determine the clinical meaningfulness of testosterone’s effects, using patient-important outcomes that are more closely aligned with testosterone-induced gains in muscle mass and strength, such as stair climbing speed and chair stand.
Supplementary Material
Research in Context.
Evidence Before This Study
The anabolic effects of testosterone on skeletal muscle mass and muscle strength are well recognized, but it is not known whether testosterone improves physical function and mobility or reduces the risk of falls in older men. In 2002 the National Institute on Aging (NIA) requested that the Institute of Medicine (IOM) assess the status of clinical research on testosterone therapy in older men. The IOM committee concluded that there was insufficient evidence that testosterone treatment of older men with low testosterone was beneficial and recommended that the NIA fund a coordinated set of efficacy trials to determine if this treatment has any benefits and to fund a larger trial to determine possible risks only if benefits were found. The NIA followed the IOM’s recommendations and funded The Testosterone Trials (The TTrials) to determine the efficacy of testosterone treatment in older men with age-related decline in testosterone levels and one or more symptoms or signs of testosterone deficiency. The primary results of the TTrials are published (19).
Added Value of this Study
The TTrials included a set of 7 coordinated trials – 3 primary and 4 secondary trials. This manuscript describes in detail the results of the Physical Function Trial (PFT), which was one of the three primary trials. This report also describes testosterone’s effects on fall frequency, which had not been studied previously. Additionally, here we characterized participant characteristics that were related to the treatment response to explain some of the surprising findings of the PFT, namely, that participants with higher gait speed at baseline appeared to show greater improvements in function than those with lower gait speed, contrary to our expectations.
The PFT is the largest controlled trial of testosterone’s effects on physical function and mobility in older men. Unlike previous trials, which often used surrogate endpoints such as lean body mass and muscle performance measures, the TTrials included physical function outcomes that were deemed patient-important and of public health significance. The TTrials included men with unequivocally low testosterone levels, measured using LC-MS/MS. By repeated monitoring of testosterone levels and blinded dose adjustments, we were able to raise and maintain testosterone levels in the mid-normal range for healthy young men. Because both self-reported as well as performance-based measures of physical function have some assets and some inherent limitations, the TTrials included both categories of outcomes to enable a more comprehensive assessment of physical function and mobility than had been conducted before. The PFT is the first to evaluate the effects of testosterone replacement on falls. The subject retention and drug adherence rates were high. With a one-year intervention period, the TTrials also are among the longest testosterone trials.
Implications of all available evidence
Testosterone treatment of older men with mobility limitation who have clearly low testosterone levels consistently improves self-reported mobility but has a modest effect on walking speed. These findings of the PFT are important in the context of the substantial pharmaceutical investment in exploring the application of androgens as function promoting therapies.
ACKNOWLEDGEMENTS
The Testosterone Trials were supported by a grant from the National Institute on Aging, National Institutes of Health (U01 AG030644), supplemented by funds from the National Heart, Lung and Blood Institute, National Institute of Neurological Diseases and Stroke, and National Institute of Child Health and Human Development. AbbVie (formerly Solvay and Abbott Laboratories) generously provided funding and donated AndroGel and placebo gel. Additional support was provided by resources of the Boston Claude D. Pepper Older Americans Independence Center grant 5P30AG031679 (SB, PI), and the Boston University’s Clinical and Translational Science Institute grant 1UL1RR025771.
TMG is the recipient of an Academic Leadership Award (K07AG3587) from the National Institute on Aging. The Yale Field Center was partially supported by the Claude D. Pepper Older Americans Independence Center (P30-AG021342) and CTSI (UL1TR000142). AMM was supported by the Department of Veterans Affairs Puget Sound Health Care System.
The funding agencies played no role in the design of the trial, analyses of data, preparation of the manuscript, or in the decision to publish.
Funding: The Testosterone Trials were supported by a grant from the National Institute on Aging, National Institutes of Health (U01 AG030644), the National Heart, Lung and Blood Institute, National Institute of Neurological Diseases and Stroke, and National Institute of Child Health and Human Development. AbbVie (provided funding and donated AndroGel and placebo gel.
