ABSTRACT
Chikungunya fever (CHIKF) is a mosquito-borne disease caused by Chikungunya virus (CHIKV). This virus is considered a priority pathogen to the UK government, the US National Institute of Allergy and Infectious Diseases (NIAID) and the US military personnel, due to the potential of CHIKV to cause major outbreaks. Nearly all CHIKV infections are symptomatic, often incapacitating and patients experience severe joint pain and inflammation that can last for more than one year with 0.4–0.5% fatality rates. Mother-to-child transmission has also been described. Despite this re-emerging disease has been documented in more than 100 countries in Europe, Oceania, Africa, Asia, the Caribbean, South and North America, no licensed vaccine is yet available to prevent CHIKF. Nevertheless, various developments have entered phase I and II trials and are now viable options to fight this incapacitating disease. This review focuses on the development of CHIKV vaccines that have reached the stage of clinical trials since the late 1960s up until 2018.
KEYWORDS: Chikungunya virus, vaccine, clinical trials
Introduction
Chikungunya Virus (CHIKV) is an arthropod-borne virus (arbovirus) transmitted to humans by the Aedes aegypti and A. albopictus mosquitoes. It is a member of the alphavirus genus, part of the Togaviridae family. The alphavirus genus comprises 29 species classified into seven antigenic complexes that include Eastern equine encephalitis (EEE), Western equine encephalitis (WEE), Venezuelan equine encephalitis (VEE), Barmah Forest (BF), Middelburg (MID), Ndumu (NDU) and Semliki Forest (SF), with CHIKV being a member of the latter complex.1
Alphaviruses have a wide geographic distribution. Infection in humans leads to two major clinical outcomes, (A) arthralgia and arthritis caused by Old World alphaviruses such as CHIKV, O’Nyong Nyong and SF viruses and (B) encephalitis, caused by the New World alphaviruses such as VEE and WEE viruses.2 Vaccines against alphavirus infections have been under development for several years and while major progress has been made for CHIKV prompting its assessment in clinical trials, most alphavirus vaccines remain in pre-clinical stage in mouse and macaque models. Nevertheless, CHIKV vaccines form a robust pipeline with promising developments from pre-clinical to phase I and II clinical trials, which have been thoroughly reviewed before.3,4 A summary of the past and current developments shows that while most vaccine candidates are in early mouse and macaque pre-clinical phases, a good number are transitioning through phase I and II trials with promising results (Figure 1).
Figure 1.
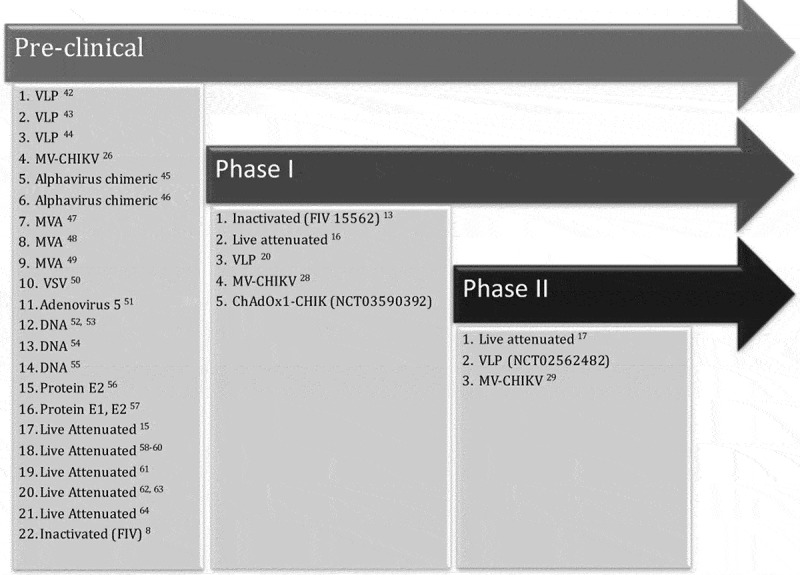
Summary of past and current CHIKV vaccines assessed in early pre-clinical phase or late phase I and II clinical trials.
Chikungunya virus (CHIKV) is one of the simplest enveloped viruses. It is a small and spherical virus of an approximate size of 60–70 nm.1,5 The genomic organisation consists of a single-stranded positive-sense RNA of approximately 11.8 kb in size that resembles the eukaryotic mRNA due to the presence of 5ʹ cap structures and a 3ʹ poly (A) tail.1 The genome size varies geographically among lineages, being longest in the Asian strains (11,777–11,999 nucleotides or nt), followed by West African (11,843–11,881 nt) and East/Central/South African (11,557–11,789).6 The genome is organised into two open reading frames, one of approximately 7.4 Kb in size encoding 4 non-structural proteins (nsP1, nsP2, nsP3 and nsP4) and one of 3.7 Kb encoding the structural proteins of the virus, including Capsid (C), peptide E3, envelope glycoprotein E2, peptide 6K/TF and envelope protein E1.1,3
Sequencing of the complete CHIKV genome has revealed the existence of four lineages: (1) West African (Waf); (2) East/Central/South African (ECSA); (3) Asian and (4) Indian Ocean Lineage (IOL).6 Whole genome sequencing has proven to be key to support identifying the IOL, since only three lineages had been identified using partial sequencing of the E1 gene in earlier studies that reported three lineages, thus underlying the inadequacy of the E gene to resolve the phylogenetic history of the CHIKV.6 These studies have been useful to trace the existence of a CHIKV ancestor within the last 500 years with indication of a divergence between the ECSA and Asian lineages occurring in the last 150 years.6 Phylogenetic studies indicate that the major Chikungunya Fever (CHIKF) outbreak in the Americas was caused by an Asian genotype, with the exception of Brazil where both ECSA and Asian lineages co-circulate, the latter has formed an Asian/American lineage defined by two amino acid substitutions in the E2 envelope glycoprotein and the 6K peptide region (E2 V368A and 6K L20M), which is evolving at a mean rate of 5 × 104 substitutions per site per year and is similar to that previously calculated for the Asian genotype.7
