Abstract
Background
Following one to five years of antibiotic mass drug administration (MDA) for the elimination of trachoma as a public health problem, programmes must conduct impact surveys to inform decisions on whether MDA is still needed. These decisions are currently based on the prevalence of trachomatous inflammation—follicular (TF), which, after MDA, correlates poorly with prevalence of ocular Chlamydia trachomatis infection.
Methodology/Principal findings
Impact surveys in six evaluation units (EUs) of Malawi were used as a platform to explore associations between the prevalence of TF, ocular C. trachomatis infection and anti-Pgp3 antibodies one year after the third annual round of MDA. Participants were examined for trachoma using the World Health Organization simplified grading system. Ocular swabs and dried blood spots (DBS) were collected from children aged 1–9 years. Swabs were tested for C. trachomatis DNA using GeneXpert. DBS were assayed for anti-Pgp3 antibodies using ELISA. EU-level prevalence of TF in children aged 1–9 years ranged from 4.7% (95% CI 3.4–6.3) to 7.2% (95% CI 5.8–8.9). Prevalence of C. trachomatis infection in children ranged from 0.1% (95% CI 0.0–0.6) to 0.7% (95% CI 0.3–1.3) while Pgp3 seroprevalence ranged from 6.9% (95% CI 5.4–8.6) to 12.0% (95% CI 10.1–14.0) and increased with age.
Conclusions/Significance
Based on current global policy, the prevalence of TF indicates that a further year of antibiotic MDA is warranted in four of six EUs yet the very low levels of infection cast doubt on the universal applicability of TF-based cut-offs for antibiotic MDA. Pgp3 seroprevalence was similar to that reported following MDA in other settings that have reached the elimination target however the predictive value of any particular level of seropositivity with respect to risk of subsequent infection recrudescence is, as yet, unknown.
Author summary
Trachoma, caused by ocular infection with the bacterium Chlamydia trachomatis, is treated at the population level with mass drug administration (MDA) of azithromycin, a broad-spectrum antibiotic. Decisions to stop MDA are subsequently made following impact surveys designed to determine the prevalence of trachoma in children aged 1–9 years. However, clinical signs of trachoma can persist in treated communities even in the absence of active C. trachomatis infection, potentially leading to continued MDA that may be unnecessary. In this study, we use the platform provided by six impact surveys to compare prevalence of clinical signs of trachoma in children with prevalence of C. trachomatis infection and anti-C. trachomatis antibodies. Based on current policy, prevalence of clinical signs of trachoma in four of six evaluation units surveyed indicated further MDA is warranted. However, tests for C. trachomatis DNA indicate extremely low levels of infection making the need for further MDA unclear. Prevalence of anti-C. trachomatis antibodies were similar to that found in other low prevalence settings, however the significance of such levels, and of levels of current C. trachomatis infection, with regard to the risk of reinfection are, as yet, unknown.
Introduction
Trachoma, caused by repeated infection with the obligate intracellular bacterium Chlamydia trachomatis, is a leading cause of preventable blindness. Elimination of trachoma as a public health problem can be achieved through implementation of the multi-faceted SAFE strategy: Surgery to correct trichiasis, Antibiotics to treat ocular C. trachomatis infection, and Facial cleanliness and Environmental improvement to reduce transmission [1–2].
The A, or antibiotic, component of SAFE is carried out at the district level through mass drug administration (MDA) of the macrolide antibiotic azithromycin. Prevalence of trachomatous inflammation—follicular (TF) in children aged 1–9 years currently guides MDA decision-making. One to five years of district-level MDA is undertaken wherever baseline prevalence of TF in children aged 1–9 years is ≥5%. The decision to stop MDA is again based on the prevalence of TF, determined through adequately powered impact surveys [3]. If the TF prevalence in children aged 1–9 years remains ≥5%, district-level MDA may continue.
The use of TF as the sole indicator to guide decisions on whether to continue or cease MDA following impact surveys may be problematic because low levels of TF can persist in the absence of ocular C. trachomatis infection [4]. Follicular conjunctivitis can be associated with non-chlamydial bacterial infection [5–6] or be of uncertain aetiology, even in apparently trachoma-endemic populations [7]. Furthermore, as active trachoma prevalence declines, it becomes increasingly difficult to train graders for surveys and to prove that graders have been trained well [8]. These issues have led to suggestions that alternate indicators for use in MDA decision-making and post-MDA surveillance be explored [9–10]. Possible alternatives include nucleic acid amplification-based tests for ocular C. trachomatis infection and assays for the presence of anti-C. trachomatis antibodies [11–12].
Malawi represents an ideal setting in which to assess the utility of alternate indicators for trachoma elimination programmes. Health officials have known Malawi to be endemic for trachoma since the 1980s [13] although an active control programme was not put in place until a decade ago. The Trachoma Action Plan was developed and launched by the Ministry of Health in 2011. The Global Trachoma Mapping Project completed mapping trachoma in Malawi in 2015 and showed, in combination with data collected previously, that 17 of 28 districts had TF prevalence between 10 and 25% [14–16]. Funding to implement the SAFE strategy in these 17 districts was made possible through the Queen Elizabeth Diamond Jubilee Trust in 2014, with the goal of eliminating trachoma by 2019. Several districts have since completed three rounds of MDA with high treatment coverage [17] and have proceeded to impact surveys, following WHO guidelines. To date, approximately half the population has received MDA and six thousand individuals have received surgery for trichiasis. The aim of the present study was to generate, in a programmatic setting, additional data on C. trachomatis infection and anti-Pgp3 antibodies as possible alternative indicators for making decisions as to whether or not to stop MDA.