Declaration of Interests
Dr. Bhasin reports receiving consulting fees from AbbVie, Novartis, and Regeneron, and grant support from AbbVie, MIB, LLC, Alivegen, Abbott, Novartis, Regeneron, and Transition Therapeutics; he also reports holding pending patent related to an algorithm for free testosterone determination. Dr. Gill reports receiving consulting fees from Novartis. Dr. Snyder reports receiving consulting fees and grant support from AbbVie. Dr. Cunningham reports receiving fees for serving as an advisor to AbbVie, Apricus Biosciences, Clarus Therapeutics, Endo Pharmaceuticals, Ferring Pharmaceuticals, Eli Lilly, Purdue Pharma, and Repros Therapeutics and grant support from Ardana. Dr. Matsumoto Dr. Matsumoto reports receiving consulting fees from AbbVie, Aytu and Eli Lilly, study medication from AbbVie, and grant support from GlaxoSmithKline. Dr. Swerdloff reports receiving consulting fees from Clarus Therapeutics, Novartis, and TesoRx and grant support from Clarus Therapeutics, Eli Lilly, Novartis, and Antares Pharma. Dr. Wang reports receiving fees for serving on an advisory board from TesoRx and grant support from Clarus Therapeutics, Lipocine, and Antares Pharma. Dr. Ensrud reports receiving fees for serving on a data and safety monitoring committee from Merck Sharp & Dohme. Dr. Farrar reports receiving fees for serving on a data and safety monitoring board from Cara Therapeutics, consulting fees from Analgesic Solutions, Aptynx, Biogen, the Campbell Consortium, Daiichi-Sankyo, Depomed, Evadera, Janssen, Mallinckrodt, Novartis, Pfizer, and Wolter Kluwer Health, and grant support from Pfizer. Dr. Cella reports receiving consulting fees from Pfizer and being the president of FACIT.org. Dr. Molitch reports receiving consulting fees from AbbVie, Eli Lilly, and Pfizer. Dr. Basaria reports receiving consulting fees from Eli Lilly and grant support from AbbVie. Dr. Ellenberg reports receiving grant support from AbbVie. No other potential conflict of interest relevant to this article was reported.
Footnotes
The TTrials Investigators
REFERENCES
- 1.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac R, Lee M, et al. Older men are as responsive to the anabolic effects of testosterone as young men. J Clin Endocrinol Metab. 2005;90(2):678–88. [DOI] [PubMed] [Google Scholar]
- 2.Storer TW, Magliano L, Woodhouse L, Lee MI, Dzekov C, Dzekov J, et al. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88(4):1478–85. [DOI] [PubMed] [Google Scholar]
- 3.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999; 84: 2647–53. [DOI] [PubMed] [Google Scholar]
- 4.Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, et al. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab. 2005; 90: 1502–10. [DOI] [PubMed] [Google Scholar]
- 5.Kenny AM, Prestwood KM, Gruman CA, Marcello KM and Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. The journals of gerontology Series A, Biological sciences and medical sciences. 2001; 56: M266–72. [DOI] [PubMed] [Google Scholar]
- 6.Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002; 288: 2282–92. [DOI] [PubMed] [Google Scholar]
- 7.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010; 363: 109–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travison TG, Basaria S, Storer TW, Jette AM, Miciek R, Farwell W, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol Medical Sciences A. 2011; 66:1090–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivas-Shankar U, Roberts SA, Connolly MJ, O’Connell MD, Adams JE, Oldham JA, et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010; 95: 639–50. [DOI] [PubMed] [Google Scholar]
- 10.Kenny AM, Bellantonio S, Gruman CA, Acosta RD and Prestwood KM. Effects of transdermal testosterone on cognitive function and health perception in older men with low bioavailable testosterone levels. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2002; 57: M321–5. [DOI] [PubMed] [Google Scholar]