History of CHIKV vaccines
Formalin-inactivated vaccines (FIV)
The history of CHIKV vaccines started with a formalin-inactivated approach in the late 60s (Figure 2) at the Walter Reed Army Institute of Research (WRAIR) in Washington D. C., when V.R Harrison, L.N. Binn and R. Randall did seminal work by assessing the immune responses elicited in mice and rhesus macaques by a formalin-inactivated CHIKV vaccine.8 The virus used to develop the vaccine was the African CHIKV strain 168, isolated from Southern Tanganyika (nowadays Tanzania) between 1952–1953.9 The experimental vaccines were prepared using chik-embryo (CE), suckling-mouse-brain (SMB) and green monkey kidney cells (GMKC). Poor immunogenicity was induced by the CE preparation, which contrasted with good immune responses induced by the SMB and GMKC preparations. The latter was selected for further development as vaccine from SMB products pose the risk of inducing encephalitis in man, thus limiting its applications. The ability of the CHIKV 168 vaccine to elicit homologous protection in mice prompted researchers to assess heterologous protection in rhesus macaques using various CHIKV strains: the African CHIKV strain 168; the CHIKV strain E.103 isolated from a pool of 78 Ae. Africanus mosquitoes at the Zika forest on June 12, 1956 by M. C. Williams;10 the Asian strain BAH-306 isolated from Thai patients11 and the Indian CHIKV strain C-266 isolated from Calcutta by K.V. Shah.12 Vaccination of eight rhesus macaques consisted of three doses of 1 ml each of GMKC-prepared CHIKV strain 168 (harvested after 72 h of infection with 105 SMICLD50) administered subcutaneously on day 0, 7 and 21. Homologous (strain 168) and heterologous challenges (strains E.103, BAH-306, C-266, mentioned above) were done 30 days after vaccination. Results yielded complete absence of viremia upon either, homologous or heterologous challenge in all vaccinated monkeys. This was the first demonstration of the homologous and heterologous protective efficacy by a formalin-inactivated CHIKV vaccine in pre-clinical models using mice and rhesus macaques.8
Figure 2.
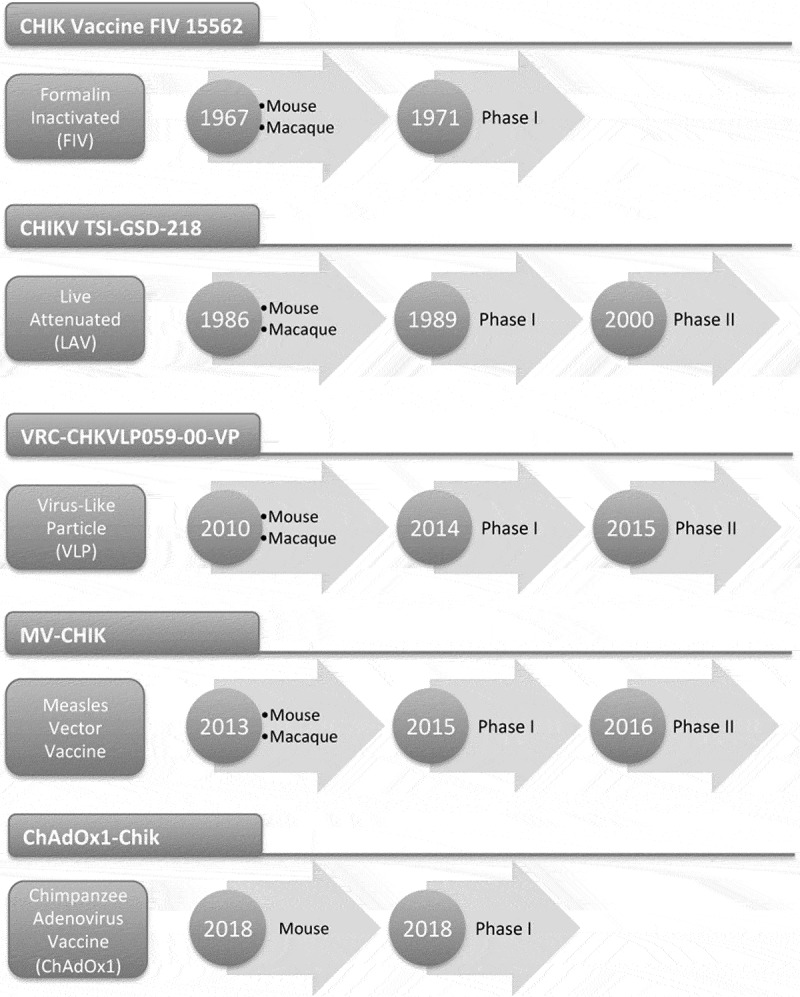
Timeline displaying the development of CHIKV vaccines that have entered clinical trials.
First CHIKV clinical trial
Four years later in 1971 at the WRAIR, V. R. Harrison, K. H. Eckels, P. J. Bartelloni and C. Hampton described the preparation of a formalin-inactivated CHIKV vaccine and its assessment in a phase I clinical trial (Figure 2).13 The vaccine seed virus was initiated from Thai human samples from the United States Army Medical Component SEATO in Bangkok, Thailand (nowadays AFRIMS), provided by Dr. E. L. Buescher. Four CHIKV isolates, designated 6348, 6461, 15561 and 23337 were grown in green monkey kidney tissue culture cells (GMKC) for 10 passages to eliminate any potential hepatitis and other adventitious viruses. Potency assays based on a mouse challenge with the CHIKV 1688 resulted in the selection of the CHIKV 15562 isolate (Table 1). A phase I clinical trial to assess the vaccine in sixteen young volunters of 21–25 years of age was performed. Two groups of eight volunteers were administered the vaccine subcutaneously (s.c.). Group I received two doses of 0.5 ml of vaccine at an interval of 28 days and group II received two doses of 1 ml under the same protocol. Local and systemic reactions were assessed for 12 days in a closed research ward. Thereafter, volunteers were observed on an outpatient basis and total absence of untoward reactions after the vaccination was observed. Most subjects developed neutralising antibodies on day 14 after the first vaccination and the second administration prompted the development of significant neutralising antibody titres with values of log10 neutralization indices of 2.7 for either dose, thus concluding that two 0.5 ml vaccine doses administered 28 days apart are sufficient to elicit good antibody responses.13
Table 1.
