Abstract
Background
Integrating primary care has been proposed to reduce fragmented care delivery for patients with complex medical needs. Because of their high rates of morbidity, healthcare use, and mortality, patients with end-stage kidney disease (ESKD) may benefit from increased access to a primary care medical home.
Objective
To evaluate the effect of integrating a primary care medical home on health-related quality of life (HRQOL) for patients with ESKD receiving chronic hemodialysis.
Design
Before–after intervention trial with repeated measures at two Chicago dialysis centers.
Participants
Patients receiving hemodialysis at either of the two centers.
Intervention
To the standard hemodialysis team (nephrologist, nurse, social worker, dietitian), we added a primary care physician, a pharmacist, a nurse coordinator, and a community health worker. The intervention took place from January 2015 through August 2016.
Main Measures
Health-related quality of life, using the Kidney Disease Quality of Life (KDQOL) measures.
Key Results
Of 247 eligible patients, 175 (71%) consented and participated; mean age was 54 years; 55% were men and 97% were African American or Hispanic. In regression analysis adjusted for individual visits with the medical home providers and other factors, there were significant improvements in four of five KDQOL domains: at 12 and 18 months, the Mental Component Score improved from baseline (adjusted mean 49.0) by 2.64 (p = 0.01) and 2.96 (p = 0.007) points, respectively. At 6 and 12 months, the Symptoms domain improved from baseline (adjusted mean = 77.0) by 2.61 (p = 0.02) and 2.35 points (p = 0.05) respectively. The Kidney Disease Effects domain improved from baseline (adjusted mean = 72.7), to 6, 12, and 18 months by 4.36 (p = 0.003), 6.95 (p < 0.0001), and 4.14 (p = 0.02) points respectively. The Physical Component Score improved at 6 months only.
Conclusions
Integrating primary care and enhancing care coordination in two dialysis facilities was associated with improvements in HRQOL among patients with ESKD who required chronic hemodialysis.
Electronic supplementary material
The online version of this article (10.1007/s11606-019-05154-9) contains supplementary material, which is available to authorized users.
KEY WORDS: medical home, primary care, hemodialysis, quality of life, kidney disease
INTRODUCTION
In 2015, nearly 450,000 Americans had end-stage kidney disease (ESKD) and were receiving chronic hemodialysis as their renal replacement therapy to sustain their lives1. Even with maintenance hemodialysis treatment, ESKD patients experience substantial morbidity, mortality, hospitalizations, and healthcare costs2–4. While survival for dialysis patients has improved, the high burden imposed by thrice-weekly hemodialysis treatments often leads to insufficient attention to other comorbid conditions, resulting in higher rates of complications, reduced health-related quality of life (HRQOL), and potentially unnecessary healthcare use4, 5. Numerous studies have demonstrated that decreases in HRQOL among ESKD patients are associated with hospitalization and mortality rates6–10.
Currently, the Centers for Medicare and Medicaid Services (CMS) sets the requirements for the dialysis care team for facility payments, including annual assessments of HRQOL11. The CMS-mandated team for hemodialysis and peritoneal dialysis comprises a nephrologist, a nurse, a social worker, and a dietitian11. With greater emphasis on improving HRQOL among ESKD12 and recognition of the need for enhanced care coordination and primary care for patients with chronic diseases13, 14, this care model may be inadequate. The current dialysis care team lacks integration with primary care5, 15 and does not include other professionals who have been recognized to improve care for other chronic illnesses16–19. Disease-oriented studies in ESKD focused on using intense and frequent dialysis have reported disease-related benefits, such as reducing left ventricular mass and hypertension, yet have not had a significant effect on dialysis patient HRQOL20, 21.
Recent attempts to address care gaps have focused on implementation of the Patient-Centered Medical Home (PCMH) model, variations of which have been implemented for patients with chronic complex illnesses such as diabetes. Although findings have been mixed, some studies show reduced hospitalizations, emergency room visits, and healthcare costs14–19, 22–24. A systematic review of integrated care models noted mixed results among a broad range of chronic conditions for quality of life outcomes, although none addressed ESKD25. Among patients with chronic kidney disease not yet requiring dialysis, the use of a multidisciplinary care team reduced the rate of kidney function decline15. A formal evaluation of a PCMH or an integrated care model in chronic hemodialysis patients has not been conducted.
Described previously26–30, our study is the first systematic design, implementation, and evaluation of an adaptation of a PCMH model for chronic hemodialysis patients. We sought to integrate PCMH with the current dialysis-mandated team by adding a general internist serving as the primary care physician (PCP), nurse coordinator, pharmacist, and community health worker (CHW). We hypothesized that this increased access to primary care would improve patient HRQOL and address unmet needs, controlling for other factors found in prior research and chronic disease models31, 32.
METHODS
Study Design
The study has been described previously26. Briefly, we used a before–after design to evaluate a PCMH-KD model of care at two dialysis centers with rolling enrollment. Comparisons were within patients over time and thus the baseline assessment served as the before measure under the current CMS-mandated dialysis care model. The start-up phase (year 1) was used for stakeholder engagement and training of all participating clinicians and staff. The 18-month intervention began in the second year. All research procedures were reviewed and approved by the University of Illinois at Chicago Office of Protection of Research Subjects.
Study Setting and Intervention
Study sites comprised two dialysis centers affiliated with one academically based nephrology group in Chicago. One site was a non-profit, university-affiliated outpatient dialysis center (University of Illinois at Chicago), and the second was a for-profit, free-standing outpatient dialysis center owned and operated by Fresenius Kidney Care (Private). Eight nephrologists from the university-affiliated medical center served both sites; dialysis center staff (e.g., nurses, dieticians, social workers) were unique to each center. Capacity at the two sites for hemodialysis was 200 patients, with turnover of about 25% per year.
The intervention included the addition of new care team members to the dialysis care teams. The CMS-required members, the new team members, and their respective roles are summarized in Table 1. The study PCPs and CHWs conducted individual patient visits in addition to participation in the weekly nephrologist-led dialysis chairside rounds, while the nurse coordinator and pharmacist roles focused on coordinating patient information and providing education during weekly nephrologist-led rounds.
Table 1.
