Abstract
The common marmoset (Callithrix jacchus) is uniquely suited for longitudinal studies of cognitive aging, due to a relatively short lifespan, sophisticated cognitive abilities, and patterns of brain aging that resemble those of humans. We examined cognitive function and fine motor skills in male and female marmosets (mean age ~5 at study entry) followed longitudinally for 2 years. Each year, monkeys were tested on a reversal learning task with three pairs of stimuli (n = 18, 9 females) and a fine motor task requiring them to grasp small rewards from two staircases (Hill and Valley test, n = 12, 6 females). There was little evidence for a decline in cognitive flexibility between the two time points, in part because of practice effects. However, independent of year of testing, females took longer than males to reach criterion in the reversals, indicating impaired cognitive flexibility. Motivation was unlikely to contribute to this effect, as males refused a greater percentage of trials than females in the reversals. With regards to motor function, females were significantly faster than males in the Hill and Valley task. From Year 1 to Year 2, a slight slowing of motor function was observed in both sexes, but accuracy decreased significantly in males only. This study (1) demonstrates that marmosets exhibit sex differences in cognitive flexibility and fine motor function that resemble those described in humans; (2) that changes in fine motor function can already be detected at middle-age; and (3) that males may experience greater age-related changes in fine motor skills than females. Additional data points will determine whether these sex and age differences persist over time.
Keywords: aging, cognition, executive function, monkey, sex differences
1 |. INTRODUCTION
The proportion of older people is steadily increasing in most parts of the world, including the United States, where the percentage of people over 65 years old is expected to rise from 15% in 2018 to 24% of the population by 2060 (US Census Bureau, 2017). Human aging is associated with a significant decline in cognitive function in most domains (Lindenberger, 2014; Reuter-Lorenz & Park, 2010; Salthouse, 2010), with fluid abilities, such as working memory and reasoning, being particularly affected, and experienced-based “crystallized” abilities, such as vocabulary, being more resistant to age-related decline. Aging is also characterized by a significant decline in fine motor function (Smith et al., 1999). Marked age-related changes are detected at every level of analysis in the human brain, from neuroanatomical structure, to neurochemistry and cellular and molecular levels (Raz, 2000; Raz & Rodrigue, 2006). However, it has become clear in recent years that the extent of age-related brain change and its functional consequences vary greatly between individuals. Longitudinal studies are particularly valuable to measure the rate of age-related change from an initial baseline performance and study the underlying mechanisms (Schaie, 2005).
Animal models are critically needed to answer key questions about the causes and consequences of cognitive and motor aging, and to identify and test potential treatments to alleviate age-related cognitive and motor impairment. Rodents possess many advantages as models for aging research (Bizon & Woods, 2009; Gallagher, Stocker, & Koh, 2011), but also have limitations with regards to the translation of the findings to humans. Nonhuman primate (NHPs), such as rhesus or cynomolgus macaques, are arguably better models for human neurocognitive and motor aging due to a higher degree of similarity with humans in cognitive and motor function, brain organization and patterns of age-related cognitive and motor decline (Baxter, 2001; Herndon, Moss, Rosene, & Killiany, 1997; Lacreuse & Herndon, 2009). However, the relatively long lifespan of macaques (~maximum 45 years) has hindered the design of longitudinal studies that can inform age-related cognitive and motor change and their biological concomitants. Shorter-lived nonhuman primates have long been proposed as complementary models for aging research (Austad, 1997). Among these, the marmoset, which has the shortest average lifespan of all anthropoids (~10 years) is particularly promising (Fischer & Austad, 2011; Ross, Davis, Dobek, & Tardif, 2012; Tardif et al., 2017; Tardif, Mansfield, Ratnam, Ross, & Ziegler, 2011). The marmoset possesses a number of behavioral and neuroanatomical features that make it particularly valuable as a model for human neurocognitive aging. Despite its small size, the marmoset has a relatively large brain, with a core neural architecture (Chaplin, Yu, Soares, Gattass, & Rosa, 2013; Liu et al., 2018) and resting state networks (Belcher et al., 2013, 2016; Silva, 2017) that show many similarities with humans’. Marmosets also have highly developed cognitive abilities (Huber & Voelkl, 2009; Spinelli et al., 2004; Strasser & Burkart, 2012) cooperative breeding and a rich social behavior with evidence of prosocial tendencies (Miller, 2017). They have relatively good manual control and are extensively used as models for motor degeneration such as Parkinson’s disease (Eslamboli, 2005). Due to its amenability to genome editing, interest in the marmoset as a model organism for neuroscience research has dramatically increased in recent years. Yet, the development of this species as a model for aging is still in infancy (Ross et al., 2012; Tardif et al., 2011). Although there is uncertainty with regards to the maximum lifespan of the marmoset in captivity (recorded as 21 years old, Nishijima et al., 2012), clear signs of aging are observed in the marmoset brain by the age of 8, as indicated by reduced neurogenesis in the dentate gyrus (Leuner, Kozorovitskiy, Gross, & Gould, 2007), β-amyloid deposition (Geula, Nagykery, & Wu, 2002), and increased abnormally phosphorylated Tau (Rodriguez-Callejas, Fuchs, & Perez-Cruz, 2016). However, age-related differences in cognitive and motor function have not been characterized. One of the greatest advantage provided by the marmoset relative to other NHPs is the ability to conduct longitudinal studies spanning the entire older lifespan of the animal (roughly 5–10 years old).
The first goal of this paper was to examine change in cognitive flexibility and fine motor function in middle-aged marmosets (~5 years old at study onset) studied at baseline and 1 year later. Cognitive flexibility is an ability that is markedly impaired with age in humans (Mell et al., 2005) and other animals (e.g., Lai, Moss, Killiany, Rosene, & Herndon, 1995; Rapp, 1990; Tapp et al., 2003). In this study, cognitive flexibility was assessed through performance on reversal learning, a task dependent on the orbitofrontal cortex (OFC) that requires the animal to adapt to changing stimulus/reward contingencies. Motor function was evaluated via performance on the Hill and Valley task, a motor task classically used in Parkinson’s disease research. The second goal of the study was to examine sex differences in the aging process. The human literature is inconsistent with regards to the existence of sex differences in the trajectories of age-related cognitive decline. Many studies have found no evidence for sex differences in age-related cognitive decline (de Frias, Nilsson, & Herlitz, 2006; Ferreira, Ferreira Santos-Galduróz, Ferri, & Fernandes Galduróz, 2014; Finkel, Reynolds, Berg, & Pedersen, 2006; Gerstorf, Herlitz, & Smith, 2006; Karlsson, Thorvaldsson, Skoog, Gudmundsson, & Johansson, 2015). However, a recent analysis of a large longitudinal dataset from the Baltimore Study on Aging reported greater age-related cognitive decline in men than women in a number of cognitive domains, including domains for which a male advantage is present at baseline (McCarrey, An, Kitner-Triolo, Ferrucci, & Resnick, 2016). A greater decline in men than in women was also found recently in the English Longitudinal Study of Ageing (Zaninotto, Batty, Allerhand, & Deary, 2018). Only one study has examined sex differences in cognition in aging NHPs. The cross-sectional comparison of Lacreuse, Kim, et al. (2005b) in rhesus monkeys showed that in young age, males outperformed females in a spatial working memory task, but that the performance of older males was no longer different from that of older females, suggesting that males may experience a greater age-related decline than females. We also reported sex differences in fine motor function in the rhesus monkey (Lacreuse, Diehl, et al., 2005), with males showing evidence of greater age-related decline than females. Longitudinal studies are critically needed to confirm these findings, track sex differences in the rate of age-related cognitive and motor decline, and understand their biological bases. The present study examines sex differences in middle-aged male and female marmosets performing a reversal learning task and the Hill and Valley task at baseline and approximately 1 year later.
