Abstract
Background:
Occupational exposure to excessive level of copper results in many adverse health effects.
Objective:
To measure pulmonary function, oxidative stress, and extent of DNA damage in workers of a copper processing industry.
Methods:
30 men working in a copper processing industry and 30 men matched for age and socioeconomic status (comparison group) were included in this study. Pulmonary function test parameters were measured for all participants. Serum malondialdehyde (MDA), ferric reducing ability of plasma (FRAP), glutathione (GSH) content in RBCs and 8-OHdG were assayed by ELISA. Extent of DNA damage in leucocytes was assayed by comet assay.
Results:
Pulmonary function parameters, FVC, FEV1, PEFR, and MVV measured in workers were significantly (p<0.05) lower than those observed in the comparison group. Compared to the comparison group, MDA was significantly (p=0.002) increased in studied workers; TAOC (p=0.017), and GSH (p=0.020) were significantly lower in workers than the comparison group. There was significant DNA damage in leucocytes in workers compared to the comparison group (difference in olive tail moment p<0.001). PEFR, FEF25-75%, and MEF50% were negatively correlated with MDA.
Conclusion:
The observed DNA damage would be due to increased oxidative stress resulting from excessive exposure to copper.
Keywords: Lung, Respiratory function tests, Oxidative stress, DNA damage, Copper, Miner
TAKE-HOME MESSAGE
Occupational exposure to copper in workers of copper industry would cause impaired lung functions.
The exposure would increase the oxidative stress in these workers.
DNA damage observed in these workers is proposed to be due to increased oxidative stress resulting from excessive exposure to copper.
Introduction
Copper is widely used in industries and for common household purposes. It is also an important environmental pollutant. Copper particulates are released into the atmosphere by windblown dust, volcanic eruptions, and anthropogenic sources—primarily copper smelters and ore processing facilities.1 Copper particles in the atmosphere will settle or be removed by precipitation, but they can be resuspended into the atmosphere in the form of dust. The general population is exposed to copper through inhalation, consumption of food and water, and dermal contact.2 Populations living near sources of copper emissions, and workers in these industries may be exposed to high levels of copper.
Exposure to excessive level of copper results in many adverse health effects including liver and kidney damage, anemia, immunotoxicity and developmental toxicity.3-5 Copper can bind to sulfhydryl groups of certain enzymes such as G6PD and glutathione reductase, thus interfering with their function, say protection of cells from free radical damage. Occupational exposure to certain metals causes pulmonary function impairment in a dose-dependent manner.6 Data on inhaled copper toxicity in humans following acute exposure to the metal are limited to a report of workers developing metal fume fever while cutting brass pipe with an electric cutting tool in a poorly ventilated area.7 Reports on chronic copper toxicity consist of two occupational exposure studies reporting respiratory and gastrointestinal irritations, hepatic effects, and possible neurological and reproductive effects.8-10 An increased risk of cancer has also been reported in copper smelters.11
There are few research studies on copper industrial workers, especially in India. Very few studies have so far been conducted on measurement and documentation of pulmonary function of copper industrial workers.12 Despite a number of studies reporting an increased risk of cancer among copper smelter workers, the data on genotoxic effects of copper in this industry are scarce.9 There is still a knowledge gap if oxidative stress in the copper industrial workers lead to DNA damage or not.13,14 Therefore, the present study was conducted to investigate the lung function, oxidative stress, and DNA damage in workers exposed to copper particulate in copper processing workers.
Materials and Methods
The present study was conducted in the Departments of Physiology and Biochemistry, UCMS & GTB Hospital, Delhi, India. The study was conducted on workers in a factory located in Jhilmil industrial area, Delhi. In this factory, copper slab was melted and redrawn into wire of desired length and thickness. During this process workers are exposed to copper dust, smoke and heat. Thirty-five male workers from the factory aged between 20 and 40 years, were first enrolled into this study. Only those male workers working for at least 4–6 hrs/day, 5–6 days/week and having exposure history in same industry for at least two years were included in the study. Those on medications that affect lung functions and those with a history of respiratory illness before joining the job were excluded from the study. Subjects with upper respiratory tract infections in preceding three weeks or with any systemic illness, such as diabetes, tuberculosis, hypertension, etc, were also excluded. Three of the workers refused to give blood and two were unable to perform the required maneuvers for pulmonary function test (PFT) correctly.