Roles of the Care Providers in the Usual Care and PCMH-KD Model
| Team member | Qualifications | Duties |
| Nephrologist | Board certified or eligible in nephrology | Manage dialysis therapy, medications, diet and fluid regimen, and care plans; facilitate transplant |
| Dialysis nurse manager | RN with significant experience in dialysis and facility management | Oversee nursing services and all direct care staff that provide dialysis and nursing care |
| Dialysis nurse | RN with training and/or experience with dialysis | Provide dialysis treatment; supervise dialysis technicians; contribute to care plans |
| Dialysis technician | High school diploma or equivalent and certification in dialysis | Provide dialysis treatment |
| Social worker | Masters degree in social work | Support social function and adjustment of patient; provide casework services; identify community social agencies and other resources; psychosocial evaluation and support; contribute to care plans |
| Dietitian | Degree in food and nutrition | Assess dietary needs; recommend dietary changes including sodium and fluid intake; refer patients to community resources; contribute to care plan |
| New Members Added with the PCMH-KD | ||
| General internist/primary care physician (PCP) | Board certified or eligible in internal medicine; training and/or experience with end stage kidney disease and dialysis | Primary care for comorbid conditions; preventive care, including age-appropriate cancer screening; coordinate subspecialty careParticipated in dialysis chairside weekly rounds and conducted individual visits |
| Nurse coordinator | Masters- or BSN-level nurse; training and/or experience with end-stage kidney disease and dialysis |
Care coordination; monitor episodic inpatient care; deliver patient education; coordinate with surgery and radiology for assessment, planning, and completion of vascular access procedures; monitor vascular access sites Participated in dialysis chairside rounds monthly |
| Pharmacist | PharmD and RPh |
Medication assessment, dosing, and safety monitoring; support medication compliance; immunizations; identify community resources for medication delivery Participated in dialysis chairside weekly rounds |
| Community health workers (CHW) | Bilingual in English and Spanish (preferred); trained in medical terminology |
Liaison between community, patient/family, and care team; bridge barriers of acculturation, language, and literacy; coordinate scheduling for transportation and other support to enable patient compliance Participated in dialysis chairside weekly rounds and conducted individual visits |
Study Population
Patient eligibility criteria required participants to be fluent in English or Spanish language, currently receiving maintenance hemodialysis at one of the two participating dialysis centers, 18 years of age or older, and able to provide informed consent for participation in the study. Patients who left the participating dialysis center or who received a kidney transplant were no longer able to continue in the study.
Patient Recruitment
Informational sessions about the study were held at each site and enrollment lasted twelve months. Patients who provided informed consent and completed baseline assessment were offered the additional services of the PCMH-KD team. Patients were initially compensated for their participation at $10 (cash) per interview and then increased to $20 per interview during the last four months of the study.
Data Collection and Measures
Briefly, demographics, medical history, social characteristics, and HRQOL were part of the initial intake26. Interviews were conducted by trained interviewers at baseline, 6, 12, and 18 months. Each interview was about 60–90 min and was conducted in either English or Spanish as per patient preference. Interviews took place in the dialysis center before, during, or after a patient’s dialysis appointment and were recorded via live web-based data entry on an Apple iPad 2 tablet using Research Electronic Data Capture (REDCap)33 hosted at the University.
Clinical measures included routine laboratory measurements already obtained for chronic hemodialysis care (anemia management (serum hemoglobin), nutrition status (serum albumin), urea reduction ratio (URR)), from dialysis records.
Patient individual visits with the study CHWs and PCPs were monitored and tracked. For the CHW visits, information about the visit purpose was documented. For the PCP visits, it was noted whether the visit was at the dialysis chairside or in an exam room. The nurse and pharmacist engaged patients on the nephrologist-led rounds; they did not have scheduled individual patient visits.
Outcome Measures: Patient-Reported Health-Related Quality of Life
To assess HRQOL, we used the Kidney Disease Quality of Life (KDQOL-SF36) questionnaire34, 35. The five domains of KDQOL-36 include physical component summary (PCS) and mental component summary (MCS), derived from the Medical Outcomes Short Form 12, and the three additional domains, i.e., burden of kidney disease (Burden), symptoms and problems of kidney disease (Symptoms), and effects of kidney disease (Effects)35.
Statistical Analysis
Sample Size Calculations
We calculated power assuming a KDQOL MCS averaging 48.6 (SD 11.3) from a prior study8. Based on our plan to compare KDQOL scores after exposure to the intervention with a baseline score assuming clustering and with the expectation to improve the score by 10%, correcting for a 10% patient loss36–38, we calculated that a minimum sample size of 150 was needed to detect 0.80 power at the α = 0.05 two-sided test.
Analysis
Descriptive analyses comprised simple means and standard deviations (SD) (continuous) and frequencies (categorical). SAS version 9.4 was used for all analyses39.
We examined change in KDQOL over time (baseline (0), 6, 12, and 18 months), using random-intercept mixed models with an AR (1) covariance pattern in the residual, with and without adjustment for selected covariates: demographics (baseline age, sex, race (African American or other), interview language, education (high school (HS) graduate or not), marital status (married or living with partner, other); clinical characteristics (dialysis vintage (months), self-reported diabetes at baseline, time-varying hemodialysis-relevant laboratory values (hemoglobin, albumin, and urea reduction ratio (URR)); and dialysis center (university or private). To assess whether components of the intervention influenced the outcome, we included in the model whether the patient had a PCP at baseline, visits with study PCPs (any/none), and follow-up visits with CHWs (above or below the median of 6 visits). We used SAS LSMESTIMATE statements to estimate adjusted KDQOL means at each visit.
RESULTS
Study Participants
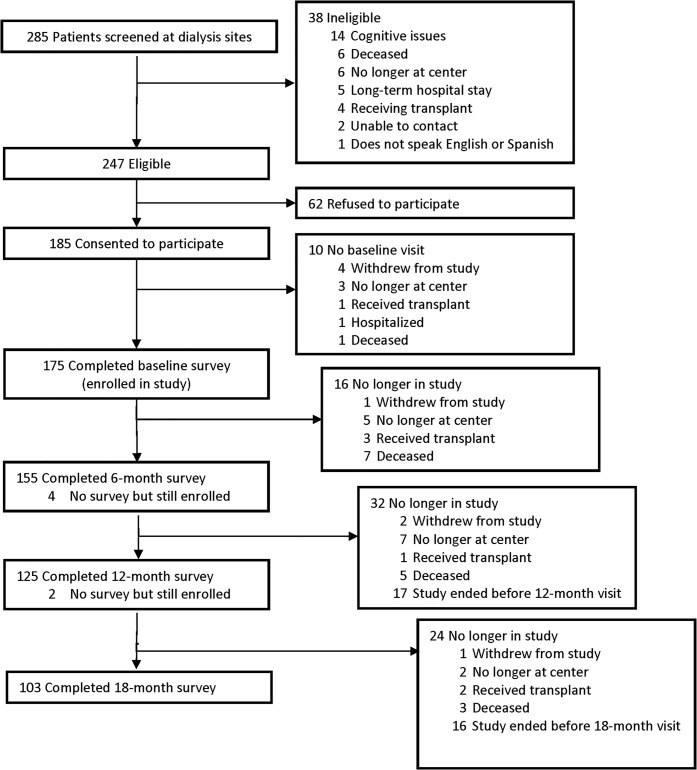
There were 285 patients who received care at the two dialysis centers and were screened for eligibility over the 12-month enrollment period (Fig. 1). Of these patients, 247 (87%) were determined to be eligible to participate in the study; 185 (75% of those eligible) consented to participate; ultimately, 175 (71% of those eligible) completed the baseline assessment and continued in the study; 155 completed the 6-month assessment; 125 completed the 12-month assessment, and 103 completed the 18-month assessment.