2 |. METHODS
2.1 |. Subjects
A total of 22 monkeys participated in this study. Eighteen marmosets (9 males, 9 females) were tested on reversal learning (Table 1) and 12 marmosets (6 males, 6 females), including 8 who performed the cognitive task, performed the Hill and Valley test (Table 2) on two occasions, approximately 1 year apart. All subjects were middle-aged, between the ages of 4 and 7 in Year 1 (mean = 4.82, SD = 0.66) and 5 and 8 years old (mean = 6.23, SD = 0.73) in Year 2. Age was not significantly different between males and females (t(16) = 1.79, p = 0.9). The monkeys were housed in opposite sex pairs in a room with a 12 h:12 h dark/light cycle. They were fed a daily diet of fresh food including fruits, vegetables, nuts and seeds, various breads, and ZuPreem marmoset food. Fruit and nuts were provided twice daily (8–9 AM and 1–3 PM) and water was available ad libitum. The monkeys were provided with daily enrichment, including foraging tubes and a variety of toys. The animals were cared for in accordance with the guidelines of the US National Research Council’s Guide for the Care and Use of Laboratory Animals, the US Public Health Service’s Policy on Humane Care and Use of Laboratory Animals, and the Guide for the Care and Use of Laboratory Animals (2011), 8th edition. The studies were approved by the University of Massachusetts Institutional Animal Care and Use Committee.
TABLE 1.
Characteristics of marmosets tested on reversal learning
| Animal ID | Sex | Age BL | Age retest | Time between tests | Test method | Hill and Valley |
|---|---|---|---|---|---|---|
| 1 | M | 3.96 | 5.33 | 1.38 | CANTAB | No |
| 2 | M | 4.65 | 5.79 | 1.14 | CANTAB | No |
| 3 | M | 4.69 | 6.37 | 1.68 | CANTAB | No |
| 4 | M | 4.77 | 5.84 | 1.07 | CANTAB | Yes |
| 5 | M | 5.01 | 6.42 | 1.41 | CANTAB | Yes |
| 6 | M | 5.21 | 6.43 | 1.23 | CANTAB | Yes |
| 7 | M | 5.21 | 6.88 | 1.66 | CANTAB | Yes |
| 8 | M | 5.56 | 7.40 | 1.84 | CANTAB | No |
| 9 | M | 6.86 | 8.12 | 1.26 | WGTA | Yes |
| Mean | Males | 5.10 | 6.51 | 1.41 | ||
| 10 | F | 4.08 | 5.38 | 1.29 | CANTAB | Yes |
| 11 | F | 4.12 | 5.81 | 1.69 | CANTAB | No |
| 12 | F | 4.21 | 5.45 | 1.24 | CANTAB | Yes |
| 13 | F | 4.41 | 5.49 | 1.08 | CANTAB | No |
| 14 | F | 4.52 | 6.31 | 1.79 | CANTAB | No |
| 15 | F | 4.78 | 5.77 | 0.99 | WGTA | No |
| 16 | F | 4.82 | 6.48 | 1.66 | CANTAB | No |
| 17 | F | 4.93 | 6.19 | 1.27 | CANTAB | No |
| 18 | F | 4.99 | 6.71 | 1.72 | CANTAB | Yes |
| Mean | Females | 4.54 | 5.95 | 1.41 | ||
| Mean | Total | 4.82 | 6.23 | 1.41 |
TABLE 2.
Characteristics of marmosets tested on the Hill and Valley test
| Animal ID | Sex | Age BL | Age retest | Time between tests | Cognitive test |
|---|---|---|---|---|---|
| 5 | M | 4.42 | 5.70 | 1.28 | Yes |
| 7 | M | 4.93 | 6.12 | 1.19 | Yes |
| 4 | M | 5.05 | 6.32 | 1.27 | Yes |
| 6 | M | 5.27 | 6.73 | 1.46 | Yes |
| 19 | M | 5.52 | 6.92 | 1.40 | NA |
| 9 | M | 6.48 | 7.67 | 1.19 | Yes |
| Mean | Males | 5.27 | 6.58 | 1.30 | |
| 10 | F | 3.96 | 5.20 | 1.25 | Yes |
| 12 | F | 4.07 | 5.33 | 1.27 | Yes |
| 20 | F | 4.47 | 5.73 | 1.26 | NA |
| 18 | F | 5.14 | 6.47 | 1.33 | Yes |
| 21 | F | 5.41 | 6.88 | 1.48 | NA |
| 22 | F | 6.09 | 7.31 | 1.22 | NA |
| Mean | Females | 4.86 | 5.92 | 1.32 | |
| Mean | Total | 5.07 | 6.36 | 1.30 |
2.2 |. Procedure
2.2.1 |. Cognitive task
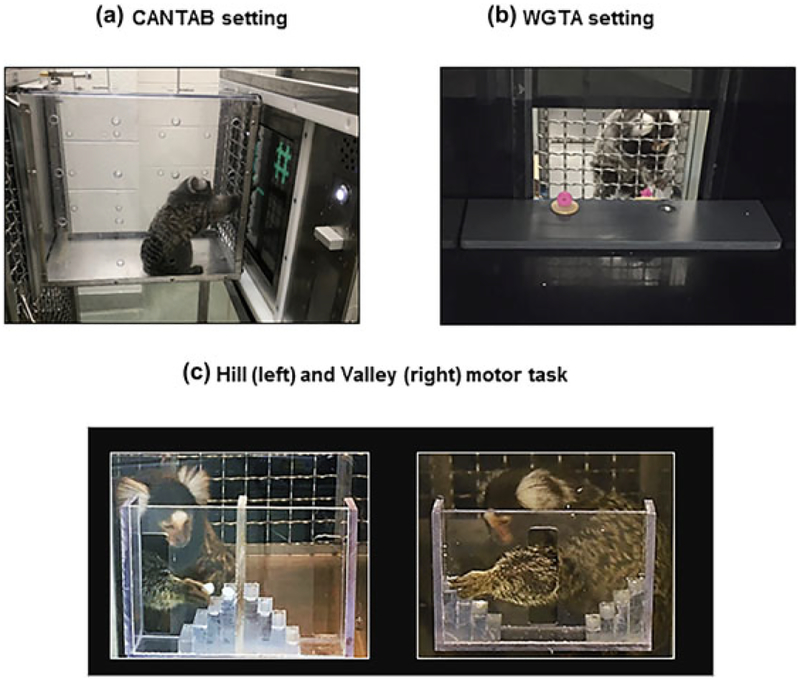
Sixteen monkeys were tested on the NHP version of the CAmbridge Neuropsychological Test Automated Battery CANTAB (Monkey CANTAB Intellistation with Liquid Reward, Model 80951A), which consisted of a touch screen panel (37.78 cm) in a stainless steel frame (56 × 38 × 30 cm) using an Intel based 1.6 GHz CPU operating system. A stainless steel sipper tube in the middle of the screen delivered the reward (banana milkshake) via a peristaltic pump, at a rate of 0.2 ml per second. To encourage participation, food and water was removed from the animals’ cages 2 hr prior to testing and replaced in the cage no later than 5 hr after removal. The marmoset was tested in their housing room. They voluntarily entered a transport box (34.1 × 20.65 × 30.8 cm) attached to the front of their homecage (Figure 1a). The CANTAB was positioned against the meshed front (2.5 × 2.5 cm openings) of the transport box, so animals could reach through to touch the screen and lick the reward from the sipper tube. Experimenters loaded CANTAB testing programs remotely from a desktop computer located outside of the marmoset housing rooms.
FIGURE 1.
(a) Marmoset in transport box performing a reversal learning trial on the CANTAB. (b) Marmoset performing a reversal learning trial in the WGTA. (c) Marmoset performing the Hill (left) and Valley (right) tasks
2.2.2 |. Reversal learning: CANTAB
CANTAB training
We followed the procedures described by Roberts, Robbins, and Everitt (1988) and Pearce, Crofts, Muggleton, and Scott (1998) for stages of tone-reinforcement associations and touch-training. Monkeys were trained to lick the milkshake from the spout, to associate a tone (41 Hz) with reward delivery (5 s), to touch the screen, touch a large static square at the center of the screen, and touch smaller squares appearing successively at random locations on the screen, before being presented with the first pair of stimuli.