Methods
Study site
The study was conducted in two districts: Chikwawa in southern Malawi (estimated population 565,000) [18] and Mchinji (est. pop. 600,000) [18] in central Malawi. Trachoma prevalence was previously assessed in both districts through surveys conducted in 2008 [14]; the prevalence of TF in children aged 1–9 years was 13.6% in Chikwawa and 21.7% in Mchinji. Prevalence of trichiasis in adults aged ≥15 years was 0.6% (Chikwawa) and 0.3% (Mchinji) [14]. Following the 2008 surveys, a trachoma elimination programme was devised and, as a part of that programme, both districts received azithromycin MDA at yearly intervals for three years (2011–2013).
Sampling strategy
For the purposes of the impact surveys, following WHO guidance, each district (Chikwawa and Mchinji) was divided into three evaluation units (EUs) [3]. To estimate a prevalence of TF, in children aged 1–9 years, of 10% in each EU, given a desired precision of ±3%, a 95% confidence limit and a design effect of 2.65, 1,019 children should be examined [8]. The average Malawian household was estimated to contain 4 to 5 people, of who 1–2 are in the 1–9 years age range. Therefore, to achieve the required sample size, taking into account non-response, 24 clusters (villages) were randomly selected in each EU and 30 households were randomly selected, from a list of households, in each cluster. Every consenting member of each selected household aged ≥1 year was examined.
Data collection
Data collection took place in June and July 2014. Prior to the onset of fieldwork, a workshop was held for trachoma graders (ophthalmic clinical officers) and enumerators. Training included quality control exercises of trachoma grading from slides in a classroom setting followed by practical tests in the field [19].
Questionnaires were used to capture demographic information and data on household-level access to water and sanitation. For the latter, questions were asked of the head of each selected household, and (where relevant) sanitation facilities observed. All household members, defined as those who slept in the household the night before, were eligible for trachoma screening. Consenting individuals were examined for clinical signs of trachoma using a 2.5× magnifying loupe and adequate sun- or torchlight. Trachoma grading was carried out according to the WHO simplified grading system [20].
Ocular swabs and dried blood spots (DBS) were collected from children aged 1–9 years. Ocular samples were collected using Dacron swabs (Puritan, Guilford, USA) passed across the upper left tarsal conjunctiva, after everting the eyelid. Field control swabs were also taken, once for every 50 swabs collected (with timing determined at random), by passing a clean swab in the air within two inches of a child’s eyes. All swabs were placed in sterile tubes and kept on cool packs in the field and frozen at -20°C within 10 hours of collection. Blood samples were collected by finger prick with a new, sterile lancet, and absorbed onto filter papers calibrated to absorb 10 μL of blood (TropBio, Townsville, Australia). These were then air-dried, sealed in individual plastic bags and placed in larger bags with desiccant before being stored at -20°C.
Laboratory methods
Swabs were tested for C. trachomatis DNA using the GeneXpert CT/NG assay (Cepheid Inc., Sunnyvale, CA, USA). Swabs were rehydrated in 800 μL nuclease-free water and vortexed vigorously. Two hundred μL of suspension from each of five swabs was then combined to generate a pool [21]. Three hundred μL of the pool was added to 900 μL of CT/NG transport media (Cepheid Inc.) and this total volume added to a GeneXpert CT/NG cartridge (Cepheid Inc.) and tested according to the manufacturer’s instructions. In the case of a positive or invalid result, all five samples making up the pool were then tested individually. Known positive and negative controls, supplied by the London School of Hygiene & Tropical Medicine (London, UK), were run every week during testing as a quality control measure.
Laboratory personnel were blind to the clinical status of subjects from whom samples were taken and were unable to distinguish between ocular swabs and swabs included as field controls.
DBS were tested, in duplicate, for antibodies against Pgp3 [22] according to the method previously described [11, 22]. Serum was eluted overnight then applied to Immulon 2HB microtiter plates (Southern Biotech, Birmingham, AL, USA) pre-sensitized with Pgp3 protein. Bound antibody was detected with HRP-labelled mouse anti-human IgG (Fc)-HRP (Southern Biotech). Plates were incubated, washed and 3,3’,5,5’-tetramethylbenzidine (KPL, Gaithersburg, MD, USA) was added before the reaction was stopped with 1N H2SO4. Absorbance was read at 450 nm (A450) using a HaloLED 96 Microplate Reader (Dynamica, London, UK). Readings were corrected for background by subtracting the average A450 of two blank wells containing no serum. Values were normalised by dividing the mean of values for the two wells against the mean for the two 200 U controls included on each plate [23].
Data entry and statistical analysis
All field data were collected using an Android smartphone app and uploaded to a password-protected cloud-based server. Laboratory data were captured electronically on the GeneXpert and HALO plate reader devices.
Data were analyzed using Stata version 14.2 (StataCorp LP, College Station, TX, USA). Prevalence of TF, C. trachomatis infection and antibodies to Pgp3 were calculated and displayed with exact binomial confidence intervals. Correlations between EU-summarised prevalence of TF, C. trachomatis infection and antibodies to Pgp3 were plotted with lines of best-fit and tested using Spearman’s rank correlation. Cluster-summarized prevalence of the three indicators was mapped using ArcMap v10.3 (Environmental Systems Research Institute, Inc. Redlands, CA, USA).
A finite mixture model was used to classify the samples as seropositive or seronegative based on normalized A450 values [22]. The cutoff for seropositivity was defined as the mean of the Gaussian distribution of the seronegative population plus three standard deviations of the seronegative population [22].
