Abstract
Secretion of very low-density lipoprotein (VLDL) by the liver is an important physiological process; however, the rate of VLDL secretion is determined by its transport from the ER to the Golgi. This transport event is facilitated by a specialized ER-derived vesicle, the VLDL transport vesicle (VTV). We have reported earlier a detailed VTV proteome which revealed that reticulon 3 (RTN3) is uniquely present in the VTV. Our immunoblotting and electron microscopic data demonstrate that RTN3 is enriched in the VTV; however, other ER-derived vesicles do not contain RTN3. Co-immunoprecipitation data coupled with confocal microscopic analyses strongly suggest that RTN3 interacts with VLDL core protein, apoB100 at the ER level. Our data show that either blocking of RTN3 using specific antibodies or RTN3 knockdown resulted in significant reduction in VTV biogenesis from hepatic ER membranes. Additionally, VLDL secretion from hepatocytes was significantly decreased when RTN3 was silenced by RTN3 siRNA. We conclude that RTN3 regulates VLDL secretion by controlling VTV-mediated ER-to-Golgi transport of nascent VLDL.
Keywords: very low-density lipoprotein (VLDL), VLDL transport vesicle (VTV), apolipoprotein B, coat complex II (COPII), reticulon 3, endoplasmic reticulum, triacylglycerol
Introduction
One of the most important functions of hepatocytes is to synthesize and secrete triglyceride-rich very low-density lipoproteins (VLDL). Imbalance in VLDL synthesis and secretion leads to dyslipidemia, which is a major risk factor for the development of a variety of severe metabolic disorders such as type 2 diabetes, hepatic steatosis, atherosclerosis etc.(Sehayek and Eisenberg, 1994; Ginsberg, 2002). The biogenesis of primordial VLDL particle occurs in hepatic endoplasmic reticulum (ER) and requires microsomal triglyceride transfer protein (MTP) that mediates the lipidation of newly synthesized apolipoproteinB-100 (apoB100), a core VLDL protein (Olofsson et al., 1999; Fisher and Ginsberg, 2002; Hussain et al., 2003). The primordial VLDL particle is transported to the Golgi where several essential modifications occur and mature VLDL is produced (Gusarova et al., 2007; Tiwari and Siddiqi, 2012). Our laboratory and others have demonstrated that the ER-to-Golgi transport of nascent VLDL particle is the rate-limiting step in its overall secretion from the liver (Gusarova et al., 2003; Tiwari and Siddiqi, 2012). It has been well established that the transport of nascent VLDL particle is facilitated by a distinct hepatic ER-derived vesicle, the VLDL transport vesicle or VTV (Gusarova et al., 2003; Siddiqi, 2008). This specialized transport carrier selectively recruits nascent VLDL particles from the ER membranes and precisely exports them to the Golgi lumen (Siddiqi, 2008; Siddiqi et al., 2010a).
Several studies have shown that VLDL containing vesicles are distinct from other ER-derived secretory protein transport vesicles (PTV) morphologically, biochemically and in their proteome; however, both types of vesicles require coat complex II (COPII) proteins for their synthesis from hepatic ER membranes (Gusarova et al., 2003; Siddiqi, 2008; Rahim et al., 2012). COPII is a budding-complex of five different cytosolic proteins (Sar1, Sec23-Sec24 and Sec13-Sec31), which is sufficient to recruit cargo proteins and vesicle formation from the ER membrane (Barlowe et al., 1993; Barlowe et al., 1994; Tang et al., 2005; Gurkan et al., 2006). Interestingly, the size of COPII-vesicles is confined to 55–70 nm in diameter and thus limiting their ability to accommodate larger cargos such as chylomicrons and VLDL (Gusarova et al., 2003; Siddiqi et al., 2003; Siddiqi, 2008; Mansbach and Siddiqi, 2010). A number of biochemical and molecular studies have clearly demonstrated that different types of ER-derived vesicles require additional proteins for cargo selection and the formation of large vesicles (Tiwari et al., 2013; Butkinaree et al., 2014; Tiwari et al., 2016).
Because VTVs are large vesicles and specifically accommodate VLDL particles, we postulated that their formation from the hepatic ER and selective packaging of VLDL particles require proteins in addition to COPII proteins. To identify additional proteins unique to the VTV, we carried out a detailed proteomic analysis that revealed several proteins which are not identified in other ER-derived transport vesicles (Rahim et al., 2012). Of these distinct VTV proteins, one protein was identified as reticulon-3 (RTN3) (Rahim et al., 2012). Reticulons (RTNs) are highly conserved membrane-associated proteins primarily localized to the ER (Yang and Strittmatter, 2007). Reticulons have been implicated in many cellular processes such as intracellular transport from the ER to other sub-cellular organelles, stabilization of ER membranes and cell division (Oertle and Schwab, 2003). To date, four types of RTN proteins (RTN1, RTN2, RTN3 and RTN4) are known to be expressed in mammalian cells and each protein has multiple isoforms. RTN3 protein is primarily expressed in liver and is localized to the ER; however, the role of RTN3 in the ER-to-Golgi transport of newly synthesized VLDL particles and their secretion from the liver remains to be investigated.
In the current study, we attempted to determine the role of RTN3 in the formation of hepatic ER-derived VTV and VLDL secretion from the hepatocytes. Our data clearly show that RTN3 is present in the VTVs and specifically interacts with apoB100 (a core VLDL protein) at the ER level. Additionally, our data suggest that either blocking the function of RTN3 or RTN3 knockdown significantly reduces the VTV biogenesis from hepatic ER membranes and VLDL secretion from the hepatocytes.
Materials and methods
Reagents and animals
[3H] oleic acid (45.5 Ci/mM) was purchased from PerkinElmer Life Sciences (Boston, MA). All immunoblotting reagents were obtained from Bio-Rad, Corp (Hercules, CA). Enhanced chemiluminescence (ECL) reagents were procured from GE Healthcare Life Sciences (Pittsburgh, PA). Tablets of protease inhibitor mixture were obtained from Roche Applied Science (Indianapolis, IN). Albumin was purchased from Sigma Chemical Co. (St. Louis, MO). All other bio-chemicals were of analytical grade. Sprague–Dawley rats, 150–200 g, were obtained from Harlan (Indianapolis, IN). All procedures involving animals were performed according to guidelines provided in the Guide for the care and Use of Laboratory Animals (Eighth Edition, 2011, published by The National Academies Press, USA) and the guidelines of the University of Central Florida’s Institutional Animal Care and Use Committee (IACUC) and an IACUC approved protocol was strictly followed.