Figure 1.
PCMH-KD participant recruitment and enrollment.
Participant Characteristics
Table 2 shows the characteristics of patients at both sites. Patients’ mean age was 54.4 years, and a majority were men (55%). Participants were nearly all African American and Hispanic (97%). One third of our subjects were interviewed in Spanish. Most had at least a high school education (65%). A large majority were not employed (82%). Income levels were low, with 68% reporting incomes less than $20,000 per year. Patients used a variety of transportation means to reach their dialysis treatments, including a personal car driven by themselves or someone else (50%) or a transport service (34%). Many patients reported a stressful life event in the past 6 months (45%). Participants’ health insurance was predominantly covered by Medicare or Medicaid; only 11% reported some private insurance coverage that is not a Medicare supplement. More than half (60%) of the patients reported at least two comorbidities.
Table 2.
Participant Characteristics by Site, Baseline
| University site (N = 109) | Private site (N = 66) | All (N = 175) | |
|---|---|---|---|
| Age in years, mean (SD) | 52.3 (15.7) | 57.8 (13.1) | 54.4 (15.0) |
| Sex, n (%) | |||
| Female | 52 (48%) | 26 (39%) | 78 (45%) |
| Male | 57 (52%) | 40 (61%) | 97 (55%) |
| Race/ethnicity, n (%) | |||
| African American | 59 (54%) | 27 (41%) | 86 (49%) |
| Hispanic | 45 (41%) | 38 (58%) | 83 (47%) |
| White or other | ≤ 11* | ≤ 11* | ≤ 11* |
| Interview language, n (%) | |||
| English | 84 (77%) | 33 (50%) | 117 (67%) |
| Spanish | 25 (23%) | 33 (50%) | 58 (33%) |
| Education, n (%) | |||
| Not HS graduate | 26 (24%) | 36 (55%) | 62 (35%) |
| HS graduate/GED | 72 (66%) | 28 (42%) | 100 (57%) |
| Bachelor’s degree | ≤ 11* | ≤ 11* | 13 (7%) |
| Marital status, n (%) | |||
| Single, never married | 51 (47%) | 19 (29%) | 70 (40%) |
| Married | 34 (31%) | 19 (29%) | 53 (30%) |
| Living with a partner | ≤ 11* | ≤ 11* | ≤ 11* |
| Widowed | 10 (9%) | 12 (18%) | 22 (13%) |
| Separated, divorced | ≤ 11* | 13 (20%) | 21 (12%) |
| Employment, n (%) | |||
| Full time | 13 (12%) | ≤ 11* | 14 (8%) |
| Part time | ≤ 11* | ≤ 11* | 15 (9%) |
| Not employed | 86 (79%) | 57 (88%) | 143 (82%) |
| Self-employed | ≤ 11* | ≤ 11* | ≤ 11* |
| Income, n (%) | |||
| < $20,000 | 60 (59%) | 48 (83%) | 108 (68%) |
| $20,000–$39,999 | 24 (24%) | 6 (10%) | 30 (19%) |
| ≥ $40,000 | 18 (18%) | ≤ 11 (7%) | 22 (14%) |
| Any Medicare or Medicaid, n (%) | |||
| Yes | 98 (90%) | 48 (73%) | 146 (83%) |
| No or do not know | ≤ 11* | 18 (27%) | 29 (17%) |
| Primary transportation to clinic, n (%) | |||
| Car (drive or ride) | 61 (56%) | 26 (39%) | 87 (50%) |
| Public Transit (elevated train or bus) | ≤ 11* | ≤ 11* | 14 (8%) |
| Medicar (Medicaid) | 19 (17%) | 23 (35%) | 42 (24%) |
| PACE paratransit/door to door | ≤ 11* | ≤ 11* | 17 (10%) |
| Other | ≤ 11* | ≤ 11* | 15 (9%) |
| Years on dialysis, mean (SD) (N = 174) | 4.8 (5.9) | 3.8 (3.6) | 4.4 (5.2) |
| Comorbidities, self-reported, n (%) | |||
| Diabetes | 50 (46%) | 43 (65%) | 93 (53%) |
| Hypertension | 89 (82%) | 55 (83%) | 144 (82%) |
| Congestive heart failure | 22 (20%) | 16 (24%) | 38 (22%) |
| Cancer (except skin) | ≤ 11* | ≤ 11* | ≤ 11* |
| Number of self-reported comorbidities, Mean (SD) | 1.7 (1.2) | 2.0 (1.0) | 1.8 (1.1) |
| Labs relevant to chronic hemodialysis, mean (SD) | |||
| Urea reduction ratio (URR) (N = 174) | 77.6 (7.3) | 75.5 (5.6) | 76.8 (6.7) |
| Hemoglobin, g/dL | 10.3 (1.4) | 10.3 (1.3) | 10.3 (1.3) |
| Albumin, g/dL | 3.5 (0.5) | 3.8 (0.5) | 3.6 (0.5) |
*N not reported for cell size less than 11
Regarding dialysis history, length of time on dialysis averaged 4.4 (SD 5.2) years, with long periods at their current dialysis center: mean 3.3 years (SD 4.4), and three quarters of patients had been at the same dialysis center for at least 6 months. Patients with prior transplants comprised 19% of the study participants. Baseline lab values for hemoglobin averaged 10.3 g/dl (SD 1.3), and 85% of patients had values considered adequate for anemia management in chronic hemodialysis patients (≥ 9 g/dl). Mean URR at baseline was 76.8% (SD 6.7), and mean albumin was 3.6 g/dl (SD 0.5). Changes in these and other lab values over time are shown in a Supplementary Table (online).
Medical Home Services
Table 3 shows the use of the study PCP and CHW in addition to their participation in nephrology-led dialysis chairside rounds during the intervention. In total, there were 348 study PCP visits occurring in exam rooms (41%), at the dialysis chairside (50%), and by phone (9%); 93 of the 175 patients had at least one study PCP visit, averaging 3.7 (SD 3.5) visits. There were 1508 CHW visits, with 11% conducted at intake, 66% as follow-up visits, and 24% as quick check-in visits; patients averaged 8.6 (SD 4.1) CHW visits. There were no differences by site (results not shown).
Table 3.
