Abstract
Introduction
Breast cancer (BC) is the most frequently reported cancer among women - reported in 2012 as 25% of all cancers. BC has been related to the increased life and activity of osteoclasts, conferring a higher risk for osteoporosis/osteopenia. This study aimed to determine a cut-off point in Hounsfield units (HU) as well as the sensitivity and specificity of computed axial tomography (CT) in the diagnosis of osteoporosis/osteopenia in Mexican women with BC.
Material and methods
We included 108 patients with a histopathological diagnosis of BC treated at the ABC Medical Center in Mexico City. All patients were subjected to both dual X-ray densitometry and CT. The receiver operating characteristic (ROC) curve was used to identify the cutoff point and sensitivity and specificity were calculated, as were confidence intervals for the diagnoses of osteoporosis/osteopenia.
Results
The mean age was 58.49 ± 11.01 years. The cutoff point with the highest sensitivity (82%) and specificity (68%) was <157 HU for osteoporosis/osteopenia in patients with BC.
Conclusions
Women with BC are exposed to several risk factors for osteoporosis/osteopenia. The CT obtained for the general evaluation of these patients can also be used to evaluate bone mineral density, avoiding additional examinations and exposure to radiation, as well as the cost it confers, offering an earlier diagnosis of osteoporosis/osteopenia for its control.
Keywords: bone mineral density, breast cancer, hounsfield units
Introduction
Breast cancer (BC) is the second most common cancer in women [1,2]. Incidence of BC increases with age; it has been reported that over 75% of BC occurs in postmenopausal women [3]. Different studies show a connection between bone mineral density and BC [4,5]. The numerous factors favoring the appearance of osteoporosis in cancer patients are related to the tumor itself and to the antitumor treatment [6]. Chemotherapy-induced ovarian failure is a common cause of bone degradation among patients with breast cancer [7].
The recognition of osteoporosis as one of the late sequelae that may appear after the cure of cancer requires an early diagnosis of this disease in patients with an oncological background to adopt the appropriate preventive measures [8,9]. Bone mineral density is inversely proportional to the risk of fracture and is currently the best predictor to evaluate this parameter [10-14]. The risk of fracture for a patient with osteoporosis is 40%, with the most frequent sites of this event being the spine, the hip, and the wrist, among others [15]. In addition to conventional radiography, there are other imaging techniques such as dual X-ray absorptiometry (DXA) and computed tomography quantification, which has been developed to evaluate bone mineral content [16]. The International Society for Clinical Densitometry (ISCD) recommends that the BMD (Bone Mineral Density) test should be carried out on all women 65 years of age or older, as well as all men over 70 years of age [16]. On the other hand, the Hounsfield Units (HU) represent the relative density of body tissues according to a calibrated level scale, based on values for air (-1000 HU), water (0 HU), and bone density (+1000 HU) [17]. Literature indicates that bone quality can also be determined through the measurements of HU, which can be calculated from a region of interest using most modern radiology programs without additional costs or radiation [18]. A sensitivity of 86% and a specificity of 94% have been described for computed axial tomography (CT) in the diagnosis of osteoporosis [18]. DXA is currently the gold standard for assessing the bone mineral density and has been correlated with fracture risk. Although it is useful for evaluating osteopenia or osteoporosis, it poses certain methodological limitations [19]. Both DXA and CT can be used for the quantification of bone mineral density. Recently, several studies have proposed a potential correlation between BMD and the result of CT. There are different conclusions regarding the usefulness of CT to evaluate BMD: the majority suggests that CT provides accurate and reliable results in this tenor. CT has been suggested to have the potential to be considered an alternative method of timely detection of osteoporosis, without radiation or additional cost since CT can be analyzed for different aspects related to the patients' pathology [20-23]. Our study is a pioneer in showing the result of a CT scan as a diagnostic test for osteoporosis/osteopenia in Mexican patients with BC.