Antibodies
Rabbit polyclonal antibodies to SVIP were raised commercially (Alpha Diagnostic, Inc. San Antonio, TX). Rabbit polyclonal antibodies to RTN3, apoB100 calnexin, Ykt6, syntaxin 5; mouse monoclonal antibodies to RTN3, GOS28, rabbit anti-rabbit IgG Texas Red and bovine anti-rabbit IgG-fluorescein isothiocyanate conjugated antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit polyclonal anti-Sar1 antibodies were generated commercially and have been described previously (Siddiqi, 2008). Rabbit polyclonal antibodies against rat VAMP7 (vesicle-associated membrane protein 7; amino acids 105–123) were described earlier (Siddiqi et al., 2006a). Rabbit anti-rat albumin antibody was purchased from Bethyl Laboratories, Inc. (Montgomery, TX). Rabbit anti-goat IgG and goat anti-rabbit IgG conjugated with agarose beads were purchased from Sigma Chemical Co. (St. Louis, MO).
Cell culture
Human hepatoma cells (HepG2) were procured from American Type Culture Collection (ATCC) and were cultured in Dulbecco’s modified Eagle’s medium (Life Technologies, Grand Island, NY) supplemented with 5% fetal bovine serum (Life Technologies, Grand Island, NY) and 1% penicillin/streptomycin (Sigma Chemical Co. St. Louis, MO). Rat primary hepatocytes were isolated from Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) as described previously (Yellaturu et al., 2009).
Isolation and purification of radio-labeled hepatic ER and Golgi
To obtain [3H]-TAG containing ER, hepatocytes were metabolically labeled using [3H]oleic acid and the ER was isolated using the same method as described earlier (Tiwari et al., 2013; Siddiqi, 2015). In brief, HepG2 or primary hepatocytes were washed with a buffer A (136 mM NaCl, 11.6 mM KH2PO4, 8mM Na2HPO4, 7.5 mM KCl, 0.5 mM dithiothreitol; pH 7.2) and incubated at 37 °C for 35–40 min in buffer A supplemented with [3H]oleic acid (100 μCi) complexed with BSA. After incubation, hepatocytes were washed to remove excess [3H]-oleic acid-BSA complex and resuspended in 0.25M sucrose in 10 mM Hepes containing 50 mM EDTA and protease inhibitors (Roche Diagnostics GmbH, Mannheim, Germany). Cell membrane was disrupted using a Parr cell disruption vessel at 1,100 psi for 40 minutes and ER and Golgi membranes were isolated and purified utilizing a sucrose step gradient as described previously (Siddiqi, 2008; Hossain et al., 2014; Siddiqi, 2015).
Cytosol preparation
Cytosol was isolated from HepG2 or primary hepatocytes using the same procedure as described previously (Siddiqi, 2008, 2015). Briefly, cells were washed with a buffer B [25 mM Hepes, 125 mM KCl, 2.5 mM MgCl2, 0.5 mM DTT and protease inhibitors; pH 7.2] and the cell membrane was disrupted using a Parr cell disruption vessel at 1,100 psi for 40 min. The cell homogenate was centrifuged at 40,000 rpm for 95 min (Beckman Rotor 70.1 Ti) and the supernatant was collected as cytosol. Cytosol was dialyzed for 8–10 hours in cold buffer B at 4°C and concentrated using a 10 kDa cut-off centricon filter (Amicon, Beverly, MA).
In vitro ER-budding assay
To generate VTV from hepatic ER, an in vitro ER-budding assay was used as reported previously (Siddiqi, 2008). In brief, ER membranes containing [3H]-TAG (500 μg prot) in transport buffer (30 mM Hepes, 250 mM sucrose, 2.5 mM MgOAc, 30 mM KCl; pH 7.2) were incubated at 37 °C for 30 minutes with cytosol (1 mg prot), an ATP-regenerating system, 5 mM Mg2+, 5 mM Ca2+, 5 mM DTT, 1 mM GTP, 1 mM diethyl-p-nitrophenylphosphate. After 30 minutes, reaction was stopped and the reaction mix was overlaid on top of a continuous sucrose-density gradient (0.1 M −1.15M). The gradient was resolved by centrifugation at 25,900 rpm for 2 hours at 4°C (Beckman rotor SW41), 20 fractions of 500 μl each were collected and [3H]-TAG dpm were measured (Siddiqi, 2008).
Immunoblotting and co-immunoprecipitation assay
Immunoblotting was performed as described earlier (Siddiqi et al., 2006b). In brief, protein samples containing equal amount of protein were separated by SDS-PAGE, transferred on to a nitrocellulose membrane (Bio-Rad, Hercules, CA) and the membrane was blocked with 10% (w/v) non-fat dried skimmed milk in PBS-T. The membrane was incubated with a specific primary (as indicated in figures) and secondary antibodies conjugated with HRP. Protein detection was performed with ECL reagents using autoradiography film (MIDSCI, St. Louis, MO).
For co-immunoprecipitation analysis, protein samples (250 μg prot) were solubilized in 2% triton X-100 in PBS followed by incubation with indicated primary antibodies for 4 hours at 4°C. Post-incubation, secondary antibodies attached to agarose beads were added and incubated for 12–14 hours at 4°C. The resulting protein complexes attached to agarose beads were washed thoroughly and co-immunoprecipitated proteins were separated by SDS-PAGE as described previously (Siddiqi et al., 2010a; Tiwari et al., 2013).
Determination of radioactivity
[3H]-TAG associated radioactivity was measured as dpm counts using the Tri-Carb 2910 TR liquid scintillation analyzer (PerkinElmer Life and Analytical Sciences, Shelton, CT) (Siddiqi, 2008).