Frequency of Individual Patient Visits with Study PCP (Primary Care Physician) and CHW (Community Health Worker) During PCMH-KD Intervention
| Total study PCP visits | N = 348 |
| Clinic | 141 (41%) |
| Chairside | 173 (50%) |
| Phone | 31 (9%) |
| Study PCP visits, all patients | N = 175 |
| Mean (SD) | 2.0 (3.1) |
| Study PCP visits, patients with visits | N = 93 |
| Mean (SD) | 3.7 (3.5) |
| Total CHW visits | N = 1508 |
| Intake visit | 159 (11%) |
| Follow-up visit | 990 (66%) |
| Check-in visit | 356 (24%) |
| CHW visits, all patients | N = 175 |
| Mean (SD) | 8.6 (4.1) |
Health-Related Quality of Life
Table 4 shows the unadjusted KDQOL domain scores at each time point. Baseline mean (SD) KDQOL domain scores were as follows: Physical Composite Scale (PCS) was 35.5 (10.2), Mental Composite Scale (MCS) was 49.2 (10.6), Burden was 46.5 (27.1), Symptoms was 76.5 (15.9), and Effects was 72.3 (20.6) (Table 4). Noteworthy is that all five domains increased at 6 months, and some continued to trend upwards (improved) over time. There were no differences across centers, although there were differences within domains by patient characteristics at baseline29.
Table 4.
Kidney Disease Quality of Life (KDQOL) Mean Scale Scores at Baseline, 6, 12, and 18 Months (Unadjusted)
| Baseline | 6 months | 12 months | 18 months | |||||
|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | |
| Physical component summary (PCS) | 172 | 35.5 (10.2) | 152 | 38.4 (11.4)† | 125 | 36.3 (11.2) | 102 | 36.8 (11.2) |
| Mental component summary (MCS) | 172 | 49.2 (10.6) | 152 | 50.4 (11.3) | 125 | 51.8 (10.2)‡ | 102 | 52.4 (9.7)† |
| Burden of kidney disease (Burden) | 174 | 46.5 (27.1) | 153 | 48.8 (26.7) | 125 | 49.6 (23.3) | 103 | 51.2 (30.3) |
| Symptoms/problems of kidney disease (Symptoms) | 174 | 76.5 (15.9) | 153 | 79.9 (14.2)‡ | 125 | 79.7 (13.6) | 103 | 76.8 (16.7) |
| Kidney disease effects (Effects) | 174 | 72.3 (20.6) | 153 | 76.3 (20.6)† | 125 | 78.3 (18.9)* | 103 | 76.1 (20.1)‡ |
Ns differ slightly from the number of interviews in the participant flow chart due to missing data for KDQOL items
*p value < 0.001 for change from baseline, from random-intercept mixed models with an AR (1) covariance pattern in the residual, visit expressed as 3 indicator variables, and no covariates
†p value < 0.01 for change from baseline
‡p value < 0.05 for change from baseline
Adjusted Regression Analysis
Adjusted analyses are presented in Table 5. The coefficient for each “Visit” variable (i.e., 6 month, 12, month, and 18 month) represents the adjusted mean change in the KDQOL domain at the point in time relative to baseline, adjusting for the other covariates. The KDQOL PCS improved significantly from baseline (adjusted mean 35.5) to 6 months by 2.59 points (7.3%, p = 0.002) (Table 5). At 12 and 18 months, the MCS improved significantly from baseline (adjusted mean 49.0) by 2.64 (5.4%, p = 0.01) and 2.96 (6.1%, p = 0.007) points respectively. The Burden domain improvement from baseline was not statistically significant at 18 months (p = 0.07). The KDQOL Symptoms domain improved significantly from baseline (adjusted mean 77.0) to 6 months by 2.61 points (3.4%, p = 0.02), but there was not a statistically significant improvement at 12 months (2.35 points, 3.0%, p = 0.051) or 18 months (p = 0.70). The KDQOL Effects domain improved significantly from baseline (adjusted mean 72.7) to 6, 12, and 18 months by 4.36 (6.0%, p = 0.003), 6.95 (9.5%, p < 0.0001), and 4.14 (5.7%, p = 0.02) points respectively, adjusting for other factors.
Table 5.
Parameter Estimates from Adjusted Random Intercept Models of Change Over Time in KDQOL Scale Scores.
|
Physical Component Score (PCS) N = 173a |
Mental Component Score (MCS) N = 173a |
|||||
| Effect | Estimate | SE | p | Estimate | SE | p |
| Intercept | 37.74 | 8.03 | < 0.0001 | 35.99 | 8.63 | < 0.0001 |
| Visit (referent = baseline) | ||||||
| 6-month visit | 2.59 | 0.82 | 0.002 | 1.22 | 0.97 | 0.21 |
| 12-month visit | 0.31 | 0.87 | 0.72 | 2.64 | 1.03 | 0.01 |
| 18-month visit | 1.02 | 0.96 | 0.29 | 2.96 | 1.10 | 0.007 |
| Age at baseline, years | − 0.12 | 0.05 | 0.03 | 0.02 | 0.05 | 0.64 |
| Sex (referent = female) | ||||||
| Male | 2.16 | 1.39 | 0.12 | 0.96 | 1.33 | 0.47 |
| Race (referent = Hispanic, other) | ||||||
| African American | 1.06 | 1.89 | 0.58 | 2.33 | 1.79 | 0.19 |
| Interview language (referent = English) | ||||||
| Spanish | − 1.27 | 2.24 | 0.57 | 0.87 | 2.12 | 0.68 |
| Dialysis vintage, months | − 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.22 |
| Site (referent = university site) | ||||||
| Private site | − 0.34 | 1.51 | 0.82 | − 2.47 | 1.44 | 0.09 |
| Education (referent = not HS graduate) | ||||||
| HS graduate | 0.19 | 1.77 | 0.92 | − 0.03 | 1.68 | 0.98 |
| Marital status (referent = not married/living with partner) | ||||||
| Married or living with partner | 2.38 | 1.39 | 0.09 | − 1.65 | 1.32 | 0.21 |
| Diabetes (referent = no self-reported diabetes) | ||||||
| Diabetes | − 3.77 | 1.49 | 0.01 | 0.26 | 1.41 | 0.86 |
| Established PCP at baseline (referent = no PCP or current PCP < 6 months) | ||||||
