Abstract
Barriers between circulation and the central nervous system (CNS) play a key role in the development and modulation of CNS immune responses. Structural variations in the vasculature traversing different anatomical regions within the CNS strongly influence where and how CNS immune responses first develop. Here, we provide an overview of cerebrovascular anatomy, focusing on the blood-CNS interface and how anatomical variations influence steady-state immunology in the compartment. We then discuss how CNS vasculature is affected by and influences the development of different pathophysiological states, such as CNS autoimmune disease, cerebrovascular injury, cerebral ischemia, and infection.
INTRODUCTION
The central nervous system (CNS) has a complex barrier system and has evolved specialized mechanisms to mount and regulate immune reactions. CNS immune responses differ based on anatomical location, and a clear distinction exists between responses that develop in the fluid spaces and membranous lining (or meninges) relative to the parenchyma (1). For example, early studies demonstrated that mouse sarcoma cells grow rapidly when injected into the brain parenchyma of rats but were immunologically rejected when placed close to a ventricle [a space filled with cerebrospinal fluid (CSF)] (2). These findings show that the development of robust immune reactions against parenchymal antigens depends on their entry into fluid spaces, which promotes egress into draining lymph nodes. Although the CNS parenchyma does not have a conventional lymphatic drainage system (3), lymphatics have been discovered in the dura mater and just outside holes in the skull bone (4-9). Once an immune response is mobilized in peripheral lymphoid tissues, leukocyte traffic into the CNS is heavily regulated by barriers that include the blood-brain barrier (BBB), blood-CSF barrier, and blood-meningeal barrier (10). Structural variations in these barriers influence the location and development of CNS immune responses that enter through vasculature. These barriers essentially control immune cell entry into different CNS compartments. Here, we review the anatomy and physiology of CNS vasculature and associated barriers and how these structures influence immune surveillance under steady-state and inflammatory conditions.
NEUROANATOMICAL OVERVIEW
Arterial blood enters the cranial cavity, primarily through two sets of blood vessels: the internal carotid arteries, which give rise to anterior brain circulation, and the vertebral arteries, which give rise to posterior circulation. These are interconnected through the circle of Willis located in subarachnoid cisterns at the base of the brain (11). The circle of Willis allows any one of these four vessels to take over brain perfusion and provides a protective mechanism against ischemia. The internal carotid arteries supply the cerebrum, whereas the vertebral arteries join to form the basilar artery that supplies the brainstem and cerebellum. Second-order branches form vessels that traverse the subarachnoid space and pia mater, giving rise to smaller arterioles (Fig. 1) that enter the brain parenchyma (Fig. 1A) (12). These penetrating vessels are surrounded by perivascular spaces that can vary in size. For example, in the cortex, a packed barrier of endothelial cells, basement membrane, and glia limitans surrounds penetrating arteries, leaving little to no fluid-filled space, whereas the lenticulostriate branches in the basal ganglia are surrounded by a larger perivascular space (13). Pia mater covers all arteries in the subarachnoid space, whereas coverage of veins is incomplete. In addition, the fluid in perivascular spaces can move directly into the subarachnoid space through fenestrations in the pia mater (Fig. 1A) (14). This allows direct communication between perivascular, subpial, and subarachnoid spaces. The communication of the perivascular compartment with the subarachnoid space plays an important role in antigen presentation, which will be described in more detail below. Venous drainage in the CNS occurs through an interconnected system of valveless veins and dural sinuses. Postcapillary venules in the CNS parenchyma drain capillaries and are surrounded by fluid-filled perivascular spaces (Figs. 1, A and B, and 2) (15). They connect into a deep and superficial venous system. Deep venous drainage occurs through the subependymal veins, internal cerebral veins, basal vein, and the great vein of Galen, which connect into the straight sinus beneath the brain. Superficial drainage occurs through cortical veins in the pia mater that drain into the dural sinuses. The venous system ultimately drains into the jugular veins or pterygoid plexus (11).
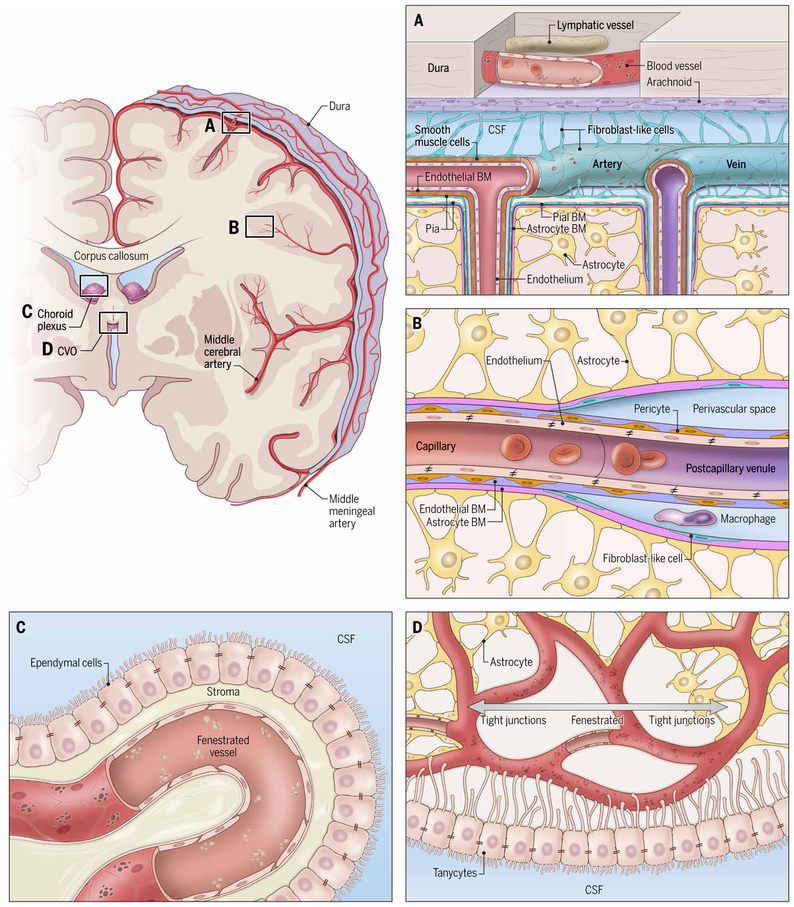
Fig. 1. Structural variations in the anatomy of cerebrovascular barriers.
Coronal depiction of cerebrum and dura mater in relation to the middle cerebral and middle meningeal arteries. (A) Meningeal and cortical vascular anatomy. The dura mater contains lymphatics and fenestrated blood vessels that lack tight junctions. The arachnoid mater is an epithelial layer that provides a barrier between the peripheral vasculature of the dura mater and the CSF through tight junctions and efflux pumps. Leptomeningeal blood vessels in the pia mater lack astrocytic ensheathment, but their endothelial cells are connected by tight junctions. There are small stomata in the connective tissue (fibroblastic reticular cells) covering pial vessels that allow an exchange of fluid between the CSF and perivascular space. Pial arteries penetrate the brain and are covered by a densely packed perivascular layer of astrocytic foot processes; astrocytic (pink), pial (gray), and endothelial (purple) basement membranes; and smooth muscle cells. Veins exiting the parenchyma have a perivascular space flanked by astrocytic foot processes as well as endothelial basement membranes (BMs). The pial BM is only present in the superficial portion of the veins. (B) Capillary and postcapillary venule within the brain parenchyma. The capillary endothelial BM (purple) is juxtaposed to the astrocytic BM (pink), whereas the postcapillary venule is surrounded by a perivascular CSF-filled space that separates the endothelial BM from the astrocytic BM. Fibroblast-like cells (green) form an interrupted extension of the pia mater in the postcapillary venules. (C) Vessels in the choroid plexus are fenestrated and lack tight junctions. The ependymal cells overlying the choroid plexus have tight junctions that are tasked with forming a blood-CSF barrier. (D) Vasculatures at the center of CVOs (such as the subfornical organ) are fenestrated and lack tight junctions, allowing exposure to solutes from the circulation. Vasculatures around the perimeter of CVOs have a traditional BBB surrounded by astrocytic foot processes and more closely resemble vessels found in the CNS parenchyma. Overlying ependymal tanycytes are highly specialized cells with tight junctions that separate CVOs from the CSF.
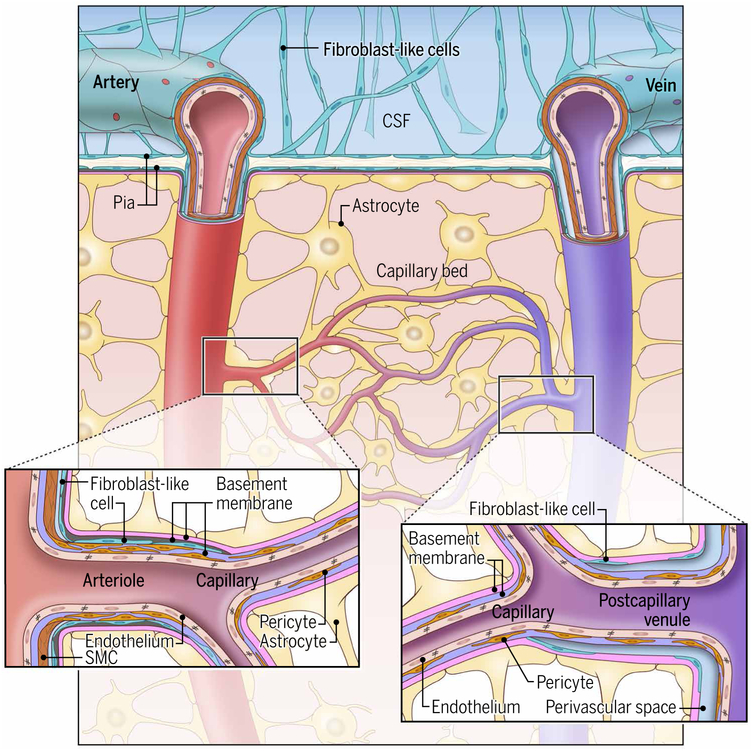
Fig. 2. Schematic representation of a cortical cerebrovascular bed.
Leptomeningeal arteries penetrate the cortex, giving rise to arterioles, capillaries, and postcapillary venules that lastly drain into cortical veins that exit the parenchyma. Leptomeningeal arteries and veins are surrounded by fibroblast-like cells and a collagen layer. Penetrating arteries are ensheathed by smooth muscle cells (SMCs) that transition to a pericyte layer at arteriole branch points. The pial layer of fibroblast-like cells ends abruptly at the transition from arteriole to capillary. In capillaries, the astrocytic BM (pink) is adjacent to the endothelial cell BM (purple). These membranes separate at the level of the postcapillary venule to form a perivascular space. Interrupted fibroblast-like cells are also present along postcapillary venules and veins as they exit the CNS parenchyma.