CANTAB reversal learning
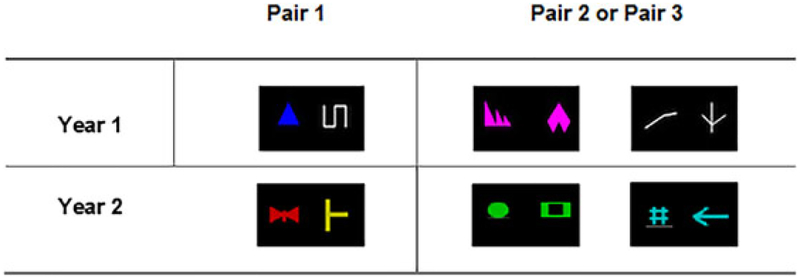
In Year 1, the marmosets were presented with three pairs of stimuli shown in Figure 2. The first pair of stimuli consisted of a blue triangle and a white line. The second pair consisted either of two different white lines or two different pink shapes (the order of presentation of pairs 2 and 3 was counterbalanced between monkeys). Pair 1 was easier to discriminate than Pairs 2 and 3, because it differed both in color and shape, while Pairs 2 and 3 consisted of different shapes of the same color. Special attention was used in selecting the colors of the visual stimuli, as female marmosets can be di- or tri-chromats while male marmosets are dichromats, that is “red-green color blind” (Caine, Osorio, & Mundy, 2010; Pessoa, Tomaz, & Pessoa, 2005). Therefore, certain combinations of colors (red/green/brown/orange) were avoided. For each pair, monkeys had to perform a simple discrimination (SD), followed by a simple reversal (SR). The two stimuli appeared in any position on the touch screen. Upon touching the correct stimulus, a positive tone was played and the liquid reward was delivered. Touching an incorrect stimulus triggered a negative tone and no reward delivery. The inter-trial interval was 3 s. Animals were tested 5 days a week and were given 40 trials a day to learn the stimulus/reward contingencies (e.g., blue triangle always rewarded). Once they reached a 90% correct criterion, the stimulus/reward contingencies were reversed (e.g., the white line was rewarded). When the 90% accuracy criterion was reached on the SR, the marmoset moved on to a new stimulus pair. The number of trials to reach criterion (TTC) was computed. In addition, the number of refusals (number of trials that the monkey refused to perform) was also recorded as an index of motivation. In Year 2, the monkeys were presented with a new set of three stimulus pairs (Figure 2). Testing procedure was identical to the one described for Year 1.
FIGURE 2.
Schematics of the stimuli used in Year 1 and Year 2
2.2.3 |. Reversal learning: WGTA
Two monkeys (1 male, 1 female) were unable to perform the tasks on the CANTAB and were tested on a manual version of the tests instead. These monkeys were tested in a Wisconsin General Testing Apparatus (WGTA)-like box which consisted of an opaque box (43.2 × 42.3 × 44.5 cm) containing a test tray (40.65 × 11.15 × 1.25 cm) with two food wells (each of diameter 2.5 cm; Figure 1b). The wells could be baited with mini-dried marshmallows and covered by stimulus objects. Between trials, the tray was concealed from view by an opaque screen.
The stimuli for the WGTA version were made of foamy material of the same shape and colors as the stimuli shown in Figure 2. Each stimulus (2 × 2 × 0.9 cm3) was glued on a 3.5 cm diameter token that completely covered a food well. The procedure was similar to the CANTAB procedure, but with important differences constrained by the WGTA setting. First, trials were given by an experimenter sitting behind the WGTA across the monkey and only 20 trials per day were given to minimize satiety effects; for each trial, the experimenter followed a 20-trial test sheet indicating the location (left or right, randomized) of the rewarded stimulus. With the door closed, the experimenter baited one of the wells with a mini-dried marshmallow and covered it with the positive stimulus, while the other well was baited with the other stimulus. The experimenter lifted the door while starting a timer to let the monkey select one of the stimuli. For each trial, the experimenter recorded the performance of each monkey (coded as 0 or 1) as well as the response times (elapsed time between door opening and monkey response) on the test sheet. The monkey had a maximum of 2 min to give a response at each trial. A lack of response was recorded as a refusal and the next trial was administered.
2.2.4 |. Motor task: Hill and valley
The Hill and Valley task is a measure of fine motor ability that has previously been used in marmosets, especially in models of stroke and Parkinson’s disease (Bihel et al., 2010; Eslamboli, Baker, Ridley, & Annett, 2003; Marshall & Ridley, 2003; Phillips et al., 2017). It assesses motor function in each limb as well as perceptual spatial impairment. The monkeys were tested in their housing room at times where they were not engaged in cognitive tasks. Similarly to CANTAB testing, they voluntarily entered the transport box attached to their home cage to access the Hill or Valley apparatus, securely attached to the front of the box via a Plexiglas screen. Each apparatus had two 5-steps (9 × 9 × 3 mm) staircases, either rising away from a central opening (Valley), or from two lateral openings (Hill); see Figure 1c. The monkeys had to reach through these openings, using either their right or left hand, to retrieve one of the mini dehydrated marshmallows (6 mm diameter) placed in the middle of each step. In the Valley version, the central vertical slot (7.7 × 2 cm) allowed the marmoset to use its left hand to reach the reward located on its right, or the right hand to reach the reward located on its left (contralateral hemifield to hand used). In the Hill version, entry was through two lateral slots (7.4 × 2 cm) on the side of each staircase so that the monkey had to use its right hand to retrieve the rewards on the right stairs and the left hand to retrieve the rewards on the left stairs (ipsilateral hemifield to hand used).
Marmosets were trained on the Hill and Valley apparatus until they successfully retrieved a marshmallow from each step with each hand. If the marmoset failed to perform the task after 10 attempts, it was excluded from further testing. For testing, marmosets were given a maximum of 5 min to retrieve all five marshmallows from one staircase of the apparatus. Each marmoset received four conditions (Hill Left, Hill Right, Valley Left, Valley Right) per session, one session per day, and performed a total of three testing sessions. The order of the Hill and Valley conditions was randomized (half received Hill first, half Valley first) and alternated each test day. If the marmoset failed to retrieve the five marshmallows within the 5-min time limit, the test session was rerun the following day. Marmosets received one point for retrieving the marshmallow on the 1st step, 2 points for retrieving from the 2nd step, and so on, for a maximal score of 15 points per hand. Marmosets lost one point each time a marshmallow was dropped (lost completely or fallen to another stair). All sessions were video recorded. A trained experimenter (KW) decoded the videotapes and computed the final scores (accuracy) and time to retrieval for each monkey, condition and year. The time to retrieval was the time elapsed from the first reaching through the opening until retrieval of the last marshmallow. The accuracy was the score obtained out of a maximum of 15.
Hand preference
Because hand preference had the potential to affect hand performance in the Hill and Valley test, we determined the hand preference of each marmoset using a simple hand reaching task. Monkeys performed 50 reaches through the central slot of the Valley apparatus to reach a mini marshmallow placed 7.7 × 2 cm from the slot. The number of Left and Right hand reaches was recorded. Any trials in which the marmoset used both hands were excluded. For each subject, a handedness index (HI) was determined by subtracting the number of left-handed responses from the number of right-handed responses and dividing by the total number of responses (Hopkins, 1999). HI values ranged from −1.0 to 1.0, with the absolute value representing the strength of the preference. The positive values indicated a right-hand bias while the negative values indicated a left-hand bias. HI values were not significantly different between males (HI = 0.167, SEM = 0.18) and females (HI = 0.173, SEM = 0.25).
2.3 |. Statistical analysis
2.3.1 |. Cognitive task
The average TTC was not significantly different between CANTAB (M = 254.33, SEM = 23.83) and WGTA monkeys (M = 215.71, SEM = 47.29, t (16) = 0.59, ns), therefore, the data of the 18 monkeys were combined for the analysis. The percentage of refusals was much lower for the WGTA monkeys (16%, SEM = 8.6) than for the CANTAB monkeys (37.6%, SEM = 2.7), however, so the analysis for this variable was performed on the 16 monkeys with CANTAB data. The TTC and the percentage of refusals were analyzed using mixed repeated measure ANOVAs with Year (1, 2), Test Type (SD, SR), and Pair Number (Pair 1, Pair 2, Pair 3) as within-subject factors and Sex as a between-subject factor. Age at onset and Interval between Tests were initially included as covariates in the models. Because none of the covariates were significant, they were not included in the final models.
2.3.2 |. Motor task
Twelve monkeys (6 males, 6 females), including 8 monkeys who also performed the cognitive test, performed the Hill and Valley at both baseline and retest (See Table 2). Because the score and time to retrieval were not significantly different between the Hill and Valley tests, the measures from both tests were averaged and analyzed with mixed measures ANOVAs with Sex, Hand, and Year as factors. As there were no indication that HI, age or interval between tests correlated with performance measures, they were not included as covariates in the models.