- 11.Storer TW, Woodhouse L, Magliano L, Singh AB, Dzekov C, Dzekov J, et al. Changes in muscle mass, muscle strength and power, but not physical function are related to testosterone dose in healthy older men. J Am Geriatrics Soc. 2008;56:1991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, Nehra A, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006. October 19;355(16):1647–59. [DOI] [PubMed] [Google Scholar]
- 13.Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, et al. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008. January 2;299(1):39–52. [DOI] [PubMed] [Google Scholar]
- 14.Storer TW, Basaria S, Traustadottir T, Harman SM, Pencina K, Li Z, Travison TG, Miciek R, Tsitouras P, Hally K, Huang G, Bhasin S. Effects of testosterone supplementation for 3 years on muscle performance and physical function in older men. J Clin Endocrinol Metab. 2017. February 1;102(2):583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basaria S, Harman SM, Travison TG, Hodis H, Tsitouras P, Budoff M, Pencina KM, Vita J, Dzekov C, Mazer NA, Coviello AD, Knapp PE, Hally K, Pinjic E, Yan M, Storer TW, Bhasin S. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: A Randomized Clinical Trial. JAMA. 2015;314:570–81. [DOI] [PubMed] [Google Scholar]
- 16.Liverman C and Blazer D. Testosterone and aging. Clinical research directions. Washington, DC: The National Academies Press, 2004. [PubMed] [Google Scholar]
- 17.Snyder PJ, Ellenberg SS, Cunningham GR, Matsumoto AM, Bhasin S, Barrett-Connor E, et al. The Testosterone Trials: Seven coordinated trials of testosterone treatment in elderly men. Clin Trials. 2014. March 31;11(3):362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cauley JA, Fluharty L, Ellenberg SS, Gill TM, Ensrud KE, Barrett-Connor E, et al. Recruitment and Screening for the Testosterone Trials. J Gerontol A Biol Sci Med Sci. 2015. September;70(9):1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, et al. Effects of testosterone on sexual function, physical function and vitality in older men. N Engl J Med. 2016;374(7):611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA 2011; 305: 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taves D. Minimization: A new method of assigning patients to treatment and control groups. Clinical Pharmacology and Therapeutics. 1974;15: 443–53. [DOI] [PubMed] [Google Scholar]
- 22.Woo J, Ho SC, Yu AL. Walking speed and stride length predicts 36 months dependency, mortality, and institutionalization in Chinese aged 70 and older. J Am Geriatr Soc. 1999;47:1257–1260 [DOI] [PubMed] [Google Scholar]
- 23.Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, Visser M, Rubin SM, Harris TB, Pahor M. Prognostic value of usual gait speed in .well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680 [DOI] [PubMed] [Google Scholar]
- 24.Melzer D, Lan TY, Guralnik JM. The predictive validity for mortality of the index of mobility-related limitation--results from the EPESE study. Age Ageing. 2003;32:619–625 [DOI] [PubMed] [Google Scholar]
- 25.Kennedy D, Stratford P, Wessel J, Gollish J and Penney D. Assessing stability and change of four performing measures: a longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC musculoskeletal disorders. 2005. 6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perera S, Mody S, Woodman R and Studenski S. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society. 2006; 54: 743–9. [DOI] [PubMed] [Google Scholar]
- 27.Redelmeier D, Bayoumi A, Goldstein R and Guyatt G. Interpreting small differences in functional status: the Six Minute Walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997; 155: 1278–82. [DOI] [PubMed] [Google Scholar]
- 28.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992; 30:473–83. [PubMed] [Google Scholar]
- 29.Santanasto AJ, Glynn NW, Lovato LC, Blair SN, Fielding RA, Gill TM, Guralnik JM, Hsu FC, King AC, Strotmeyer ES, Manini TM, Marsh AP, McDermott MM, Goodpaster BH, Pahor M, Newman AB; LIFEStudy Group. Effect of physical activity versus health education on physical function, grip strength and mobility. J Am Geriatr Soc. 2017;65(7):1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996. 4;335(1):1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.