Thirty-two age-matched men served as the comparison group, were also enrolled into the study; two of them were unable to perform the PFT maneuver correctly, and thus were excluded from the study. The comparison group members were recruited from the same locality having same socioeconomic status not working in copper processing units. They were either rickshaw puller or laborers.
Sample Size
From a previous report, the mean DNA damage for copper smelters and controls differed by 13.0 (damage index/100 leukocytes).15 To detect a difference of this magnitude, considering a SD of 10.0, type I error of 0.05, and study power of 90%, the minimum sample size for each group (case and the comparison group) was calculated to be 22. To ascertain normalcy of data, we decided to study at least 30 cases and 30 controls.
Procedure
A comprehensive modified questionnaire developed based on American Thoracic Society (ATS), National Heart and Lung Institute (NHLI) division of lung diseases-1978 recommendations (American thoracic society guidelines) suitable to Indian scenario, was used for epidemiological research.16
The questionnaire was filled in for each participant. Height, weight, chest circumference, and body mass index (BMI) were measured. The standing height of each subject was measured without shoes to the nearest 1 cm; weight was measured to the nearest 1 kg.
Pulmonary Function Test
The pulmonary function test was carried out on participants using a Sibelmed Datospir 120B precision portable spirometer with a built-in computer program.17-19 The program provides a set of predicted normal values for all the lung function parameters assessed, based on age, height, weight, gender and ethnicity of the subject. The software analyzes and provides a detailed interpretation of flow-volume loop. The participants were tested on relatively empty stomach, ie, about 2–3 hrs after a light meal. In order to alley anxiety, an apprehension associated with testing, the participants were familiarized with the procedure and apparatus to use. The ambient temperature on different days varied from 18–24 °C. Lung function test was done for all participants. The end-point of the test was assessed by the shape and size of the flow-volume loop. Participants were asked to do the test for three consecutive times. The best results of the three fulfilling the criteria of reproducibility and validity was considered for analysis. Those with inadequate respiratory efforts were excluded from the study. All the pulmonary function tests mentioned above were carried out under the guidelines, as specified in American thoracic society (ATS) statement (Snowbird workshop on standardization of spirometry, 1979).20
Estimation of Oxidative Stress Markers in Blood
Serum level of malondialdehyde (MDA), ferric reducing ability of plasma (FRAP), glutathione (GSH) content in RBCs, and 8-OHdG were assayed by ELISA.
Assessment of DNA Damage
The extent of DNA damage in leucocytes was measured by comet assay.21,22 Agarose-precoated slides were prepared by smearing 600 μL of 1% agarose over the slides by using the pipette tip horizontally and spread gently over the slide. The slides were allowed to air dry to form a thin film. Slides were labeled at the frosted end, using a diamond tipped pen. After agarose was solidified, the slides were submerged in a covered dish containing lysis solution. Slides were gently handled by holding them horizontally and lowering into solutions. Samples were lysed for 30 min in lysis solution (pH>13) at 4 °C. After lysis, the slides were removed carefully and submerged in electrophoresis buffer for electrophoresis. Slides were stained by 30 μL of a 2-μg/mL stock solution of EtBr directly onto the slide. The staining was performed in dark. The slides were immediately analyzed under fluorescent microscope (BX51, Olympus, Japan). The slides were analyzed by examining at least 50 comet images from each slide. Doublets or comets at slide edges were avoided. To quantify DNA damage, parameters like percent of DNA content in the head and tail, olive tail moment (OTM), and tail length (TL) were evaluated using Komet 5.0 software (Kinetic Imaging, Liverpool, UK).
Ethics
The study was approved by Institutional Ethics Committee–Human Research (IEC-HR), of UCMS. A written informed consent was taken from all participants.
Statistical Analysis
SPSS® for Windows® ver 20 was used for data analysis. The lung function, oxidative stress markers, and DNA damage were compared between the workers and the comparison group by Student's ttest for independent data. For analysis of the results of comet assay, either means or medians, depending on population distribution, was used. Error bars typically represent the between-experiment variability, not the within-slide variability for a specific parameter. A p value <0.05 was considered statistically significant.