Characteristics of Chikungunya vaccines that have entered clinical trials.
| Vaccine | Platform | Lineage | CHIKV Immunogen |
Adjuvant | Number of Doses (interval) |
Vaccination Route | Stage of Development |
|---|---|---|---|---|---|---|---|
| CHIK Vaccine FIV 15562 |
Formalin-Inactivated Virus (FIV) |
Asian Strain 15,561 |
Whole inactivated virus | No | 2 (28 days) |
Subcutaneous | Phase I Trial |
| CHIKV TSI-GSD-218 | Live Attenuated Virus (LAV) |
Asian Strain 15,561 |
Whole attenuated virus | No | 1 | Subcutaneous | Phase II Trial |
| VRC-CHKVLP059-00-VP | Virus-Like Particle (VLP) |
West African (Waf) Strain 37,997 |
Capsid E3 E2 6K E1 |
No | 3 (0, 4, 20 weeks) |
Intramuscular | Phase II Trial |
| MV-CHIK | Measles Viral Vectored Vaccine (VVV) |
Indian Ocean Lineage (IOL) |
Capsid E3 E2 6K E1 |
No | 2 (28 days) |
Intramuscular | Phase II Trial |
| ChAdOx1-Chik | Chimpanzee Adenoviral Vector (ChAdOx1) |
Consensus sequence from multiple lineages | Capsid E3 E2 6K E1 |
No | 1 | Intramuscular | Phase I Trial |
CHIKV live-attenuated vaccines (LAV)
Production of formalin-inactivated CHIKV vaccines generates cost and safety concerns due to the requirement of BSL-3 facilities and the associated risks posed by producing large amounts of infectious CHIKV prior to being subjected to the inactivation process.14 These concerns prompted the development in the 1980s of a Live-attenuated CHIKV vaccine by scientists at the U. S. Army Medical Research Institute of Infectious Diseases (USAMRIID) in Maryland.15
Studies with the formalin-inactivated CHIKV 15561 generated extensive knowledge on the vaccine production and assessment in pre-clinical and clinical trials.13 Hence, the vaccine produced after 10 passages was subjected to an 11th passage in GMKC to be subsequently transferred from WRAIR to USARMRIID to proceed to attenuation by culturing virus in human embryonic lung MRC-5 cells. 347 plaques were subjected to 18 passages in MRC-5 cells. Clone 181 produced small, 2–3 mm homogeneous plaques and the CHIK 181/clone 25 was finally chosen as the vaccine seed for subsequent vaccine production. The selected clone provided complete protective efficacy to weanling mice using an intracerebral (i.c.) challenge with CHIKV. Following these results, rhesus macaques were administered the vaccine subcutaneously and challenged with CHIKV approximately 5 weeks after vaccination. All vaccinated macaques had complete, sterile protection (Table 1).15 Induction of neutralizing antibodies was evident and peaked at day 14, with an average of PRNT80 titers of 165, 440 and 800 following vaccinations with doses of 3.5, 4.5 and 5.5 log10 pfu of immunizing dose.
Following these results, the CHIK 181/clone 25 was manufactured at The Salk Institute-Government Services Division (TSI-GSD) and an investigational new drug application was filed by the U.S. Department of Defense with the FDA in 1986.14 The CHIKV TSI-GSD-218 vaccine entered clinical trials at both, USAMRIID and the University of Maryland Center for Vaccine development (Figure 2),16 showing safety and immunogenicity in a phase I trial in 15 volunteers. The vaccine was subsequently tested in a phase II, randomized, double blind, placebo controlled in 59 healthy volunteers (35 men, 24 women) receiving a single s.c. vaccine dose of 0.5 ml of the reconstituted vaccine, containing 105 pfu/dose. 14 volunteers (12 men, 2 women) received placebo vaccine.17 Vaccination resulted in 98% of volunteers developing neutralising antibodies and importantly, 5 (8%) CHIKV vaccinees developed transient arthralgias that were absent in the placebo group. The authors noted that the incidence of arthralgia in vaccinees compared to controls would have reached statistical significance in a study with larger vaccine and control groups.17
Developing a CHIKV vaccine with the traditional methodologies using inactivated or live-attenuated viruses present some challenges, as mentioned earlier, due to the production of large amounts of virus requiring the appropriate biosafety levels to prevent accidents before achieving inactivation, or induction of adverse events like arthralgia upon vaccination with live-attenuated viruses. Therefore, the field of CHIKV vaccine development presents opportunities for the development of new sub-unit vaccines, Virus-Like Particles, replication-defficient viral vectors, DNA vaccines and proteins that are considered safe options, albeit potentially less immunogenic thus requiring the use of adjuvants or the administration of multiple doses to enhance immunity against CHIKV. Intermitent, but major outbreaks contributed to maintaining the interests in the development of a CHIKV vaccine, prompting efforts to assess new vaccine platforms.