The meningeal vasculature is a major point of entry into the CNS and should be considered separately from those that traverse the CNS parenchyma. The meninges are three membranes that envelop the CNS and consist of dura, arachnoid, and pia mater (Fig. 1A). In humans, the dura is a ~1-mm-thick fibrous structure with an inner and outer layer that separate to form large venous sinuses. The outer layer is attached to the inner surface of the skull and extends vascular connections into the bone. Blood vessels within the dura mater are fenestrated and do not have tight junctions, leaving this structure open to peripheral circulation (Fig. 1A). The arachnoid mater is a ~200-μm translucent avascular membrane that is structurally contiguous to the dura mater and is composed of two layers of squamous epithelial cells that express varying amounts of laminin α5+ basement membrane. The inner cell layer consists of ramified epithelial cells with long cytoplasmic processes, and these cells are connected by tight junctions (16-18). Beneath the sealed arachnoid mater is the subarachnoid space, which is filled with CSF. Fibroblast-like cells and sheet-like collagenous trabeculae connect the inner arachnoid membrane to the pia mater, dividing the subarachnoid space into relatively distinct CSF compartments (Fig. 1A) (19). The pia mater is composed of epithelial cells that produce a laminin α1+ basement membrane and fibroblast-like cells and separates the subarachnoid space from brain parenchyma and perivascular spaces (18, 20). This structure does not have tight junctions like the arachnoid mater. Together, the arachnoid and pia mater comprise the leptomeninges.
Most of the blood supply to the cranial dura mater comes from meningeal arteries originating from the internal carotid artery, vertebral artery, and external carotid artery (Fig. 1). The dura mater is supplied anteriorly by the anterior meningeal arteries that arise from the ophthalmic artery. The middle meningeal arteries, on the other hand, arise from the maxillary artery, and the posterior meningeal arteries arise from the occipital and vertebral arteries (11). There is also blood supply to the dura mater from the aforementioned pial vessels, which include olfactory branches and pericallosal branches, the anterior falcine artery, the medial dural tentorial branch, the subarcuate artery, and the posterior meningeal artery (21). The arachnoid and pia mater are relatively avascular when compared with the dura mater (16).
An abundant anastomotic arterial network covers the dura mater and is in the outer layer of this structure. Venous drainage occurs through satellite veins that accompany the arteries and through an irregular network of veins and venous lakes that drain into the venous sinuses (22). There are also venous connections between the dura mater and skull bone. Diploic veins are large veins with irregular dilatations that connect skull bone marrow pockets to meningeal veins, dural sinuses, and pericranial veins. Emissary veins, on the other hand, traverse the entire skull bone and make connections between venous sinuses in the dura mater and extracranial veins in the scalp (23, 24). Two additional CNS structures that require special consideration from a vascular perspective are the choroid plexus within the ventricles (Fig. 1C) and the circumventricular organs (CVOs) (Fig. 1D). Blood vessels within these two structures have endothelia that lack tight junctions and are open to peripheral circulation (25, 26). The ventricles are a communicating system of five cavities within the brain parenchyma, lined with ependymal cells and filled with cerebrospinal fluid (Fig. 1C). The choroid plexus is a spongy, vascularized structure that produces CSF and is attached to the walls of ventricles. It contains capillaries of choroidal arteries that are covered by an ependymal epithelial layer. The ependymal cells express tight junctions, providing a barrier between the fenestrated blood vessels and the CSF (Fig. 1C). The choroid plexus extends from the inferior horn of the lateral ventricle to the interventricular foramen where it connects to the choroid plexus of the roof of the third ventricle. There is also choroid plexus located at the roof of the fourth ventricle. The choroid plexus of the lateral and third ventricles is supplied by branches of the internal carotid artery and the posterior cerebral artery. The choroid plexus of the fourth ventricle is supplied by the posterior circulation (23).
The CVOs of the brain are a distinct group of structures bordering the third and fourth ventricles that perform homeostatic and neurosecretory functions and lack a BBB (Fig. 1D). The CVOs provide a connection between the CNS and peripheral blood and are usually divided into two categories: sensory and secretory. Sensory CVOs include the subfornical organ, area postrema, and vascular organ of the lamina terminalis. These sample the contents of the systemic circulation and relay information to the CNS. By contrast, secretory CVOs—such as the subcommissural organ, median eminence, posterior pituitary, and pineal gland—secrete substances such as peptides directly into the circulation (25).
Apart from these classical neuroanatomical descriptions, it is important to understand the structural changes in CNS blood vessels as they progress from leptomeningeal arteries to parenchymal arterioles, capillaries, postcapillary venules, venules, and, lastly, veins (Fig. 2). The surface of the CNS parenchyma consists of glia limitans basement membrane and pia mater that follow penetrating arteries to points of arteriole branching (18). More specifically, laminin α1+ basement membrane and fibroblast-like cells are observed before these branch points in the parenchyma. Fibroblast-like cells are also found along postcapillary venules, where they are sparse and not accompanied by a fully formed basement membrane. Postcapillary venules and veins have a more pronounced CSF-filled perivascular space than the other types of parenchyma blood vessels (15), and these spaces support a lot of immune activity. Mural cells in the form of pericytes and smooth muscle cells are present throughout the arteriovenous continuum. Arterial smooth muscle cells gradually transition to pericytes at the level of the arteriole. Recent single-cell transcriptomic (27) and immunohistochemical studies (18) have provided molecular definitions for the principal cell types along the CNS arteriovenous continuum and have also mapped the distinct forms of basement membrane.
BLOOD-CNS INTERFACE
P. Ehrlich was the first to recognize that there is a barrier between the circulation and brain that differs from most peripheral organs. He injected coerulean-S sulfate intravenously into rodents and observed lack of extravasation into the brain (28). Since then, it has been observed that although intravascular tracers do not enter the brain parenchyma, they are found in the leptomeninges, choroid plexus, and perivascular spaces (29). Therefore, it is of importance to consider the differential permeability of these different anatomical compartments. The BBB should be considered a summation of mechanisms that control the exchange of substances and cells between the circulation and CNS. Collectively, the BBB is composed of endothelial cells, basement membrane, pericytes, glia limitans, and microglia. Interactions among these different cells and structures along with neurons refine the functions of the BBB and together act as a neurovascular unit (30). BBB endothelial cells selectively restrict movement of substances into the CNS by means of tight junctions and enzymatic reactions. However, these cells can also selectively transport small and large molecules into the CNS through passive diffusion, facilitated diffusion, and active transport. The endothelial cells comprising most CNS blood vessels, including subpial and parenchymal vessels, lack fenestrations and have diminished pinocytosis. They are also connected by tight junctions, which consist of occludin and claudins, that link to the cytoskeleton through scaffolding proteins [such as zonula occluden-1 (ZO-1), ZO-2, ZO-3, and cingulin] (Fig. 3). Adherens junctions, including cadherin, also link endothelial cells and connect to actin filaments through α-, β-, and γ-catenins; vinculin; and actinin (31). Activation of Rho guanosine triphosphatases (GTPases) regulates the length of these actin fibers, thus controlling the integrity of both adherens junctions and tight junctions (32).
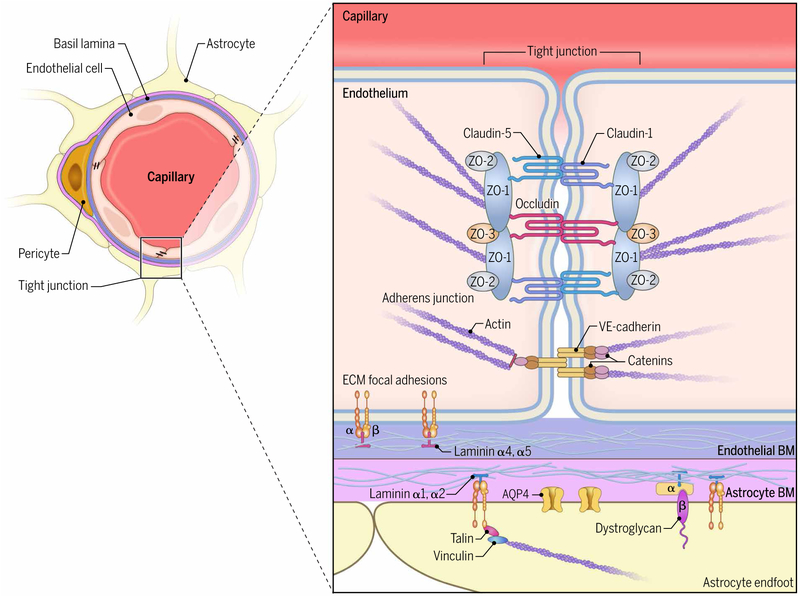
Fig. 3. Anatomy of parenchymal capillary barriers.
Tight junction proteins—such as occludin; claudin-3, claudin-5, and claudin-12; and ZO-1 and ZO-2—and JAMs form tight junctions between cerebrovascular endothelial cells that limit the passage of materials from the blood into the CNS. These are linked to the actin cytoskeleton within endothelial cells. Vascular endothelial cadherins and intracellular catenins form adherens junctions that provide tensile force between endothelial cells through linkage to the actin cytoskeleton. The endothelial BM is primarily composed of laminins α4 and α5 and is juxtaposed to the astrocytic BM, which is composed of laminins α1 and α2. The endothelial cells interact with the basal lamina through α and β integrins that bind to laminins and fibronectin. Similarly, astrocytic endfeet interact with BM through integrins and dystroglycan.
The matrix components of the basal lamina—which include laminins, proteoglycans, fibronectin, and type IV collagen—also play an important role in the regulation of the BBB and contribute to the interactions between endothelial cells and astrocytic endfeet (Fig. 3). This is mediated by integrin αβ receptors bound to laminin and fibronectin as well as dystroglycan, which binds to laminins, agrin, and perlecan (33). The endothelial and astrocytic basement membranes have a similar structure but differ in their composition of laminin isoforms. The endothelial basement membrane is composed primarily of laminins α4 and α5, whereas the astrocytic basement membrane is composed of laminin α2 and, to a lesser extent, laminin α1 (Fig. 3) (18). Pericytes are undifferentiated contractile connective tissue cells embedded within the endothelial basement membrane (Fig. 2) (34). For capillary endothelial cells, basement membrane is either directly attached to or closely juxtaposed to the basement membrane of astrocytic endfeet (18). This negatively charged tight structure permits free migration of macromolecules with low charge, such as ferritin with a 13-nm-diameter (35) and intrathecally injected immunoglobulin G (36). In postcapillary venules, there is a better defined fluid-filled perivascular space surrounding the basement membrane (Fig. 2) (37).
Astrocytic endfeet form the glia limitans, which covers the entire surface of the brain and spinal cord (glia limitans superficialis) and separates the perivascular space from the brain parenchyma (glia limitans perivascularis) (Figs. 1, A and B, and 2). They cover >99% of the vascular surface and densely surround tight junctions, creating an additional barrier that limits entry into the CNS parenchyma (38). Molecules from 0.8 to 70 kDa have been shown to penetrate the glia limitans to varying degrees, whereas molecules from 150 to 2000 kDa are confined within the perivascular space (18, 39, 40). The 20-nm gaps between astrocytic endfeet likely generate a crucial cutoff for diffusion of molecules into the interstitial fluid (ISF) (40); however, it is unclear whether astrocytic coverage is complete (41), and the tightly compacted basement membrane may also play an important role in preventing solute diffusion into the brain parenchyma (18). The glia limitans and basement membranes form the rate-limiting barrier between CSF and ISF. Molecule exchange between these two fluids depends on size, lipophilicity, concentration gradients, and astrocytic transport mechanisms, among others. The glia limitans and basement membranes also create a barrier between circulating components of the immune system and the CNS parenchyma, a division that is integral to the neuroimmune axis (42). The close proximity of astrocytic endfeet and endothelial cells has led to the notion that the glia limitans can directly affect BBB permeability (43). Although all the astrocytic factors that promote permeability are not known, those often implicated in the process are glutamate, aspartate (43), interleukin-1β (IL-1β) (44, 45), endothelin-1, nitric oxide (46), interferon-γ (45), and tumor necrosis factor-α (TNFα) (45, 47).