Materials and methods
A total of 108 patients diagnosed with BC (clinical and histopathological diagnosis) - with densitometry and computed tomography studies using multidetector helical equipment, cross-sections were made with abdomen computed tomography protocol, detector coverage (mm) 40.0, helical thickness (mm) 2.5, pitch and speed (mm/rot) 1.375: 1, 55.00, rotation time (s) 0.6, mA 520, KV 120, with an approximate total dose report per patient CTD vol 11.14 (mGy), DLP 578.93 (mGy-cm) conducted at the ABC Cancer Center, Mexico City - were included in this study. Exclusions comprised patients with hypothyroidism, with treatments based on steroid corticosteroids, and with incomplete clinical records; unreliable DXA or CT results as patients with metallic material in vertebral bodies were also eliminated. Medical records were collected using the clinical file with prior approval from ABC Medical Center's ethics committee (folio TABC-17-22). Baseline characteristics were collected from the clinical history, including data on risk and comorbidity as well as hereditary family history, smoking habits, and excess weight or obesity by body mass index. The CT scan results were collected in HU from the evaluation of the vertebral body of L3 (trabecula) using PACS. A DXA analysis was performed by an observer-blinded from the DXA results on the lumbar spine and both coxofemoral joints. Results were classified according to the World Health Organization in standard deviations (SD), according to the parameter for age in our population (Osteopenia: -1 to -2.5 SD. Osteoporosis: -2.5 SD. Normal: up to -1 SD).
The densitometer's brand was Hologic, model Discovery WI with serial number 87039. The tomography equipment employed was a 64-slice GE CT scanner, lightspeed VCT model with serial number 5230VCTABC.
Means, standard deviations, frequencies, and percentages were determined. The diagnostic test parameters were determined for qualitative data as well as the receiver operating characteristic (ROC) curve to obtain a cut-off point in HU for osteoporosis/osteopenia. Kolmogorov-Smirnov, chi-square, ANOVA and the Pearson and Spearman correlation tests were applied. The data analysis was performed with the program SPSS v19 and Epidat 3.1. A confidence interval of 95% was taken for all tests and determinations.
Results
A total of 108 patients diagnosed with BC were studied. The average age was 58.49 ± 11.01 years, with a 48 years range (34 to 82 years). The frequencies of risk factors related to lifestyle and comorbidities were as follows: 29.6% of the patients were overweight and 16.7% were obese. The minimum BMI was 18 kg/m2 and the maximum was 39 kg/m2. Regarding the gynecological-obstetric history, the frequency of early menarche was 3.7% (four cases), none of the patients had late menopause and only 4.6% (five cases) was nulliparous. Stage III-IV had a frequency of 22.2% (24 cases), metastasis at the time of diagnosis was 20.4% (22 cases). Women who were in stage III-IV had 18 times more risk (95% confidence interval of 5.83-56.14); that is, metastasis was related in 68.2% to stage III-IV (p = 0.0001). Only 9.3% (10 cases) were treated with hormone therapy and 37.4% (40 cases) with surgery. Regarding the immunohistochemical markers, 48.1% of cases had triple-negative profile (Negative estrogen receptor/negative progesterone receptor/negative HER2).
The bone density evaluated by DXA showed that 55.6% of the patients presented osteoporosis/osteopenia (45.4% Osteopenia, 10.2% Osteoporosis). The DXA result was related to the risk age for menopause and stage III-IV (Table 1).
Table 1. DXA result related to the risk age for menopause and stage III-IV.
*Statistical significance, P-value <0.05, Chi square
DXA: Dual X-ray absorptiometry
| DXA Study Results | |||||||
| Normal (n = 38) | Osteopenia (n = 56) | Osteoporosis (n = 14) | P -value | ||||
| Age >48 years | 38% (32) | 50% (42) | 12% (10) | 0.04* | |||
| Stage III-IV | 25% (6) | 58.3% (14) | 16.7% (4) | 0.03* | |||
Table 2 shows the HU global averages obtained through CT in relation to the qualitative result of the DXA (normal, osteopenia, osteoporosis). This same table also shows the mean HU values obtained through the CT scan in the risk age for menopause and relation to the categorical result of the DXA (normal, osteopenia, osteoporosis).