| Current PCP ≥ 6 months at BL | − 0.08 | 1.42 | 0.95 | − 0.11 | 1.34 | 0.93 |
| Visits with study PCP (referent = none) | ||||||
| Any visits with study PCP | − 5.13 | 1.49 | 0.0006 | − 1.35 | 1.41 | 0.34 |
| Follow-up visits with CHW (referent ≤ 6) | ||||||
| ≥ 6 follow-up visits with CHW (median or above) | 2.41 | 1.51 | 0.11 | − 0.88 | 1.43 | 0.54 |
| Urea reduction ratio (URR) | − 0.04 | 0.07 | 0.54 | − 0.07 | 0.08 | 0.35 |
| Hemoglobin, g/dL | 0.84 | 0.34 | 0.01 | 0.88 | 0.38 | 0.02 |
| Albumin, g/dL | 0.45 | 1.15 | 0.69 | 2.37 | 1.24 | 0.06 |
|
Burden of kidney disease N = 173a |
Symptoms/problems N = 173a |
|||||
| Effect | Estimate | SE | p | Estimate | SE | p |
| Intercept | 75.90 | 19.84 | 0.0002 | 55.31 | 11.44 | < 0.0001 |
| Visit (referent = baseline) | ||||||
| 6-month visit | 2.62 | 1.95 | 0.18 | 2.61 | 1.13 | 0.02 |
| 12-month visit | 3.25 | 2.06 | 0.12 | 2.35 | 1.20 | 0.05 |
| 18-month visit | 3.86 | 2.16 | 0.07 | −0.51 | 1.30 | 0.70 |
| Age at baseline, years | 0.05 | 0.14 | 0.71 | − 0.07 | 0.08 | 0.40 |
| Sex (referent = female) | ||||||
| Male | 1.44 | 3.70 | 0.70 | 2.38 | 2.08 | 0.25 |
| Race (referent = Hispanic, other) | ||||||
| African American | 8.90 | 5.07 | 0.08 | 0.60 | 2.84 | 0.83 |
| Interview language (referent = English) | ||||||
| Spanish | −5.35 | 6.00 | 0.37 | 1.49 | 3.37 | 0.66 |
| Dialysis vintage, months | 0.00 | 0.03 | 0.96 | 0.01 | 0.02 | 0.67 |
| Site (referent = university site) | ||||||
| Private site | − 5.69 | 4.02 | 0.16 | − 0.35 | 2.26 | 0.88 |
| Education (referent = not HS graduate) | ||||||
| HS graduate | − 3.25 | 4.72 | 0.49 | 0.80 | 2.65 | 0.76 |
| Marital status (referent = not married/living with partner) | ||||||
| Married or living with partner | 0.88 | 3.73 | 0.81 | 0.98 | 2.09 | 0.64 |
| Diabetes (referent = no self-reported diabetes) | ||||||
| Diabetes | − 3.97 | 3.98 | 0.32 | − 2.63 | 2.23 | 0.24 |
| Established PCP at baseline (referent = no PCP or current PCP < 6 months) | ||||||
| Current PCP ≥ 6 months at BL | 2.85 | 3.80 | 0.45 | − 2.19 | 2.13 | 0.31 |
| Visits with study PCP (referent = none) | ||||||
| Any visits with study PCP | − 6.78 | 3.99 | 0.09 | − 4.15 | 2.24 | 0.06 |
| Follow-up visits with CHW (referent ≤ 6) | ||||||
| ≥ 6 follow-up visits with CHW (median or above) | 2.64 | 4.02 | 0.51 | 0.03 | 2.26 | 0.99 |
| Urea reduction ratio (URR) | − 0.44 | 0.17 | 0.01 | 0.00 | 0.10 | 0.98 |
| Hemoglobin, g/dL | − 0.08 | 0.82 | 0.92 | 1.36 | 0.48 | 0.005 |
| Albumin, g/dL | 1.59 | 2.79 | 0.57 | 3.44 | 1.62 | 0.03 |
|
Kidney disease effects (Effects) N= 173a |
||||||
| Effect | Estimate | SE | p | |||
| Intercept | 70.56 | 14.98 | < 0.0001 | |||
| Visit (referent = baseline) | ||||||
| 6-month visit | 4.36 | 1.47 | 0.003 | |||
| 12-month visit | 6.95 | 1.56 | < 0.0001 | |||
| 18-month visit | 4.14 | 1.73 | 0.02 | |||
| Age at baseline, years | 0.02 | 0.11 | 0.83 | |||
| Sex (referent = female) | ||||||
| Male | − 0.35 | 2.73 | 0.90 | |||
| Race (referent = Hispanic, other) | ||||||
| African American | 7.94 | 3.73 | 0.03 | |||
| Interview language (referent = English) | ||||||
| Spanish | − 0.22 | 4.43 | 0.96 | |||
| Dialysis vintage, months | 0.00 | 0.02 | 0.87 | |||
| Site (referent = university site) | ||||||
| Private site | − 2.34 | 2.97 | 0.43 | |||
| Education (referent = not HS graduate) | ||||||
| HS graduate | − 6.45 | 3.48 | 0.06 | |||
| Marital status (referent = not married/living with partner) | ||||||
| Married or living with partner | − 2.03 | 2.75 | 0.46 | |||
| Diabetes (referent = no self-reported diabetes) | ||||||
| Diabetes | − 1.02 | 2.94 | 0.73 | |||
| Established PCP at baseline (referent = no PCP or current PCP < 6 months) | ||||||
| Current PCP ≥ 6 months at BL | − 0.35 | 2.80 | 0.90 | |||
| Visits with study PCP (referent = none) | ||||||
| Any visits with study PCP | − 12.11 | 2.94 | < 0.0001 | |||
| Follow-up visits with CHW (referent ≤ 6) | ||||||
| ≥ 6 follow-up visits with CHW (median or above) | 2.46 | 2.97 | 0.41 | |||
| Urea reduction ratio (URR) | − 0.18 | 0.13 | 0.17 | |||
| Hemoglobin, g/dL | 0.29 | 0.63 | 0.65 | |||
| Albumin, g/dL | 5.12 | 2.12 | 0.02 | |||
aNumber of participants contributing at least one observation to the analysis. Two participants with a missing value for a baseline covariate were excluded
We show the regression coefficients and statistics for covariates for each of the KDQOL domain regression models (Table 5). For the PCS model, being on dialysis longer (dialysis vintage) or having diabetes was negatively associated with HRQOL. Neither variable was significant in the other HRQOL models, indicating that these factors have a greater impact on the physical health domain than on the other HRQOL domains, adjusting for other factors.
Noteworthy is that in the PCS and Effects domain models, patients who had any visits to the study PCP had significantly lower HRQOL scores. A non-significant negative relationship was observed for the MCS (p = 0.34), Symptoms (p = 0.06), and Burden (p = 0.09) domain models as well.
There was also a significant positive association between lab values for hemoglobin on PCS, MCS, and Symptoms and for albumin levels with the Symptoms and Effects domains.