Blocking an ER-protein function and siRNA transfection
An equal amount of indicated antibodies was incubated with the ER containing [3H]-TAG or 1 hour at 4°C (Siddiqi et al., 2010a; Tiwari et al., 2013). Post-incubation, unbound antibodies were removed by washing the ER membranes with cold 0.1 M sucrose in Hepes buffer. The ER was resuspended in transport buffer (30 mM Hepes, 0.25 M sucrose, 2.5 mM MgOAc, 30 mM KCl; pH7.2) to use in in vitro assays.
For siRNA-mediated silencing, HepG2 or primary hepatocytes were transfected with siRNA using the same protocol as described before (Tiwari et al., 2013). Hepatocytes were transfected with RTN3 siRNA, 5’CAAUGGUGCAUGUCAACAAtt3’, (Silencer select Custom-designed siRNA, Life Technologies, Grand Island, NY; #4390827) using Lipofectamine RNAiMax following the manufacturer’s instructions (Life Technologies, Grand Island, NY).
Immuno-electron microscopy and Immunocytochemistry
We used negative-staining approach of immuno-electron microscopy to visualize the localization of RTN3 to the VTVs and used the same protocol for immuno-gold labeling as reported previously (Mukherjee et al., 2000; Tiwari et al., 2013; Tiwari et al., 2016).
To demonstrate the co-localization of RTN3 with apoB100 and calnexin, we performed confocal microscopy using Zeiss Spinning Disk Confocal microscope and volocity image analyzer software as described earlier (Tiwari et al., 2013; Tiwari et al., 2016).
Determination of VLDL secretion
To determine VLDL secretion, we measured [3H]-TAG and apoB100 in the media as described before (Thibeaux et al., 2017). For [3H]-TAG, 100 μl of media was collected in triplicates and [3H]-TAG dpm were measured using scintillation counter. For apoB100 secretion, apoB100 was immunoprecipitated from the media, that was collected at different time points, using anti-apoB100 antibodies. Immunoprecipitated apoB100 was detacted by immunoblotting and its amount was determined protein-band density analysis using NIH Image J program.
Statistical analysis
Data were compared using a GraphPad Software (GraphPad Prism 7 Software for Mac OS X version) utilizing a one-way analysis of variance (ANOVA).
Results
Hepatic ER-derived VTVs contain RTN3 protein
Because the biogenesis of VLDL occurs in the ER and nascent VLDL is transported to the Golgi for its maturation followed by its secretion via plasma membrane, we first sought to determine the distribution of RTN3 in various subcellular organelles in hepatocytes. To accomplish this goal, we isolated the ER, Golgi and cytosol from hepatocytes and determined their purity by immunoblotting using marker proteins specific to subcellular organelles. The data presented in Fig. 1A, show that calnexin, an ER marker protein, is enriched in the ER; however, GOS28 which is a marker for the Golgi is not present in the ER. As shown in Fig. 1A, Golgi membrane did not contain recognizable calnexin, whereas GOS28 was enriched in the Golgi. Additionally, we found that both the ER and the Golgi did not contain Rab11, an endosomal/lysosomal marker protein (data not shown). These results confirm the purity of our ER and Golgi membranes, which allowed us to use these sub-cellular organelles for in vitro assays. Using purified hepatic ER membranes, we carried out an in vitro ER-budding assay to generate ER-derived VTVs, which were purified on sucrose density gradient and characterized by marker proteins. To ascertain that our vesicular fraction contains authentic ER-derived VTVs, we tested for the VTV-marker protein, apoB100 and the ER-derived vesicle marker protein, p58. Consistent with prior published data, the results shown in Fig. 1A clearly demonstrate that the VTVs concentrate apoB100 and contain p58 protein suggesting that they are bona fide ER-derived VLDL containing vesicles (Gusarova et al., 2003; Rahim et al., 2012). To determine that RTN3 is present in the VTV, ER, Golgi, and cytosol, we carried out immunoblotting analysis using antibodies specific to RTN3. As demonstrated in Fig. 1A, hepatic ER, VTV, Golgi and cytosol contain RTN3; however, RTN3 was found to be the least in the cytosol. Interestingly, RTN3 is enriched in the ER and the VTVs as compared to the Golgi membranes (Fig. 1A).
FIGURE 1.
RTN3 is present in the VTV. A. 40 μg of protein from each purified fraction of ER, VTV, Golgi and cytosol were resolved by 12% SDS-PAGE, transblotted on to a nitrocellulose membrane and probed with specific antibodies to indicated proteins. Protein detection was performed using ECL reagents. The data are representative of three independent experiments. The amount of RTN3, p58 and ApoB100 per 40 μg fraction protein (as shown in Fig. 1A) was determined by analyzing protein band density using NIH Image J program. The results are the mean ±SD (n =3). Bars labeled with asterisk show p values using one-way ANOVA; ** p < 0.005; *** p < 0.001. Bars labeled with ns show non-significant p values. B. Immuno-electron microscopic illustration of RTN3 localization to the VTVs employing the negative staining. VTVs were adsorbed on formvar-carbon-coated nickel grids, treated with either (I) anti-rabbit pre-immune IgG; or (II) rabbit polyclonal anti-RTN3 antibodies and detected with anti-rabbit IgG conjugated with 15 nm gold particles. Bars size = 150 nm.
Because RTN3 has never been shown to be present in ER-derived VTVs, we wished to visualize the localization of RTN3 to the VTVs morphologically. We performed immunogold labeling of RTN3 on the VTVs and examined by electron microscopy adopting the negative-staining method. Data presented in the Fig. 1B (panel II) clearly demonstrate that RTN3 is present on the surface of the VTVs as determined by immunogold labeling, whereas no immunogold labeling (Fig. 1B, panel I) was observed when we used pre-immune IgG as control. These biochemical and morphological evidences clearly demonstrate that RTN3 is not only present but enriched in the VTVs.