Seroconversion rates (SCR) were calculated by fitting a simple reversible catalytic model to the measured seroprevalence, stratified into yearly age-groups, using maximum likelihood methods, assuming no seroreversion [24].
Case management
All individuals with TF were provided with two tubes of 1% tetracycline ointment and instructions for twice-daily use for six weeks. Cases of trichiasis were referred for surgery.
Ethics statement
The study adhered to the tenets of the Declaration of Helsinki and was approved by Malawi’s Ministry of Health National Health Sciences Ethics and Research Committee. Written, informed consent was obtained from the parent or guardian of all children. In the case of adults whose eyes were examined but from whom no samples were taken, verbal consent was obtained and documented electronically. CDC staff did not have contact with study participants and were determined to be non-engaged in the study.
Results
Overall, 13,556 individuals, representing 94.3% of the censused population, participated in the study. Of these, 6,314 (43.9%) were children aged 1–9 years. Demographic characteristics of individuals and households are given in Table 1.
Table 1. Characteristics of study participants.
| Characteristic | No. (%) |
|---|---|
| Total censused population | 14,379 |
| Gender | |
| Male | 6,097 (42.4) |
| Female | 8,282 (57.6) |
| Age (years) | |
| 1–9 | 6,314 (43.9) |
| 10–14 | 1,452 (10.1) |
| ≥15 | 6,613 (46.0) |
| Total population examined | |
| Yes | 13,556 (94.3) |
| No–absent | 771 (5.4) |
| No–refused | 48 (0.3) |
| No–other | 4 (0.0) |
| Household characteristics | |
| Main source of drinking water | |
| Piped water into dwelling | 62 (0.4) |
| Piped water into yard | 165 (1.2) |
| Public tap | 1,400 (9.7) |
| Borehole | 9,920 (69.0) |
| Protected dug well | 552 (3.8) |
| Unprotected dug well | 1,698 (11.8) |
| Unprotected spring | 175 (1.2) |
| Surface water | 382 (2.7) |
| Other | 25 (0.2) |
| Habitual time to fetch water | |
| Water source in yard | 420 (2.9) |
| Less than 30 minutes | 10,081 (70.1) |
| 30 minutes to 1 hour | 3,232 (22.5) |
| More than 1 hour | 646 (4.5) |
| Access to latrine | |
| Shared or public latrine | 3,513 (24.4) |
| Private latrine | 9,686 (67.4) |
| No structure, outside house | 106 (0.7) |
| No structure, in bush | 1,066 (7.4) |
| Other | 8 (0.1) |
| Type of latrine (observed) | |
| Flush/pour | 86 (0.5) |
| Ventilated, improved pit latrine | 33 (0.2) |
| Pit latrine | 13,221 (91.9) |
| No facilities, bush or field | 1,036 (7.2) |
| Other | 23 (0.2) |
The majority of households reported accessing their drinking water at boreholes (69%) and 70% of households were reportedly within 30 minutes of their water source (Table 1). Access to sanitation was good, with over 90% of households reporting access to either shared or private latrines (Table 1).
District-level prevalence of trichiasis in participants 15 years of age and older was 0.43% in Chikwawa (13/2,999) and 0.12% (4/3,251) in Mchinji. There was no difference in the prevalence of TT amongst female (11/4,179; 0.26%) and male (6/2,071; 0.29%) participants.
Prevalence of TF amongst children aged 1–9 years ranged from 4.7% (95% CI 3.4–6.3) in the EU of Kasisi/DHO to 7.2% (95% CI 5.8–8.9) in the EU of Makanda Gumba (Table 2). Overall TF prevalence in children aged 1–9 years was 5.1% (95% CI 4.3–6.0) in Chikwawa district and 6.4% (95% CI 5.6–7.3) in Mchinji (Table 2).
Table 2. Prevalence of trachomatous inflammation—follicular (TF), C. trachomatis infection and anti-Pgp3 antibodies in participants aged 1–9 years.