Within the meninges, blood vessels differ based on their anatomical location. For example, endothelial cells comprising blood vessels in the dura mater lack tight junctions and allow extravasation of solutes as large as 43 kDa (Fig. 1A) (48). The dura mater has lymphatic drainage (4-8) and in many ways resembles a peripheral tissue. However, the arachnoid mater, which has tight junctions and efflux pumps, serves as an important barrier between the fenestrated peripheral vasculature of the dura and the CSF (49). The arachnoid and pia mater are sometimes referred to together as the leptomeninges, and blood vessels within this structure have endothelial cells connected by tight junctions but lack astrocytic foot processes (Fig. 1A). The paracellular junctions between these endothelial cells vary in tightness. Some are similar to those found on parenchymal vessels, whereas others have 2.8-nm gaps (50). Nevertheless, pial vessels are not permeable to the majority of molecules, including 44-kDa horseradish peroxidase and 0.4-kDa fluorescein (51). Pial vessels do have 1- to 3-μm stomata in the adventitia (a layer of connective tissue covering the vessels) (Fig. 1A), potentially allowing an exchange of macromolecules between the CSF and perivascular space (36).
The blood-CSF barrier within the choroid plexus has fundamentally different properties than those of the BBB. The endothelium on choroid plexus vessels is fenestrated and lacks tight junctions and a glia limitans (Fig. 1C). However, ependymal cells overlying these vessels do have tight junctions and are tasked with forming a blood-CSF barrier composed of transmembrane proteins such as occludin, claudin-1, claudin-2, and claudin-11 (52, 53). Large proteins in plasma can diffuse out of choroidal capillaries but are restricted by the tight junction–expressing ependyma. The choroid plexus, which is located within the ventricles, has a very narrow connection to surrounding parenchyma at the choroid vessel entry point (11). Therefore, solutes extravasating from choroid plexus capillaries cannot travel directly into the brain parenchyma.
Like vessels in the choroid plexus, CVO vasculature is also fenestrated, with circular pores 40 to 60 nm in diameter that allow exposure to solutes from circulation (54). More specifically, blood vessels at the center of CVOs lack tight junction molecules, whereas vasculature in the periphery of these organs more closely resembles vessels with a traditional BBB in the brain parenchyma (Fig. 1D). In addition, the capillaries at the center of the CVO lack a glia limitans (25). Therefore, capillaries in central CVO subdivisions have higher vascular permeability compared with those around the perimeter. Within the CVO, the endothelial cell basement membrane on all capillaries is covered by an outer basement membrane. After intravenous administration, 10-kDa dextran was observed in the perivascular spaces of CVO capillaries but did not enter the organ. By contrast, tracers with a smaller molecular weight (such as fluorescein and 3-kDa dextran) were able to enter the CVO but not the surrounding brain tissue because of a densely packed perimeter of astrocytes-tanycytes that express tight junction proteins (25). Similar to the choroid plexus, the CVO has highly specialized ependymal cells (referred to as tanycytes) that extend processes into the CVO parenchyma and form a barrier between the CVO parenchyma and the CSF. Tanycytes are connected with tight junctions that include ZO-1, occludin, claudin-5, and claudin-1 that limit diffusion of macromolecules from the CSF into the CVO (55).
CNS IMMUNE SURVEILLANCE
The elaborate barrier system between the brain parenchyma and circulation combined with restricted afferent and efferent communication with lymphatic tissue limits immune surveillance of the CNS relative to other peripheral tissues. CNS immune privilege differs substantially between the brain parenchyma and CSF-filled spaces such as the meninges (56). The glia limitans separates the brain parenchyma from the CSF/perivascular spaces and plays a major role in compartmentalizing CNS immune reactions.
The perivascular space plays a role in antigen drainage from the CNS parenchyma. Only a small fraction of ISF (15%) is secreted into the CSF (57), and the role of an intraparenchymal convective flow (the glymphatic system) (40) in this process remains controversial (58, 59). Movement of ISF from the brain parenchyma occurs primarily between basement membranes in the perivascular spaces of capillaries and arteries. This continues along basement membranes in the tunica media of cerebral and leptomeningeal arteries (13). Antigen, antibody, and complement complexes can become entrapped in this arterial basement membrane drainage pathway and impair perivascular lymphatic drainage (60).
As noted previously, the inner and outer basement membranes of parenchymal capillaries are directly apposed and have minimal space for perivascular fluid. This limits the ability of immune cells to traffic from the brain parenchyma to draining lymph nodes. In fact, fluorescent tracers (3-kDa dextran and 40-kDa ovalbumin) were shown to move from the brain parenchyma along basement membranes, whereas particulates (0.2 to 1.0 μm in diameter) remained trapped in the parenchyma. By contrast, injection of these same particulates into the CSF resulted in egress to the cervical lymph nodes (61). This result explains why intracerebral injection of BCG (62) or influenza virus (56) directly into the brain parenchyma does not elicit an adaptive immune response. Antigens are known to move from the CSF compartment to mandibular and deep cervical lymph nodes by exiting through perineural pathways—including the olfactory, optic, and trigeminal nerves (9, 63)—into an extensive network of lymphatic vessels that reside outside the CNS. Antigen drainage from the dura mater occurs in a similar fashion by way of lymphatic vessels traversing the dura mater and draining to the deep cervical lymph nodes (4, 5, 64).
Immune surveillance and the initiation of CNS immune responses is dependent on antigen-presenting cells (APCs) that reside alongside important barriers such as perivascular spaces, leptomeningeal vessels, the choroid plexus, and the subarachnoid space (65-68). These spaces contain bone marrow-derived dendritic cells (DCs) and relatively long-lived macrophages, although the DCs are generally more efficient at presenting antigen (67, 69, 70). Like microglia, most macrophages residing in the leptomeninges and perivascular spaces are derived from early yolk sac–derived erythromyeloid progenitors, and during steady state, these cells have minimal turnover. This contrasts with macrophages in the choroid plexus and dura mater, which do turn over and receive some input from peripheral blood monocytes during adulthood (71-73). This is likely because both compartments contain fenestrated blood vessels and are open to peripheral circulation. In fact, the choroid plexus is proposed to serve as an immune gateway (74, 75), and the dura mater similarly hosts a high amount of immune traffic.
To survey peptides displayed on the surface of CNS APCs, T cells must access the spaces in which these APCs reside. Whereas diffusion of solutes in the CNS parenchyma is regulated mostly at the level of capillaries, immune cell extravasation often occurs along postcapillary venules (Fig. 4) (76). Leukocytes can traverse vascular endothelium through trans- or paracellular routes (77). To access the brain parenchyma, these cells need to migrate across endothelial cells and inner basement membrane as well as the glia limitans and outer basement membrane, which is a two-step process (78). Under steady state, brain endothelial cells do not support myeloid cell adhesion because of a lack of surface P-selectin expression (79). In fact, leukocyte migration across the BBB is limited to very few activated CD8+ and CD4+ T cells but not innate immune cells (80). α4-Integrins mediate capture of activated CD4+ T cells on BBB endothelium by binding to adhesion molecules such as vascular cell adhesion molecule–1 (VCAM-1). This is followed by G protein–dependent strengthening of the interaction and eventual extravasation (Fig. 4) (81). Activated T cells can enter the perivascular space independent of antigen specificity (82), but are unable to traverse the astrocytic basement membrane and glia limitans. CXCL12 expression by endothelial cells contributes, in part, to retaining activated CD4+ T cells within the perivascular space through CXCR4 binding (Fig. 4) (83). In addition, activated T cells need to recognize cognate antigen presented by perivascular APCs to enter the CNS parenchyma (65, 84); otherwise, they undergo apoptosis or reenter the circulation. The importance of this T cell–dependent surveillance process is exemplified by the development of progressive multifocal leukoencephalopathy, a CNS disease induced by John Cunningham virus infection, in some patients that receive therapeutic antibodies directed against α4-integrins (85).
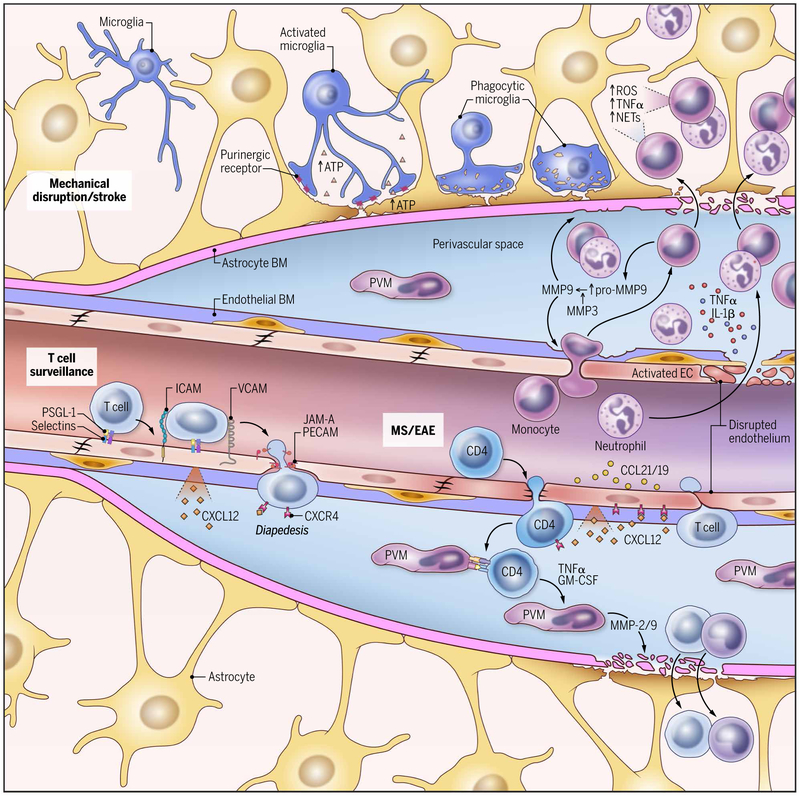
Fig. 4. Blood-CNS interface during inflammation.