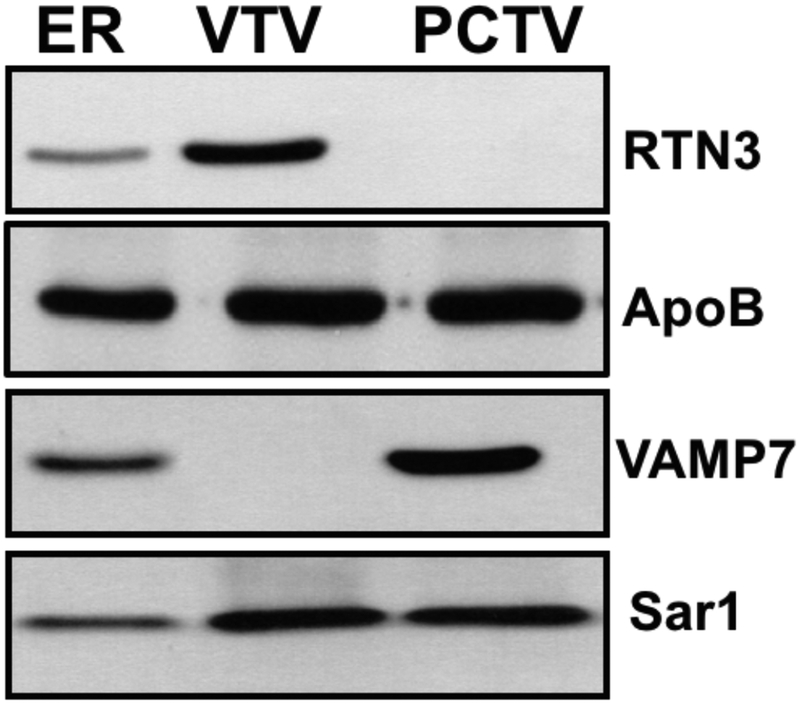
RTN3 does not localize to small intestinal ER-derived pre-chylomicron transport vesicle (PCTV)
Since both VTVs and PCTVs bud off the ER membranes and export lipoprotein particles from the ER to the Golgi in liver and small intestine, respectively, we decided to find out if RTN3 is present in the PCTV. As controls, we probed for the maker proteins of ER-derived vesicles (Sar1) and lipoprotein containing vesicles (apoB). Both Sar1 and apoB proteins were present in VTVs and PCTVs (Fig. 2) indicating that our vesicular fractions are authentic ER-derived lipoprotein containing vesicles. The results presented in Fig. 2 show that RTN3 is present the ER and VTV whereas PCTV does not contain recognizable amount of RTN3. Next, we examined for the presence of a PCTV marker protein, VAMP7 (Siddiqi et al., 2006a; Siddiqi et al., 2006b). Our results demonstrate that VAMP7 is enriched in the PCTV fraction; however consistent with previous reports, VTVs were devoid of VAMP7 (Siddiqi et al., 2010a). These findings suggest that RTN3 is specifically localized to the hepatic ER-derived VTVs.
FIGURE 2.
RTN3 is present in VTV but not in PCTV. 40 μg of protein each purified fraction of ER, VTV was resolved by 12% SDS-PAGE, transblotted on to a nitrocellulose membrane and probed with specific antibodies to the indicated proteins. ECL reagents were used for protein detection. The data are representative of four independent experiments.
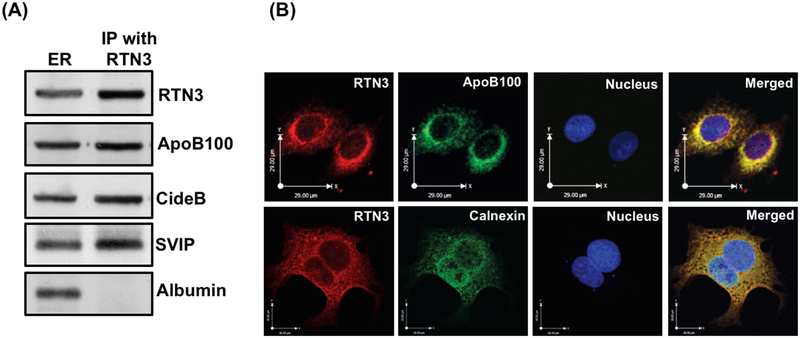
RTN3 interacts with VLDL core protein and components of VTV-budding complex
Because our prior published data strongly suggest that RTN3 is specifically present in the VTVs and not in other ER-derived vesicles (Rahim et al., 2012), we decided to find out whether RTN3 interacts with VTV-cargo protein, apoB100, and with proteins of VTV-budding complex (cideB and SVIP), which is required to generate VTV from hepatic ER membranes (Tiwari et al., 2013; Tiwari et al., 2016). To answer this question, we performed co-immunoprecipitation experiments using specific RTN3 antibodies and solubilized hepatic ER membranes and the co-immunoprecipitated proteins were detected by immunoblotting. As shown in Fig. 3A, RTN3 co-immunoprecipitates apoB100, cideB and SVIP. In contrast, RTN3 did not interact with albumin (Fig. 3A), which is cargo protein for ER-derived protein transport vesicle (PTV). These results clearly illustrate that RTN3 specifically interacts with VTV cargo and budding complex proteins at the ER level. These data further suggest a potential role of RTN3 in the ER-to-Golgi transport of nascent VLDL.
FIGURE 3.
RTN3 interacts with apoB100, cideB and SVIP but not with albumin. A. ER membranes (250 μg protein) were solubilized in 2% (v/v) Triton X-100 and treated with anti-rabbit RTN3 antibodies (10 μg) for 4h at 4°C. Anti-rabbit IgGs bound to agarose beads were added and incubated overnight at 4°C. Immune-complexes bound to agarose beads were isolated and washed 10 times with ice cold PBS to remove unbound proteins. Protein sample was separated by SDS-PAGE (8–16% gel) and probed with anti-RTN3, anti-apoB100, anti-cideB, anti-SVIP and anti-albumin antibodies. B. RTN3 co-localizes with apoB100 in hepatic ER. Hepatocytes were double labeled with either (upper panel) RTN3 (Texas red) and apoB100 (FITC, green) or (lower panel) RTN3 (Texas red) and calnexin, an ER marker (FITC, green. The nucleus is stained with DAPI (blue). Merged figures show colocalization of RTN3 with apoB100 and calnexin.