| TF | Ocular Ct infection | Anti-Pgp3 antibodies | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | % | 95% CI1 | N | n | % | 95% CI1 | N | N | % | 95% CI1 | |
| Overall | 6,075 | 354 | 5.8 | 5.3–6.4 | 5,866 | 18 | 0.3 | 0.2–0.5 | 5,917 | 570 | 9.6 | 8.9–10.4 |
| EU | ||||||||||||
| Chapananga2 | 937 | 46 | 4.9 | 3.6–6.5 | 924 | 2 | 0.2 | 0.0–0.8 | 916 | 104 | 11.4 | 9.4–13.6 |
| Ngabu Ngokwe2 | 914 | 52 | 5.7 | 4.3–7.4 | 881 | 1 | 0.1 | 0.0–0.6 | 897 | 88 | 9.8 | 7.9–11.9 |
| Kasisi/DHO2 | 869 | 41 | 4.7 | 3.4–6.3 | 863 | 2 | 0.2 | 0.0–0.8 | 830 | 67 | 8.1 | 6.3–10.1 |
| Makanda Gumba3 | 1,120 | 81 | 7.2 | 5.8–8.9 | 1,085 | 7 | 0.7 | 0.3–1.3 | 1,117 | 134 | 12.0 | 10.1–14.0 |
| Luzi Kochilira3 | 1,183 | 77 | 6.5 | 5.2–8.1 | 1,119 | 3 | 0.3 | 0.1–0.8 | 1,139 | 107 | 9.4 | 7.8–11.2 |
| DHO Nkwazi3 | 1,052 | 57 | 5.4 | 4.1–7.0 | 994 | 3 | 0.3 | 0.1–0.9 | 1,018 | 70 | 6.9 | 5.4–8.6 |
| District | ||||||||||||
| Chikwawa | 2,720 | 139 | 5.1 | 4.3–6.0 | 2,668 | 5 | 0.2 | 0.1–0.4 | 2,643 | 259 | 9.8 | 8.7–11.0 |
| Mchinji | 3,355 | 215 | 6.4 | 5.6–7.3 | 3,198 | 13 | 0.4 | 0.2–0.7 | 3,274 | 311 | 9.5 | 8.5–10.6 |
| Gender | ||||||||||||
| Female | 3,151 | 192 | 6.1 | 5.3–7.0 | 3,033 | 12 | 0.4 | 0.2–0.7 | 3,076 | 293 | 9.5 | 8.5–10.6 |
| Male | 2,924 | 162 | 5.5 | 4.7–6.4 | 2,833 | 6 | 0.2 | 0.1–0.5 | 2,841 | 277 | 9.8 | 8.7–10.9 |
| Age (years) | ||||||||||||
| 1 | 650 | 37 | 5.7 | 4.0–7.8 | 626 | 2 | 0.3 | 0.0–1.1 | 637 | 24 | 3.8 | 2.4–5.6 |
| 2 | 910 | 85 | 9.3 | 7.5–11.4 | 852 | 2 | 0.2 | 0.0–0.8 | 882 | 39 | 4.4 | 3.2–6.0 |
| 3 | 800 | 55 | 6.9 | 5.2–8.9 | 765 | 2 | 0.3 | 0.0–0.9 | 780 | 56 | 7.2 | 5.5–9.2 |
| 4 | 738 | 53 | 7.2 | 5.4–9.3 | 710 | 1 | 0.1 | 0.0–0.8 | 716 | 48 | 6.7 | 5.0–8.8 |
| 5 | 724 | 39 | 5.4 | 3.9–7.3 | 703 | 2 | 0.3 | 0.0–1.0 | 708 | 64 | 9.0 | 7.0–11.4 |
| 6 | 603 | 43 | 7.1 | 5.2–9.5 | 589 | 2 | 0.3 | 0.0–1.2 | 591 | 73 | 12.4 | 9.8–15.3 |
| 7 | 596 | 20 | 3.4 | 2.0–5.1 | 585 | 4 | 0.7 | 0.2–1.7 | 581 | 76 | 13.1 | 10.4–16.1 |
| 8 | 453 | 13 | 2.9 | 1.5–4.9 | 442 | 2 | 0.5 | 0.1–1.6 | 436 | 73 | 16.7 | 13.4–20.6 |
| 9 | 601 | 9 | 1.5 | 0.7–2.8 | 594 | 1 | 0.2 | 0.0–0.9 | 586 | 117 | 20.0 | 16.8–23.4 |
1Exact binomial confidence interval
2EU in Chikwawa
3EU in Mchinji
Ocular swabs were collected from 6,075 children. Of these, 185 ocular swabs gave invalid results, indicating they did not contain detectable amounts of human DNA. Swabs from 24 children were missing. A positive or negative PCR result was obtained for the remaining 5,866 swabs (96.6% of the total). The prevalence of C. trachomatis infection was low, ranging from 0.1% (95% CI 0.0–0.6) in the EU of Ngabu Ngokwe to 0.7% in Makanda Gumba (95% CI 0.3–1.3) (Table 2). District-level prevalence of infection was 0.2% (95% CI 0.1–0.4) in Chikwawa and 0.4% (95% CI 0.2–0.7) in Mchinji (Table 2). Of the 264 negative field controls tested, none gave a positive result for C. trachomatis DNA.
Pgp3 ELISA results were obtained for 97.4% of recruited children (5,917/6,075) aged 1–9 years. The mean absorbance value was 0.3581 (standard deviation 0.2765). Seroprevalence was lowest in DHO Nkwazi [6.9% (95% CI 5.4–8.6)] and highest in Makanda Gumba [12.0% (95% CI 10.1–14.0)] and increased with age. The lowest seroprevalence levels were found in children aged 1 year [3.8%, (95% CI 2.4–5.6)] while the highest levels were in children aged 9 years [20.0%, (95% CI 16.8–23.4)] (Table 2). Seroprevalence was similar in both districts; 9.8% (95% CI 8.7–11.0) in Chikwawa and 9.5% (95% CI 8.5–10.6) in Mchinji (Table 2). The seroconversion rate (SCR) was 0.0283 (95% CI: 0.0275–0.029) per year for Chikwawa and 0.021 (95% CI: 0.020–0.021) for Mchinji.