This schematic of a CNS blood vessel summarizes three different immunological scenarios: steady-state T cell surveillance, CNS autoimmune disease, and cerebrovascular injury and stroke. T cell surveillance: During steady state, leukocyte extravasation across the BBB is limited to few activated T lymphocytes that interact with ICAM-1 and VCAM-1 expressed on the lumen of vascular endothelial cells. CXCL12 expression by endothelial cells on the abluminal side contributes to sequestering these activated CD4+ T cells in the perivascular space through binding to CXCR4 on the T cell. EAE: During CNS autoimmune diseases such as EAE, the efficiency of leukocyte diapedesis is increased. CCL19, CCL21, and CXCL12 are up-regulated by cerebrovascular endothelial cells that promote the recruitment and adhesion of encephalitogenic CD4+ T cells. Vascular adherence and extravasation are also facilitated by selectins (P-selectin–PSGL-1 interactions) and integrins (LFA-1–ICAM-1 and VLA-4–VCAM-1 interactions). CXCR7, expressed on the abluminal surface of endothelial cells, binds to CXCL12 and reduces T cell sequestration in the perivascular space. After extravasation, T cells interact with APCs, including perivascular macrophages (PVMs), in the perivascular space. Recognition of cognate-peptide MHC complexes results in production of chemokines and cytokines (such as TNFα and granulocyte-macrophage colony-stimulating factor) that promote recruitment of myelomonocytic cells from the blood. This is followed by production of metalloproteinases (such as MMP-2 and MMP-9) that selectively cleave dystroglycan in the astrocytic foot processes, allowing penetration of effector T cells into the CNS parenchyma. Vascular injury and stroke: Mechanical disruption of the glia limitans leads to a rapid release of ATP that is detected by purinergic receptors expressed on microglia. The microglia provide immediate barrier support and debris clearance. Cerebrovascular injury can also cause resident and infiltrating monocyte-derived macrophages to release cytokines (TNFα and IL-1β), chemokines, ROS, and metalloproteinases (pro–MMP-9). TNFα and IL-1β trigger endothelial cell activation, promoting further myelomonocytic cell invasion. Pro–MMP-9 becomes activated by MMP-3, causing additional destruction of the glia limitans. Once in the parenchyma, neutrophils can release ROS and NETs in an attempt to control pathogens that are not present. These effector mechanisms contribute to tissue damage.
Subarachnoid and pial vessels also restrict extravasation of leukocytes; however, migration across these vessels is more efficient relative to parenchymal vessels (86). This is because meningeal, unlike parenchymal, vascular endothelial cells constitutively express P-selectin during steady state, which is stored in Weibel-Palade bodies, allowing rapid release to the endothelial surface (79, 86). Also, CCR7-dependent extravasation of leukemic T cells in the leptomeninges suggests that local expression of CCL19 can promote memory CCR7+ T cell extravasation across leptomeningeal blood vessels (87). In general, CNS compartments such as the meninges, choroid plexus, and CVOs are more permissive to steady-state immune cell surveillance. Steady-state immune cell traffic through the choroid plexus is influenced by type I and type II interferon signaling. With age, a type I interferon–induced gene expression is observed in the choroid plexus that negatively affects brain function (88, 89). Blockade of type I interferon improves brain function and restores the choroid plexus to a homeostatic type II interferon–dependent signature. Immune traffic through the choroid plexus appears to rely partly on expression of intercellular adhesion molecule–1 (ICAM-1) and VCAM-1, which are expressed on the apical surface of choroid plexus epithelial cells (90). CVOs such as the area postrema have also been shown to support T cell traffic in the healthy brain (91). Because the choroid plexus, CVOs, and dura mater are open to peripheral circulation, elevated T cell surveillance is likely required to keep these compartments free of infections.
The outermost layer of the meninges (or dura mater) is quite different than most other parts of the CNS enveloped by the underlying arachnoid mater. The dura mater resembles the periphery with its permeable vasculature, lymphatic drainage, and diverse assortment of resident immune cells (such as macrophages, DCs, mast cells, innate lymphoid cells, B cells, and T cells) (92). CD4+ T cells can traffic into the meninges from circulation and scan the tissue for antigens before departing to the deep cervical lymph nodes (93). In addition, the dura mater contains a high concentration of perivascular macrophages that continuously sample the tissue. On the basis of high-parameter mass cytometry and single-cell RNA-sequencing studies, the dural macrophages appear to be distinct from those residing in the pia and underlying parenchyma (73, 94).
There is also vasculature (diploic veins) that connects bone marrow pockets residing in the cancellous layer of the skull bone to dural vessels. These vessels provide an additional point of entry for immune cells, which can travel directly from the skull bone marrow to the dura mater through 100-μm bone channels (95). Similarly, cancer cells were also shown to use this route of entry. During acute lymphoblastic leukemia, lymphocytes use α6-integrins to enter the leptomeninges by migrating in the perivascular space along diploic and emissary veins that run through the skull bone. These cancer cells never traverse the BBB but do enter the subarachnoid space (96). During steady state, the arachnoid mater typically serves as a barrier between the dura mater and underlying CNS; however, the study of acute lymphoblastic leukemia cells has uncovered a means to breach this barrier.
BLOOD-CNS INTERFACE DURING INFLAMMATION
Cytokines
The CNS is affected by cytokines (97), and this can occur through a variety of different mechanisms, including direct transfer across the BBB, stimulation of CVOs, nerve stimulation, or release from infiltrating or resident immune cells, among others (98). Cytokines are transported into the CNS by using saturable transport mechanisms. This can occur in a cytokine receptor–dependent [for example, TNFα (99)] or cytokine receptor–independent (for example, IL-1) manner. Moreover, circulating cytokines can promote abluminal expression of soluble mediators such as prostaglandin E2 by binding to the luminal surface of cerebrovascular endothelial cells (100). In this way, the BBB can actively serve as a relay station for immune signaling between the blood and CNS. The dura mater, choroid plexus, and CVOs are particularly exposed to the effects of circulating cytokines given their fenestrated blood vessels and relative openness to factors in the blood (25). For example, ablation of the vascular organ of the lamina terminalis (a CVO) reduces cytokine-induced fever after injection of lipopolysaccharide (LPS) (101). LPS administration is also known to promote rapid expression of pro-inflammatory cytokines such as TNFα in all sensory CVOs as well as the meninges and choroid plexus (102). CVOs and other CNS barrier tissues can, in turn, respond to various inflammatory stimuli through expression of cytokine receptors (103), Toll-like receptors (TLRs) (104), and CD14 (105). As mentioned, CVOs are separated from adjacent neural tissue in the brain parenchyma by tanycytic astrocytes that limit diffusion of solutes from the CVO to the surrounding tissue (25). However, bidirectional neuronal projections to the hypothalamus, hippocampus, and amygdala may relay inflammatory signals from CVOs to other areas of the CNS. In addition, lipophilic molecules such as prostaglandins, produced within the CVO in response to cytokines, can traverse the CVO barrier and affect surrounding neural tissue (106).
Experimental autoimmune encephalomyelitis
Experimental autoimmune encephalomyelitis (EAE) is often used as a model for human multiple sclerosis (MS) and to study autoreactive immune cell traffic into the CNS (107). Because a detailed summary of MS literature is beyond the scope of this review, the reader should refer to other excellent reviews on this topic (108, 109). EAE is usually induced by triggering an autoreactive T cell response against myelin, either through myelin peptide immunization or through adoptive transfer of myelin-reactive T cells. Similar to steady state, the process of diapedesis during CNS autoimmune disease involves T cell interactions with endothelial selectins through P-selectin glycoprotein ligand–1 (PSGL-1), although the requirement for selectins is not absolute. This results in T cell rolling and eventual arrest mediated by LFA-1-ICAM-1 and VLA-4-VCAM-1 interactions (Fig. 4), as observed by using the proteolipid protein (PLP139-151) immunization model of EAE (110). Activated leukocyte cell adhesion molecule (CD166) can also foster diapedesis of monocytes and, to a lesser extent, CD4 T cells during EAE (111, 112). In addition to adhesion molecules, T cell arrest is often linked to G protein signaling in vessels (110), which is facilitated by chemokines such as CCL19 and CCL21 that are produced by vascular endothelial cells (113). CXCL12 expression is elevated during EAE as well and is not confined to the abluminal surface of blood vessels (114). Using the myelin oligodendrocyte glycoprotein (MOG35-55) immunization model of EAE, it was shown that CXCR7 expression on the abluminal side of endothelial cells binds CXCL12, limiting the ability of this chemokine to trap leukocytes in the perivascular space, as it does during steady state (Fig. 4) (115).
The process of T cell diapedesis across CNS blood vessels involves firm attachment to endothelial cells (dependent on ICAM-1, ICAM-2, and VCAM-1) to resist the shear forces of vascular flow. This then allows LFA-1–dependent T cell crawling against blood flow until a location permissive to diapedesis is found (116). Prolonged T cell crawling before diapedesis is thought to depend on the properties of CNS vascular endothelial cells because the process is more rapid with peripheral endothelial cells (117). Early CD4+ T cell extravasation was observed primarily through leptomeningeal vasculature in the Lewis rat model of EAE (66) as well as the choroid plexus in the MOG35-55 model (118) and, to a less extent, postcapillary venules in the CNS parenchyma. Upon extravasation, the autoreactive T cells interact with peptide–major histocompatibility complex (MHC) II–bearing APCs (such as DCs) in the perivascular spaces, triggering up-regulation of chemokines that recruit myeloid cells and release of TNFα that increases production of metalloproteinases MMP-2 and MMP-9 by resident and recruited myeloid cells (Fig. 4) (65, 67, 84, 119, 120). The metalloproteinases selectively cleave dystroglycan, which tethers astrocytic foot processes to the basement membrane (Fig. 3), allowing penetration of effector T cells and myeloid cells into the CNS parenchyma in the MOG35-55 EAE model (121). This is a rate-limiting step for the initiation of EAE and the eventual destruction of parenchyma white matter. In the choroid plexus, encephalitogenic CD4+ T cells enter the CSF in a CCR6-dependent manner, which is likely linked to expression of the chemoattractant CCL20 produced by choroid plexus epithelial cells (118). After entering the CSF through the choroid plexus, CD4+ T cells accumulate in leptomeningeal spaces, suggesting that the choroid plexus might be one of the earliest CNS entry points for autoreactive T cells during the development of EAE (118). Another adhesion molecule that can facilitate the accumulation of autoreactive T cells in the CNS during MOG33-55 EAE is melanoma cell adhesion molecule (MCAM) (122, 123). MCAM+ effector T cells migrate more efficiently across the BBB than their MCAM-negative counterparts and appear to do so through homotypic binding with MCAM expressed on vascular endothelial cells (123) and through interactions with laminin-411 (124). Injection of anti-MCAM antibodies into mice also reduces the severity of EAE, demonstrating the importance of this adhesion molecule in disease pathogenesis (122, 123).
Traumatic cerebrovascular injury
Traumatic cerebrovascular injury is an important aspect of traumatic brain injury (TBI) and contributes to a subsequent inflammatory response. TBI can disrupt vascular integrity in the meninges and brain parenchyma, resulting in leakage of materials from the blood supply into CNS (Fig. 4) (125-127). In fact, ultrastructural studies in primates have revealed substantial cerebrovascular disruption, with widening of intercellular junctions between endothelial cells and swelling of perivascular astrocytes (128). The duration of the leakage varies from patient to patient and can extend for months or even years after the initial injury. In animal models of TBI, reduced microvascular densities were observed in the brain within a day of injury, although the density returned to normal within 2 weeks, suggesting engagement of vascular repair mechanisms (129). Vascular repair was also observed in the meninges after TBI and was dependent on the recruitment of nonclassical monocytes from the blood (127). Thus, it is possible to repair CNS vasculature after TBI; however, it is unclear whether the anatomy is ever completely restored to its original configuration.