In an attempt to further substantiate our co-immunoprecipitation data, we decided to examine the RTN3-apoB100 interaction at the ER level morphologically employing a double-labeled immunofluorescence technique of confocal microscopy. Our data presented in Fig. 3B show that RTN3 co-localizes with apoB100 in reticular pattern, which is a characteristic of the ER membranes. Moreover, we found RTN3-apoB100 co-staining in punctate structures which is a characteristic pattern of the ER-derived VTVs (upper panel, Fig. 3B) (Tiwari et al., 2016). We used an ER marker, calnexin, to demonstrate that RTN3 interacts with apoB100 at the ER level. Our data illustrate that RTN3 co-localizes with calnexin (lower panel, Fig.3B). Our biochemical and morphological evidence presented in Fig. 3 strongly suggest that RTN3 interacts with apob100 at the ER level.
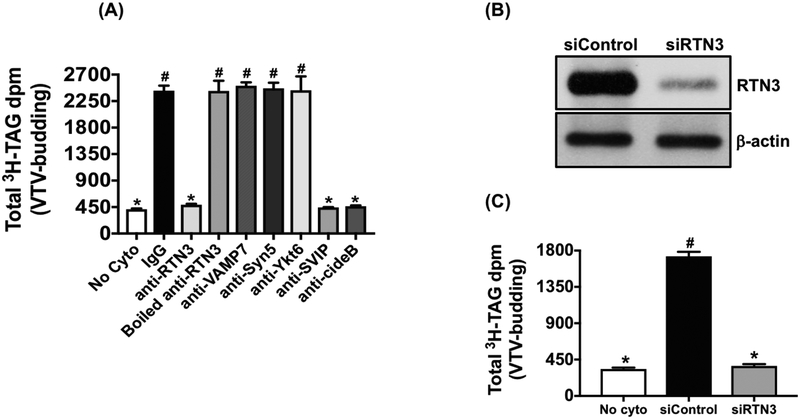
VTV biogenesis reduces in the absence of RTN3
Because we have shown that RTN3 is present on the surface the VTV (Fig. 1) and interacts with the components of VTV-budding complex (cideB and SVIP) and apoB100, a VTV cargo-protein at the ER level (Fig. 3), we questioned if RTN3 plays a functional role in the process of VTV-biogenesis from hepatic ER membranes. To address this question, RTN3 was blocked by incubating the ER with either specific antibodies against RTN3 or appropriate IgGs as control at 4 °C for 1 hour. Unbound antibodies were removed after 1-hour incubation. Additionally, we immunodepleted the traces of RTN3 from hepatic cytosol using anti-RTN3 antibodies as described (Tiwari et al., 2016). Our results demonstrate that the generation of the VTV was significantly decreased as a result of RTN3 blockade; however, there was no effect on VTV biogenesis when we treated ER and cytosol similarly with preimmune IgGs (Fig. 4A). These data indicate that RTN3 plays an active role in VTV formation from hepatic ER. To rule out the possibility that the observed reduction in VTV generation is a result of steric hindrance caused by anti-RTN3 antibodies, we boiled RTN3 antibodies before treating the ER and cytosol. We found that the VTV generation continued as normal when we treated the ER and cytosol with boiled RTN3 antibodies (Fig. 4A).
FIGURE 4.
Impact of either RTN3 blockade or RTN3 knockdown on VTV formation. VTV budding assays were carried out as described in Methods. A. Prior to budding assay, the ER containing [3H]-TAG was incubated at 4°C for 2 hours separately with each of pre-immune IgG, anti-RTN3, boiled anti-RTN3, anti-syntaxin5, anti-ykt6, anti-VAMP7, anti-cideB or anti-SVIP antibodies and antibodies in each case were removed by washing. Cytosol was pre-treated at 4°C for 2 hours with pre-immune IgG, anti-RTN3, boiled anti-RTN3, anti-syntaxin5, anti-ykt6, anti-VAMP7, anti-cideB or anti-SVIP antibodies bound to agarose beads and the antibodies were removed by centrifugation. As a negative control, ER containing [3H]-TAG isolated from untreated hepatocytes was incubated in the absence of cytosol (No cyto). Results are mean ±SD (n =4). Bars labeled with different symbols show P < 0.005 using one-way ANOVA. B. Protein samples of whole cell lysate (WCL) from untreated (Untreated) hepatocytes or treated with either RTN3 siRNA (siRTN3) or control siRNA (siControl) were probed with specific anti-RTN3 and anti-βactin antibodies. C. VTV budding assays were carried out using [3H]-TAG containing ER membranes and cytosol that were prepared from hepatocytes treated with either RTN3 siRNA (siRTN3) or control siRNA (siControl). As a negative control, ER containing [3H]-TAG isolated from untreated hepatocytes was incubated in the absence of cytosol (No cyto). Bars labeled with different symbols show P < 0.001 using one-way ANOVA.
To further support our observations and to eliminate the possibility of non-specific blocking effects of RTN3 antibodies on VTV formation, we blocked proteins that are known to be involved in ER-to-Golgi transport such as syntaxin5, ykt6 and VAMP7, and examined the VTV-biogenesis. Our data clearly demonstrate that the blockade of syntaxin5, ykt6 and VAMP7 at the ER level did not have any effect on VTV biogenesis from hepatic ER (Fig. 4A). These data indicate the specific nature of the observed reduction in VTV formation as a result of blocking RTN3. Because we observed that RTN3 interacts with other known budding-components of the VTV, cideB and SVIP, we blocked their function similarly using specific antibodies against cideB and SVIP. Consistent with our previous published data, blocking of either cideB or SVIP significantly reduced VTV formation from the ER (Fig. 4A). These findings strongly suggest that RTN3 interacts with apoB100, cideB and SVIP and regulate the formation of VTV from hepatic ER.