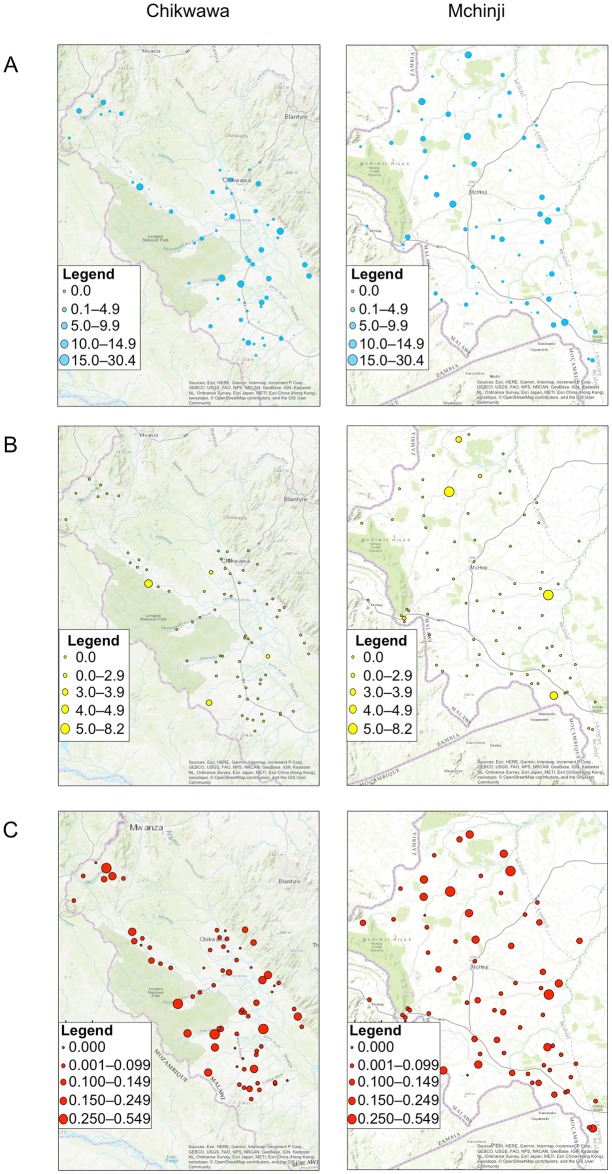
Geographical distributions of TF, C. trachomatis infection and prevalence of anti-Pgp3 antibodies in children aged 1–9 years are shown in Fig 1. The highest prevalence of all three indicators were found in the EU of Makanda Gumba (Table 2).
Fig 1. Mapping of indicators of trachoma in Chikwawa and Mchinji.
Cluster level prevalence of (A) TF, (B) C. trachomatis infection and (C) anti-Pgp3 antibodies.
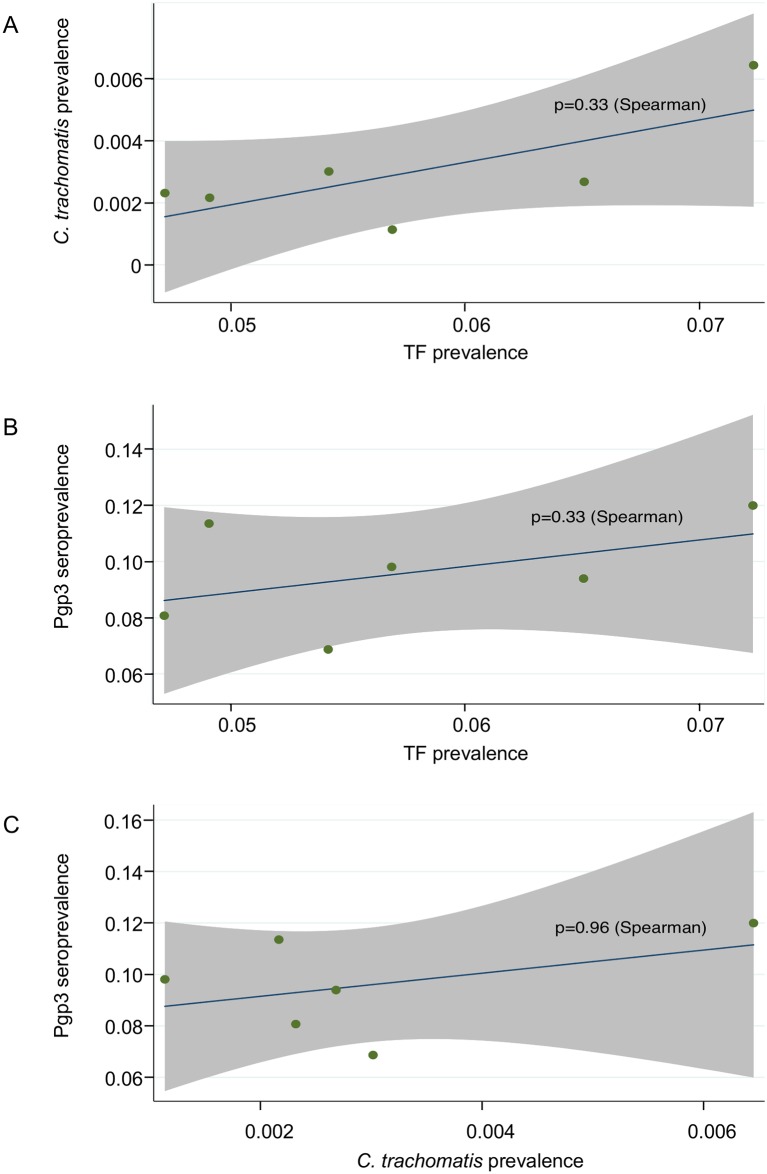
No association was seen between any of EU-summarised prevalence of TF and C. trachomatis infection (Fig 2A), EU-summarised prevalence of TF and anti-Pgp3 antibodies and EU-summarised prevalence of C. trachomatis infection and anti-Pgp3 antibodies (Fig 2C).
Fig 2. Correlations between EU-summarised prevalence of TF, C. trachomatis infection and anti-Pgp3 antibodies.
(A) Correlation of EU-summarised prevalence of TF and C. trachomatis infection. (B) Correlation of EU-summarised prevalence of TF and anti-Pgp3 antibodies. (C) Correlation of EU-summarised prevalence of C. trachomatis infection and anti-Pgp3 antibodies. EUs are represented by green dots. Lines of best fit are shown in blue. Shaded areas denote 95% confidence intervals.