TBI and the associated CNS vascular damage is a type of sterile injury [recently reviewed in (130)]. A great deal is known about sterile injuries; they involve release of alarmins detected by damage-associated molecular pattern (DAMP) sensors such as Toll-like and purinergic receptors (131). This, in turn, triggers a rapid inflammatory response characterized by inflammasome assembly, nuclear factor-κβ signaling, resident immune cell activation, cytokine and chemokine production (such as IL-6, TNFα, CXCL1, CXCL2, and CCL2), and recruitment of peripheral myelomonocytic cells, among others (Fig. 4) (130). TLR4 is up-regulated after TBI and recognizes LPSs and endogenous proteins, such as high-mobility group box 1 protein, heat shock proteins, and low-density lipoprotein (132). Purinergic receptors also play an important role in TBI by detecting adenosine 5′-triphosphate (ATP) and uridine 5′-diphosphate, which are released after tissue damage (133).
The speed of this reaction is crucial for the maintenance of CNS barriers, including the glia limitans and cerebrovasculature. For example, damage to the glia limitans superficialis after TBI results in a profound morphological transformation of the underlying microglia that depends on ATP release by surface-associated astrocytes and subsequent detection by microglial purinergic receptors (126). Real-time imaging studies revealed that microglia transform into honeycomb- and jellyfish-like morphologies that provide barrier support and debris clearance (126). In the absence of this transformation, the glia limitans leaked profoundly. Similar findings were made in a laser-induced model of focal BBB breakdown. In this model, focal cerebrovascular damage resulted in a rapid, purinergic receptor–dependent projection of microglia processes that walled off the damaged vessel, limiting the extent of BBB leakage (134). These findings demonstrate that the immune system has quick-response elements in place to deal with acute brain trauma and restore CNS barriers.
Cerebral ischemia
Ischemic stroke results from permanent or transient reduction in regional cerebral blood flow. A commonly used animal model involves occlusion of the middle cerebral artery (MCAO) (135). Neurons are especially susceptible to ischemia and the reperfusion injury that often follows an ischemic stroke. The acute phase after ischemia is characterized by the rapid-release DAMPs, which are sensed by endothelial cells as well as resident and circulating immune cells. Resident macrophages and invading myelomonocytic cells release cytokines (such as TNFα and IL-1β), chemokines, reactive oxygen species (ROS), neutrophil extracellular traps (NETs), and MMP-9, which lead to further disruption of the BBB, brain edema, neuronal death, and hemorrhagic transformation (Fig. 4) (136).
Neutrophils are among the first peripheral immunes recruited to the brain after an ischemic-reperfusion event and are thought to contribute to the injury in a least some brain regions (137). The process of neutrophil recruitment in most peripheral tissues involves engagement of E- and P-selectins through PSGL-1. Inflammatory cytokines such as TNFα and IL-1β promote cell-surface translocation of P-selectin from intracellular storage in the Weibel-Palade bodies as well as expression of E-selectin, ICAM-1, and VCAM-1 on the surface of endothelial cells (138). Engagement of selectins by neutrophils allow them to slow down and detect chemoattractants (such as CXCL1 and CXCL2) bound to endothelial cells by CXCR2, which subsequently triggers activation of LFA-1 and macrophage-1 antigen (Mac-1) (139). LFA-1 interaction with ICAM-1 results in neutrophil arrest and Mac-1-facilitated neutrophil diapedesis. Paracellular diapedesis is mediated by platelet endothelial cell adhesion molecule–1, CD99, and junctional adhesion molecule–A (JAM-A) (140). After extravasation, neutrophils are programmed to eradicate pathogens by means of release of free radicals and NETs (141).
In contrast to most peripheral vasculature, blood vessels in the CNS parenchyma lack P-selectin in Weibel-Palade bodies and only express low levels of this protein under steady state (79). In fact, constitutive expression of P-selectin is only present in meningeal vessels. Although expression of P-selectin can be up-regulated in meningeal and parenchymal vessels during inflammation, the delayed availability of this protein in the CNS leads to slower leukocyte recruitment kinetics when compared with the periphery. After cerebral ischemia or subarachnoid hemorrhage, neutrophils rapidly accumulate in the leptomeningeal space (142). However, these cells become confined by the astrocytic basement membrane and glia limitans to the perivascular space (143, 144).
Neutrophil extravasation into the brain parenchyma after ischemia is dependent on MMP activity (145). Astrocytes secrete MMP-2 from their endfeet, and pro–MMP-9 and pro–MMP-3 are produced by endothelial cells, microglia, and pericytes. Up-regulation of hypoxia-inducible factor–1α after hypoxia leads to furin production, which activates MMP-14 that, in turn, activates MMP-2. In addition, TNFα and IL-1β induce expression of pro–MMP-9 and pro–MMP-3, and the latter is responsible for activating MMP-9. This combination of inflammatory events leads to tight junction and glia limitans degradation as well as leukocyte infiltration of the brain parenchyma and vasogenic edema (Fig. 4) (145). Pro-inflammatory monocytes eventually follow neutrophils into the brain parenchyma and enter in a CCR2-dependent manner, eventually differentiating into Ly6Clow phagocytes. However, depletion of these cells has a negative impact on stroke outcome and increases the incidence of hemorrhagic transformation (146). To date, a number of preclinical and clinical trials have failed to improve ischemic stroke outcomes or lesion size by preventing leukocyte diapedesis (142, 147), indicating that our knowledge of the inflammatory response to this condition is incomplete.
Infection
Differences in the anatomy of CNS vasculature affect the tropism and pathophysiology of infectious diseases. Pathogens can more easily enter the CNS through fenestrated blood vessels that lack tight junctions, such as those residing in the dura mater, choroid plexus, and CVOs (Fig. 1) (148). Subpial and parenchymal vascular endothelial cells have tight junctions and low pinocytotic activity that greatly restrict the access of pathogens to CNS tissue. Even if pathogens do enter the dura mater, choroid plexus, or CVOs, they must still traverse tight junction–expressing barriers such as the arachnoid mater, choroid plexus ependyma, and tanycytes, respectively. Moreover, once within the CSF, drainage of antigenic material to peripheral lymph nodes is a crucial component of the CNS defense against invading microbes.
Although one way to traverse CNS barriers is to infect the cells that comprise them, another strategy is to alter barrier integrity. For example, group B streptococcus, a microbe that causes bacterial meningitis, promotes expression of a transcriptional repressor (Snail1) in CNS vascular endothelial cells, causing down-regulation of tight junction proteins (occluding, ZO-1, and claudin-5) and a loss of barrier integrity (149). This type of mechanism enables microbes to more easily invade the CNS. Inflammatory cytokines released in the defense against microbes can also modulate CNS barrier integrity. During West Nile virus (WNV) infection, type I and type III interferon signaling promotes tightening of the BBB in CNS vascular endothelial cells, restricting virus from accessing the CNS (150, 151). At least for type I interferon, BBB tightening is mediated by activation of small GTPases, Rac1 and RhoA, which counterbalance endothelial cells against the barrier-opening effects of cytokines such as TNFα and IL-1β (150). Endothelial TAM receptors also have barrier-sealing properties in the context of viral infection. TAM receptors (Tyro3, Axl, and Mertk) are immune-dampening tyrosine kinases that recognize ligands (Gas6 and protein S) that bind to phosphatidylserine on apoptotic cells. Enveloped viruses sometimes incorporate phosphatidylserine into their outer membrane. Detection of this ligand by the TAM receptor Mertk in CNS vascular endothelial cells results in barrier tightening because of synergy with type I interferon signaling (152). Deficiency in this receptor renders the CNS more susceptible to infection by neuroinvasive viruses such as La Crosse encephalitis virus and WNV.
Unless a pathogen is injected directly into the CNS parenchyma (56), infection typically results in a rapid recruitment of peripheral blood leukocytes, especially to the dura mater, leptomeninges, and choroid plexus. After coronavirus infection, endothelial and fibroblastic reticular cells in the meninges release CCL19 and CCL21 to recruit and reactivate antiviral CCR7+ CD8+ T cells (153). Timely recruitment of these T cells is required to prevent fatal CNS disease after infection with this virus. Although infiltrating leukocytes are usually necessary in the fight against microbes, these cells can sometimes cause great harm to CNS barriers. For example, infection of the meninges with a noncytopathic pathogen such as lymphocytic choriomeningitis (LCMV) causes fatal meningitis (154). During this disease, recruited CD8+ T cells and myelomonocytic cells damage meningeal vasculature and the walls of the ventricular system, causing severe edema and brainstem herniation (154, 155). Similar damage to meningeal and parenchymal vasculature is observed during cerebral malaria. This potentially fatal disease is initiated by infection with Plasmodium falciparum in humans and Plasmodium berghei ANKA in rodents. Similar to LCMV meningitis, death from cerebral malaria results from the accumulation of water in the cranium and brainstem herniation (156, 157). In rodents, it was revealed that BBB breakdown and fatal edema are caused by parasite-specific CD8+ T cell engagement of cerebrovascular endothelial cells that cross-present parasite antigen (157, 158). Displacement of these T cells from CNS vasculature by means of therapeutic administration of antibodies to LFA-1 and VLA-4 prevented the disease (157). Collectively, these findings demonstrate that the inflammatory response to pathogens has the potential to tighten and harm CNS barriers depending on the context.
CONCLUSIONS
CNS vasculature serves as the entry point for peripheral immune cells, and their entry is influenced by structural variations in the barriers surrounding blood vessels. The CNS parenchyma contains a highly fortified barrier system (the BBB) and is consequently very selective when it comes to peripheral immune cell entry. In general, peripheral immune cells do not enter the CNS parenchyma during steady state. However, they can traverse the BBB during pathophysiological conditions (such as autoimmune disease, injury, stroke, or infection) but only after acquiring the ability to move from the perivascular space across the glia limitans. The glia limitans serves as a barrier to peripheral immune cells and can be disrupted mechanically (such as injury) or enzymatically (such as metalloproteinases). Both allow peripheral immune cells to enter the parenchyma. The meninges are generally more supportive of peripheral immune traffic than the CNS parenchyma and serve as the initiation site for many CNS immune responses that involve peripheral immune cells. Blood vessels beneath the arachnoid mater have tight junctions and are more secure than those in the meningeal layer above (the dura mater). In fact, the dura mater, with its fenestrated blood vessels and lymphatic drainage, in many ways resembles a peripheral tissue. The choroid plexus and CVOs also contain fenestrated blood vessels that lack tight junctions and are supportive of peripheral immune cell traffic. These vascular beds are walled off from the CNS by specialized epithelial cells and/or astrocytes. Similarly, the arachnoid mater keeps the dura mater separate from the underlying subarachnoid space, which contains CSF. All these barriers are composed of biological materials and are by no means absolute, but they do compartmentalize peripheral immune cells until the barriers are breached. It is essential that we acquire a more detailed understanding of the mechanisms that underlie the degradation and reassembly of blood-CNS barriers if we want to restore homeostasis in the CNS after injury, ischemia, autoimmune disease, and infection. Much emphasis over the years has been placed on the BBB; however, as discussed in this review, several other CNS barriers exist that require detailed studies as well. The blood-CNS interface is incredibly sophisticated and should be considered from the perspective of each anatomical subdivision for all CNS inflammatory diseases.