Results
Out of 30 workers who completed the study, 18 (60%) were nonsmokers, 8 (27%) were taking 2–4 cigarettes per day, and 4 (13%) were taking 5–10 cigarettes per day. Of 30 studied workers, 19 (63%) were working for less than five years; 11 (37%) for more than five years.
The mean age, height, weight and BMI was not significantly different between the studied groups (Table 1). FVC, FEV1, FEV1/FVC, ERV, PEFR, MEF50%, MIF50%, and FEF25-75% were significantly copper processing workers compared with those in the comparison group (Table 2). Regarding oxidative stress parameters, while serum MDA was found to be significantly (p=0.002) higherthe workers compared with the camparison group, T-AOC and GSH levels in the workers were significantly (p<0.02) lower than in the comparison group (Table 3). Comet assay showed that the mean percent of DNA in tail, olive tail moment (Fig 1), and tail length in copper processing workers were significantly higher than that in the comparison group (Table 4). The percentage of DNA in the head was significantly (p=0.016) lower in the workers (64.66%) than in the comparison group (72.72%).
| Table 1: Anthropometric measured parameters in copper processing workers and the comparison group. Figures are mean (SD). | |||
| Parameters |
Comparison group (n=30) |
Workers (n=30) |
p value |
| Age (yrs) | 30.0 (5.3) | 30.1 (5.2) | 0.942 |
| Ht (cm) | 165.1 (6.2) | 163.0 (5.1) | 0.148 |
| Wt (kg) | 68.5 (5.3) | 68.2 (5.5) | 0.831 |
| BMI (kg/m2) | 25.2 (2.8) | 25.7 (2.7) | 0.474 |
| Table 2: Pulmonary function test parameters in copper processing workers and the comparison group. Figures are mean (SD). | |||
| Variable | Comparison group (n=30) | Workers (n=30) | Mean difference (95% CI) |
| FVC (L) | 3.80 (0.30) | 3.51 (0.45) | -0.28 (-0.48 to -0.08) |
| FVC (% pred*) | 85.13 (3.97) | 88.180 (4.88) | -3.33 (-5.64 to -1.03) |
| FEV1 (L) | 3.24 (0.28) | 2.88 ( 0.38) | -0.36 (-0.53 to -0.18) |
| FEV1(% pred) | 84.57 (3.07) | 1.07 (4.73) | -3.43 (-5.50 to -1.37) |
| FEV1/FVC (%) | 85.58 (5.07) | 82.26 (5.63) | -3.31 (-6.09 to -0.55) |
| FEV1/FVC (% pred) | 97.30 (3.30) | 98.57 (5.46) | 1.26 (-1.07 to 3.60) |
| VC (L) | 3.39 (0.41) | 3.25 (0.50) | -0.142 (-0.38 to 0.09) |
| TV (L) | 0.54 (0.04) | 0.55 (0.048) | 0.006 (-0.02 to 0.03) |
| ERV (L) | 1.35 (0.20) | 1.26 (0.12) | -0.092 (-0.18 to -0.01) |
| PEFR (L/s) | 6.46 (0.50) | 5.30 (0.61) | -1.160 (-1.45 to -0.87) |
| PEFR (% pred) | 97.47 (5.15) | 88.17 (6.80) | -9.30 (-12.42 to -6.18) |
| MEF50%(L/s) | 4.03 (0.43) | 3.59 (0.50) | -0.441 (-0.68 to -0.20) |
| MEF50% (% pred) | 91.03 (4.95) | 89.03 (4.76) | -2.00 (-4.51 to 0.51) |
| MIF50% (L/s) | 3.67 (0.15) | 3.28 (0.24) | -0.39 (-0.49 to -0.28) |
| FEF25-75% (L/s) | 4.02 (0.31) | 3.59 (0.38) | -0.43 (-0.61 to -0.25) |
| FEF25-75% (% pred) | 85.80 (3.67) | 84.30 (3.05) | -1.50 (-3.25 to 0.25) |
| MVV (L/min) | 100.19 (17.47) | 95.00 (16.71) | -5.19 (-14.02 to 3.64) |
| *Percent predicted value | |||
| Table 3: Oxidative stress parameters measured in copper processing workers and the comparison group. Figures are mean (SD). | |||
| Oxidative stress parameters | Comparison group (n=30) | Workers (n=30) | Mean difference (95% CI) |
| MDA (nM/mL) | 446.76 (82.34) | 527.85 (107.97) | 81.08 (31.46 to 130.71) |
| T-AOC (mIU/L) | 39.50 (16.39) | 30.18 (12.88) | -9.31 (-16.93 to -1.69) |
| GSH (µg/mL) | 38.34 (13.56) | 30.47 (11.86) | -7.87 (-14.46 to -1.29) |
| 8-OHdG (pg/mL) | 3294.51 (996.31) | 3130.40 (1379.03) | -164.11 (-785.86 to 457.64) |
Figure 1.