Discussion
Our data show that prevalence of TF one year following the completion of three annual rounds of MDA was low in all six EUs surveyed. Two EUs have reached the elimination TF prevalence threshold of less than 5% in children aged 1–9 years [25] while the remaining four EUs are nearing this target. The prevalence of ocular C. trachomatis infection in the same children was 0.3%, considerably lower than that of active disease. The persistence of low-level rates of TF in communities where ocular infection is rare has previously been clearly documented in trachoma-endemic populations approaching elimination [4] and has been responsible, in part, for prompting the discussion of whether TF is the most appropriate marker on which to base decisions to stop MDA.
According to current guidelines, antibiotic MDA should be stopped only when impact surveys in EUs (population 100,000–250,000 people) indicate the prevalence of TF in children aged 1–9 years is below 5% [25]. The present results therefore indicate that another year of antibiotic MDA is warranted in four of the six EUs before re-survey. Yet, when viewed alongside PCR data that indicate a virtual absence of C. trachomatis infection [prevalence 0.3% (95% CI 0.2–0.5)], doubt is cast on the need for further antibiotic use. There is, however, no current recommendation for programmatic application of data on infection and PCR testing remains expensive. Serology, which can be conducted at much lower cost and multiplexed for integrated surveillance of multiple infectious diseases [10], may prove a viable alternate indicator for stopping MDA and undertaking post-MDA surveillance.
Overall seroprevalence in the current study (9.6%) was somewhat higher than prevalence of TF (5.8%), which is consistent with the notion that anti-Pgp3 antibody responses are markers of cumulative exposure rather than of persistent infection [26–27]. Furthermore, there was an increase in seropositivity with age seen in the current study, which also reflects cumulative exposure.
The cut-off for determining seropositivity with the ELISA assay used in this study was calculated using the finite mixture model, following the approach recommended by Migchelsen et al. [22]. This approach may over-estimate prevalence relative to other methods, such as the receiver operating characteristic (ROC) curves [22–23] used elsewhere [28–30]. Comparison to other studies is further complicated by the varying means of detecting Pgp3 antibodies. Many studies have used bead-based immunoassays [12, 26–27, 30–31], which are more expensive and technically challenging than the ELISA-based method employed here. However, a direct comparison of the two assays (EISA versus bead-based immunoassay) in a pre-validation survey in Ghana showed similar population-level prevalence [5.5% (95% CI: 4.8–6.3) for ELISA; 4.3% (95% CI: 3.7–4.9) for bead-based assay] [32] with calculated SCRs being virtually identical [0.013 (95% CI: 0.011–0.016) for ELISA; 0.012 (95% CI: 0.009–0.016) for bead-based assay). While further direct comparisons of platforms in large-scale surveys are needed, initial studies suggest good agreement of data from different assay platforms, especially if the SCR is the output of greatest interest.
Before serology could be adopted as an alternative or adjunct to TF prevalence for stopping MDA and post-MDA decision-making, seropositivity thresholds would need to be established. One approach might be to focus on the youngest children–those born during MDA or after its cessation and presumably very infrequently exposed to ocular C. trachomatis infection. Because the current survey was conducted in the year following completion of the third round of MDA and because children under 1 year of age, who might have had transplacentally-transferred maternal antibodies, were not eligible for inclusion, we do not have data from children born after the cessation of MDA. The youngest children in this study (aged 1–3 years) were born during the three-year MDA campaign and had similar seroprevalence to 1–3-year-olds in elimination settings; in post-validation settings, 1–3-year-olds are reported to have a prevalence of anti-Pgp3 antibodies of <6%, as measured primarily by bead-based assays [12, 32–34].
A second approach would be to look at overall seroprevalence in 1–9-year-olds. While the prevalence of anti-Pgp3 antibodies here was much lower than that in populations that have high transmission rates [27–28], the antibody prevalence was higher than in communities where longitudinal follow-up and tests for infection have documented sustained elimination of ocular C. trachomatis infection [12, 29–30]. For example, a study conducted 10 years after cessation of antibiotic MDA in Rombo district of Tanzania found just 3.5% (95% CI: 1.4–7.1) of 1–9-year-olds to have anti-Pgp3 antibodies [12], less than the 9.6% seropositivity (95% CI: 8.9–10.4) seen in the children in the current study. In The Gambia, 4.2% (95% CI: 2.9–5.9) of children aged 1–9 years were positive for Pgp3 antibodies 5 years after MDA [29], while in Nepal 3.4% (95% CI: 1.4–6.9) of children aged 9 years were seropositive 4 years following cessation of MDA [30].
A third approach would be to look at trends in antibody responses with age. The SCR estimated for the two districts here [0.0283 (95% CI: 0.0275–0.029) per year for Chikwawa and 0.021 (95% CI: 0.020–0.021) for Mchinji] were higher than that predicted to be associated with <5% TF [SCR of 0.015 (95% CI: 0.0–049)] [35], but the confidence intervals were overlapping. An algorithm combining these two metrics–seroprevalence and SCR–may have utility as well, particularly in settings like the Malawi districts studied here, where SCR estimates are close to but not below predictions for TF<5%. While it is unclear that this level of seropositivity is definitively associated with local elimination, the current data add to the evidence base that will be needed to determine appropriate thresholds for potential use of serology for surveillance.
Supporting information
(CSV)
(CSV)
(DOC)
Acknowledgments
We thank the community ophthalmic clinical officers and field workers involved in data collection; the community leaders and villagers for their participation in the study; Godwin Tembo, who assisted with the GeneXpert assay; and Pat Lammie, who provided valuable critical review of the manuscript.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Data Availability
Relevant data are within the manuscript and its Supporting Information files. Household GPS coordinates have been removed from these data to protect the identify of study participants.