3 |. RESULTS
3.1 |. Cognitive task
3.1.1 |. Trials to criterion (TTC)
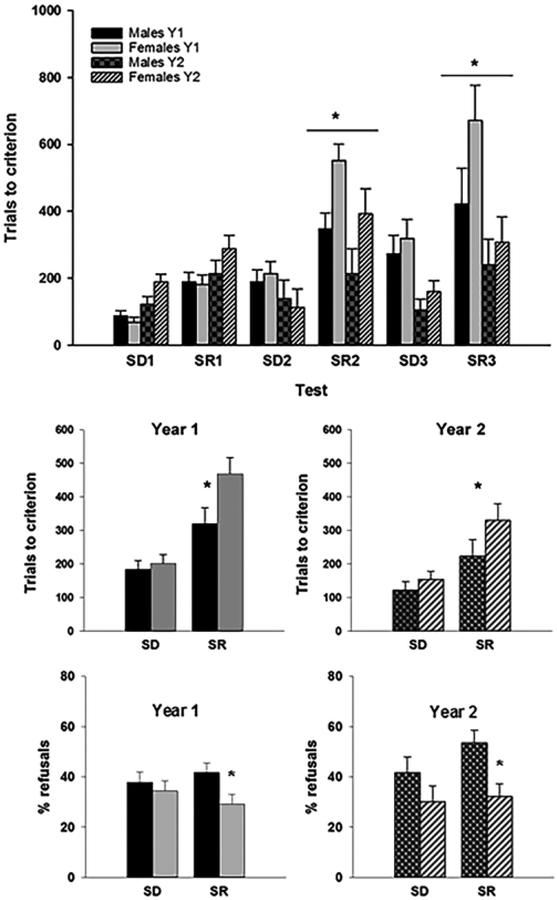
The ANOVA revealed a significant effect of Year (F (1, 16) = 7.99, p = .012, partial η2 = 0.33) on TTC, with animals taking significantly less trials to perform the discriminations in Year 2 (m = 207.037, SEM = 24.87) than in Year 1 (M = 293.04, SEM = 25.67). Additionally, test Type was also significant (F(1, 16) = 89.81, p = .0001, partial η2 = 0.85), indicating that, independent of year of testing, monkeys took longer to learn the SRs (M = 335.06, SEM = 27.95) than the SDs (m = 165.02, SEM = 13.96). TTC varied also according to Pair Number (F (2, 32) = 14.24, p = .001, partial η2 = 0.47) with animals taking significantly fewer trials on the 1st pair (M = 167.51, SEM = 12.45) than on the 2nd (M = 270.32, SEM = 26.34) and 3rd (M = 312.29, SEM = 33.95; Figure 3a). The main effect of Sex on TTC was not significant (F (1, 16) = 3.57, p = 0.077, partial η2 = 0.18), however, a significant interaction between Sex and Test Type (F (1, 16) = 8.26, p = 0.011, partial η2 = .34) revealed that females needed more trials (M = 398.96, SEM = 39.52) than males (M = 271.17, SEM = 39.52) to reach criterion on the SRs, while there was no sex difference on the SDs (M males = 152.68, SEM = 19.74; M females = 177.35, SEM = 19.74; Figure 3b). A significant interaction between Year and test Type (F(1,16) = 4.89, p = .042, partial η2 = 0.23) indicated that the performance improvement from Year 1 to Year 2 was significant for the SRs (F(1,16) = 7.98, p = .012) but not for the SDs (F(1,16) = 0.87, ns). Pair Number also interacted with test Type (F(2, 16) = 4.84, p = 0.15, partial η2 = 0.23), and Year (F(2, 16) = 11.60, p = .001, partial η2 = 0.42) reflecting differences in stimulus pairs complexity. A marginal Sex × Test Type × Pair Number (F (2,32) = 2.86, p = .072, partial η2 = 0.15) suggested that females were especially impaired for the more complex pairs.
FIGURE 3.
(a) Trials to criterion of males and female marmosets as a function of stimulus pair in each year of testing. (b) Trials to criterion averaged by test Type and Sex in each Year of testing. (c) Percentage of refusals per test Type and Sex in each Year of testing. SD, simple discrimination; SR, simple Reversal. *p < .05
The speed of acquisition of the SDs varied greatly between monkeys, which also contributed to their ability to perform the reversals. In order to account for these individual differences, we computed a Reversal Index (RI) (Rajalakshmi & Jeeves, 1965) for each year of testing, as follow: RI = mean (TTCSR1 + TTCSR2 + TTCSR3)/mean (TTCSD1 + TTCSD2 + TTCSD3). The RI evaluates how many more trials were needed to complete each of the three reversals, relative to the three simple discriminations, with higher values indicating poorer performance. A repeated measure ANOVA with Sex and Year as independent variables indicated that RI did not vary significantly with Year of testing (F(1, 16) = .035, ns). This showed that even though the TTC decreased between Y1 and Y2, reflecting improved performance, the ability to perform the reversals relative to the discriminations remained stable across years. Additionally, the RI was significantly higher in females (M = 2.31, SEM = 0.14) than in males (M = 1.84, SEM = 0.14; F (1, 16) = 5.77, p < .05), reflecting the poorer performance of females in the reversals in both years. The interaction between Year and Sex was not significant (F(1, 16) = 0.45, ns).
3.1.2 |. Refusals
The two animals who performed the WGTA version of the task were excluded from this analysis. The remaining marmosets (n = 16) refused a large percentage of trials (37.6%) on the CANTAB. Interestingly, males refused significantly more trials (M = 43.7%, SEM = 3.3) than females (M = 31.5%, SEM = 3.3; F(1, 14) = 8.47, p = .011, partial η2 = 0.38) and this sex difference depended on test Type (F(1, 14) = 4.89, p = .044, partial η2 = 0.26). As can be seen in Figure 3c, the sex difference was specific to the reversals (F(1, 14) = 12.02, p = .004), with no significant difference detected for the SDs (F(1, 14) = 2.20, ns). The main effect of Year (F(1, 16) = 0.89, ns) and the interactions were not significant. There was no correlation between the TTC and the % of refusals (r (16) = −0.16, p = .55), even after controlling for sex (r(13) = 0.11, p = 0.70).
3.1.3 |. Individual trajectories
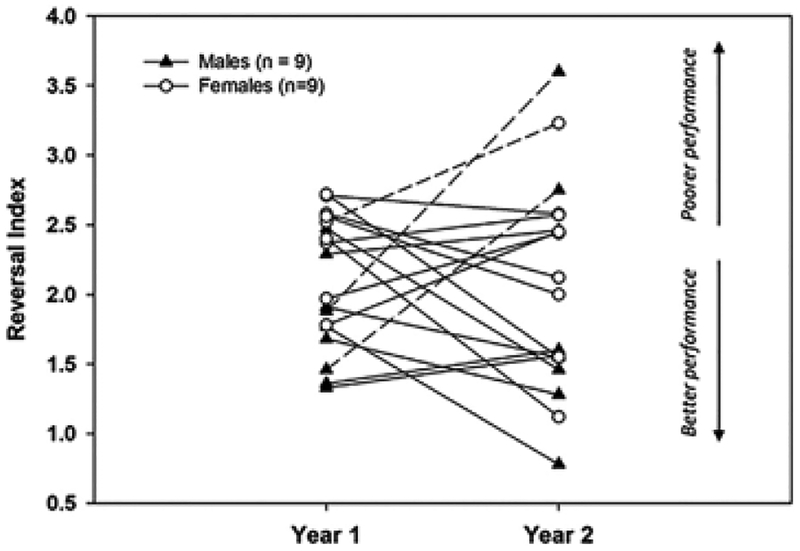
One of the advantages of longitudinal designs is the ability to investigate individual trajectories. Figure 4 represents individual RIs for each year of testing in males and females. As can be seen from the figure, independently of sex, most individuals maintained or improved performance from Year 1 to Year 2, but a few individuals (dotted line in the figure) experienced a decline (i.e., higher RI). Our n was too small to examine this issue statistically, but we speculate that such individuals may follow a trajectory of pathological aging. Additional longitudinal points will be needed to confirm this interpretation.