DISCUSSION
We conducted a before–after study with repeated measures of an adaptation of the PCMH for kidney disease focused on chronic hemodialysis patients at two dialysis centers in an urban area with a racially and ethnically diverse patient population. The PCMH-KD model added additional healthcare providers to the current CMS-mandated team. Results from the study revealed that multiple domains of HRQOL improved from baseline, especially mental health (MCS) and kidney disease effects (Effects), which maintained significant positive change from baseline (usual care) at 18 months. To our knowledge, this is the first study to adapt a PCMH model for chronic hemodialysis care. There are several noteworthy findings from our work.
We observed heterogeneity in the HRQOL component trend patterns. We found that three of the five domains were significantly improved at 6 months (PCS, Symptoms, Effects), two domains improved at 12 months (MCS and Effects) and two domains improved at 18 months (MCS and Effects). For the Symptoms and Burden domains, the improvements never reached statistical significance at the p < 0.05 level. The magnitude of the changes observed are within the ≥ 3–5-point change criterion often considered to be clinically meaningful, although not specific to dialysis patients40–42. Our findings suggest that some HRQOL domains may be more sensitive to health system changes than others. Another consideration is that the measurement properties for some HRQOL domains could be unstable over time, and some have reported ceiling effects with the MCS and PCS domains for some populations10, 42, 43. More recent cross-sectional evaluation of the psychometric properties offer assurance about the factor structure, reliability, and construct validity of the KDQOL44, although measurement invariance over time remains an area for further investigation. Yet the KDQOL has been shown to be a strong predictor of morbidity and mortality among dialysis patients6–10, and there is increasing emphasis by stakeholders on using HRQOL and other patient-centered measures that are reliable and actionable for providers45, 46. Further understanding about population-specific measures and over time is critical, especially with CMS implementation of new dialysis models47–49.
The HRQOL domain scores for our study population are consistent with other studies of ESKD among veterans3, 4. Our results for chronic hemodialysis patients are slightly lower than those reported by Peipert and colleagues (2018) for peritoneal and hemodialysis patients combined (mean scores for PCS, MCS, Burden, Symptoms, and Effects at 38, 51, 52, 79, and 74, respectively)44. To date, longitudinal data on HRQOL among ESKD hemodialysis patients have not been published, and which would enable comparisons. Our findings of a positive association between lab values for hemoglobin on PCS, MCS, and Symptoms and for albumin levels with the Symptoms and Effects domains are consistent with prior literature and our previous baseline report29, 49–51. Also consistent with prior research, we found a negative relationship between HRQOL domain scores for physical health (PCS) and diabetes52. That we did not find a significant association for the presence of diabetes for other HRQOL domains may be due to the additional socioeconomic factors we included26, 52.
Previous studies of racial/ethnic differences in HRQOL in ESKD patients have shown that African Americans report better HRQOL than non-African Americans53–55. In contrast, when controlling for other factors, we found that only for the Effects domain did African Americans have a higher score compared to our predominantly Hispanic subjects, while there was no significant relationship for the other four HRQOL domains. In the Effects domain, we found that those who identified as Hispanic or White, who had a less than high school education and lower serum albumin scores (suggesting poorer nutritional status), experienced significantly less increase in their score. These three factors—race/ethnicity, education, and nutrition—point to a need that might be addressed together, such as through an education program focusing on dietary health and overall dialysis care effectiveness and incorporating diverse perspectives across racial, cultural, and socioeconomic backgrounds. Understanding the broader needs of specific patient populations should be carefully considered in designing interventions that aim to improve patients’ well-being in a dialysis setting.
We found that HRQOL domain scores for PCS and Effects were significantly lower among patients who had visits with the study PCP, adjusting for other factors. This negative association may indicate that patients with greater needs sought out the study PCP more than those with fewer needs. Underscoring the accessibility of PCPs to patients in our study, nearly half of the overall visits were performed at the chairside during dialysis. Mandel and colleagues reported that some chronic hemodialysis patients opted to have conversations with physicians, including PCPs, during dialysis56 treatments. Some of our patients continued to see another PCP in addition to the study PCP, and we were unable to capture interactions with physicians outside of the study. In earlier analyses, we found no association between HRQOL and having another PCP at baseline29, although we did not explore the intensity of the relationship (e.g., longevity, or visit frequency). We are not aware of other studies that have examined the relationship of HRQOL in dialysis or other chronic disease populations with the frequency of PCP visits. While visits with the CHWs did not independently influence changes in KDQOL scores, prior analysis suggests the CHWs facilitated access to the PCPs and is consistent with the CHW role as a clinical liaison30. Our sample size did not afford a comparison of the impact CHWs on Spanish-speaking versus English-speaking participants.
Our study had limitations. As a before–after design and limited to two intervention sites with one group of academically based nephrologists, we cannot solely attribute the observed changes in HRQOL to the intervention with absolute certainty. Although those who withdrew consent during the study was small (4%), an additional 22% were lost due to transplant, leaving the center, or death and the impact on our results is not known. Also, although there was a team assembled, we did not collect detailed process measures on how the team interacted with the PCP or each other. Therefore, we cannot be entirely certain how other components of the intervention (e.g., pharmacist, nurse coordinator) contributed to HRQOL. Enhanced dialog between all the team members may have contributed to improved HRQOL. Future studies should consider approaches to capture team communications and related additional patient-reported outcomes.
CONCLUSION
We conducted a before–after health system intervention study with repeated measures aimed at integrating primary care and enhancing care coordination in an interdisciplinary dialysis care team and comparing outcomes with care provided under the current Medicare-mandated model of care. Several aspects of HRQOL improved over time. The addition of a PCP in our model appeared to meet a previously unmet need for some patients with low HRQOL. With increased emphasis on improving patient experience with care46, 47, 57, there is an urgent need for novel healthcare interventions that address these issues among chronic hemodialysis patients. Systematic evaluation of new care models is needed to facilitate comparison of results across studies. Ultimately, we hope that findings from our study will inform healthcare reorganization efforts aimed at improving care and outcomes for chronic hemodialysis patients, as well as other patients with kidney disease.
Electronic Supplementary Material
(DOCX 15 kb)
Contributors
We appreciate the time and effort of those who helped to develop and implement this study, especially the pre-implementation training team, the clinical study team, the dialysis center staff at both sites, and the research personnel. We are grateful to the patients and their families who volunteered their time to participate in the study.
Funders
This work was supported by funding from the Patient-Centered Outcomes Research Institute (PCORI), contract no. IH-12-11-5420. Infrastructure support was provided by Fresenius Medical Care through its companies Frenova Renal Research and Fresenius Kidney Care. Additional support for this project was provided by the Office of the Vice President for Health Affairs at the University of Illinois at Chicago. Data for this study was provided in part by the University of Illinois at Chicago Center for Clinical and Translational Science, funded by National Center for Advancing Translational Sciences, National Institutes of Health (NIH) (UL1TR002003). Dr. Hynes was supported on a US Department of Veterans Affairs Research Career Scientist Award (RCS-98-352). Ms. Chukwudozie was supported by the GUIDE Cancer Research Training Project (NCI: 1P20CA202908). The manuscript content is solely the responsibility of the authors and does not necessarily reflect the views of PCORI or the US Department of Veterans Affairs or the NIH.