Table 2. Bone density assessed by CT (HU) in relation to DXA results with the age of risk for menopause.
*Statistical significance
SD: Standard deviation; CT: Computed axial tomography; DXA: Dual X-ray absorptiometry.
| Age of Risk for Menopause | |||
| DXA results | Global Mean ± SD | >48 years Mean ± SD | <48 years Mean ± SD |
| Normal | 187.84 ± 64.4 | 166.03 ± 56.71 | 231.46 ± 59.4 |
| Osteopenia | 139.19 ± 40.1 | 134.39 ± 38.3 | 164.42 ± 44.6 |
| Osteoporosis | 93.73 ± 21.6 | 95.74 ± 21.73 | 73.63 |
| P value | 0.0001* | 0.0001* | 0.007* |
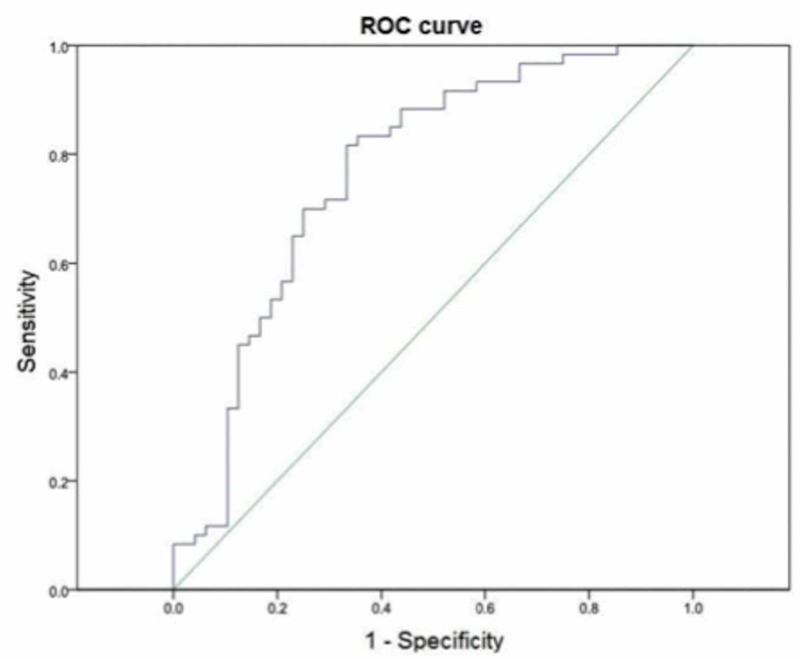
Figure 1 shows the ROC curve, where the maximum point of sensitivity and specificity for CT (HU) is shown in relation to the result of osteoporosis/osteopenia according to DXA. The optimal cut-off point was <157 HU for osteoporosis/osteopenia in patients with BC, reaching a sensitivity of 82% and a specificity of 68%, with an area under the curve of 0.76 (p = 0.0001) and a confidence interval of 95% from 0.67 to 0.86.
Figure 1. ROC curve of the CT study (HU) to evaluate bone mineral density (BMD) in patients diagnosed with BC.
ROC: Receiver operating characteristic; BC: Breast cancer.
A contingency table was made between the diagnosis of osteoporosis/osteopenia (cut-off point of <157 HU) evaluated by CT and by DXA. Table 3 shows 35 cases of true positives between both diagnostic methods.
Table 3. CT scan results with a cut-off point of 157 HU in relation to the DXA results in patients with BC.
CT: Computed axial tomography; DXA: Dual X-ray absorptiometry; BC: Breast cancer.
| DXA | Total (n = 108) | ||||
| Osteoporosis/osteopenia (N = 60) | Normal (n = 48) | ||||
| CT | <157 HU | Osteoporosis/osteopenia | 58.3% (35) | 64.6% (31) | 61.1% (66) |
| >157 HU | Normal | 41.7% (25) | 35.4% (17) | 38.9% (42) | |
Table 4 shows the CT scan's diagnostic test parameters, employing the cutoff point of 157 HU, and the gold standard (DXA characterized in healthy patients).