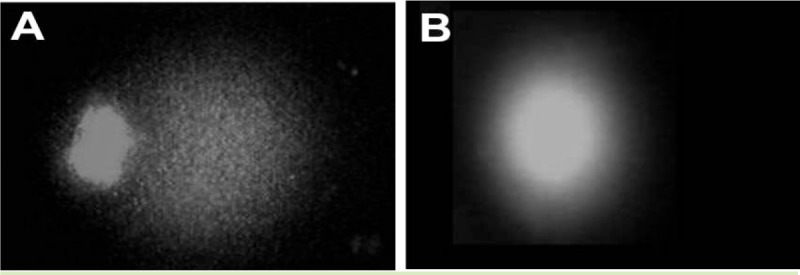
Comet tail in copper processing (A) and the comparison group (B)
| Table 4: Comparison of DNA damage in copper processing workers and the comparison group. Figures are mean (SD). | ||||
| DNA damage | Comparison group (n=30) | Workers (n=30) | Mean difference (95% CI) | |
| Head DNA | 72.72 (16.55) | 64.66 (5.50) | -8.05 (-14.42 to -1.67) | |
| Tail DNA | 30.47 (3.89) | 35.33 (5.50) | 4.85 (2.39 to 7.31) | |
| Olive tail moment | 9.44 (1.22) | 12.01 (1.75) | 2.56 (1.78 to 3.34) | |
| Tail length | 77.75 (7.35) | 85.56 (8.02) | 7.80 (3.82 to 11.78) | |
No significant positive correlation was found between the oxidative stress parameters and lung function variables. However, MDA had a significant (p<0.05) negative correlation with PEFR, MEF50%, and FEF25-75%. PEFR, and FEF25-75% had significant (p<0.05) with olive tail moment. MDA had a significant negative correlation with olive tail moment. Similarly, PEFR and MVV had a significant positive correlation with the tail length.
Discussion
The present study was conducted to determine the lung functions, oxidative stress, and extent of DNA damage in a group of copper processing workers.
Many of the pulmonary function parameters (ERV, FVC, FEV1, FEV1/FVC, PEFR, MEF50%, MIF50%, and FEF25-75%) were significantly decreased in the copper processing industry workers compared to the comparison group. Previous studies on copper industry workers showed similar findings of mixed obstructive and restrictive ventilatory defects.1 However, some studies did not find any significant difference in the respiratory parameters of the two groups.23 In earlier cross-sectional study on workers exposed to several metals, variables such as FEV1/FVC, PEFR25-75%, and FEF75-85% were reduced significantly in the exposed group, which was quite similar to our findings.8 Decrease in respiratory parameters especially FEV1 is the first measurable sign of the initiation of bronchitis and obstructive lung disease.24,25 In the present study, obstructive pattern of lung involvement was the predominate type observed, which might be due to inflammatory reactions, respiratory irritation, and the resultant narrowing of the airways caused by copper dust and mist.1
The flow rates at low volumes, ie, FEF25-75% and MEF50%, are sensitive indices of flow in small airways—those with internal diameters of <2 mm. These parameters were lower at low lung volumes, both in restrictive and obstructive diseases.26 Therefore, our findings reflected more involvement of small airways. Particles generated during copper processing are probably small in size. These small particles, by virtue of their greater surface area to mass ratio, can carry a much larger fraction of toxic compounds, such as hydrocarbons and metals on their surface. Importantly, they remain airborne for long periods and deposit in greater numbers and deeper into the lungs than larger particles do.27 Therefore, chronic exposure to these small particles can lead to chronic inflammation of the respiratory tract and lung parenchyma.