Compliance with Ethical Standard
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.US Renal Data System . USRDS 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD; 2017. [Google Scholar]
- 2.Stroupe KT, Fischer MJ, Kaufman JS, O’Hare AM, Sohn MW, Browning MM, Huo Z, Hynes DM. Predialysis nephrology care and costs in elderly patients initiating dialysis. Med Care. 2011;49(3):248–256. doi: 10.1097/MLR.0b013e31820192ba. [DOI] [PubMed] [Google Scholar]
- 3.Saban KL, Bryant FB, Reda DJ, Stroupe KT, Hynes DM. Measurement invariance of the kidney disease and quality of life instrument (KDQOL-SF) across veterans and non-veterans. Health Qual Life Outcomes. 2010;8(1):120. doi: 10.1186/1477-7525-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hynes DM, Stroupe KT, Fischer MJ, et al. Comparing VA and private sector healthcare costs for end-stage renal disease. Med Care. 2012;50(2):161–170. doi: 10.1097/MLR.0b013e31822dcf15. [DOI] [PubMed] [Google Scholar]
- 5.Zimmerman DL, Selick A, Singh R, Mendelssohn DC. Attitudes of Canadian nephrologists, family physicians and patients with kidney failure toward primary care delivery for chronic dialysis patients. Nephrol Dial Transplant. 2003;18(2):305–309. doi: 10.1093/ndt/18.2.305. [DOI] [PubMed] [Google Scholar]
- 6.Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64:339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 7.Lowrie EG, Curtin RB, LePain N, Schatell D. Medical outcomes study short form-36: A consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis. 2003;41:1286–1292. doi: 10.1016/s0272-6386(03)00361-5. [DOI] [PubMed] [Google Scholar]
- 8.Wingard RL, Pupim LB, Krishnan M, Shintani A, Alp Ikizler T, Hakim RM. Early Intervention Improves Mortality and Hospitalization Rates in Incident Hemodialysis Patients: RightStart Program. Clin J Am Soc Nephrol. 2007;2:1170–1175. doi: 10.2215/CJN.04261206. [DOI] [PubMed] [Google Scholar]
- 9.Lacson E, Xu J, Lin S-F, Dean SG, Lazarus JM, Hakim RM. A comparison of SF-36 and SF-12 composite scores and subsequent hospitalization and mortality risks in long-term dialysis patients. Clin J Am Soc Nephrol. 2010;5(2):252–260. doi: 10.2215/CJN.07231009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebman S, Li NC, Lacson E. Change in quality of life and one-year mortality risk in maintenance dialysis patients. Qual Life Res. 2016;25(9):2295–306. doi: 10.1007/s11136-016-1257-y. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Conditions for coverage for ESRD facilities, final rule. 42 C.F.R. part 494.80. Condition: Patient Assessment. Fed Register. 2008; p. 20393.
- 12.Finkelstein FO, Arsenault KL, Taveras A, Awuah K, Finkelstein SH. Assessing and improving the health-related quality of life of patients with ESRD. Nat Rev Nephrol. 2012;8(12):718–724. doi: 10.1038/nrneph.2012.238. [DOI] [PubMed] [Google Scholar]
- 13.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 14.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 15.Bayliss EA, Bhardwaja B, Ross C, Beck A, Lanese DM. Multidisciplinary team care may slow the rate of decline in renal function. Clin J Am Soc Nephrol. 2011;6(4):704–710. doi: 10.2215/CJN.06610810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter BL, Bosworth HB, Green BB. The hypertension team: The role of the pharmacist, nurse, and teamwork in hypertension therapy. J Clin Hypertens (Greenwich) 2012;14(1):51–65. doi: 10.1111/j.1751-7176.2011.00542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philis-Tsimikas A, Walker C, Rivard L, et al. Improvement in diabetes care of underinsured patients enrolled in project dulce – A community-based, culturally appropriate, nurse case management and peer education diabetes care model. Diabetes Care. 2004;27(1):110–115. doi: 10.2337/diacare.27.1.110. [DOI] [PubMed] [Google Scholar]
- 18.Beckham S, Kaahaaina D, Voloch K, Washburn A. A community-based asthma management program: Effects on resource utilization and quality of life. Hawaii Med J. 2004;63(4):121–126. [PubMed] [Google Scholar]
- 19.Norris SL, Chowdhury FM, Van Le K, et al. Effectiveness of community health workers in the care of persons with diabetes. Diabet Med. 2006;23(5):544–556. doi: 10.1111/j.1464-5491.2006.01845.x. [DOI] [PubMed] [Google Scholar]
- 20.Unruh M, Benz R, Greene T, et al. HEMO Study Group: Effects of hemodialysis dose and membrane flux on health-related quality of life in the HEMO Study. Kidney Int. 2004;66:355–366. doi: 10.1111/j.1523-1755.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- 21.Chertow GMLN, Levin NW, Beck GJ, et al. FHN Trial Group: Incenter hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussey PS, Schneider EC, Rudin RS, Fox DS, Lai J, Pollack CE. Continuity and the costs of care for chronic disease. JAMA Intern Med. 2014;174(5):742–748. doi: 10.1001/jamainternmed.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sweeney L, Halpert A, Waranoff J. Patient-centered management of complex patients can reduce costs without shortening life. Am J Manag Care. 2007;13(1936–2692; 1088–0224; 2):84–92. [PubMed] [Google Scholar]
- 24.Anvari E, Mojazi Amiri H, Aristimuno P, Chazot C, Nugent K. Comprehensive and personalized care of the hemodialysis patient in Tassin, France: a model for the patient-centered medical home for subspecialty patients. ISRN Nephrol 2013:1–6. [DOI] [PMC free article] [PubMed]
- 25.Flanagan S, Damery S, Combes G. The effectiveness of integrated care interventions in improving patient quality of life for patients with chronic conditions. An overview of the systematic review evidence. Health Qual Life Outcomes. 2017;15:188. doi: 10.1186/s12955-017-0765-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter AC, Fitzgibbon ML, Fischer MJ, et al. Rationale and design of a patient-centered medical home intervention for patients with end-stage renal disease on hemodialysis. Contemp Clin Trials. 2015;42:1–8. doi: 10.1016/j.cct.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hynes DM, Fischer MJ, Schiffer LA, et al. Evaluating a Novel Health System Intervention for Chronic Kidney Disease Care Using the RE-AIM Framework: Insights After Two Years. Contemp Clin Trials. 2017;52:20–26. doi: 10.1016/j.cct.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Cukor D, Cohen L, Cope E, et al. Patient and stakeholder engagement in kidney diseases related research. Clin J Am Soc Nephrol. 2016;11(9):1703–12. doi: 10.2215/CJN.09780915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porter AC, Fitzgibbon ML, Fischer MJ, et al. Quality of Life among Participants in a Patient-Centered Medical Home Intervention for Patients with End-stage Renal Disease on Hemodialysis. Insights in Internal Medicine. 2017; [Version 1, Approved]. 1:3.1.