In consideration to clearly establish the role of RTN3 in VTV biogenesis, we decided to knockdown RTN3 in hepatocytes utilizing specific RTN3 siRNA and lipofectamine as a delivery system. After transfecting the hepatocytes with either control or RTN3 siRNA, we first determined the expression level of RTN3 protein by immunoblotting. We found that RTN3 siRNA significantly decreased the expression of RTN3 in hepatocytes, whereas RTN3 expression did not change when control siRNA was used (Fig. 4B). As a control, we probed for β-actin in the same samples and our data show that there was no effect of RTN3 siRNA on the expression level of β-actin in hepatocytes suggesting the specificity of RTN3 siRNA-mediated reduction in RTN3 protein expression (Fig. 4B). To find out the impact of RTN3 silencing on VTV biogenesis, we first isolated the ER membranes and cytosol from RTN3 knocked down hepatocytes and then carried out an in vitro VTV budding assay. Consistent with RTN3 blockade results, knockdown of RTN3 significantly reduced the VTV-biogenesis whereas VTV generation continued uninterrupted when control siRNA was utilized for transfection (Fig.4C). Taken together, our data strongly suggest a functional role of RTN3 in the VTV biogenesis from hepatic ER membranes.
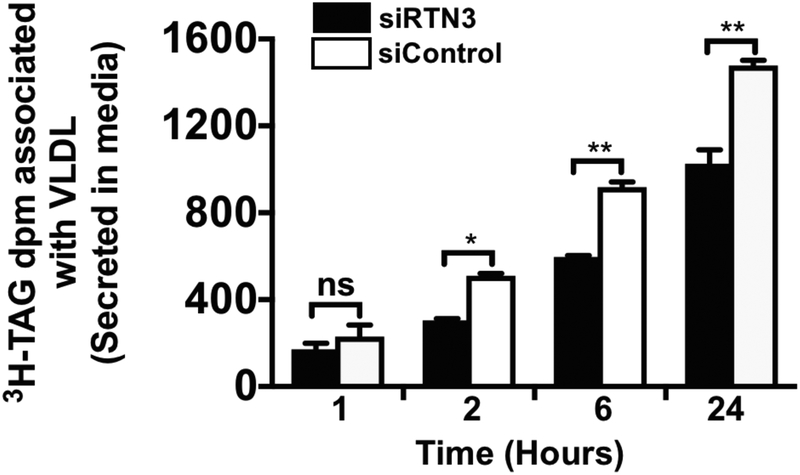
siRNA-mediated silencing of RTN3 reduces VLDL secretion
Because we and other have shown that VTV-mediated ER-to-Golgi transport of nascent VLDL is the rate-limiting step in its secretion from hepatocytes, we decided to examine the impact of RTN3 knockdown on VLDL secretion from hepatocytes. To attain this goal, we silenced RTN3 in hepatocytes using specific RTN3 siRNA as discussed above and observed VLDL secretion by determining [3H]TAG levels secreted in the media as reported earlier (Tiwari et al., 2016). RTN3 knocked down hepatocytes were incubated with [3H]oleic acid complexed to BSA for one hour. After one hour, oleic acid-BSA was removed by replacing fresh media without oleic acid-BSA complex and samples of media were collected at different time points to determine [3H]TAG levels secreted in the media. As expected, RTN3 knocked down hepatocytes secreted significantly decreased amount of [3H]TAG as compared to control hepatocytes which were transfected with control siRNA (Fig 5). We found that the observed reduction in [3H]TAG secretion was highest at the 24-hour time-point (Fig. 5). These data clearly demonstrate that knockdown of RTN3 negatively impacts VLDL secretion from hepatocytes.
FIGURE 5.
RTN3 silencing reduces VLDL secretion. Hepatocytes were transfected with either RTN3 siRNA (50 nM) or control siRNA (silencer select siRNA, Ambion). RTN3 knockdown hepatocytes and control hepatocytes were washed with warm (37°C) PBS and incubated with [3H]oleic acid-BSA complex at a final concentration of 0.4 mM oleic acid for one hour. After incubation, oleic acid-BSA was removed by replacing with media without oleic acid-BSA complex. Media samples were collected at different time points and associated [3H]TAG dpm were determined. Results are the mean ±SD (n =4). Bars labeled with asterisk show p values compared with control siRNA using one-way ANOVA; * p < 0.02; ** p < 0.005; ns = non-significant.
Discussion
Normal VLDL production and secretion are essential physiological processes that maintain overall lipid homeostasis; however, any abnormality associated with these processes can lead to the development of a variety of debilitating metabolic disorders (Ginsberg, 2002; Choi and Ginsberg, 2011; Sparks et al., 2012). VLDL is the end product of dietary lipid metabolism by the liver and its synthesis occurs in the ER. However, nascent VLDL needs to be transported to the Golgi for its maturation and this process is facilitated by a specialized hepatic ER-derived vesicle, the VTV (Siddiqi, 2008; Rahim et al., 2012). It has been well established that VTV-mediated ER-to-Golgi transport of nascent VLDLs governs the overall secretion of VLDL particles from the liver (Tiwari and Siddiqi, 2012). We have shown previously that VTVs are different from other ER-derived vesicles in size, cargo and protein composition (Gusarova et al., 2003; Siddiqi, 2008; Rahim et al., 2012). The VTV proteome reveals that these vesicles specifically contain cideB, SVIP, RTN3 and LPCAT3 proteins (Rahim et al., 2012). Our previous reports have demonstrated that cideB and SVIP are essential for VTV-mediated VLDL transport and secretion (Tiwari et al., 2013; Tiwari et al., 2016); however, the role of RTN3 in VLDL export remains to be explored. In this study, we aimed to define the role of RTN3 in VTV-biogenesis and VLDL secretion from hepatocytes. Like other reticulon proteins, RTN3 is primarily localized to the ER; however, its role in the intracellular transport and secretion of VLDL from the liver has never been studied. The data presented in this report provide strong biochemical and morphological evidence in support of a new functional role of RTN3 in VTV biogenesis and VLDL secretion from the liver.