Virus-like particles (vlps)
VLPs are produced by the expression of viral structural proteins that self-assemble to produce structures similar to the original virus but lacking the capacity to infect and replicate. By mimicking the virus structure without the viral genomes, they resemble ‘empty shells’.18
The Vaccine Research Center (VRC) at NIH’s National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, has developed a CHIKV VLP. The vaccine is composed of the structural CHIKV proteins Capsid, E3, E2, 6K and E1 sequences of the CHIKV strain 37,997 from a CHIKV Waf lineage. The VLP vaccine has been tested in macaques (n = 6), using intramuscular regimes consisting of 20 μg of VLPs in 1mL PBS at weeks 0, 4 and 24. Immune responses were evident after a primary immunisation and increased after subsequent boosts at 4 and 24 weeks. All the immunised macaques were able to control viraemia and inflammation after a challenge with 1010 PFU of CHIKV (strain LR2006 opy-1) doses through the induction of neutralising antibodies.19
The VLP, known as VRC-CHKVLP059-00-VP was GMP produced by transfection of VRC293 cells (suspension-adapted, serum-free HEK293 cells) with plasmid DNA expressing the CHIKV structural genes (Table 1). The VLP has been assessed in the VRC 311 phase I clinical trial in a dose-escalation, open label trial to evaluate safety, tolerability and immunogenicity of the CHIKV VLP in 25 adults of 18 to 50 years of age.20 The vaccine was administered intramuscularly (i.m.) to humans at doses of 10 μg, 20 μg and 40 μg given at weeks 0, 4 and 20 weeks with no adjuvant included. The vaccine was well tolerated and no serious adverse events were reported. Antibody responses by ELISA were positive upon measurement of an endpoint ELISA titer technique agains the CHIKV VLP antigen strain 37,997, reaching an average of 4,457, 5,881 and 8,611 (after receiving 10, 20 and 40 μg of VLP, respectively) on week 44 after receiving three vaccinations. Most participants showed induction of neutralising antibodies after the first VLP vaccination and all participants had neutralising antibodies 4 weeks after the second vaccination, reaching titers of 188, 236 and 346 elicited by 10, 20 and 40 μg of VLP, respectively.20 The VRC-CHKVLP059-00-VP has entered phase II trials in 2015 in a multicenter study to evaluate safety and immunogenicity using two vaccine doses in 400 healthy adults between 18 to 60 years of age in locations including Dominican Republic, Guadeloupe, Haiti, Martinique and Puerto Rico ClinicalTrials.gov (NCT02562482).
Viral-vectored vaccines (VVV)
Measles viral vector
A member of the Morbillivirus genus within the family of Paramixovirus, Measles virus (MV) has been developed as a viral vectored vaccine for various diseases at the Institut Pasteur in Paris in a seminal work lead by the group of Frédéric Tangy.21–25
In 2013, Samantha Brandler et al. reported the development of a recombinant Measles viral-vectored (MVV) vaccine expressing the heterologous structural genes of CHIKV (Figure 2).26 The cDNA CHIKV structural cassette encodes the C-E3-E2-6K-E1 which in turn forms Virus-Like Particles upon expression in cell culture.26 The CHIKV sequence corresponded to an Indian Ocean Lineage (IOL) isolate from a patient sampled on 2 December 2005 in the Southern locality of St. Louis, La Réunion (CHIKV strain 06–49, GenBank accession no. AM258994).27
Immune responses have been evaluated in genetically modified mice expressing the human MV receptor hCD46 and no IFN-α/β receptor (CD46-IFNAR) to assess vaccine efficacy in a mouse model highly susceptible to CHIKV infection. Antibody responses were assessed after a single or a double injection one month apart with 103, 104 or 105 MV TCID50. A single immunisation gave ELISA endpoint titres between of 1,350, 4,050 and 12,150 that increased after a boost to 2,700, 12,150 or 48,600 for each respective MV dose. Neutralising antibodies were quantified using a plaque reduction neutralisation test (PRNT) to calculate reduction of plaque number in at least 50% (PRNT50) or 90% (PRNT90). PRNT50 titres after a single vaccination were of 50, 150 and 450 for each increasing MV-CHIK priming dose and 450, 1,350 and 4,050 after a boost with 103, 104 or 105 MV TCID50. Cellular responses quantified by an ex vivo IFN-gamma ELISPOT yielded responses of a mean of 150 sfu/106 splenocytes after a single immunisation with a higher MV dose of 106 TCID50. Survival of mice after a challenge with 100 PFU of CHIKV-06–49 showed protection of 83% of the mice vaccinated with 103 TCID50, while complete protection was achieved with prime/boost using the two highest doses or upon a single prime with 105 TCID50, thus demonstrating the potential of the MV-CHIK as a vaccine for CHIKV and other arboviruses26 and prompting its assessment in a phase I trial that was reported two years later.
The MV-CHIKV was assessed for safety and immunogenicity in a phase I clinical trial (Table 1 and Figure 2),28 which enrolled 42 participants with 12 volunteers each group to receive a low (1.5x104 TCID50), intermediate (7.5x104 TCID50) or high (3.0x105 TCID50) dose of the vaccine and one control group (n = 6) receiving Priorix (GSK MMR vaccine containing MV). Seroconversion was 44%, 92% and 90% for the low, intermediate and high dose group after a single immunisation and geometric mean titres of PRNT50 neutralising antibodies were 10, 48 and 46 for the low, intermediate and high vaccine priming doses with Priorix giving a control titre of 7. A homologous prime boost with an interval of 28 days yielded GM antibody titres of 73, 150 and 433 that peaked at day 56 post boost. 100% seroconversion required a vaccine boost. In general, the vaccine showed a good safety profile with no serious adverse events recorded.28
A subsequent double-blind, randomised, placebo-controlled and active-controlled phase 2 trial has recently been completed. 263 participants were recruited to evaluate safety and immunogenicity after vaccination with low (5xE4 TCID50) or high (5xE5 TCID50) doses in 0.3 ml of solution at either, an interval of 28 (D0 and D28) or 168 (D28 and D196) days between prime and boost. Presence of neutralizing antibodies at day 56 was the primary endpoint and results showed that a low vaccine dose induced a PRNT50 titer of 50.16 and 12.87 (short and long interval, respectively), while the high dose induced titers of 174.80 and 33.64 (short and long interval, respectively), with excellent safety and tolerability.29
Adenoviral vectors
Adenoviruses are members of the family Adenoviridae. One of the genus belonging to this family, the Mastadenovirus have mammals and vertebrates as natural hosts and it includes the human adenoviruses. There are 51 human adenovirus serotypes which are classified in six subgroups, from A to F. Chimpanzee adenoviruses are considered part of subgroup E.30
Most people have been exposed to common human adenovirus from early childhood, generating immune responses that can neutralise homologous serotypes. Neutralizing antibodies to the human serotype AdHu5, for instance, vary from 34% in the USA to 76% in Thailand and up to 89% in Nigeria.31 Thus, vaccine efficiency using viral vectors derived from common human serotypes can be negatively affected. Amongst approaches to circumvent pre-existing immunity to human adenovirus are the use of non-human adenoviruses.32,33 Similarity of chimpanzee and human adenovirus has prompted the development of simian adenoviral vectors which have become widely used. The first report using a chimpanzee adenovirus as viral vectored vaccine was made by the group lead by Hildegund Ertl at the Wistar Institute in Philadelphia, USA, who demonstrated the induction of immune responses to a rabies glycoprotein expressed by the chimpanzee adenovirus serotype 68.34
Recently, a new ChAdOx1 viral vector was derived from a chimpanzee adenovirus known Y25 that belongs to subgroup E adenoviruses. This adenoviral vector was shown to induce similar immunogenicity to other chimpanzee adenoviruses and low seroprevalence of pre-existing immunity in populations from the UK and Gambia.35 ChAdOx1 was subsequently engineered in Oxford to express the Chikungunya structural proteins Capsid, E3, E2, 6K and E1 encoded by a consensus sequence from various CHIKV lineages. The ChAdOx1-Chik is able to induce high titres of neutralising antibodies and high frequencies of CHIKV-specific T cells in mouse pre-clinical models (manuscript in preparation).