FIGURE 4.
Reversal index in Year 1 and Year 2 for each subject. The dotted line highlights individuals with RI increasing (declining performance) in Year 2
3.2 |. Motor task
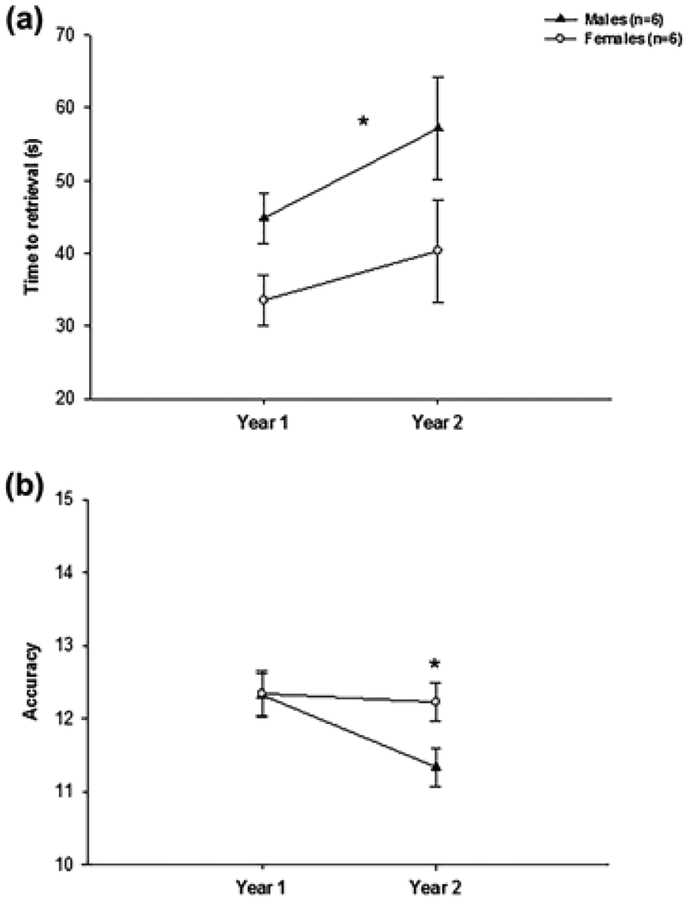
Monkeys retrieved the marshmallows in an average of 44 s (SEM = 3.02) and obtained an average score of 12.05 (SEM = 0.16) out of the maximum of 15. No significant difference was detected between the left and right hands for either the scores or the time to retrieval. The scores dropped slightly but significantly from Year 1 to Year 2 (F(1, 10) = 5.26, p < .045, partial η2 = 0.34) while the time to retrieval tended to increase during the same time period (F(1, 10) = 3.65, p < .085, partial η2 = 0.27). Females were significantly faster than males in performing the tests (F(1, 10) = 5.41, p = 0.042, partial η2 = 0.35) but there was not sex differences in accuracy (F(1, 10) = 1.96, p = 0.19, partial η2 = 0.16). The interaction between Sex and Year was not significant either for the time to retrieval F(1, 10) = 0.31, p = .59, partial η2 = 0.03) or for the score F(1, 10) = 3.26, p = .10, partial η2 = 0.25). To examine this latter interaction, we conducted follow up analyses for each sex separately. As can be seen from Figure 5, females maintained a score around 12 between the 2 years (12.34 vs. 12.22, t (5) = 0.29, ns), while males experienced a significant drop in performance from year 1 to Year 2 (12.32 vs. 11.33, t(5) = 3.84, p = .012).
FIGURE 5.
Mean time to retrieval (s) (a) and accuracy (b) in the Hill and Valley test (combined) in males and females in each year of testing. *p < .05
4 |. DISCUSSION
We examined 1-year change in cognitive flexibility and fine motor function in middle-aged marmosets of both sexes. We found minimal age-related change in cognitive flexibility but detected significant declines in fine motor function with age. In addition, we found sex differences in both domains, underlining the importance of incorporating sex as a variable in NHP studies. We discuss these results in details below.
4.1 |. Sex and age differences in cognitive flexibility
Cognitive flexibility is a dimension of executive function that is severely affected by age in a number of species including rodents (Mizoguchi, Shoji, Tanaka, & Tabira, 2010), mouse lemurs (Joly, Ammersdörfer, Schmidtke, & Zimmermann, 2014), rhesus monkeys (Lai et al., 1995; Moore, Killiany, Herndon, Rosene, & Moss, 2006), great apes (Lacreuse, Parr, Chennareddi, & Herndon, 2018; Manrique & Call, 2015), and humans (Berry et al., 2016). Age-related decline in this domain can already be observed at middle-age in chimpanzees (Lacreuse et al., 2018) and rhesus monkeys (Moore et al., 2006), but most studies have been cross-sectional, precluding an assessment of cognitive change with age. We assessed cognitive flexibility through performance on reversal learning, a task that requires the monkey to adapt to changing stimulus/reward contingencies. Decades of work in a variety of species, including marmosets, have shown reversal learning to be dependent on the OFC (Dias, Robbins, & Roberts, 1996; Hornak et al., 2004; Izquierdo, Brigman, Radke, Rudebeck, & Holmes, 2017; Marquardt, Sigdel, & Brigman, 2017; Rudebeck & Murray, 2008; Rygula, Walker, Clarke, Robbins, & Roberts, 2010), a brain region that undergoes significant changes in structure and function with age in humans (Resnick, Lamar, & Driscoll, 2007). Reversal learning has been used extensively in marmosets, but aging studies are lacking. A small study in four aged marmosets reported that aged animals were only impaired in the initial discrimination/reversal pair, catching up to the performance of historical young controls in later discriminations (Munger, Takemoto, Raghanti, & Nakamura, 2017), but larger samples are needed to confirm these findings.
Our study focused on longitudinal change in reversal learning in middle-aged marmosets tested at baseline and 1 year later. We used different stimuli between the two time points to minimize practice effects. Yet, the absolute TTC decreased significantly from Year 1 to Year 2, reflecting a test–retest effect inherent to longitudinal designs. However, as noted in early work (Rumbaugh & Jeeves, 1966), a better approach to capture reversal learning performance is to assess reversal performance relative to pre-reversal performance. This allows to circumvent differences in motivation, perceptual and/or motor skills or anxiety that contribute to individual differences in discrimination abilities. Using a Reversal Index, RI, as such a ratio, we found that reversal performance remained stable between the two time points. That is, despite overall performance improving from baseline to re-test, the proportion of trials needed to perform a reversal relative to a simple discrimination remained unchanged, with approximately twice as many trials needed to perform a reversal relative to an initial discrimination. It is possible that cognitive flexibility in the marmoset declines later in life. As we continue our longitudinal research, it will be important to investigate individual trajectories, as a few individuals showed declining performance between the two time points, a pattern that may represent a path towards pathological aging.
A robust finding from our study is the presence of sex differences in reversal learning. Relative to males, females showed slower acquisition of the reversals across the two time points. This sex difference was clearly related to task difficulty, as it was specific to reversal performance in the more complex pairs (i.e., SR2 and SR3). It is important to note that potential differences in color vision between females (di- or tri-chomats) and males (dichromats) cannot explain these results, since the same pairs of stimuli were presented for the discriminations and the reversals. In addition, the visual stimuli used in the complex pairs were of the same color. Using the refusals (% of aborted trials) as an index of motivation, we were also able to rule out lesser motivation in females as a contributing factor. Indeed, males did refuse a greater percentage of trials than females on the reversals, a finding in agreement with our previous observation that males may be more sensitive to punishment than females (LaClair & Lacreuse, 2016). The possibility that other factors, such as greater anxiety or stress reactivity in females (e.g., Johnson et al., 1996), may underlie the sex difference in reversal learning needs to be examined in future studies. However, sex differences in reversal learning have also been reported in humans, where a female impairment in reversal learning has been described in both children (Overman, Bachevalier, Schuhmann, & Ryan, 1996) and adults (Evans & Hampson, 2015). These effects are likely related to both the organizational and activational influences of sex steroids (Overman et al., 1996). Indeed, early in life, androgens facilitate the development of the OFC/better reversal performance in males and masculinized female monkeys (Clark & Goldman-Rakic, 1989). The activational effects in adulthood are less clear, but we showed previously that ovariectomized female marmosets exhibit an impairment in reversal learning following estradiol (E2) replacement (Lacreuse et al., 2014). Although we were unable to assess the relationships between endogenous E2 and performance in the present study (learning was confounded with cycle), based on these previous findings, we hypothesize that E2 has a detrimental effect on cognitive flexibility and associated brain substrates (OFC circuitry) in the female marmoset.