In the studied copper factory, the first step was mining and crushing the metal. This was followed by grinding, smelting, electrolytic refining, electro-winning, annealing, and electroplating. Heat and reducing agents are used in the process for changing the oxidation state of copper. During different stages of the process, a lot of smoke, dust or mist of copper is disseminated into the workplace environment.
In the present study, those suffering from upper respiratory diseases were excluded. No respiratory signs or symptoms (eg, chest tightness, wheezing, or breathlessness) were observed in our study groups. Those suffering from systemic illness (ie, diabetes, tuberculosis, and hypertension), which increases the oxidative stress and may thereby confound the results, were not included in the present study.
In the workers, serum level of MDA was significantly higher than the comparison group. FRAP, and GSH levels were significantly lower in the workers. Increased level of MDA reflects increased free radical load that leads to lipid peroxidation in the workers. Reactive oxygen species degrades polyunsaturated fatty acids and MDA formation. Animal study done by Ozcelik, et al, showed that plasma MDA levels are higher in rats that were exposed to copper.28 Therefore, MDA is used as a biomarker to measure the level of oxidative stress. GSH, an antioxidant, functions directly as a free radical scavenger. Serum levels of GSH in copper processing workers were also significantly lesser than that in the comparison group, indicating decreased free radical scavenging ability and thus, increased oxidative stress. An earlier study revealed an increase in GSSG/GSH ratio in cells exposed to copper oxide, indicating that reactive oxygen species generated by copper oxide could induce oxidative stress in HEP-2 cells.29 A study on dogs done by Spee, et al, also showed an increase in GSSG/GSH ratio in chronic inflammatory and cholestatic liver disease induced by the copper.30
Serum level of FRAP reflects the total antioxidant power or total reducing power of electron-donating antioxidants. Decreased levels of FRAP reflects decreased antioxidant power of plasma, and thus, increased propensity for oxidative stress with consequent damage at cellular and molecular level in copper processing workers. Serum level of FRAP was significantly lower in the exposed group compared with the non-exposed group, indicating reduced antioxidant capacity. To the best of our knowledge, this is the first study that reports a correlation between GSH levels, FRAP and 8-OHdG in copper processing workers.
A significant negative correlation was observed between serum level of MDA and FEV1, PEFR and FEF25-75%, and MEF50%. Therefore, it can be inferred that serum MDA, an oxidative stress marker, may also act as a potential biomarker for pulmonary function deficits.31 We have not come across any study correlating oxidative stress parameters and lung function test parameters in copper processing industrial workers.
Exposure to copper is associated with the generation of reactive oxygen species (ROS)32 that can directly attack biomolecules, with the consequent enhancement in membrane lipid peroxidation, DNA damage, and protein oxidation.33 The ROS generated by this process could attack DNA, leading to base damage and DNA strand excision. The true strand breaks and/or the strand breaks formed as an intermediate step in excision repair of altered bases could explain the increased DNA damage in the comet assay. These results are in accordance with those of other authors who associated metal exposition and single strand break.34,35 De Olivera, et al, showed a significant increase in DNA damage in peripheral blood lymphocytes of smelter workers compared to the controls.15
Shubber, et al, studied women using the copper-containing intrauterine devices (IUDs). They observed a combination of high copper plasma level, chromosomal aberrations, and an increased frequency of sister chromatid exchange with a positive correlation between the long-term use of the IUDs and DNA damage.36
In conclusion, our results indicated that occupational exposure of workers to copper would cause impaired lung functions and DNA damage. These may be due to increased oxidative stress resulting from excessive exposure to copper.
Conflicts of Interest:
None declared.
Financial Support:
None.
Cite this article as: Kumar S, Khaliq F, Singh S, et al. Pulmonary functions, oxidative stress and DNA damage in workers of a copper processing industry. Int J Occup Environ Med 2016;7:107-115.
References
- 1. Occupational Health Guideline for copper dusts and mists. US Department of labour: Occupational Safety and Health Administration 1978. Available from www.cdc.gov/niosh/docs/81-123/pdfs/0150.pdf (Accessed April 27, 2015).