ChAdOx1-Chik has been developed under Good Manufacturing Practices and has entered clinical trials in 2018 ClinicalTrials.gov (NCT03590392). This is a phase I, open label, dose escalation clinical trial to assess safety and immunogenicity in healthy volunteers between 18–50 years of age. The vaccine will be administered intramuscularly at three different doses: 5x109, 2.5 × 1010 and 5 × 1010 that have been selected based on previous results using the ChAdOx1 viral vector as a flu vaccine.36 ChAdOx1 Chik is a replication-deficient adenovirus that does not require adjuvants to induce strong immune responses and it has been administered successfully to more than 160 volunteers in various clinical trials with no serious adverse events reported. Results of the CHIK001 trial are expected in 2019.
A major consideration for vaccine approaches will be their ability to provide protection against heterologous lineages. Vaccines mentioned above have used sequences from different isolates. Table 1 shows that some are based on Asian strains, others on Waf, IOL or consensus sequences from all lineages. CHIKV has the ability to evolve into novel variants within a short period of time when entering a naive population as evidenced recently in the Americas37. Nevertheless, CHIKV still keeps a high percent in amino acid identity, ranging from 95–99.9% within the structural proteins, which implies a limited diversity between CHIKV isolates. Despite the sequence and virulence diversity in animal models, vaccines based on one lineage (e.g. IOL) can provide long-lasting cross protection in heterologous CHIKV challenges in mice and macaques despite high virulence presented by some isolates in naive animals.38 These conclusions have been reinforced by studies evaluating the neutralisation capacity of VLP-induced nAbs in clinical trial samples when tested against 9 CHIKV strains that represent all CHIKV genotypes.39 These results indicate that CHIKV variability may not affect cross- or heterologous protection when developing vaccines based on different isolates or consensus sequences.
Another important aim for vaccine developers will consist of establishing a correlate of protection for CHIKV infection to support a swift transition towards licensure. Experience indicates that the lack of a reliable correlate of protection is a major roadblock for developing and improving vaccines such as tuberculosis40 that rely on expensive and complex phase IIb and III trials to assess efficacy. This objective may be easier to achieve for CHIKV, as evidence indicates that the presence of IgG antibodies correlate with virus clearance.41 As CHIKV outbreaks have an unpredictable nature with rapid and unexpected movements affecting large populations to then be followed by years of relative infectious silence, efficacy trials will face a major challenge in their design to evaluate vaccine efficacy and scientists may have to find alternative and smarter strategies. Epidemiology studies will be of major importance to determine the interplay between viruses transmitted by the same Aedes mosquito species to contribute not only to the design of trials but also to vaccination strategies as CHIKV, Zika and Dengue vaccines reach the stage of licensure.
In summary, 51 years have passed since the first report of the development of a CHIKV vaccine. The unpredictable epidemiology of CHIKV with sudden massive outbreaks followed by years of relative silence, has played a major role on the development of CHIKV vaccines, which seem to gain or loose momentum depending on the emergence or disappearence of outbreaks. Initial clinical developments focused on Formalin-Inactivated Vaccines (FAV) during the late 1960s and a Live-Attenuated Vaccines (LAV) in the late 1980s. Despite promising results, efforts waned due to the unpredictability of the CHIKV epidemiology, difficulty to demonstrate protective efficacy in the field and limited funding availability.14 A CHIKV epidemic in La Réunion in 2006, maintained the interest and activities on LAV and renewed the interest in the development of new vaccine approaches, such as Virus-Like Particles (VLP) and Measles Vectored Vaccines (MVV). A more recent oubreak in the Americas starting in the Caribbean in 2013 and expanding to South, Central and North America, particularly in Mexico, contributed to the latest development based on Chimpanzee Adenoviral vectored vaccines for CHIKV that are the most recent vaccine platforms entering CHIKV clinical trials.
Funding Statement
This work was supported by the Innovate UK [972212]. https://gtr.ukri.org/projects?ref=972212
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Development of a ChAdOx1-Chik is independent research funded by the UK Department of Health through Innovate UK “New vaccines for global epidemics: development and manufacture” grant No. 972212 (A.R-S).