4.2 |. Motor function
Age-related slowing in fine motor function has been well characterized in cross-sectional studies in both humans (Smith et al., 1999) and rhesus monkeys (Lacreuse, Diehl, et al., 2005; Zhang et al., 2000). Age-related slowing has been related to changes in the striatum, which undergoes significant atrophy with age in humans (Raz et al., 2003) and macaques (Lacreuse, Diehl, et al., 2005; Matochik et al., 2000). Interestingly, the age-related decline in striatal volume across a 5 years period is uniform across age (20–77 years old), indicating a steady lifespan shrinkage of this structure in humans (Raz et al., 2003). In contrast to this linear decline, there is evidence that age-related motor slowing accelerates between the ages of 47 and 62 in humans (Smith et al., 2005).
Marmosets have long been used as models for stroke and Parkinson’s disease, with several studies assessing motor deficits in the Hill and Valley test (Bihel et al., 2010; Eslamboli et al., 2003; Marshall & Ridley, 2003; Phillips et al., 2017). However, a comparison of motor function based on age has not been conducted. In part because of its standard use in marmosets, we used the Hill and Valley test to assess changes in fine motor function in marmosets of both sexes. Although our sample size was small, the analysis revealed interesting findings. First, with respect to age, we found a small, but significant drop in accuracy from baseline to re-test. Interestingly, this drop in performance was significant in males only. Females were faster than males overall, but there was a marginal trend for a slowing of motor function in both sexes. Additional subjects and longitudinal data points are needed to confirm these findings. However, we note that both the speed advantage of females and the greater age-related decline in males are consistent with prior findings in humans and macaques. Indeed, a female advantage in fine motor function has been noted in several human studies (Agnew, Bolla-Wilson, Kawas, & Bleecker, 1988; Jennings, Janowsky, & Orwoll, 1998; Kennedy & Raz, 2005). In addition, we previously found evidence for greater age-related slowing in males than in females, in a cross-sectional study comparing the performance of young (7 years old) and older rhesus monkeys (22 years old) on the Lifesaver Test, a task requiring monkeys to remove a lifesaver candy from shapes of different complexities (Lacreuse, Diehl, et al., 2005).
The only other longitudinal investigation of motor function in aging NHPs examined 1-year change in motor performance in young (4–6 years old) and older (21–26 years old) female rhesus monkeys tested on the lifesaver test (Walton, Scheib, McLean, Zhang, & Grondin, 2008). In contrast to our results in marmosets, Walton et al. (2008) found improved performance at re-test in both young and older monkeys, supporting the idea that procedural memories are well preserved over time. It is likely that task differences underlie in part the discrepancies with our findings. Indeed, the lifesaver test emphasizes the speed of hand movement per se, while the Hill and Valley measures larger movements of the upper limbs, visual-hand coordination and grasping ability. An additional motor test would be useful to better characterize the nature of the observed deficits in our study. In addition, the Walton et al. (2008) study included only female subjects, leaving the question of sex differences, that is, a potential decline in males, unanswered.
5 |. CONCLUSION
Having the shortest lifespan of all anthropoids, the marmoset is an NHP ideally suited for longitudinal investigations of the aging process on cognition and motor function. This report suggests that middle-aged marmosets re-tested one year after baseline assessments (1) do not show a significant age-related decline in reversal learning; (2) exhibit robust sex differences in reversal learning, with a female impairment observed across the two time points; (3) show a decrease in motor accuracy in males only, and a slight motor slowing in both sexes (4) exhibit sex differences in motor speed, with females being faster than males overall.
These data underscore the importance of sex differences in both cognitive and motor ability and suggest that motor function may be particularly sensitive to aging, as the age-related motor decline can already be observed at middle-age, as in humans. Further longitudinal data points will allow us to confirm whether males show a greater decline in motor ability with age and whether cognitive flexibility declines later in life in either one or both sexes.
ACKNOWLEDGMENTS
This study was supported by NIH grant R01 AG046266. We thank the UMass animal care staff and the veterinary staff for their excellent assistance and the UMass Center for Research on Families for statistical consultation. We are grateful to the Psychology shop for building the apparatuses and to all the students who participated in data collection.
Funding information
National Institute on Aging, Grant number: R01 AG046266
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest.
REFERENCES
- Agnew J, Bolla-Wilson K, Kawas CH, & Bleecker ML (1988). Purdue Pegboard age and sex norms for people 40 years old and older. Developmental Neuropsychology, 4, 29–35. 10.1080/87565648809540388 [DOI] [Google Scholar]
- Austad SN (1997). Small nonhuman primates as potential models of human aging. ILAR Journal, 38, 142–147. 10.1093/ilar.38.3.142 [DOI] [PubMed] [Google Scholar]
- Baxter MG (2001). Cognitive aging in nonhuman primates In Hof PR & Mobbs CV (Eds.), Functional neurobiology of aging (pp. 407–420). San Diego: Academic Press, 10.1016/b978-012351830-9/50028-7 [DOI] [Google Scholar]
- Belcher AM, Yen CC-C, Notardonato L, Ross TJ, Volkow ND, Yang Y, … Tomasi D (2016). Functional connectivity hubs and networks in the awake marmoset brain. Frontiers in Integrative Neuroscience, 10, 9 10.3389/fnint.2016.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher AM, Yen CC, Stepp H, Gu H, Lu H, Yang Y, … Stein EA (2013). Large-scale brain networks in the awake, truly resting marmoset monkey. Journal of Neuroscience, 33, 16796–16804. 10.1523/JNEUROSCI.3146-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Shah VD, Baker SL, Vogel JW, O’Neil JP, Janabi M, … Jagust WJ (2016). Aging affects dopaminergic neural mechanisms of cognitive flexibility. The Journal of Neuroscience, 36, 12559–12569. 10.1523/jneurosci.0626-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihel E, Pro-Sistiaga P, Letourneur A, Toutain J, Saulnier R, Insausti R, … Touzani O (2010). Permanent or transient chronic ischemic stroke in the non-human primate: Behavioral, neuroimaging, histological, and immunohistochemical investigations. Journal of Cerebral Blood Flow and Metabolism, 30, 273–285. 10.1038/jcbfm.2009.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, & Woods A (Eds.). (2009). Animal models of human cognitive aging. New York, NY: Humana Press, 10.1007/978-1-59745-422-3 [DOI] [Google Scholar]
- Caine NG, Osorio D, & Mundy NI (2010). A foraging advantage for dichromatic marmosets (Callithrix geoffroyi) at low light intensity. Biology Letters, 6, 36–38. 10.1098/rsbl.2009.0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin TA, Yu H-H, Soares JGM, Gattass R, & Rosa MGP (2013). A conserved pattern of differential expansion of cortical areas in simian primates. The Journal of Neuroscience, 33, 15120–15125. 10.1523/jneurosci.2909-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AS, & Goldman-Rakic PS (1989). Gonadal hormones influence the emergence of cortical function in nonhuman primates. Behavioral Neuroscience, 103, 1287–1295. 10.1037/0735-7044.103.6.1287 [DOI] [PubMed] [Google Scholar]
- de Frias CM, Nilsson LG, & Herlitz A (2006). Sex differences in cognition are stable over a 10-year period in adulthood and old age. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 13, 574–587. 10.1080/13825580600678418 [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, & Roberts AC (1996). Dissociation in prefrontal cortex of affective and attentional shifts. Nature, 380, 69–72. 10.1038/380069a0 [DOI] [PubMed] [Google Scholar]