- 2.Zietz BP, Dieter HH, Lakomek M. et al. Epidemiological investigation on chronic copper toxicity to children exposed via the public drinking water supply. Sci Total Environ. 2003;302:127–44. doi: 10.1016/s0048-9697(02)00399-6. [DOI] [PubMed] [Google Scholar]
- 3.Suciu I, Prodan L, Lazar V. et al. Research on copper poisoning. Med Lav. 1981;3:190–7. [PubMed] [Google Scholar]
- 4.Brewer GJ. Copper toxicity in the general population. Clin Neurophysiol. 2010;121:459–60. doi: 10.1016/j.clinph.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Uauy R, Maass A, Araya M. Estimating risk from copper excess in humanpopulations. Am J Clin Nutr. 2008;88:867S–71S. doi: 10.1093/ajcn/88.3.867S. [DOI] [PubMed] [Google Scholar]
- 6.Kusaka Y, Yokoyama K, Sera Y. et al. Respiratory diseases in hard metal workers: an occupational hygiene study in a factory. Br J Ind Med. 1986;43:474–85. doi: 10.1136/oem.43.7.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong CW, Moore LW Jr, Hackler RL. et al. An outbreak of metal fume fever Diagnostic use of urinary copper and zinc determinations. J Occup Med. 1983;25:886–8. [PubMed] [Google Scholar]
- 8.Askergren A, Mellgren M. Changes in the nasal mucosa after exposure to copper salt dust A preliminary report. Scand J Work Environ Health. 1975;1:45–9. doi: 10.5271/sjweh.2861. [DOI] [PubMed] [Google Scholar]
- 9.Suciu I, Prodan L, Lazar V. et al. Research on copper poisoning. Med Lav. 1981;3:190–7. [PubMed] [Google Scholar]
- 10.Brewer GJ. The risks of copper toxicity contributing to cognitive decline in the aging population and to Alzheimer's disease. J Am Coll Nutr. 2009;28:238–42. doi: 10.1080/07315724.2009.10719777. [DOI] [PubMed] [Google Scholar]
- 11.Mizukami S, Ichimura R, Kemmochi S. et al. Tumor promotion by copper-overloading and its enhancement by excess iron accumulation involving oxidative stress response in the early stage of a rat two stage hepatocarcinogenesis model. Chem Biol Interact. 2010;185:189–201. doi: 10.1016/j.cbi.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Rastogi SK, Gupta BN, Husain T. et al. A cross-sectional study of pulmonary function among workers exposed to multimetals in the glass bangle industry. Am J Ind Med. 1991;20:391–99. doi: 10.1002/ajim.4700200311. [DOI] [PubMed] [Google Scholar]
- 13.Midander K, Cronholm P, Karlsson HL. et al. Surface characteristics, copper release, and toxicity of nano- and micrometer-sized copper and copper(II) oxide particles: a cross-disciplinary study. Small. 2009;5:389–99. doi: 10.1002/smll.200801220. [DOI] [PubMed] [Google Scholar]
- 14.Boveris A, Musacco-Sebio R, Ferrarotti N. et al. The acute toxicity of iron and copper: biomolecule oxidation and oxidative damage in rat liver. J Inorg Biochem. 2012;116:63–9. doi: 10.1016/j.jinorgbio.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 15.De Olivera JV, Boufleur LA, Dos Santos CE. et al. Occupational genotoxicity among copper smelters. Toxicol Ind Health. 2012;28:789–95. doi: 10.1177/0748233711422735. [DOI] [PubMed] [Google Scholar]
- 16.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 17.Sati PC, Kaushik R, Kumar V. et al. Oxidative status in workers engaged in recycling of plastic: occupational hazard. Indian J Physiol Pharmacol. 2012;56:234–8. [PubMed] [Google Scholar]
- 18.Khaliq F, Singh P, Chandra P. et al. Pulmonary functions in plastic factory workers: a preliminary study. Indian J Physiol Pharmacol. 2011;55:60–6. [PubMed] [Google Scholar]