Table 4. Diagnostic test parameters for CT scan with a 157 HU cut-off point and DXA results in patients with BC.
CT: Computed axial tomography; DXA: Dual X-ray absorptiometry; BC: Breast cancer; PPV: Positive prognostic value; NPV: Negative prognostic value.
| Sensitivity (CI 95%) | Specificity (CI 95%) | PPV (CI 95%) | NPV (CI 95%) | Prevalence (CI 95%) | |
| CT | 58.33% (45.03 to 71.64) | 35.42% (20.84 to 49.99) | 53.03% (40.23 to 65.83) | 40.48% (24.44 to 56.51) | 55.56% (45.72 to 65.39) |
Finally, we observed that the HU obtained by CT scan negatively correlated with age (r = -0.53, p = 0.0001) and the DXA result (r = -0.53, p = 0.0001).
Discussion
The average age our patients presented at the time of diagnosis of BC was similar to that previously reported for the Mexican population [24-27]. Regarding excess weight and obesity, in our study, we observed lower frequencies concerning other studies conducted in our country [28]. The prevalence of osteoporosis found in our population was similar to that described in studies of osteoporosis in Europe: it was only 1.8% lower than that described in the European population [28]. According to the findings in the DXA values, the patients with osteoporosis were mostly menopausal women; when comparing clinical stages, those located in stage III-IV had the highest percentage of osteoporosis (36.4%).
Regarding the results obtained by CT pre-menopausal patients with normal DXA presented 65.43 less HU than those found in menopausal patients with normal DXA, as expected. Likewise, when comparing the results in patients on clinical stages III-IV but with a normal DXA, these were higher (86.4 more) than that obtained in abnormal DXA patients but with early clinical stages (I-II-III). This can presumably be due to the presence of metastatic lesions as well as the biochemical processes of the activation of beta adrenergic receptors [29]. Through the CT scan, a cut-off level for osteoporosis/osteopenia equal to or <157 HU was obtained with a sensitivity of 82%, which is very similar to that reported by Pickhardt et al. in 2013. They reported a 90% sensitivity for a cut-off level of 160 HU, even though their study was of the North American population and included both sexes [21]. Other studies exist where authors correlate DXA with CT: Batawil and Sabiq in 2012 described a cut-off level of 203 HU to exclude osteopenia/osteoporosis [30]. This may be because this study was conducted in the country of Saudi Arabia, with a group of women with various previous diagnoses and only some of them with BC.
According to the results from this clinical investigation, values were obtained using CT with Hounsfield units (HU) for osteoporosis/osteopenia and normal bone density. The mean for patients with BC with a normal DXA was 187.84 HU; it was 139.19 HU for patients with a DXA showing osteopenia, and 93.73 HU for patients with a DXA for osteoporosis. This difference in HU values was both statistically significant and similar to other studies. We conclude that computed tomography could provide a bone density profile employing Hounsfield units in all patients who undergo this study for other medical reasons. It is relevant for the clinician since the state of bone metabolism should not be forgotten in the evaluation of the patient with breast cancer, the timely detection will allow him to reduce fracture risks.
Mexican patients with breast cancer are a good example: by taking advantage of the studies that are practiced for follow-up and/or approach during clinical staging, the time-cost-radiation of dual X-ray absorptiometry can be avoided. The optimal cut-off point for osteoporosis/osteopenia through CT was equal to or less than 157 HU, reaching a sensitivity of 82% and a 68% specificity, with a 95% CI. The resulting HU correlated with age: pre-menopausal patients showed an average of 231.46 HU, unlike patients with BC and menopausal women who had an average of 166.03 HU. These results show that normal bone density parameters should be established in cancer patients with both DXA and CT studies. Normal bone density parameters should also be reestablished in the gold standard that is densitometry when it is determined in oncological patients.
Limitations
One limitation of our study was that it was not possible for us to validate our results, we only reported the descriptive findings and we proposed a cut-off point, for our population but we need to perform further studies.