- Eslamboli A (2005). Marmoset monkey models of Parkinson’s disease: Which model, when and why? Brain Research Bulletin, 68, 140–149. 10.1016/j.brainresbull.2005.08.005 [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Baker HF, Ridley RM, & Annett LE (2003). Sensorimotor deficits in a unilateral intrastriatal 6-OHDA partial lesion model of Parkinson’s disease in marmoset monkeys. Experimental Neurology, 183, 418–429. https://doi.org/S0014488603001390 [DOI] [PubMed] [Google Scholar]
- Evans KL, & Hampson E (2015). Sex differences on prefrontally-dependent cognitive tasks. Brain and Cognition, 93, 42–53. 10.1016/j.bandc.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Ferreira L, Ferreira Santos-Galduróz R, Ferri CP, & Fernandes Galduróz JC (2014). Rate of cognitive decline in relation to sex after 60 years-of-age: A systematic review. Geriatrics & Gerontology International, 14, 23–31. 10.1111/ggi.12093 [DOI] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, Berg S, & Pedersen NL (2006). Surprising lack of sex differences in normal cognitive aging in twins. The International Journal of Aging and Human Development, 62, 335–357. 10.2190/c39x-9qhy-49dm-x9gj [DOI] [PubMed] [Google Scholar]
- Fischer KE, & Austad SN (2011). The development of small primate models for aging research. ILAR journal, 52, 78–88. 10.1093/ilar.52.1.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Stocker AM, & Koh MT (2011). Mindspan: Lessons from rat models of neurocognitive aging. ILAR Journal, 52, 32–40. 10.1093/ilar.52.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstorf D, Herlitz A, & Smith J (2006). Stability of sex differences in cognition in advanced old age: The role of education and attrition. The Journals of Gerontology: Series B, 61, P245–P249. 10.1093/geronb/61.4.P245 [DOI] [PubMed] [Google Scholar]
- Geula C, Nagykery N, & Wu CK (2002). Amyloid-beta deposits in the cerebral cortex of the aged common marmoset (Callithrix jacchus): Incidence and chemical composition. Acta Neuropathology, 103, 48–58. 10.1007/s004010100429 [DOI] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, & Killiany RJ (1997). Patterns of cognitive decline in aged rhesus monkeys. Behavioural Brain Research, 87, 25–34. 10.1016/s0166-4328(96)02256-5 [DOI] [PubMed] [Google Scholar]
- Hopkins WD (1999). On the other hand: Statistical issues in the assessment and interpretation of hand preference data in nonhuman primates. International Journal of Primatology, 20, 851–866. 10.1023/a:1020822401195 [DOI] [Google Scholar]
- Hornak J, O’Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, & Polkey CE (2004). Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience, 16, 463–478. 10.1162/089892904322926791 [DOI] [PubMed] [Google Scholar]
- Huber L, & Voelkl B (2009). Social and physical cognition in marmosets and tamarins In Ford SM, Porter LM, & Davis LC (Eds.), The smallest anthropoids (pp. 183–201). Boston, MA: Springer, 10.1007/978-1-4419-0293-1_10 [DOI] [Google Scholar]
- Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, & Holmes A (2017). The neural basis of reversal learning: An updated perspective. Neuroscience, 345, 12–26. 10.1016/j.neuroscience.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings PJ, Janowsky JS, & Orwoll E (1998). Estrogen and sequential movement. Behavioral Neuroscience, 112, 154–159. 10.1037/0735-7044.112.1.154 [DOI] [PubMed] [Google Scholar]
- Johnson EO, Kamilaris TC, Carter CS, Calogero AE, Gold PW, & Chrousos GP (1996). The biobehavioral consequences of psychogenic stress in a small, social primate (Callithrix jacchus jacchus). Biological Psychiatry, 40, 317–337. 10.1016/0006-3223(95)00397-5 [DOI] [PubMed] [Google Scholar]
- Joly M, Ammersdörfer S, Schmidtke D, & Zimmermann E (2014). touchscreen-based cognitive tasks reveal age-related impairment in a primate aging model, the grey mouse lemur (Microcebus murinus). PLoS ONE, 9, e109393 10.1371/journal.pone.0109393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson P, Thorvaldsson V, Skoog I, Gudmundsson P, & Johansson B (2015). Birth cohort differences in fluid cognition in old age: Comparisons of trends in levels and change trajectories over 30 years in three population-based samples. Psychology and Aging, 30, 83–94. 10.1037/a0038643 [DOI] [PubMed] [Google Scholar]
- Kennedy KM, & Raz N (2005). Age, sex and regional brain volumes predict perceptual-motor skill acquisition. Cortex, 41, 560–569. 10.1016/S0010-9452(08)70196-5 [DOI] [PubMed] [Google Scholar]
- LaClair M, & Lacreuse A (2016). Reversal learning in gonadectomized marmosets with and without hormone replacement: Are males more sensitive to punishment? Animal Cognition, 19, 619–630. 10.1007/s10071-016-0966-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Chang J, Metevier CM, Laclair M, Meyer JS, & Ferris CM (2014). Oestradiol modulation of cognition in adult female marmosets (Callithrix jacchus). Journal of Neuroendocrinoly, 26, 296–309. 10.1111/jne.12147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Diehl MM, Goh MY, Hall MJ, Volk AM, Chhabra RK, & Herndon JG (2005). Sex differences in age-related motor slowing in the rhesus monkey: Behavioral and neuroimaging data. Neurobiology of Aging, 26, 543–551. https://doi.org/S0197-4580(04)00231-3 [DOI] [PubMed] [Google Scholar]
- Lacreuse A, & Herndon JG (2009). Nonhuman primate models of cognitive aging In Bizon JL & Woods A (Eds.), Animal models of human cognitive aging (pp. 1–30). New York, NY: Humana Press, 10.1007/978-1-59745-422-3_2 [DOI] [Google Scholar]
- Lacreuse A, Kim CB, Rosene DL, Killiany RJ, Moss MB, Moore TL, … Herndon JG (2005). Sex, age, and training modulate spatial memory in the rhesus monkey (Macaca mulatta). Behavioral Neuroscience, 119, 118–126. https://doi.org/2005-01705-012 [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Parr A, Chennareddi L, & Herndon JG (2018). Age-related decline in cognitive flexibility in female chimpanzees. Neurobiology of Aging, 72, 83–88. 10.1016/j.neurobiolaging.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ZC, Moss MB, Killiany RJ, Rosene DL, & Herndon JG (1995). Executive system dysfunction in the aged monkey: Spatial and object reversal learning. Neurobiology of Aging, 16, 947–954. https://doi.org/0197458095020144 [DOI] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, & Gould E (2007). Diminished adult neurogenesis in the marmoset brain precedes old age. Proceedings of the National Academy of Sciences of the United States of America, 104, 17169–17173. 10.1073/pnas.0708228104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U (2014). Human cognitive aging: Corriger la fortune? Science, 346, 572–578. 10.1126/science.1254403 [DOI] [PubMed] [Google Scholar]
- Liu C, Ye FQ, Yen CC, Newman JD, Glen D, Leopold DA, & Silva AC (2018). A digital 3D atlas of the marmoset brain based on multi-modal MRI. Neuroimage, 169, 106–116. 10.1016/j.neuroimage.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique HM, & Call J (2015). Age-dependent cognitive inflexibility in great apes. Animal Behaviour, 102, 1–6. 10.1016/j.anbehav.2015.01.002 [DOI] [Google Scholar]
- Marquardt K, Sigdel R, & Brigman JL (2017). Touch-screen visual reversal learning is mediated by value encoding and signal propagation in the orbitofrontal cortex. Neurobiology of Learning and Memory, 139, 179–188. https://doi.org/S1074-7427(16)30301-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JWB, & Ridley RM (2003). Assessment of cognitive and motor deficits in a marmoset model of stroke. ILAR Journal, 44, 153–160. 10.1093/ilar.44.2.153 [DOI] [PubMed] [Google Scholar]
- Matochik JA, Chefer SI, Lane MA, Woolf RI, Morris ED, Ingram DK, … London ED (2000). Age-related decline in striatal volume in monkeys as measured by magnetic resonance imaging. Neurobiology of Aging, 21, 591–598. 10.1016/s0197-4580(00)00134-2 [DOI] [PubMed] [Google Scholar]
- McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, & Resnick SM (2016). Sex differences in cognitive trajectories in clinically normal older adults. Psychology and Aging, 31, 166–175. 10.1037/pag0000070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mell T, Heekeren HR, Marschner A, Wartenburger I, Villringer A, & Reischies FM (2005). Effect of aging on stimulus-reward association learning. Neuropsychologia, 43, 554–563. 10.1016/j.neuropsychologia.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Miller CT (2017). Why marmosets? Developmental Neurobiology, 77, 237–243. 10.1002/dneu.22483 [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Shoji H, Tanaka Y, & Tabira T (2010). Orbitofrontal dopaminergic dysfunction causes age-related impairment of reversal learning in rats. Neuroscience, 170, 1110–1119. 10.1016/j.neuroscience.2010.08.037 [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, & Moss MB (2006). Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiology of Aging, 27, 1484 10.1016/j.neurobiolaging.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Munger EL, Takemoto A, Raghanti MA, & Nakamura K (2017). Visual discrimination and reversal learning in aged common marmosets (Callithrix jacchus). Neuroscience Research, 124, 57–62. 10.1016/j.neures.2017.06.002 [DOI] [PubMed] [Google Scholar]
- Nishijima K, Saitoh R, Tanaka S, Ohsato-Suzuki M, Ohno T, & Kitajima S (2012). Life span of common marmoset (Callithrix jacchus) at CLEA Japan breeding colony. Biogerontology, 13, 439–443. 10.1007/s10522-012-9388-1 [DOI] [PubMed] [Google Scholar]
- Overman WH, Bachevalier J, Schuhmann E, & Ryan P (1996). Cognitive gender differences in very young children parallel biologically based cognitive gender differences in monkeys. Behavioral Neuroscience, 110, 673–684. 10.1037/0735-7044.110.4.673 [DOI] [PubMed] [Google Scholar]
- Pearce PC, Crofts HS, Muggleton NG, & Scott EA (1998). Concurrent monitoring of EEG and performance in the common marmoset: A methodological approach. Physiology and Behavior, 63, 591–599. 10.1016/s0031-9384(97)00494-0 [DOI] [PubMed] [Google Scholar]
- Pessoa DM, Tomaz C, & Pessoa VF (2005). Color vision in marmosets and tamarins: Behavioral evidence. American Journal of Primatology, 67, 487–495. 10.1002/ajp.20202 [DOI] [PubMed] [Google Scholar]
- Phillips KA, Ross CN, Spross J, Cheng CJ, Izquierdo A, Biju K, … Tardif SD (2017). Behavioral phenotypes associated with MPTP induction of partial lesions in common marmosets (Callithrix jacchus). Behavioural Brain Research, 325, 51–62. 10.1016/j.bbr.2017.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajalakshmi R, & Jeeves MA (1965). The relative difficulty of reversal learning (reversal index) as a basis of behavioural comparisons. Animal Behaviour, 13, 203–211. 10.1016/0003-3472(65)90035-7 [DOI] [PubMed] [Google Scholar]
- Rapp PR (1990). Visual discrimination and reversal learning in the aged monkey (Macaca mulatta). Behavioral Neuroscience, 1043, 876–884. 10.1037/0735-7044.104.6.876 [DOI] [PubMed] [Google Scholar]
- Raz N (2000). Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings In Craik FIM & Salthouse TA (Eds.), The handbook of aging and cognition (pp. 1–90). Mahwah, NJ, US: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Raz N, & Rodrigue KM (2006). Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews, 30, 730 10.1016/j.neubiorev.2006.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, & Acker JD (2003). Differential aging of the human striatum: Longitudinal evidence. American Journal of Neuroradiology, 24, 1849–1856. [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Lamar M, & Driscoll I (2007). Vulnerability of the orbitofrontal cortex to age-associated structural and functional brain changes. Annals of the New York Academy of Sciences, 1121, 562–575. 10.1196/annals.1401.027 [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, & Park DC (2010). Human neuroscience and the aging mind: A new look at old problems. Journal of Gerontology Series B Psychological Sciences and Social Sciences, 65, 405–415. 10.1093/geronb/gbq035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AC, Robbins TW, & Everitt BJ (1988). The effects of intradimensional and extradimensional shifts on visual discrimination learning in humans and non-human primates. Quaterly Journal of Experimental Psychology B, 40, 321–341. [PubMed] [Google Scholar]
- Rodriguez-Callejas JD, Fuchs E, & Perez-Cruz C (2016). Evidence of tau hyperphosphorylation and dystrophic microglia in the common marmoset. Frontiers in Aging Neuroscience, 8, 315 10.3389/fnagi.2016.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CN, Davis K, Dobek G, & Tardif SD (2012). Aging phenotypes of common marmosets (Callithrix jacchus). Journal of Aging Research, 2012, 10.1155/2012/567143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, & Murray EA (2008). Amygdala and orbitofrontal cortex lesions differentially influence choices during object reversal learning. Journal of Neuroscience, 28, 8338–8343. 10.1523/jneurosci.2272-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh DM, & Jeeves MA (1966). A comparison of two discrimination-reversal indices intended for use with diverse groups of organisms. Psychonomic Science, 6, 1–2. 10.3758/bf03327927 [DOI] [Google Scholar]
- Rygula R, Walker SC, Clarke HF, Robbins TW, & Roberts AC (2010). Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. Journal of Neuroscience, 30, 14552–14559. 10.1523/jneurosci.2631-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2010). Major issues in cognitive aging. New York, NY: Oxford University Press, 10.1093/acprof:oso/9780195372151.001.0001 [DOI] [Google Scholar]
- Schaie KW (2005). What can we learn from longitudinal studies of adult development? Research in Human Development, 2, 133–158. 10.1207/s15427617rhd0203_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC (2017). Anatomical and functional neuroimaging in awake, behaving marmosets. Developmental Neurobiology, 77, 373–389. 10.1002/dneu.22456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Umberger G, Manning E, Slevin J, Wekstein D, Schmitt F, … Kryscio R (1999). Critical decline in fine motor hand movements in human aging. Neurology, 53, 1458–1458. 10.1212/wnl.53.7.1458 [DOI] [PubMed] [Google Scholar]
- Smith CD, Walton A, Loveland AD, Umberger GH, Kryscio RJ, & Gash DM (2005). Memories that last in old age: Motor skill learning and memory preservation. Neurobiology of Aging, 26, 883–890. 10.1016/j.neurobiolaging.2004.08.014 [DOI] [PubMed] [Google Scholar]
- Spinelli S, Pennanen L, Dettling AC, Feldon J, Higgins GA, & Pryce CR (2004). Performance of the marmoset monkey on computerized tasks of attention and working memory. Cognitive Brain Research, 19, 123–137. 10.1016/j.cogbrainres.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Strasser A, & Burkart JM (2012). Can we measure brain efficiency? An empirical test with common marmosets (Callithrix jacchus). Brain Behavior Evolution, 80, 26–40. 10.1159/000338014 [DOI] [PubMed] [Google Scholar]
- Tapp PD, Siwak CT, Estrada J, Head E, Muggenburg BA, Cotman CW, & Milgram NW (2003). Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learning and Memory, 10, 64–73. 10.1101/lm.54403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, & Ziegler TE (2011). The marmoset as a model of aging and age-related diseases. ILAR Journal, 52, 54–65. 10.1093/ilar.52.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Richardson A, Lanford R, Gelfond JA, Forney LJ, Brown CJ, … Ross CN (2017). The development of a specific pathogen free (SPF) barrier colony of marmosets (Callithrix jacchus) for aging research. Aging., 10.18632/aging.101340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton A, Scheib JL, McLean S, Zhang Z, & Grondin R (2008). Motor memory preservation in aged monkeys mirrors that of aged humans on a similar task. Neurobiology of Aging, 29, 1556–1562. 10.1016/j.neurobiolaging.2007.03.016 [DOI] [PubMed] [Google Scholar]
- Zaninotto P, Batty GD, Allerhand M, & Deary IJ (2018). Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English longitudinal study of ageing. Journal of Epidemiology and Community Health, 72, 685–694. 10.1136/jech-2017-210116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Andersen A, Smith C, Grondin R, Gerhardt G, & Gash D (2000). Motor slowing and parkinsonian signs in aging rhesus monkeys mirror human aging. Journals of Gerontology Series A-Biological Sciences & Medical Sciences, 55, B473–B480. 10.1093/gerona/55.10.b473 [DOI] [PubMed] [Google Scholar]