References
- 1.Solignat M, Gay B, Higgs S, Briant L, Devaux C.. Replication cycle of chikungunya: a re-emerging arbovirus. Virology. 2009;393:183–97. doi: 10.1016/j.virol.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers AM, Brault AC, Shirako Y, Strauss EG, Kang W, Strauss JH, Weaver SC. Evolutionary relationships and systematics of the alphaviruses. J Virol. 2001;75:10118–31. doi: 10.1128/JVI.75.21.10118-10131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers AM. Vaccine and therapeutic options to control chikungunya virus. Clin Microbiol Rev. 2018;31(1). doi: 10.1128/CMR.00104-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsauer K, Tangy F. Chikungunya virus vaccines: viral vector-based approaches. J Infect Dis. 2016;214:S500–S5. doi: 10.1093/infdis/jiw369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erasmus JH, Rossi SL, Weaver SC. Development of Vaccines for Chikungunya Fever. J Infect Dis. 2016;214:S488–S96. doi: 10.1093/infdis/jiw271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volk SM, Chen R, Tsetsarkin KA, Adams AP, Garcia TI, Sall AA, Nasar F, Schuh AJ, Holmes EC, Higgs S, et al. Genome-scale phylogenetic analyses of chikungunya virus reveal independent emergences of recent epidemics and various evolutionary rates. J Virol. 2010;84:6497–504. doi: 10.1128/JVI.01603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahadeo NSD, Allicock OM, De Salazar PM, Auguste AJ, Widen S, Olowokure B, Gutierrez C, Valadere AM, Polson-Edwards K, Weaver SC, et al. Understanding the evolution and spread of chikungunya virus in the Americas using complete genome sequences. Virus Evol. 2017;3:vex010. doi: 10.1093/ve/vex010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison VR, Binn LN, Randall R. Comparative immunogenicities of chikungunya vaccines prepared in avian and mammalian tissues. Am J Trop Med Hyg. 1967;16:786–91. [PubMed] [Google Scholar]
- 9.Mason PJ, Haddow AJ. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952-53; an additional note on Chikungunya virus isolations and serum antibodies. Trans R Soc Trop Med Hyg. 1957;51:238–40. [DOI] [PubMed] [Google Scholar]
- 10.Weinbren MP, Haddow AJ, Williams MC. The occurrence of Chikungunya virus in Uganda. I. Isolation from mosquitoes. Trans R Soc Trop Med Hyg. 1958;52:253–57. [DOI] [PubMed] [Google Scholar]
- 11.Halstead SB, Buescher EL. Hemorrhagic disease in rodents infected with virus associated with Thai hemorrhagic fever. Science. 1961;134:475–76. [DOI] [PubMed] [Google Scholar]
- 12.Shah KV, Gibbs CJ Jr., Banerjee G. Virological investigation of the epidemic of haemorrhagic fever in calcutta: isolation of three strains of chikungunya virus. Indian J Med Res. 1964;52:676–83. [PubMed] [Google Scholar]
- 13.Harrison VR, Eckels KH, Bartelloni PJ, Hampton C. Production and evaluation of a formalin-killed Chikungunya vaccine. J Immunol. 1971;107:643–47. [PubMed] [Google Scholar]
- 14.Hoke CH Jr., Pace-Templeton J, Pittman P, Malinoski FJ, Gibbs P, Ulderich T, Mathers M, Fogtman B, Glass P, Vaughn DW. US Military contributions to the global response to pandemic chikungunya. Vaccine. 2012;30:6713–20. doi: 10.1016/j.vaccine.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE Jr., Lupton HW. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine. 1986;4:157–62. [DOI] [PubMed] [Google Scholar]
- 16.Malinoski FJ, Ksiazek T, Ramsburg H, Lupton HW, Meadors GF. Safety and immunogenicity of a new chikungunya virus vaccine: double-blind, placebo controlled human trial. 38th Annual Meeting of the Amer Soc Trop Med Hyg. 1989;Section 8.2. [Google Scholar]
- 17.Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg. 2000;62:681–85. [DOI] [PubMed] [Google Scholar]
- 18.Ding X, Liu D, Booth G, Gao W, Lu Y. Virus-like particle engineering: from rational design to versatile applications. Biotechnol J. 2018;13:e1700324. doi: 10.1002/biot.v13.5. [DOI] [PubMed] [Google Scholar]
- 19.Akahata W, Yang ZY, Andersen H, Sun S, Holdaway HA, Kong WP, Lewis MG, Higgs S, Rossmann MG, Rao S, et al. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334–38. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang LJ, Dowd KA, Mendoza FH, Saunders JG, Sitar S, Plummer SH, Yamshchikov G, Sarwar UN, Hu Z, Enama ME, et al. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. Lancet. 2014;384:2046–52. doi: 10.1016/S0140-6736(14)61185-5. [DOI] [PubMed] [Google Scholar]
- 21.Brandler S, Lucas-Hourani M, Moris A, Frenkiel MP, Combredet C, Fevrier M, Bedouelle H, Schwartz O, Desprès P, Tangy F, et al. Pediatric measles vaccine expressing a dengue antigen induces durable serotype-specific neutralizing antibodies to dengue virus. PLoS Negl Trop Dis. 2007;1:e96. doi: 10.1371/journal.pntd.0000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brandler S, Marianneau P, Loth P, Lacote S, Combredet C, Frenkiel MP, Desprès P, Contamin H, Tangy F. Measles vaccine expressing the secreted form of West Nile virus envelope glycoprotein induces protective immunity in squirrel monkeys, a new model of West Nile virus infection. J Infect Dis. 2012;206:212–19. doi: 10.1093/infdis/jis328. [DOI] [PubMed] [Google Scholar]
- 23.Brandler S, Ruffie C, Najburg V, Frenkiel MP, Bedouelle H, Despres P, Tangy F. Pediatric measles vaccine expressing a dengue tetravalent antigen elicits neutralizing antibodies against all four dengue viruses. Vaccine. 2010;28:6730–39. doi: 10.1016/j.vaccine.2010.07.073. [DOI] [PubMed] [Google Scholar]
- 24.Despres P, Combredet C, Frenkiel MP, Lorin C, Brahic M, Tangy F. Live measles vaccine expressing the secreted form of the West Nile virus envelope glycoprotein protects against West Nile virus encephalitis. J Infect Dis. 2005;191:207–14. doi: 10.1086/426824. [DOI] [PubMed] [Google Scholar]