Distinct biochemical and morphological nature of VLDL containing VTVs warrants detailed studies at the molecular level in order to precisely delineate the mechanisms that control the selection of VLDL and the genesis of a large vesicle, which can house VLDL particle. It has been established that the formation of VTV from hepatic ER membranes requires proteins in addition to COPII proteins, which were originally considered sufficient to trigger the packaging of different cargos and capable of forming ER-derived vesicles of expanded sizes (Barlowe et al., 1994; Miller and Barlowe, 2010; D’Arcangelo et al., 2013). However, a number of recent studies focusing on ER-to-Golgi transport of macromolecules demonstrate that proteins in addition to COPII components are needed for the selection of specific cargos and the generation of larger vesicle from the ER (Siddiqi et al., 2010b; Tiwari et al., 2013; Bajaj Pahuja et al., 2015; Tiwari et al., 2016). Because we reported earlier that RTN3 is specifically present in the VTVs but not in the PCTVs of small intestinal origin, we postulated that RTN3 may be involved in the VTV-mediated export of nascent VLDL from hepatic ER membranes. Our biochemical and morphological data presented in this report clearly indicate that RTN3 interacts with VTV-cargo protein apoB100 and previously identified proteins in the VTV-budding complex, cideB and SVIP. The interaction between RTN3 and apoB100, a VLDL core protein, raises the possibility that RTN3 might be playing a role in specific packaging of nascent VLDL particle into VTVs. Additionally, interaction between RTN3 and cideB or SVIP suggest its involvement in VTV formation.
A variety of transport vesicles bud off the ER membranes that are involved in dedicated export of specific cargos from the ER to different intracellular destinations and a number of proteins have been identified that meticulously regulate cargo-selection and formation of appropriate-sized transport carriers from the ER membranes. The classical example of such proteins is COPII proteins that are comprised of five different cytosolic proteins, Sar1, Sec23–24 and Sec13–31 (Barlowe et al., 1994). These proteins are sufficient to select a cargo and generate a vesicle from the ER membrane; however, the size of COPII vesicles range between 50–70 nm limiting their ability to accommodate macromolecules such as chylomicrons, VLDL and collagen; however, a number of proteins have been identified that interact with COPII proteins and form a larger cage required for the formation larger vesicle (Siddiqi et al., 2010b; Tiwari et al., 2013; Butkinaree et al., 2014; Bajaj Pahuja et al., 2015; Tiwari et al., 2016). To date three proteins, cideB, KLHL12 and SVIP, have been identified that are actively involved in intracellular VLDL trafficking and secretion (Tiwari et al., 2013; Butkinaree et al., 2014; Tiwari et al., 2016).
Our data strongly demonstrate that both blockade of RTN3 with specific antibodies and siRNA-mediated knockdown of RTN3, significantly decreased VTV-generation from hepatic ER revealing its new functional role in vesicle formation. The thesis that the transport of nascent VLDL from the ER to the Golgi is essential in VLDL secretion from the liver is further supported by the current data demonstrating a marked reduction in VLDL secretion from RTN3 knocked down hepatocytes. Several lines of evidence suggest that we have described a new physiological function of RTN3 in intracellular VLDL transport and secretion.
In conclusion, we have identified RTN3 as a new protein component of the VTV-budding complex, which is essential for the biogenesis of VTV from hepatic ER membranes. As revealed by co-immunoprecipitation data, a clear interaction between RTN3 and cideB/SVIP strongly suggest that RTN3 facilitate the formation of a large cage which is necessary for the formation of VTV. This study uncovered a novel functional role of RTN3 in the biogenesis of VLDL transport vesicles from hepatic ER membranes and eventual VLDL secretion from the liver. These findings are not only novel but physiologically important as they provide new mechanistic insights on intracellular VLDL transport and secretion. Additionally, the identification of RTN3 as a regulator of VTV biogenesis provides a potential target in controlling VLDL secretion from the liver.
Acknowledgements
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) R01 DK-81413 (to SAS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or NIH.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Bajaj Pahuja K, Wang J, Blagoveshchenskaya A, Lim L, Madhusudhan MS, Mayinger P, Schekman R, 2015. Phosphoregulatory protein 14-3-3 facilitates SAC1 transport from the endoplasmic reticulum. Proc Natl Acad Sci U S A 112, E3199–3206. doi: 10.1073/pnas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C, d’Enfert C, Schekman R, 1993. Purification and characterization of SAR1p, a small GTP-binding protein required for transport vesicle formation from the endoplasmic reticulum. J Biol Chem 268, 873–879. [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R, 1994. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77, 895–907. 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Butkinaree C, Guo L, Ramkhelawon B, Wanschel A, Brodsky JL, Moore KJ, Fisher EA, 2014. A regulator of secretory vesicle size, Kelch-like protein 12, facilitates the secretion of apolipoprotein B100 and very-low-density lipoproteins--brief report. Arterioscler Thromb Vasc Biol 34, 251–254. doi: 10.1161/ATVBAHA.113.302728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Ginsberg HN, 2011. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab 22, 353–363. doi: 10.1016/j.tem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcangelo JG, Stahmer KR, Miller EA, 2013. Vesicle-mediated export from the ER: COPII coat function and regulation. Biochimica et biophysica acta 1833, 2464–2472. doi: 10.1016/j.bbamcr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher EA, Ginsberg HN, 2002. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem 277, 17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN, 2002. New perspectives on atherogenesis: role of abnormal triglyceride-rich lipoprotein metabolism. Circulation 106, 2137–2142. [DOI] [PubMed] [Google Scholar]