Funding Statement
This work received financial support from the Wellcome Trust (Grant number 098521) and the Coalition for Operational Research on Neglected Tropical Diseases, which is funded at The Task Force for Global Health primarily by the Bill & Melinda Gates Foundation, by the United States Agency for International Development through its Neglected Tropical Diseases Program and with UK aid from the British people. Use of the GeneXpert was made possible with a loan from the International Trachoma Initiative. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Francis, V, Turner, V, World Health Organization Programme for the Prevention of Blindness. Achieving community support for trachoma control: a guide for district health work. World Health Organization. 1995; WHO/PBL/93.36. http://www.who.int/iris/handle/10665/59567
- 2.Ngondi J, Gebre T, Shargie EB, Adamu L, Ejigsemahu Y, Teferi T, et al. Evaluation of three years of the SAFE strategy (Surgery, Antibiotics, Facial cleanliness and Environmental improvement) for trachoma control in five districts of Ethiopia hyperendemic for trachoma. Trans R Soc Trop Med Hyg. 2009; 103(10): 1001–1010. 10.1016/j.trstmh.2008.11.023 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization, Strategic and Technical Advisory Group on Neglected Tropical Diseases. Technical consultation on trachoma surveillance. September 11−12, 2014, Task Force for Global Health, Decatur, USA. World Health Organization. 2015; WHO/HTM/NTD/2015.02. http://www.who.int/iris/handle/10665/174085
- 4.Ramadhani AM, Derrick T, Macleod D, Holland MJ and Burton MJ. The relationship between active trachoma and ocular Chlamydia trachomatis infection before and after mass antibiotic treatment. PLoS Negl Trop Dis. 2016;10(10): e0005080 10.1371/journal.pntd.0005080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton MJ, Hu VH, Massae P, Burr SE, Cheallier C, Afwamba IA, et al. What is causing active trachoma? The role of non-chlamydial bacterial pathogens in a low prevalence setting. Invest Ophthalmol Vis Sci. 2011;52(8): 6012–6017. 10.1167/iovs.11-7326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burr SE, Hart J, Edwards T, Baldeh I, Bojang E, Harding-Esch EM, et al. Association between ocular bacterial carriage and follicular trachoma following mass azithromycin distribution in The Gambia. PLoS Negl Trop Dis. 2013; 7(7): e2347 10.1371/journal.pntd.0002347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butcher RMR, Sokana O, Jack K, Kalae E, Sui L, Russell C, et al. Active trachoma cases in the Solomon Islands have varied polymicrobial community structures but do not associate with individual non-chlamydial pathogens of the eye. Front Med. 2018;4: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon AW, Pavluck A, Courtright P, Aboe A, Adamu L, Alemayehu W, et al. The Global Trachoma Mapping Project: methodology of a 34-country population-based study. Ophthalmic Epidemiol. 2015;22(3): 214–25. 10.3109/09286586.2015.1037401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solomon AW, Foster A, Mabey DC. Clinical examination versus Chlamydia trachomatis assays to guide antibiotic use in trachoma control programmes. Lancet Infect Dis. 2006;6(1): 5–6; author reply 7–8. 10.1016/S1473-3099(05)70304-2 [DOI] [PubMed] [Google Scholar]
- 10.Solomon AW, Engels D, Bailey RL, Blake IM, Brooker S, Chen JX, et al. A diagnostics platform for the integrated mapping, monitoring, and surveillance of neglected tropical diseases: rationale and target product profiles. PLoS Negl Trop Dis. 2012;6(7): e1746 10.1371/journal.pntd.0001746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodhew EB, Priest JW, Moss DM, Zhong G, Munoz B, Mkocha H, et al. CT694 and pgp3 as serological tools for monitoring trachoma programs. PLoS Negl Trop Dis. 2012;6(11): e1873 10.1371/journal.pntd.0001873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin DL, Bid R, Sandi F, Goodhew EB, Massae PA, Lasway A, et al. Serology for trachoma surveillance after cessation of mass drug administration. PLoS Negl Trop Dis. 2015;9(2): e3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tielsch JM, West KP, Katz J, Keyvan-Larijani E, Tizazu T, Schwab L, et al. The epidemiology of trachoma in southern Malawi. Am J Trop Med Hyg. 1988;38: 393–399. 10.4269/ajtmh.1988.38.393 [DOI] [PubMed] [Google Scholar]
- 14.Kalua K, Chirwa T, Kalilani L, Abbenyi S, Mukaka M, Bailey R. Prevalence and risk factors for trachoma in central and southern Malawi. PloS One. 2010;5(2): e9067 10.1371/journal.pone.0009067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalua K, Phiri M, Kumwenda I, Masika M, Pavluck AL, Willis R, et al. Baseline Trachoma Mapping in Malawi with the Global Trachoma Mapping Project (GTMP). Ophthalmic Epidemiol. 2015;22(3): 176–183. 10.3109/09286586.2015.1035793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalua K, Chisambi A, Chinyanya D, Kamwendo Z, Masika M, Willis R, et al. Completion of Baseline Trachoma Mapping in Malawi: Results of Eight Population-Based Prevalence Surveys Conducted with the Global Trachoma Mapping Project. Ophthalmic Epidemiol. 2016;23(sup1): 32–38. 10.1080/09286586.2016.1230224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalua K, Balakasi S, Chisambi A, Kanjado T, Masika M, Singano R, et al. Report of the 2015 Malawi trachoma mass drug administration (MDA) coverage survey in 9 districts. J Ophthalmol Vis Neurosci. 2016;1(3): 1–4. [Google Scholar]