- 25.Lorin C, Mollet L, Delebecque F, Combredet C, Hurtrel B, Charneau P, Brahic M, Tangy F. A single injection of recombinant measles virus vaccines expressing human immunodeficiency virus (HIV) type 1 clade B envelope glycoproteins induces neutralizing antibodies and cellular immune responses to HIV. J Virol. 2004;78:146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brandler S, Ruffie C, Combredet C, Brault JB, Najburg V, Prevost MC, Habel A, Tauber E, Desprès P, Tangy F. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine. 2013;31:3718–25. doi: 10.1016/j.vaccine.2013.05.086. [DOI] [PubMed] [Google Scholar]
- 27.Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes J-M, Pettinelli F, et al. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsauer K, Schwameis M, Firbas C, Mullner M, Putnak RJ, Thomas SJ, Desprès P, Tauber E, Jilma B, Tangy F. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect Dis. 2015;15:519–27. doi: 10.1016/S1473-3099(15)70043-5. [DOI] [PubMed] [Google Scholar]
- 29.Reisinger EC, Tschismarov R, Beubler E, Wiedermann U, Firbas C, Loebermann M, Pfeiffer A, Muellner M, Tauber E, Ramsauer K. Immunogenicity, safety, and tolerability of the measles-vectored chikungunya virus vaccine MV-CHIK: a double-blind, randomised, placebo-controlled and active-controlled phase 2 trial. Lancet. 2018. doi: 10.1016/S0140-6736(18)32488-7. [DOI] [PubMed] [Google Scholar]
- 30.Lasaro MO, Ertl HC. New insights on adenovirus as vaccine vectors. Mol Ther. 2009;17:1333–39. doi: 10.1038/mt.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang Z, Li Y, Cun A, Yang W, Ellenberg S, Switzer WM, Kalish ML, Ertl HCJ. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg Infect Dis. 2006;12:1596–99. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majhen D, Calderon H, Chandra N, Fajardo CA, Rajan A, Alemany R, Custers J. Adenovirus-based vaccines for fighting infectious diseases and cancer: progress in the field. Hum Gene Ther. 2014;25:301–17. doi: 10.1089/hum.2013.235. [DOI] [PubMed] [Google Scholar]
- 33.Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM, Ertl HC. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J Virol. 2003;77:10780–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang Z, Gao G, Reyes-Sandoval A, Cohen CJ, Li Y, Bergelson JM, Wilson JM, Ertl HCJ. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J Virol. 2002;76:2667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dicks MD, Spencer AJ, Edwards NJ, Wadell G, Bojang K, Gilbert SC, Hill AVS, Cottingham MG, Kremer EJ. A novel chimpanzee adenovirus vector with low human seroprevalence: improved systems for vector derivation and comparative immunogenicity. PLoS One. 2012;7:e40385. doi: 10.1371/journal.pone.0040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antrobus RD, Coughlan L, Berthoud TK, Dicks MD, Hill AV, Lambe T, Gilbert SC. Clinical assessment of a novel recombinant simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved Influenza A antigens. Mol Ther. 2014;22:668–74. doi: 10.1038/mt.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stapleford KA, Moratorio G, Henningsson R, Chen R, Matheus S, Enfissi A, Weissglas-Volkov D, Isakov O, Blanc H, Mounce BC, et al. Whole-genome sequencing analysis from the chikungunya virus caribbean outbreak reveals novel evolutionary genomic elements. PLoS Negl Trop Dis. 2016;10:e0004402. doi: 10.1371/journal.pntd.0004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langsjoen RM, Haller SL, Roy CJ, Vinet-Oliphant H, Bergren NA, Erasmus JH, Livengood JA, Powell TD, Weaver SC, Rossi SL. Chikungunya virus strains show lineage-specific variations in virulence and cross-protective ability in murine and nonhuman primate models. MBio. 2018;9(2) e02449-17. doi: 10.1128/mBio.02449-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goo L, Dowd KA, Lin TY, Mascola JR, Graham BS, Ledgerwood JE, Pierson TC. A virus-like particle vaccine elicits broad neutralizing antibody responses in humans to all chikungunya virus genotypes. J Infect Dis. 2016;214:1487–91. doi: 10.1093/infdis/jiw431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatt K, Verma S, Ellner JJ, Salgame P. Quest for correlates of protection against tuberculosis. Clin Vaccine Immunol. 2015;22:258–66. doi: 10.1128/CVI.00721-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kam YW, Lum FM, Teo TH, Lee WW, Simarmata D, Harjanto S, Chua C-L, Chan Y-F, Wee J-K, Chow A, et al. Early neutralizing IgG response to Chikungunya virus in infected patients targets a dominant linear epitope on the E2 glycoprotein. EMBO Mol Med. 2012;4:330–43. doi: 10.1002/emmm.201200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metz SW, Gardner J, Geertsema C, Le TT, Goh L, Vlak JM, Suhrbier A, Pijlman GP, Powers AM. Effective chikungunya virus-like particle vaccine produced in insect cells. PLoS Negl Trop Dis. 2013;7:e2124. doi: 10.1371/journal.pntd.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metz SW, Martina BE, van Den Doel P, Geertsema C, Osterhaus AD, Vlak JM, Pijlman GP. Chikungunya virus-like particles are more immunogenic in a lethal AG129 mouse model compared to glycoprotein E1 or E2 subunits. Vaccine. 2013;31:6092–96. doi: 10.1016/j.vaccine.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 44.Saraswat S, Athmaram TN, Parida M, Agarwal A, Saha A, Dash PK. Expression and characterization of yeast derived Chikungunya Virus Like Particles (CHIK-VLPs) and its evaluation as a potential vaccine candidate. PLoS Negl Trop Dis. 2016;10:e0004782. doi: 10.1371/journal.pntd.0004782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang E, Volkova E, Adams AP, Forrester N, Xiao SY, Frolov I, Weaver SC. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine. 2008;26:5030–39. doi: 10.1016/j.vaccine.