- Gurkan C, Stagg SM, Lapointe P, Balch WE, 2006. The COPII cage: unifying principles of vesicle coat assembly. Nat Rev Mol Cell Biol 7, 727–738. doi: 10.1038/nrm2025. [DOI] [PubMed] [Google Scholar]
- Gusarova V, Brodsky JL, Fisher EA, 2003. Apolipoprotein B100 exit from the endoplasmic reticulum (ER) is COPII-dependent, and its lipidation to very low density lipoprotein occurs post-ER. J Biol Chem 278, 48051–48058. doi: 10.1074/jbc.M700475200. [DOI] [PubMed] [Google Scholar]
- Gusarova V, Seo J, Sullivan ML, Watkins SC, Brodsky JL, Fisher EA, 2007. Golgi-associated maturation of very low density lipoproteins involves conformational changes in apolipoprotein B, but is not dependent on apolipoprotein E. J Biol Chem 282, 19453–19462. doi: 10.1074/jbc.M700475200. [DOI] [PubMed] [Google Scholar]
- Hossain T, Riad A, Siddiqi S, Parthasarathy S, Siddiqi SA, 2014. Mature VLDL triggers the biogenesis of a distinct vesicle from the trans-Golgi network for its export to the plasma membrane. Biochem J 459, 47–58. doi: 10.1042/BJ20131215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain MM, Shi J, Dreizen P, 2003. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res 44, 22–32. doi: 10.1194/jlr.R200014-JLR200. [DOI] [PubMed] [Google Scholar]
- Mansbach CM, Siddiqi SA, 2010. The biogenesis of chylomicrons. Annu Rev Physiol 72, 315–333. doi: 10.1146/annurev-physiol-021909-135801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EA, Barlowe C, 2010. Regulation of coat assembly--sorting things out at the ER. Current Opin Cell Biol 22, 447–453. doi: 10.1016/j.ceb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K, Siddiqi SA, Hashim S, Raje M, Basu SK, Mukhopadhyay A, 2000. Live Salmonella recruits N-ethylmaleimide-sensitive fusion protein on phagosomal membrane and promotes fusion with early endosome. J Cell Biol 148, 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertle T, Schwab ME, 2003. Nogo and its paRTNers. Trends Cell Biol 13, 187–194. 10.1016/S0962-8924(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Olofsson SO, Asp L, Boren J, 1999. The assembly and secretion of apolipoprotein B-containing lipoproteins. Curr Opin Lipidol 10, 341–346. [DOI] [PubMed] [Google Scholar]
- Rahim A, Nafi-valencia E, Siddiqi S, Basha R, Runyon CC, Siddiqi SA, 2012. Proteomic analysis of the very low density lipoprotein (VLDL) transport vesicles. J Proteomics 75, 2225–2235. doi: 10.1016/j.jprot.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehayek E, Eisenberg S, 1994. The role of native apolipoprotein B-containing lipoproteins in atherosclerosis: cellular mechanisms. Curr Opin Lipidol 5, 350–353. [DOI] [PubMed] [Google Scholar]
- Siddiqi S, Mani AM, Siddiqi SA, 2010a. The identification of the SNARE complex required for the fusion of VLDL-transport vesicle with hepatic cis-Golgi. Biochem J 429, 391–401. doi: 10.1042/BJ20100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi S, Saleem U, Abumrad NA, Davidson NO, Storch J, Siddiqi SA, Mansbach CM 2nd, 2010b. A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J Lipid Res 51, 1918–1928. doi: 10.1194/jlr.M005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi SA, 2008. VLDL exits from the endoplasmic reticulum in a specialized vesicle, the VLDL transport vesicle, in rat primary hepatocytes. Biochem J 413, 333–342. doi: 10.1042/BJ20071469. [DOI] [PubMed] [Google Scholar]
- Siddiqi SA, 2015. In Vitro Analysis of the Very-Low Density Lipoprotein Export from the Trans-Golgi Network. Curr Protoc Cell Biol 67, 11 21 11–17. doi: 10.1002/0471143030.cb1121s67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi SA, Gorelick FS, Mahan JT, Mansbach CM 2nd, 2003. COPII proteins are required for Golgi fusion but not for endoplasmic reticulum budding of the pre-chylomicron transport vesicle. J Cell Sci 116, 415–427. doi: 10.1242/jcs.00215. [DOI] [PubMed] [Google Scholar]
- Siddiqi SA, Mahan J, Siddiqi S, Gorelick FS, Mansbach CM 2nd, 2006a. Vesicle-associated membrane protein 7 is expressed in intestinal ER. J Cell Sci 119, 943–950. doi: 10.1242/jcs.02803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi SA, Siddiqi S, Mahan J, Peggs K, Gorelick FS, Mansbach CM 2nd, 2006b. The identification of a novel endoplasmic reticulum to Golgi SNARE complex used by the prechylomicron transport vesicle. J Biol Chem 281, 20974–20982. doi: 10.1074/jbc.M601401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks JD, Sparks CE, Adeli K, 2012. Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler Thromb Vasc Biol 32, 2104–2112. doi: 10.1161/ATVBAHA.111.241463. [DOI] [PubMed] [Google Scholar]
- Tang BL, Wang Y, Ong YS, Hong W, 2005. COPII and exit from the endoplasmic reticulum. Biochim Biophys Acta 1744, 293–303. doi: 10.1016/j.bbamcr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Thibeaux S, Siddiqi S, Zhelyabovska O, Moinuddin F, Masternak MM, Siddiqi SA, 2018. Cathepsin B Regulates Hepatic Lipid Metabolism by Cleaving Liver Fatty Acid Binding Protein. J Biol Chem. 293(6):1910–1923. doi: 10.1074/jbc.M117.778365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, Siddiqi S, Siddiqi SA, 2013. CideB protein is required for the biogenesis of very low density lipoprotein (VLDL) transport vesicle. J Biol Chem 288, 5157–5165. doi: 10.1074/jbc.M112.434258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, Siddiqi S, Zhelyabovska O, Siddiqi SA, 2016. Silencing of Small Valosin-containing Protein-interacting Protein (SVIP) Reduces Very Low Density Lipoprotein (VLDL) Secretion from Rat Hepatocytes by Disrupting Its Endoplasmic Reticulum (ER)-to-Golgi Trafficking. J Biol Chem 291, 12514–12526. doi: 10.1074/jbc.M115.705269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, Siddiqi SA, 2012. Intracellular trafficking and secretion of VLDL. Arterioscler Thromb Vasc Biol 32, 1079–1086. doi: 10.1161/ATVBAHA.111.241471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YS, Strittmatter SM, 2007. The reticulons: a family of proteins with diverse functions. Genome Biol 8, 234. doi: 10.1186/gb-2007-8-12-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellaturu CR, Deng X, Cagen LM, Wilcox HG, Mansbach CM 2nd, Siddiqi SA, Park EA, Raghow R, Elam MB, 2009. Insulin enhances post-translational processing of nascent SREBP-1c by promoting its phosphorylation and association with COPII vesicles. J Biol Chem 284, 7518–7532. doi: 10.1074/jbc.M805746200. [DOI] [PMC free article] [PubMed] [Google Scholar]