Conclusions
According to the results obtained by CT in our clinical research, the average for breast cancer patients with normal densitometry was 187.84 HU, for patients with osteopenia in densitometry was 139.19 HU and 93.73 HU for osteoporotic patients by densitometry; this difference in values of HU was statistically significant. The optimal cut-off point obtained by ROC curve for osteoporosis/osteopenia by CT was equal or less than 157 HU, reaching a sensitivity of 82% and 68% specificity with a 95% CI. Therefore, we conclude that computed tomography can provide a bone density profile using the Hounsfield units.
Acknowledgments
We would like to express our gratitude to the participants of this study. To MD Marco Antonio Téliz Meneses, Head of the Radiology and Image Department of the ABC Medical Center.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Ferlay J, Soerjomataram I, Dikshit R, et al. Int J Cancer. 2015;136:359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Does adjuvant chemotherapy change bone mineral density and related serum biomarkers in women with breast cancer? Safaei-Nodehi R, Esmaili J, Sharifian R, Movaseghi S, Parkhideh S. Caspian J Intern Med. 2017;8:91–98. doi: 10.22088/cjim.8.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bone mineral density and breast cancer risk factors among premenopausal and postmenopausal women - A systematic review. Zain NM, Seriramulu VP, Chelliah KK. https://www.ncbi.nlm.nih.gov/pubmed/27509955. Asian Pac J Cancer Prev. 2016;17:3229–3234. [PubMed] [Google Scholar]
- 4.Association between bone mineral density and incidence of breast cancer. Fraenkel M, Novack V, Liel Y, et al. PLoS One. 2013;8:70980. doi: 10.1371/journal.pone.0070980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calcium and vitamin D supplementation and loss of bone mineral density in women undergoing breast cancer therapy. Datta M, Schwartz GG. Crit Rev Oncol Hematol. 2013;88:613–624. doi: 10.1016/j.critrevonc.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minireview: the OPG/RANKL/RANK system. Khosla S. Endocarinology. 2001;142:5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- 7.Antineoplastic treatment effect on bone mineral density in Mexican breast cancer patients. Monroy-Cisneros K, Esparza-Romero J, Valencia ME, Guevara-Torres AG, Méndez-Estrada RO, Anduro-Corona I, Astiazarán-García H. BMC Cancer. 2016;16:860. doi: 10.1186/s12885-016-2905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical management of osteoporosis in women with a history of breast carcinoma. Van Poznac C, Sauter NP. Cancer. 2005;104:443–456. doi: 10.1002/cncr.21201. [DOI] [PubMed] [Google Scholar]
- 9.Osteoporosis in breast and prostate cancer survivors. Hoff AO, Gagel RF. https://www.ncbi.nlm.nih.gov/pubmed/15945345. Oncology. 2005;19:651–658. [PubMed] [Google Scholar]
- 10.Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis Diagnosis, and Therapy. JAMA. 2001;285:785–795. [Google Scholar]
- 11.Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. Siris ES, Miller PD, Barrett-Connor E, et al. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 12.Osteoporosis and skeletal fractures. Gardner MJ, Demetrakopoulos D, Shindle MK, Griffith MH, Lane JM. HSS J. 2006;2:62–69. doi: 10.1007/s11420-005-0137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.BMD, clinical risk factors and their combination for hip fracture prevention. Johansson H, Kanis JA, Oden A, Johnell O, McCloskey E. Osteoporos Int. 2009;20:1675–1682. doi: 10.1007/s00198-009-0845-x. [DOI] [PubMed] [Google Scholar]