- 18.National Statistical Office. 2018 Malawi population and housing census preliminary report. Government of Malawi. 2018. https://malawi.unfpa.org/sites/default/files/resource-pdf/2018%20Census%20Preliminary%20Report.pdf
- 19.Courtright P, MacArthur C, Macleod C, Dejene M, Gass K, Lewallen S, et al. Tropical data: training system for trachoma prevalence surveys (version 1). International Coalition for Trachoma Control. 2016. http://tropicaldata.knowledgeowl.com/help/training-system-for-trachoma-prevalence-surveys
- 20.Thylefors B, Dawson CR, Jones BR, West SK, Taylor HR. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65: 477–483. [PMC free article] [PubMed] [Google Scholar]
- 21.Diamant J, Benis R, Schachter J, Moncada J, Pang F, Jha HC, et al. Pooling of Chlamydia laboratory tests to determine the prevalence of ocular Chlamydia trachomatis infection. Ophthalmic Epidemiol. 2001;8(2–3): 109–117. [DOI] [PubMed] [Google Scholar]
- 22.Migchelsen SJ, Martin DL, Southisombath K, Turyaguma P, Heggen A, Rubangakene PP, et al. Defining seropositivity thresholds for use in trachoma elimination studies. PLoS Negl Trop Dis. 2017;11(1): e0005230 10.1371/journal.pntd.0005230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gwyn S, Cooley G, Goodhew B, Kohlhoff S, Banniettis N, Wiegand R, et al. Comparison of platforms for testing antibody responses against the Chlamydia trachomatis antigen Pgp3. Am J Trop Med Hyg. 2017;97(6): 1662–1668. 10.4269/ajtmh.17-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonal SL, Carneiro I, et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102(14): 5108–5113. 10.1073/pnas.0408725102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. Validation of elimination of trachoma as a public health problem. World Health Organization. 2016; WHO/HTM/NTD/2016.8. http://www.who.int/iris/handle/10665/208901
- 26.West SK, Munoz B, Weaver J, Mrango Z, Dize L, Gaydos C, et al. Can we use antibodies to Chlamydia trachomatis as a surveillance tool for national trachoma control programs? Results from a district survey. PLoS Negl Trop Dis. 2016;10(1): e0004352 10.1371/journal.pntd.0004352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodhew EB, Morgan SM, Switzer AJ, Munoz B, Dize L, Gaydos C, et al. Longitudinal analysis of antibody responses to trachoma antigens before and after mass drug administration. BMC Infect Dis. 2014;14: 216 10.1186/1471-2334-14-216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cama A, Müller A, Taoaba R, Butcher RMR, Itibita I, Migchelsen SJ, et al. Prevalence of signs of trachoma, ocular Chlamydia trachomatis infection and antibodies to Pgp3 in residents of Kiritimati Island, Kiribati. PLoS Negl Trop Dis. 2017;11(9): e0005863 10.1371/journal.pntd.0005863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migchelsen SJ, Sepúlveda N, Martin DL, Cooley G, Gwyn S, Pickering H, et al. Serology reflects a decline in the prevalence of trachoma in two regions of The Gambia. Sci Rep. 2017;7(1): 15040 10.1038/s41598-017-15056-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zambrano AI, Sharma S, Crowley K, Dize L, Muñoz BE, Mishra SK, et al. The World Health Organization recommendations for trachoma surveillance, experience in Nepal and added benefit of testing for antibodies to Chlamydia trachomatis pgp3 protein: NESTS Study. PLoS Negl Trop Dis. 2016;10(9): e0005003 10.1371/journal.pntd.0005003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pant BP, Bhatta RC, Chaudhary JS, Awasthi S, Mishra S, Sharma S, et al. Control of trachoma from Achham district, Nepal: a cross-sectional study form the Nepal National Trachoma Program. PLoS Negl Trop Dis. 2016;10(2): e0004462 10.1371/journal.pntd.0004462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Senyonjo LG, Debrah O, Martin DL, Asante-Poku A, Migchelsen SJ, Gwyn S, et al. Serological and PCR-based markers of ocular Chlamydia trachomatis transmission in northern Ghana after elimination of trachoma as a public health problem. PLoS Negl Trop Dis. 2018;12(12): e0007027 10.1371/journal.pntd.0007027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gwyn SE, Xiang L, Kandel RP, Dean D, Gambhir M, Martin DL. Prevalence of Chlamydia trachomatis-Specific Antibodies before and after Mass Drug Administration for Trachoma in Community-Wide Surveys of Four Communities in Nepal. Am J Trop Med Hyg. 2018;98(1): 216–220. 10.4269/ajtmh.17-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West SK, Zambrano AI, Sharma S, Mishra SK, Muñoz BE, Dize L, et al. Surveillance Surveys for Reemergent Trachoma in Formerly Endemic Districts in Nepal From 2 to 10 Years After Mass Drug Administration Cessation. JAMA Opthalmol. 2017:135(11): 1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinsent A, Solomon AW, Bailey RL, Bid R, Cama A, Dean D, et al. The utility of serology for elimination surveillance of trachoma. Nat Commun. 2018; 9: 5444 10.1038/s41467-018-07852-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
(CSV)
(DOC)
Data Availability Statement
Relevant data are within the manuscript and its Supporting Information files. Household GPS coordinates have been removed from these data to protect the identify of study participants.