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang E, Kim DY, Weaver SC, Frolov I. Chimeric Chikungunya viruses are nonpathogenic in highly sensitive mouse models but efficiently induce a protective immune response. J Virol. 2011;85:9249–52. doi: 10.1128/JVI.00844-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Den Doel P, Volz A, Roose JM, Sewbalaksing VD, Pijlman GP, van Middelkoop I, Duiverman V, van de Wetering E, Sutter G, Osterhaus ADME, et al. Recombinant modified vaccinia virus Ankara expressing glycoprotein E2 of Chikungunya virus protects AG129 mice against lethal challenge. PLoS Negl Trop Dis. 2014;8:e3101. doi: 10.1371/journal.pntd.0003101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weger-Lucarelli J, Chu H, Aliota MT, Partidos CD, Osorio JE. A novel MVA vectored Chikungunya virus vaccine elicits protective immunity in mice. PLoS Negl Trop Dis. 2014;8:e2970. doi: 10.1371/journal.pntd.0002970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Arriaza J, Cepeda V, Hallengard D, Sorzano CO, Kummerer BM, Liljestrom P, Esteban M. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against chikungunya infection. J Virol. 2014;88:3527–47. doi: 10.1128/JVI.03418-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chattopadhyay A, Wang E, Seymour R, Weaver SC, Rose JK. A chimeric vesiculo/alphavirus is an effective alphavirus vaccine. J Virol. 2013;87:395–402. doi: 10.1128/JVI.01860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang D, Suhrbier A, Penn-Nicholson A, Woraratanadharm J, Gardner J, Luo M, Le TT, Anraku I, Sakalian M, Einfeld D, et al. A complex adenovirus vaccine against chikungunya virus provides complete protection against viraemia and arthritis. Vaccine. 2011;29:2803–09. doi: 10.1016/j.vaccine.2011.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muthumani K, Block P, Flingai S, Muruganantham N, Chaaithanya IK, Tingey C, Wise M, Reuschel EL, Chung C, Muthumani A, et al. Rapid and long-term immunity elicited by dna-encoded antibody prophylaxis and DNA vaccination against chikungunya virus. J Infect Dis. 2016;214:369–78. doi: 10.1093/infdis/jiw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muthumani K, Lankaraman KM, Laddy DJ, Sundaram SG, Chung CW, Sako E, Wu L, Khan A, Sardesai N, Kim JJ, et al. Immunogenicity of novel consensus-based DNA vaccines against Chikungunya virus. Vaccine. 2008;26:5128–34. doi: 10.1016/j.vaccine.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mallilankaraman K, Shedlock DJ, Bao H, Kawalekar OU, Fagone P, Ramanathan AA, Ferraro B, Stabenow J, Vijayachari P, SG Sundaram, et al. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl Trop Dis. 2011;5:e928. doi: 10.1371/journal.pntd.0001370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tretyakova I, Hearn J, Wang E, Weaver S, Pushko P. DNA vaccine initiates replication of live attenuated chikungunya virus in vitro and elicits protective immune response in mice. J Infect Dis. 2014;209:1882–90. doi: 10.1093/infdis/jiu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar M, Sudeep AB, Arankalle VA. Evaluation of recombinant E2 protein-based and whole-virus inactivated candidate vaccines against chikungunya virus. Vaccine. 2012;30:6142–49. doi: 10.1016/j.vaccine.2012.07.072. [DOI] [PubMed] [Google Scholar]
- 57.Khan M, Dhanwani R, Rao PV, Parida M. Subunit vaccine formulations based on recombinant envelope proteins of Chikungunya virus elicit balanced Th1/Th2 response and virus-neutralizing antibodies in mice. Virus Res. 2012;167:236–46. doi: 10.1016/j.virusres.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Chu H, Das SC, Fuchs JF, Suresh M, Weaver SC, Stinchcomb DT, Partidos CD, Osorio JE. Deciphering the protective role of adaptive immunity to CHIKV/IRES a novel candidate vaccine against Chikungunya in the A129 mouse model. Vaccine. 2013;31:3353–60. doi: 10.1016/j.vaccine.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plante K, Wang E, Partidos CD, Weger J, Gorchakov R, Tsetsarkin K, Borland EM, Powers AM, Seymour R, Stinchcomb DT, et al. Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog. 2011;7:e1002142. doi: 10.1371/journal.ppat.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roy CJ, Adams AP, Wang E, Plante K, Gorchakov R, Seymour RL, Vinet-Oliphant H, Weaver SC. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J Infect Dis. 2014;209:1891–99. doi: 10.1093/infdis/jiu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piper A, Ribeiro M, Smith KM, Briggs CM, Huitt E, Nanda K, Spears CJ, Quiles M, Cullen J, Thomas ME, et al. Chikungunya virus host range E2 transmembrane deletion mutants induce protective immunity against challenge in C57BL/6J mice. J Virol. 2013;87:6748–57. doi: 10.1128/JVI.03357-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hallengard D, Kakoulidou M, Lulla A, Kummerer BM, Johansson DX, Mutso M, Lulla V, Fazakerley JK, Roques P, Le Grand R, et al. Novel attenuated Chikungunya vaccine candidates elicit protective immunity in C57BL/6 mice. J Virol. 2014;88:2858–66. doi: 10.1128/JVI.03453-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roques P, Ljungberg K, Kummerer BM, Gosse L, Dereuddre-Bosquet N, Tchitchek N, Rives M, Georges N, Garcia-Bonnet N, Sylla AI, et al. Attenuated and vectored vaccines protect nonhuman primates against Chikungunya virus. JCI Insight. 2017;2:e83527. doi: 10.1172/jci.insight.88864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gardner CL, Hritz J, Sun C, Vanlandingham DL, Song TY, Ghedin E, Higgs S, Klimstra WB, Ryman KD, Weaver SC. Deliberate attenuation of chikungunya virus by adaptation to heparan sulfate-dependent infectivity: a model for rational arboviral vaccine design. PLoS Negl Trop Dis. 2014;8:e2719. doi: 10.1371/journal.pntd.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]