- 30.Chukwudozie IB, Fitzgibbon ML, Schiffer L, et al. Factors contributing to uptake of primary care provider visits among chronic hemodialysis patients in a patient-centered medical home intervention study. Transl Behav Med. 2018;8(3):341–350. doi: 10.1093/tbm/iby021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen Ronald M. Revisiting the Behavioral Model and Access to Medical Care: Does it Matter? Journal of Health and Social Behavior. 1995;36(1):1. [PubMed] [Google Scholar]
- 32.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: Translating evidence into action. Health Aff (Millwood) 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 33.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL™) instrument. Qual Life Res. 1994;3(5):329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 35.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Dahmen G, Ziegler A. Generalized estimating equations in controlled clinical trials: Hypotheses testing. Biom J. 2004;46(2):214–232. [Google Scholar]
- 37.Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med. 1998;17(14):1643–1658. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 38.Dahmen G, Ziegler A. Independence estimating equations for controlled clinical trials with small sample sizes - interval estimation. Methods Inf Med. 2006;45(4):430–434. [PubMed] [Google Scholar]
- 39.SAS [computer program]. Version 9.4. SAS institute; 2014.
- 40.Samsa G, Edelman D, Rothman ML, et al. Determining clinically important differences in health status measures: A general approach with illustration to the health utilities index mark II. Pharmacoeconomics. 1999;15(2):141–155. doi: 10.2165/00019053-199915020-00003. [DOI] [PubMed] [Google Scholar]
- 41.Hays R, Woolley JM. The concept of clinically meaningful difference in health-related quality-of-life research. How meaningful is it? Pharmacoeconomics. 2000;18(5):419–423. doi: 10.2165/00019053-200018050-00001. [DOI] [PubMed] [Google Scholar]
- 42.Simon GE, Revicki DA, Grothaus L, Vonkorff M. SF-36 summary scores: Are physical and mental health truly distinct? Med Care. 1998;36(4):567–572. doi: 10.1097/00005650-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Hall YN, Larive B, Painter P, et al. Effects of six versus three times per week hemodialysis on physical performance, health, and functioning: Frequent hemodialysis network (FHN) randomized trials. Clin J Am Soc Nephrol. 2012;7(5):782–794. doi: 10.2215/CJN.10601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peipert JD, Bentler PM, Klicko K, Hays RD. Psychometric Properties of the Kidney Disease Quality of Life 36-Item Short-Form Survey (KDQOL-36) in the United States. Am J Kidney Dis. 2018;71(4):461–468. doi: 10.1053/j.ajkd.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 45.Moss AH, Davison SN. How the ESRD Quality Incentive Program Could Potentially Improve Quality of Life for Patients on Dialysis. Clin J Am Soc Nephrol. 2015;10(5):888–893. doi: 10.2215/CJN.07410714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Hare AM, Armistead N, Schrag WL, Diamond L, Moss AH. Patient-centered care: an opportunity to accomplish the "three aims" of the national quality strategy in the Medicare ESRD program. Clin J Am Soc Nephrol. 2014;9(12):2189–2194. doi: 10.2215/CJN.01930214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.CMS End Stage Renal Disease Care Model, Centers for Medicare and Medicaid Services. 2019. https://innovation.cms.gov/initiatives/comprehensive-esrd-care/ and Comprehensive ESRD Care Model (CEC) Model Fact Sheet https://innovation.cms.gov/Files/fact-sheet/cec-fs.pdf. Accessed 4-1-2019.
- 48.Berns JS, Glickman JD, Reese PP. Dialysis Payment Model Reform: Managing Conflicts Between Profits and Patient Goals of Care Decision Making. Am J Kidney Dis. 2018;71(1):133–136. doi: 10.1053/j.ajkd.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 49.Lacson E, Xu J, Lin SF, Dean SG, Lazarus JM, Hakim R. Association between achievement of hemodialysis quality-of-care indicators and quality-of-life scores. Am J Kidney Dis. 2009;54:1098–1107. doi: 10.1053/j.ajkd.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 50.Leaf DE, Goldfarb DS. Interpretation and review of health-related quality of life data in CKD patients receiving treatment for anemia. Kidney Int. 2009;75:15–24. doi: 10.1038/ki.2008.414. [DOI] [PubMed] [Google Scholar]
- 51.Farag YM, Keithi-Reddy SR, Mittal BV, Surana SP, Addabbo F, Goligorsky MS, Singh AK. Anemia, inflammation and health-related quality of life in chronic kidney disease patients. Clin Nephrol. 2011;75(6):524–33. doi: 10.5414/cnp75524. [DOI] [PubMed] [Google Scholar]
- 52.Kaysen GA, Larive B, Painter P, et al. on behalf of the FHN Trial Group. Baseline Physical Performance, Health, and Functioning of Participants in the Frequent Hemodialysis Network (FHN) Trial. Am J Kidney Dis. 2011;57(1):101–112. doi: 10.1053/j.ajkd.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feroze U, Noori N, Kovesdy CP, et al. Quality-of-life and mortality in hemodialysis patients: roles of race and nutritional status. Clin J Am Soc Nephrol. 2011;6(5):1100–1111. doi: 10.2215/CJN.07690910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Unruh M, Miskulin D, Yan G, et al. Racial differences in health-related quality of life among hemodialysis patients. Kidney Int. 2004;65(4):1482–1491. doi: 10.1111/j.1523-1755.2004.00529.x. [DOI] [PubMed] [Google Scholar]
- 55.Hicks LS, Cleary PD, Epstein AM, Ayanian JZ. Differences in health-related quality of life and treatment preferences among black and white patients with end-stage renal disease. Qual Life Res. 2004;13(6):1129–1137. doi: 10.1023/B:QURE.0000031350.56924.cc. [DOI] [PubMed] [Google Scholar]
- 56.Mandel EI, Bernacki RE, Block SD. Serious Illness Conversations in ESRD. Clin J Am Soc Nephrol. 2017;12(5):854–863. doi: 10.2215/CJN.05760516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weisbord SD. Patient-Centered Dialysis Care: Depression, Pain, and Quality of Life. Semin Dial. 2016;29(2):158–64. doi: 10.1111/sdi.12464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 15 kb)