Acknowledgments:
We thank A. Hoofring in the NIH Medical Arts Design Section for help with all the illustrations.
Funding: This work was supported by the intramural program at the National Institute of Neurological Disorders and Stroke (NINDS), NIH.
REFERENCES AND NOTES
- 1.Engelhardt B, Vajkoczy P, Weller RO, The movers and shapers in immune privilege of the CNS. Nat. Immunol 18, 123–131 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Murphy JB, Sturm E, Conditions determining the transplantability of tissues in the brain. J. Exp. Med 38, 183–197 (1923). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galea I, Bechmann I, Perry VH, What is immune privilege (not)? Trends Immunol. 28, 12–18 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J, Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K, A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med 212, 991–999 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mascagni P, Vasorum Lymphaticorum Corporis Humani Historia et Ichnographia (P. Carli, 1787). [Google Scholar]
- 7.Waggener JD, Beggs J, The membranous coverings of neural tissues: An electron microscopy study. J. Neuropathol. Exp. Neurol 26, 412–426 (1967). [DOI] [PubMed] [Google Scholar]
- 8.Andres KH, von Düring M, Muszynski K, Schmidt RF, Nerve fibres and their terminals of the dura mater encephali of the rat. Anat. Embryol 175, 289–301 (1987). [DOI] [PubMed] [Google Scholar]
- 9.Ma Q, Ineichen BV, Detmar M, Proulx ST, Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun 8, 1434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes Pinheiro MA, Kooij G, Mizee MR, Kamermans A, Enzmann G, Lyck R, Schwaninger M, Engelhardt B, de Vries HE, Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim. Biophys. Acta 1862, 461–471 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Youmans JR, Neurological Surgery: A Comprehensive Reference Guide to the Diagnosis and Management of Neurosurgical Problems (Saunders, ed. 3, 1990). [Google Scholar]
- 12.Jones EG, On the mode of entry of blood vessels into the cerebral cortex. J. Anat 106, 507–520 (1970). [PMC free article] [PubMed] [Google Scholar]
- 13.Morris AWJ, MacGregor Sharp M, Albargothy NJ, Fernandes R, Hawkes CA, Verma A, Weller RO, Carare RO, Vascular basement membranes as pathways for the passage of fluid into and out of the brain. Acta Neuropathol. 131, 725–736 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichimura T, Fraser PA, Cserr HF, Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 545, 103–113 (1991). [DOI] [PubMed] [Google Scholar]
- 15.Zhang ET, Inman CB, Weller RO, Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J. Anat 170, 111–123 (1990). [PMC free article] [PubMed] [Google Scholar]
- 16.Alcolado R, Weller RO, Parrish EP, Garrod D, The cranial arachnoid and pia mater in man: Anatomical and ultrastructural observations. Neuropathol. Appl. Neurobiol 14, 1–17 (1988). [DOI] [PubMed] [Google Scholar]
- 17.Oda Y, Nakanishi I, Ultrastructure of the mouse leptomeninx. J. Comp. Neurol 225, 448–457 (1984). [DOI] [PubMed] [Google Scholar]
- 18.Hannocks M-J, Pizzo ME, Huppert J, Deshpande T, Abbott NJ, Thorne RG, Sorokin L, Molecular characterization of perivascular drainage pathways in the murine brain. J. Cereb. Blood Flow Metab 38, 669–686 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weller RO, Microscopic morphology and histology of the human meninges. Morphologie 89, 22–34 (2005). [DOI] [PubMed] [Google Scholar]
- 20.Hutchings M, Weller RO, Anatomical relationships of the pia mater to cerebral blood vessels in man. J. Neurosurg 65, 316–325 (1986). [DOI] [PubMed] [Google Scholar]
- 21.Bhogal P, Makalanda HLD, Brouwer PA, Gontu V, Rodesch G, Mercier P, Söderman M, Normal pio-dural arterial connections. Interv. Neuroradiol 21, 750–758 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roland J, Bernard C, Bracard S, Czorny A, Floquet J, Race JM, Forlodou P, Picard L, Microvascularization of the intracranial dura mater. Surg. Radiol. Anat 9, 43–49 (1987). [DOI] [PubMed] [Google Scholar]
- 23.Spetzler RF, Kalani MYS, Nakaji P, Neurovascular Surgery (Thieme, 2015). [Google Scholar]
- 24.Mortazavi MM, Tubbs RS, Riech S, Verma K, Shoja MM, Zurada A, Benninger B, Loukas M, Cohen Gadol AA, Anatomy and pathology of the cranial emissary veins: A review with surgical implications. Neurosurgery 70, 1312–1318; discussion 1318–1319 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Morita S, Furube E, Mannari T, Okuda H, Tatsumi K, Wanaka A, Miyata S, Heterogeneous vascular permeability and alternative diffusion barrier in sensory circumventricular organs of adult mouse brain. Cell Tissue Res. 363, 497–511 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Kaur C, Rathnasamy G, Ling E-A, The choroid plexus in healthy and diseased brain. J. Neuropathol. Exp. Neurol 75, 198–213 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Vanlandewijck M, He L, Mäe MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Laviña B, Gouveia L, Sun Y, Raschperger E, Räsänen M, Zarb Y, Mochizuki N, Keller A, Lendahl U, Betsholtz C, A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Ehrlich M, Königthum und staatswesen der alten Hebräer: Nach biblischen und talmudischen quellen bearbeitet (Gedruckt bei J. v. Bertalanffy, Steinamanger, 1885). [Google Scholar]
- 29.Obersteiner H, Ueber einige Lymphräume im Gehirn. wien.acad. Sitzungsber. LXI. 1. Abthl (1870). [Google Scholar]
- 30.Abbott NJ, Rönnbäck L, Hansson E, Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci 7, 41–53 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Tietz S, Engelhardt B, Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell Biol 209, 493–506 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stamatovic SM, Keep RF, Kunkel SL, Andjelkovic AV, Potential role of MCP-1 in endothelial cell tight junction ‘opening’: Signaling via Rho and Rho kinase. J. Cell Sci 116, 4615–4628 (2003). [DOI] [PubMed] [Google Scholar]
- 33.del Zoppo GJ, Milner R, Integrin-matrix interactions in the cerebral microvasculature. Arterioscler. Thromb. Vasc. Biol 26, 1966–1975 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Allt G, Lawrenson JG, Pericytes: Cell biology and pathology. Cells Tissues Organs 169, 1–11 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Brightman MW, The brain’s interstitial clefts and their glial walls. J. Neurocytol 31, 595–603 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Pizzo ME, Wolak DJ, Kumar NN, Brunette E, Brunnquell CL, Hannocks M-J, Abbott NJ, Meyerand ME, Sorokin L, Stanimirovic DB, Thorne RG, Intrathecal antibody distribution in the rat brain: Surface diffusion, perivascular transport and osmotic enhancement of delivery. J. Physiol 596, 445–475 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krstić RV, Die Gewebe des Menschen und der Säugetiere: ein Atlas zum Studium für Mediziner und Biologen (Springer-Verlag, ed. 2, korrigierter Nachdruck der 1. Aufl., 1984). [Google Scholar]
- 38.Kacem K, Lacombe P, Seylaz J, Bonvento G, Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: A confocal microscopy study. Glia 23, 1–10 (1998). [PubMed] [Google Scholar]
- 39.Bedussi B, van Lier MGJTB, Bartstra JW, de Vos J, Siebes M, VanBavel E, Bakker ENTP, Clearance from the mouse brain by convection of interstitial fluid towards the ventricular system. Fluids Barriers CNS 12, 23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M, A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med 4, 147ra111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korogod N, Petersen CCH, Knott GW, Ultrastructural analysis of adult mouse neocortex comparing aldehyde perfusion with cryo fixation. eLife 4, e05793 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horng S, Therattil A, Moyon S, Gordon A, Kim K, Argaw AT, Hara Y, Mariani JN, Sawai S, Flodby P, Crandall ED, Borok Z, Sofroniew MV, Chapouly C, John GR, Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J. Clin. Invest 127, 3136–3151 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbott NJ, Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat 200, 629–638 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Smith C, Howlett C, Stanimirovic D, Inflammatory activation of human brain endothelial cells by hypoxic astrocytes in vitro is mediated by IL-1β. J. Cereb. Blood Flow Metab 20, 967–978 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Lau LT, Yu AC-H, Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. J. Neurotrauma 18, 351–359 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, McCarron RM, Azzam N, Bembry J, Reutzler C, Lenz FA, Spatz M, Endothelin-1 and nitric oxide affect human cerebromicrovascular endothelial responses and signal transduction. Acta Neurochir. Suppl 76, 131–135 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Chung IY, Benveniste EN, Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J. Immunol 144, 2999–3007 (1990). [PubMed] [Google Scholar]
- 48.Balin BJ, Broadwell RD, Salcman M, El-Kalliny M, Avenues for entry of peripherally administered protein to the central nervous system in mouse, rat, and squirrel monkey. J. Comp. Neurol 251, 260–280 (1986). [DOI] [PubMed] [Google Scholar]
- 49.Yasuda K, Cline C, Vogel P, Onciu M, Fatima S, Sorrentino BP, Thirumaran RK, Ekins S, Urade Y, Fujimori K, Schuetz EG, Drug transporters on arachnoid barrier cells contribute to the blood-cerebrospinal fluid barrier. Drug Metab. Dispos 41, 923–931 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cassella JP, Lawrenson JG, Firth JA, Development of endothelial paracellular clefts and their tight junctions in the pial microvessels of the rat. J. Neurocytol 26, 567–575 (1997). [DOI] [PubMed] [Google Scholar]
- 51.Nag S, Pathophysiology of blood-brain barrier breakdown. Methods Mol. Med 89, 97–119 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Cserr HF, Bundgaard M, Blood-brain interfaces in vertebrates: A comparative approach. Am. J. Physiol 246, R277–R288 (1984). [DOI] [PubMed] [Google Scholar]
- 53.Wolburg H, Wolburg-Buchholz K, Liebner S, Engelhardt B, Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci. Lett 307, 77–80 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Ciofi P, Garret M, Lapirot O, Lafon P, Loyens A, Prévot V, Levine JE, Brain-endocrine interactions: A microvascular route in the mediobasal hypothalamus. Endocrinology 150, 5509–5519 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B, Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J. Comp. Neurol 521, 3389–3405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevenson PG, Hawke S, Sloan DJ, Bangham CR, The immunogenicity of intracerebral virus infection depends on anatomical site. J. Virol 71, 145–151 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF, Drainage of interstitial fluid from different regions of rat brain. Am. J. Physiol 246, F835–F844 (1984). [DOI] [PubMed] [Google Scholar]