- 14.An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Johnell O, Kanis JA. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 15.New horizons in osteoporosis. Rachner TD, Khosla S, Hofbauer LC. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The perspective of the International Osteoporosis Foundation on the official positions of the International Society for Clinical Densitometry. Kanis JA, Seeman E, Johnell O, Rizzoli R, Delmas P. https://www.ncbi.nlm.nih.gov/pubmed/15908701. J Clin Densitom. 2005;8:145–147. doi: 10.1385/jcd:8:2:145. [DOI] [PubMed] [Google Scholar]
- 17.Bone density: comparative evaluation of Hounsfield units in multislice and cone-beam computed tomography. Silva IM, Freitas DQ, Ambrosano GM, Bóscolo FN, Almeida SM. https://www.ncbi.nlm.nih.gov/pubmed/23184166. Braz Oral Res. 2012;26:550–556. doi: 10.1590/s1806-83242012000600011. [DOI] [PubMed] [Google Scholar]
- 18.Using Hounsfield units to assess osteoporotic status on wrist computed tomography scans: comparison with dual energy X-ray absorptiometry. Johnson CC, Gausden EB, Weiland AJ, Lane JM, Schreiber JJ. J Hand Surg Am. 2016;41:767–774. doi: 10.1016/j.jhsa.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Preoperative assessment of the cancellous bone mineral density of the proximal humerus using CT data. Krappinger D, Roth T, Gschwentner M, Suckert A, Blauth M, Hengg C, Kralinger F. Skeletal Radiol. 2012;41:299–304. doi: 10.1007/s00256-011-1174-7. [DOI] [PubMed] [Google Scholar]
- 20.Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. J Bone Joint Surg Am. 2011;93:1057–1063. doi: 10.2106/JBJS.J.00160. [DOI] [PubMed] [Google Scholar]
- 21.Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Ann Intern Med. 2013;158:588–595. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Validation of a CT-derived method for osteoporosis screening in IBD patients undergoing contrast-enhanced CT enterography. Weber NK, Fidler JL, Keaveny TM, et al. Am J Gastroenterol. 2014;109:401–408. doi: 10.1038/ajg.2013.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The use of routine non density calibrated clinical computed tomography data as a potentially useful screening tool for identifying patients with osteoporosis. Burke CJ, Didolkar MM, Barnhart HX, Vinson EN. Clin Cases Miner Bone Metab. 2016;13:135–140. doi: 10.11138/ccmbm/2016.13.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CYP3A4 expression in breast cancer and its association with risk factors in Mexican women. Floriano-Sanchez E, Rodriguez NC, Bandala C, Coballase-Urrutia E, Lopez-Cruz J. Asian Pac J Cancer Prev. 2014;15:3805–3809. doi: 10.7314/apjcp.2014.15.8.3805. [DOI] [PubMed] [Google Scholar]
- 25.Metastasis risk reduction related with beta-blocker treatment in Mexican women with breast cancer. Parada-Huerta E, Alvarez-Dominguez T, Uribe-Escamilla R, et al. https://www.ncbi.nlm.nih.gov/pubmed/27356717. Asian Pac J Cancer Prev. 2016;17:2953–2957. [PubMed] [Google Scholar]
- 26.Relation of alcohol/tobacco use with metastasis, hormonal (estrogen and progesterone) receptor status and c-erbB2 protein in mammary ductal carcinoma. Leon-Hernandez SR, Padilla EL, Algara AC, et al. Asian Pac J Cancer Prev. 2014;15:5709–5714. doi: 10.7314/apjcp.2014.15.14.5709. [DOI] [PubMed] [Google Scholar]
- 27.RNA expression of cytochrome P450 in Mexican women with breast cancer. Bandala C, Floriano-Sánchez E, Cárdenas-Rodríguez N, López-Cruz J, Lara-Padilla E. Asian Pac J Cancer Prev. 2012;13:2647–2653. doi: 10.7314/apjcp.2012.13.6.2647. [DOI] [PubMed] [Google Scholar]
- 28.Integrated imaging approach to osteoporosis: state-of-the-art review and update. Guglielmi G, Muscarella S, Bazzocchi A. Radiographics. 2011;31:1343–1364. doi: 10.1148/rg.315105712. [DOI] [PubMed] [Google Scholar]
- 29.Molecular pathways: beta-adrenergic signaling in cancer. Cole SW, Sood AK. Clin Cancer Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hounsfield unit for the diagnosis of bone mineral density disease: a proof of concept study. Batawil N, Sabiq S. Radiography. 2016;22:93–98. [Google Scholar]