- 19.Sati PC, Khaliq F, Vaney N. et al. Pulmonary function and oxidative stress in workers exposed to styrene in plastic factory: occupational hazards in styrene-exposed plastic factory workers. Hum Exp Toxicol. 2011;30:1743–50. doi: 10.1177/0960327111401436. [DOI] [PubMed] [Google Scholar]
- 20.ATS statement. Snowbird workshop on standardization of spirometry. Am Rev Respir Dis. 1979;119:831–8. doi: 10.1164/arrd.1979.119.5.831. [DOI] [PubMed] [Google Scholar]
- 21.Ostling O, Johanson K. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun. 1984;123:291–8. doi: 10.1016/0006-291x(84)90411-x. [DOI] [PubMed] [Google Scholar]
- 22.Singh NP, McCoy MT, Tice RR. et al. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 23.Marcisz C, Jonderko G, Wieczorek-Latka U. et al. The Respiratory system of workers employed in the casting and processing of copper. Pneumonol Alergol Pol. 1998;66:433–9. [PubMed] [Google Scholar]
- 24.Beaty TH, Cohen BH, Newill CA. et al. Impaired pulmonary function as a risk factor for mortality. Am J Epidemiol. 1982;116:102–13. doi: 10.1093/oxfordjournals.aje.a113385. [DOI] [PubMed] [Google Scholar]
- 25.Tockman MS, Pearson JD, Fleg JL. et al. Rapid decline in FEV1 A new risk factor for coronary heart disease mortality. Am J Respir Crit Care Med. 1995;151:390–8. doi: 10.1164/ajrccm.151.2.7842197. [DOI] [PubMed] [Google Scholar]
- 26. Cotes JE, Chinn DJ, Miller MR. How individual diseases affect lung functions. In Lung function: Physiology measurement and application in medicine. 6th ed. Blackwell Scientific Publications,2006;560-92.
- 27. Harrison RM, Brimblecombe P, Dervent RG. Health effects of non biological particles. In Airborne Particulate Matter in the United Kingdom: Third Report of the Quality of Urban Air Review Group. Department of Environment, London 1996;115-8.
- 28.Ozcelik D, Ozaras R, Gurel Z. et al. Copper-mediated oxidative stress in rat liver. Biol Trace Elem Res. 2003;96:209–15. doi: 10.1385/BTER:96:1-3:209. [DOI] [PubMed] [Google Scholar]
- 29.Fahmy B, Cormier SA. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol In Vitro. 2009;23:1365–71. doi: 10.1016/j.tiv.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spee B, Arends B, van den Ingh TS. et al. Copper metabolism and oxidative stress in chronic inflammatory and cholestatic liver diseases in dogs. J Vet Intern Med. 2006;20:1085–92. doi: 10.1892/0891-6640(2006)20[1085:cmaosi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Schünemann HJ, Muti P, Freudenheim JL. et al. Oxidative stress and lung function. Am J Epidemiol. 1997;146:939–48. doi: 10.1093/oxfordjournals.aje.a009220. [DOI] [PubMed] [Google Scholar]
- 32.Flora SJ, Mittal M, Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res. 2008;128:501–23. [PubMed] [Google Scholar]
- 33.Videla LA, Fernández V, Tapia G. et al. Oxidative stress-mediated hepatotoxicity of iron and copper: role of Kupffer cells. Biometals. 2003;16:103–11. doi: 10.1023/a:1020707811707. [DOI] [PubMed] [Google Scholar]
- 34.Hengstler JG, Bolm-Audorff U, Faldum A. et al. Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis. 2003;24:63–73. doi: 10.1093/carcin/24.1.63. [DOI] [PubMed] [Google Scholar]
- 35.Villela IV, de Oliveira IM, Silveira JC. et al. Assessment of environmental stress by the micronucleus and comet assays on Limnoperna fortunei exposed to Guaíba hydrographic region samples (Brazil) under laboratory conditions. Mutat Res. 2007;628:76–86. doi: 10.1016/j.mrgentox.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Shubber E, Amin NS, El-Adhami BH. Cytogenetic effects of copper-containing intrauterine contraceptive device (IUCD) on blood lymphocytes. Mutat Res. 1998;417:57–63. doi: 10.1016/s1383-5718(98)00090-4. [DOI] [PubMed] [Google Scholar]