- 58.Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG, The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 135, 387–407 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Smith AJ, Yao X, Dix JA, Jin B-J, Verkman AS, Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. eLife 6, e27679 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carare RO, Teeling JL, Hawkes CA, Püntener U, Weller RO, Nicoll JAR, Perry VH, Immune complex formation impairs the elimination of solutes from the brain: Implications for immunotherapy in Alzheimer's disease. Acta Neuropathol. Commun 1, 48 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JAR, Perry VH, Weller RO, Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol 34, 131–144 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Matyszak MK, Perry VH, Bacillus Calmette-Guérin sequestered in the brain parenchyma escapes immune recognition. J. Neuroimmunol 82, 73–80 (1998). [DOI] [PubMed] [Google Scholar]
- 63.Kida S, Pantazis A, Weller RO, CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol. Appl. Neurobiol 19, 480–488 (1993). [DOI] [PubMed] [Google Scholar]
- 64.Louveau A, Herz J, Alme MN, Salvador AF, Dong MQ, Viar KE, Herod SG, Knopp J, Setliff JC, Lupi AL, Da Mesquita S, Frost EL, Gaultier A, Harris TH, Cao R, Hu S, Lukens JR, Smirnov I, Overall CC, Oliver G, Kipnis J, CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat. Neurosci 21, 1380–1391 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B, Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med 11, 328–334 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Bartholomäus I, Kawakami N, Odoardi F, Schläger C, Miljkovic D, Ellwart JW, Klinkert WEF, Flügel-Koch C, Issekutz TB, Wekerle H, Flügel A, Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462, 94–98 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Mundt S, Mrdjen D, Utz SG, Greter M, Schreiner B, Becher B, Conventional DCs sample and present myelin antigens in the healthy CNS and allow parenchymal T cell entry to initiate neuroinflammation. Sci. Immunol 4, eaau8380 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Nayak D, Zinselmeyer BH, Corps KN, McGavern DB, In vivo dynamics of innate immune sentinels in the CNS. Intravital 1, 95–106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, Liu K, Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J. Exp. Med 208, 1695–1705 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lauterbach H, Zuniga EI, Truong P, Oldstone MBA, McGavern DB, Adoptive immunotherapy induces CNS dendritic cell recruitment and antigen presentation during clearance of a persistent viral infection. J. Exp. Med 203, 1963–1975 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldmann T, Wieghofer P, Costa Jordão MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FMV, Bechmann I, Kerschensteiner M, Linnarsson S, Jung S, Prinz M, Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol 17, 797–805 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rua R, Lee JY, Silva AB, Swafford IS, Maric D, Johnson KR, McGavern DB, Infection drives meningeal engraftment by inflammatory monocytes that impairs CNS immunity. Nat. Immunol 20, 407–419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, De Prijck S, Vandamme N, De Schepper S, Van Isterdael G, Scott CL, Aerts J, Berx G, Boeckxstaens GE, Vandenbroucke RE, Vereecke L, Moechars D, Guilliams M, Van Ginderachter JA, Saeys Y, Movahedi K, A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci 22, 1021–1035 (2019). [DOI] [PubMed] [Google Scholar]
- 74.Kunis G, Baruch K, Rosenzweig N, Kertser A, Miller O, Berkutzki T, Schwartz M, IFN-γ-dependent activation of the brain's choroid plexus for CNS immune surveillance and repair. Brain 136, 3427–3440 (2013). [DOI] [PubMed] [Google Scholar]
- 75.Baruch K, Schwartz M, CNS-specific T cells shape brain function via the choroid plexus. Brain Behav. Immun 34, 11–16 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Raine CS, Cannella B, Duijvestijn AM, Cross AH, Homing to central nervous system vasculature by antigen-specific lymphocytes. II. Lymphocyte/endothelial cell adhesion during the initial stages of autoimmune demyelination. Lab. Invest 63, 476–489 (1990). [PubMed] [Google Scholar]
- 77.Wolburg H, Wolburg-Buchholz K, Engelhardt B, Diapedesis of mononuclear cells across cerebral venules during experimental autoimmune encephalomyelitis leaves tight junctions intact. Acta Neuropathol. 109, 181–190 (2005). [DOI] [PubMed] [Google Scholar]
- 78.Owens T, Bechmann I, Engelhardt B, Perivascular spaces and the two steps to neuroinflammation. J. Neuropathol. Exp. Neurol 67, 1113–1121 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Barkalow FJ, Goodman MJ, Gerritsen ME, Mayadas TN, Brain endothelium lack one of two pathways of P-selectin-mediated neutrophil adhesion. Blood 88, 4585–4593 (1996). [PubMed] [Google Scholar]
- 80.Engelhardt B, Ransohoff RM, Capture, crawl, cross: The T cell code to breach the blood-brain barriers. Trends Immunol. 33, 579–589 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Vajkoczy P, Laschinger M, Engelhardt B, Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J. Clin. Invest 108, 557–565 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kawakami N, Nägerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flügel A, Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J. Exp. Med 201, 1805–1814 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS, CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J. Immunol 177, 8053–8064 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Lodygin D, Odoardi F, Schläger C, Körner H, Kitz A, Nosov M, van den Brandt J, Reichardt HM, Haberl M, Flügel A, A combination of fluorescent NFAT and H2B sensors uncovers dynamics of T cell activation in real time during CNS autoimmunity. Nat. Med 19, 784–790 (2013). [DOI] [PubMed] [Google Scholar]
- 85.Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, Curfman B, Miszkiel K, Mueller-Lenke N, Sanchez E, Barkhof F, Radue E-W, Jäger HR, Clifford DB, Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N. Engl. J. Med 354, 924–933 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carrithers MD, Visintin I, Kang SJ, Janeway CA Jr., Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain 123 (Pt. 6), 1092–1101 (2000). [DOI] [PubMed] [Google Scholar]
- 87.Buonamici S, Trimarchi T, Ruocco MG, Reavie L, Cathelin S, Mar BG, Klinakis A, Lukyanov Y, Tseng J-C, Sen F, Gehrie E, Li M, Newcomb E, Zavadil J, Meruelo D, Lipp M, Ibrahim S, Efstratiadis A, Zagzag D, Bromberg JS, Dustin ML, Aifantis I, CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature 459, 1000–1004 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, Amit I, Schwartz M, Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 346, 89–93 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deczkowska A, Baruch K, Schwartz M, Type I/II interferon balance in the regulation of brain physiology and pathology. Trends Immunol. 37, 181–192 (2016). [DOI] [PubMed] [Google Scholar]
- 90.Wolburg K, Gerhardt H, Schulz M, Wolburg H, Engelhardt B, Ultrastructural localization of adhesion molecules in the healthy and inflamed choroid plexus of the mouse. Cell Tissue Res. 296, 259–269 (1999). [DOI] [PubMed] [Google Scholar]
- 91.Loeffler C, Dietz K, Schleich A, Schlaszus H, Stoll M, Meyermann R, Mittelbronn M, Immune surveillance of the normal human CNS takes place in dependence of the locoregional blood-brain barrier configuration and is mainly performed by CD3+/CD8+ lymphocytes. Neuropathology 31, 230–238 (2011). [DOI] [PubMed] [Google Scholar]
- 92.Rua R, McGavern DB, Advances in meningeal immunity. Trends Mol. Med 24, 542–559 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Radjavi A, Smirnov I, Derecki N, Kipnis J, Dynamics of the meningeal CD4+ T-cell repertoire are defined by the cervical lymph nodes and facilitate cognitive task performance in mice. Mol. Psych 19, 531–533 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mrdjen D, Pavlovic A, Hartmann FJ, Schreiner B, Utz SG, Leung BP, Lelios I, Heppner FL, Kipnis J, Merkler D, Greter M, Becher B, High-dimensional single-cell mapping of central nervous system immune cells reveals distinct myeloid subsets in health, aging, and disease. Immunity 48, 599 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, Vandoorne K, Wojtkiewicz GR, Masson GS, Vinegoni C, Kim J, Kim D-E, Weissleder R, Swirski FK, Moskowitz MA, Nahrendorf M, Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci 21, 1209–1217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao H, Price TT, Cantelli G, Ngo B, Warner MJ, Olivere L, Ridge SM, Jablonski EM, Therrien J, Tannheimer S, McCall CM, Chenn A, Sipkins DA, Leukaemia hijacks a neural mechanism to invade the central nervous system. Nature 560, 55–60 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ching S, Zhang H, Belevych N, He L, Lai W, Pu X.-a., Jaeger LB, Chen Q, Quan N, Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J. Neurosci 27, 10476–10486 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Banks WA, Blood-brain barrier transport of cytokines: A mechanism for neuropathology. Curr. Pharm. Des 11, 973–984 (2005). [DOI] [PubMed] [Google Scholar]
- 99.Pan W, Kastin AJ, TNFα transport across the blood-brain barrier is abolished in receptor knockout mice. Exp. Neurol 174, 193–200 (2002). [DOI] [PubMed] [Google Scholar]
- 100.Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB, EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci 10, 1131–1133 (2007). [DOI] [PubMed] [Google Scholar]
- 101.Blatteis CM, Bealer SL, Hunter WS, Llanos J-Q, Ahokas RA, Mashburn TA Jr., Suppression of fever after lesions of the anteroventral third ventricle in guinea pigs. Brain Res. Bull 11, 519–526 (1983). [DOI] [PubMed] [Google Scholar]
- 102.Nadeau S, Rivest S, Regulation of the gene encoding tumor necrosis factor alpha (TNF-α) in the rat brain and pituitary in response in different models of systemic immune challenge. J. Neuropathol. Exp. Neurol 58, 61–77 (1999). [DOI] [PubMed] [Google Scholar]
- 103.Ericsson A, Liu C, Hart RP, Sawchenko PE, Type 1 interleukin-1 receptor in the rat brain: Distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J. Comp. Neurol 361, 681–698 (1995). [DOI] [PubMed] [Google Scholar]
- 104.Laflamme N, Rivest S, Toll-like receptor 4: The missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. FASEB J. 15, 155–163 (2001). [DOI] [PubMed] [Google Scholar]
- 105.Lacroix S, Feinstein D, Rivest S, The bacterial endotoxin lipopolysaccharide has the ability to target the brain in upregulating its membrane CD14 receptor within specific cellular populations. Brain Pathol. 8, 625–640 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Komaki G, Arimura A, Koves K, Effect of intravenous injection of IL-1 beta on PGE2 levels in several brain areas as determined by microdialysis. Am. J. Physiol 262, E246–E251 (1992). [DOI] [PubMed] [Google Scholar]
- 107.Lassmann H, Models of multiple sclerosis: New insights into pathophysiology and repair. Curr. Opin. Neurol 21, 242–247 (2008). [DOI] [PubMed] [Google Scholar]
- 108.Dendrou CA, Fugger L, Friese MA, Immunopathology of multiple sclerosis. Nat. Rev. Immunol 15, 545–558 (2015). [DOI] [PubMed] [Google Scholar]
- 109.Reich DS, Lucchinetti CF, Calabresi PA, Multiple sclerosis. N. Engl. J. Med 378, 169–180 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piccio L, Rossi B, Scarpini E, Laudanna C, Giagulli C, Issekutz AC, Vestweber D, Butcher EC, Constantin G, Molecular mechanisms involved in lymphocyte recruitment in inflamed brain microvessels: Critical roles for P-selectin glycoprotein ligand-1 and heterotrimeric Gi-linked receptors. J. Immunol 168, 1940–1949 (2002). [DOI] [PubMed] [Google Scholar]
- 111.Lyck R, Lécuyer M-A, Abadier M, Wyss CB, Matti C, Rosito M, Enzmann G, Zeis T, Michel L, García Martín AB, Sallusto F, Gosselet F, Deutsch U, Weiner JA, Schaeren-Wiemers N, Prat A, Engelhardt B, ALCAM (CD166) is involved in extravasation of monocytes rather than T cells across the blood-brain barrier. J. Cereb. Blood Flow Metab 37, 2894–2909 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, Haqqani AS, Kreymborg K, Krug S, Moumdjian R, Bouthillier A, Becher B, Arbour N, David S, Stanimirovic D, Prat A, Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat. Immunol 9, 137–145 (2008). [DOI] [PubMed] [Google Scholar]
- 113.Alt C, Laschinger M, Engelhardt B, Functional expression of the lymphoid chemokines CCL19 (ELC) and CCL 21 (SLC) at the blood-brain barrier suggests their involvement in G-protein-dependent lymphocyte recruitment into the central nervous system during experimental autoimmune encephalomyelitis. Eur. J. Immunol 32, 2133–2144 (2002). [DOI] [PubMed] [Google Scholar]
- 114.McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, Klein RS, Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. Am. J. Pathol 172, 799–808 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cruz-Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, Wright M, McCandless EE, Patel JR, Luker GD, Littman DR, Russell JH, Klein RS, CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J. Exp. Med 208, 327–339 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steiner O, Coisne C, Cecchelli R, Boscacci R, Deutsch U, Engelhardt B, Lyck R, Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood-brain barrier endothelium. J. Immunol 185, 4846–4855 (2010). [DOI] [PubMed] [Google Scholar]
- 117.Steiner O, Coisne C, Engelhardt B, Lyck R, Comparison of immortalized bEnd5 and primary mouse brain microvascular endothelial cells as in vitro blood-brain barrier models for the study of T cell extravasation. J. Cereb. Blood Flow Metab 31, 315–327 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F, C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol 10, 514–523 (2009). [DOI] [PubMed] [Google Scholar]
- 119.Song J, Wu C, Korpos E, Zhang X, Agrawal SM, Wang Y, Faber C, Schäfers M, Körner H, Opdenakker G, Hallmann R, Sorokin L, Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. 10, 1040–1054 (2015). [DOI] [PubMed] [Google Scholar]
- 120.Jordao MJC, Sankowski R, Brendecke SM, Sagar, Locatelli G, Tai Y-H, Tay TL, Schramm E, Armbruster S, Hagemeyer N, Groß O, Mai D, Çiçek Ö, Falk T, Kerschensteiner M, Grün D, Prinz M, Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363, eaat7554 (2019). [DOI] [PubMed] [Google Scholar]
- 121.Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, Sorokin LM, Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J. Exp. Med 203, 1007–1019 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duan H, Xing S, Luo Y, Feng L, Gramaglia I, Zhang Y, Lu D, Zeng Q, Fan K, Feng J, Yang D, Qin Z, Couraud P-O, Romero IA, Weksler B, Yan X, Targeting endothelial CD146 attenuates neuroinflammation by limiting lymphocyte extravasation to the CNS. Sci. Rep 3, 1687 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Larochelle C, Cayrol R, Kebir H, Alvarez JI, Lécuyer M-A, Ifergan I, Viel É, Bourbonnière L, Beauseigle D, Terouz S, Hachehouche L, Gendron S, Poirier J, Jobin C, Duquette P, Flanagan K, Yednock T, Arbour N, Prat A, Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system. Brain 135, 2906–2924 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Flanagan K, Fitzgerald K, Baker J, Regnstrom K, Gardai S, Bard F, Mocci S, Seto P, You M, Larochelle C, Prat A, Chow S, Li L, Vandevert C, Zago W, Lorenzana C, Nishioka C, Hoffman J, Botelho R, Willits C, Tanaka K, Johnston J, Yednock T, Laminin-411 is a vascular ligand for MCAM and facilitates TH17 cell entry into the CNS. PLOS ONE 7, e40443 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shlosberg D, Benifla M, Kaufer D, Friedman A, Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol 6, 393–403 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB, Transcranial amelioration of inflammation and cell death after brain injury. Nature 505, 223–228 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Russo MV, Latour LL, McGavern DB, Distinct myeloid cell subsets promote meningeal remodeling and vascular repair after mild traumatic brain injury. Nat. Immunol 19, 442–452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maxwell WL, Irvine A, Adams JH, Graham DI, Gennarelli TA, Response of cerebral microvasculature to brain injury. J. Pathol 155, 327–335 (1988). [DOI] [PubMed] [Google Scholar]
- 129.Park E, Bell JD, Siddiq IP, Baker AJ, An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: A role for hypoxia-inducible factors in traumatic brain injury. J. Cereb. Blood Flow Metab 29, 575–584 (2008). [DOI] [PubMed] [Google Scholar]
- 130.Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J, Neuroimmunology of traumatic brain injury: Time for a paradigm shift. Neuron 95, 1246–1265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Russo MV, McGavern DB, Immune surveillance of the CNS following infection and injury. Trends Immunol. 36, 637–650 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee H, Lee S, Cho I-H, Lee SJ, Toll-like receptors: Sensor molecules for detecting damage to the nervous system. Curr. Protein Pept. Sci 14, 33–42 (2013). [DOI] [PubMed] [Google Scholar]
- 133.Corps KN, Roth TL, McGavern DB, Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 72, 355–362 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lou N, Takano T, Pei Y, Xavier AL, Goldman SA, Nedergaard M, Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc. Natl. Acad. Sci. U.S.A 113, 1074–1079 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lo EH, Experimental models, neurovascular mechanisms and translational issues in stroke research. Br. J. Pharmacol 153 (suppl. 1), S396–S405 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kriz J, Inflammation in ischemic brain injury: Timing is important. Crit. Rev. Neurobiol 18, 145–157 (2006). [DOI] [PubMed] [Google Scholar]
- 137.Beray-Berthat V, Croci N, Plotkine M, Margaill I, Polymorphonuclear neutrophils contribute to infarction and oxidative stress in the cortex but not in the striatum after ischemia-reperfusion in rats. Brain Res. 987, 32–38 (2003). [DOI] [PubMed] [Google Scholar]
- 138.Min J-K, Kim Y-M, Kim SW, Kwon M-C, Kong Y-Y, Hwang IK, Won MH, Rho J, Kwon Y-G, TNF-related activation-induced cytokine enhances leukocyte adhesiveness: Induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-κB activation in endothelial cells. J. Immunol 175, 531–540 (2005). [DOI] [PubMed] [Google Scholar]
- 139.Sanz M-J, Kubes P, Neutrophil-active chemokines in in vivo imaging of neutrophil trafficking. Eur. J. Immunol 42, 278–283 (2012). [DOI] [PubMed] [Google Scholar]
- 140.Woodfin A, Voisin M-B, Imhof BA, Dejana E, Engelhardt B, Nourshargh S, Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood 113, 6246–6257 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A, Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 (2004). [DOI] [PubMed] [Google Scholar]
- 142.Enzmann G, Kargaran S, Engelhardt B, Ischemia-reperfusion injury in stroke: Impact of the brain barriers and brain immune privilege on neutrophil function. Ther. Adv. Neurol. Disord 11, 1756286418794184 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Enzmann G, Mysiorek C, Gorina R, Cheng Y-J, Ghavampour S, Hannocks M-J, Prinz V, Dirnagl U, Endres M, Prinz M, Beschorner R, Harter PN, Mittelbronn M, Engelhardt B, Sorokin L, The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury. Acta Neuropathol. 125, 395–412 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, Urra X, Chamorro A, Planas AM, Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 129, 239–257 (2015). [DOI] [PubMed] [Google Scholar]
- 145.Yang Y, Rosenberg GA, Matrix metalloproteinases as therapeutic targets for stroke. Brain Res. 1623, 30–38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gliem M, Mausberg AK, Lee J-I, Simiantonakis I, van Rooijen N, Hartung H-P, Jander S, Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann. Neurol 71, 743–752 (2012). [DOI] [PubMed] [Google Scholar]
- 147.Elkins J, Veltkamp R, Montaner J, Johnston SC, Singhal AB, Becker K, Lansberg MG, Tang W, Chang I, Muralidharan K, Gheuens S, Mehta L, Elkind MSV, Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): A randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 16, 217–226 (2017). [DOI] [PubMed] [Google Scholar]
- 148.Schwerk C, Tenenbaum T, Kim KS, Schroten H, The choroid plexus—A multi-role player during infectious diseases of the CNS. Front. Cell. Neurosci 9, 80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim BJ, Hancock BM, Bermudez A, Cid ND, Reyes E, van Sorge NM, Lauth X, Smurthwaite CA, Hilton BJ, Stotland A, Banerjee A, Buchanan J, Wolkowicz R, Traver D, Doran KS, Bacterial induction of Snail1 contributes to blood-brain barrier disruption. J. Clin. Invest 125, 2473–2483 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Daniels BP, Holman DW, Cruz-Orengo L, Jujjavarapu H, Durrant DM, Klein RS, Viral pathogen-associated molecular patterns regulate blood-brain barrier integrity via competing innate cytokine signals. mBio 5, e01476–14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lazear HM, Daniels BP, Pinto AK, Huang AC, Vick SC, Doyle SE, Gale M Jr., Klein RS, Diamond MS, Interferon-lambda restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci. Transl. Med 7, 284ra59 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Miner JJ, Daniels BP, Shrestha B, Proenca-Modena JL, Lew ED, Lazear HM, Gorman MJ, Lemke G, Klein RS, Diamond MS, The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat. Med 21, 1464–1472 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cupovic J, Onder L, Gil-Cruz C, Weiler E, Caviezel-Firner S, Perez-Shibayama C, Rülicke T, Bechmann I, Ludewig B, Central nervous system stromal cells control local CD8+ T cell responses during virus-induced neuroinflammation. Immunity 44, 622–633 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim JV, Kang SS, Dustin ML, McGavern DB, Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 457, 191–195 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Matullo CM, O'Regan KJ, Hensley H, Curtis M, Rall GF, Lymphocytic choriomeningitis virus-induced mortality in mice is triggered by edema and brain herniation. J. Virol 84, 312–320 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Seydel KB, Kampondeni SD, Valim C, Potchen MJ, Milner DA, Muwalo FW, Birbeck GL, Bradley WG, Fox LL, Glover SJ, Hammond CA, Heyderman RS, Chilingulo CA, Molyneux ME, Taylor TE, Brain swelling and death in children with cerebral malaria. N. Engl. J. Med 372, 1126–1137 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Swanson II PA, Hart GT, Russo MV, Nayak D, Yazew T, Peña M, Khan SM, Janse CJ, Pierce SK, McGavern DB, CD8+ T cells induce fatal brainstem pathology during cerebral malaria via luminal antigen-specific engagement of brain vasculature. PLOS Pathog. 12, e1006022 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Howland SW, Poh CM, Gun SY, Claser C, Malleret B, Shastri N, Ginhoux F, Grotenbreg GM, Rénia L, Brain microvessel cross-presentation is a hallmark of experimental cerebral malaria. EMBO Mol. Med 5, 984–999 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]