Abstract
Background
Birth cohort screening is recommended for hepatitis C virus (HCV) and underserved populations are disproportionally affected by HCV. Little is known about the influence of race on the HCV care continuum in this population.
Objective
To assess the cascade of HCV care in a large racially diverse and underserved birth cohort.
Design
Retrospective cohort study using electronic medical record data abstracted until August 31, 2017.
Patients
34,810 patients born between 1945 and 1965 engaged in primary care between October 1, 2014, and October 31, 2016, within the safety-net clinics of the San Francisco Health Network.
Main Measures
Rate of hepatitis C testing, hepatitis C treatment, and response to therapy.
Results
Cohort characteristics were as follows: median age 59 years, 57.6% male, 25.5% White (20.6% Black, 17.7% Latino, 33.0% Asian/Pacific Islander (API), 2% other), and 32.6% preferred a non-English language. 99.7% had an HCV test (95.4% HCV antibody, 4.3% HCVRNA alone). Among HCV antibody-positive patients (N = 4587), 22.9% were not tested for confirmatory HCVRNA. Among viremic patients (N = 3673), 20.8% initiated HCV therapy, 90.6% achieved sustained virologic response (SVR) and 8.1% did not have a SVR test. HCV screening and treatment were highest in APIs (98.7 and 34.7% respectively; p < 0.001). Blacks had the highest chronic HCV rate (22.2%; p < 0.001). Latinos had the lowest SVR rate (81.3%; p = 0.01). On multivariable analysis, API race (vs White, OR 1.20; p = 0.001), presence of HIV co-infection (OR 1.58; p = 0.02), presence of chronic kidney disease (OR 0.47; p < 0.001), English (vs non-English) as preferred language (OR 0.54; p = 0.002), ALT (OR 0.39 per doubling; p < 0.001), and HCVRNA (OR 0.83 per 10-fold increase; p < 0.001) were associated with HCV treatment.
Conclusions
Despite near-universal screening, gaps in active HCV confirmation, treatment, and verification of cure were identified and influenced by race. Tailored interventions to engage and treat diverse and underserved populations with HCV infection are needed.
Electronic supplementary material
The online version of this article (10.1007/s11606-018-4649-6) contains supplementary material, which is available to authorized users.
KEY WORDS: direct-acting antivirals, health disparity, vulnerable populations, linkage to care, African American
INTRODUCTION
Hepatitis C virus (HCV) infection affects over 3.5 million Americans and untreated HCV is associated with increased mortality, loss of productivity, and healthcare costs,1,2 whereas HCV viral eradication with treatment improves health.3–5 Adult patients in the 1945–1965 birth cohort account for nearly 75% of HCV cases in the USA6,7 and are at increased risk of mortality and hepatocellular carcinoma.6,8 Consequently, in 2012 and 2013, the Centers for Disease Control and the U.S. Preventive Services Task Force recommended one-time birth cohort HCV screening irrespective of risk factors to improve identification of chronic HCV cases.9,10
HCV screening in the birth cohort is cost-effective when patients with chronic HCV are successfully linked to treatment11,12 and can prevent up to 121,000 deaths among those undiagnosed.13 Furthermore, the ASCEND study recently described the important role primary care providers can play in improving rates of HCV treatment and cure.14 However, despite availability of safe and effective direct-acting antiviral (DAA) treatments, linkage to HCV treatment remains suboptimal.15 Thus, improving HCV treatment uptake is critical to reducing the burden of disease.
The American Association for the Study of Liver Disease and Infectious Disease Society of America (AASLD-IDSA) recent guidelines identified access to HCV therapy, lack of provider and patient education, competing priorities, and loss to follow-up as potential barriers to HCV treatment initiation.16 Furthermore, these barriers to care are exacerbated by underserved and socioeconomically disadvantaged patient status. While the introduction of the Affordable Care Act has resulted in a higher proportion of public insurance among underserved populations, patients with Medicaid coverage are still less likely to receive HCV treatment compared to other types of insurance.17 Moreover, HCV screening, prevalence, and treatment rates may vary significantly by race and gender,18,19 and minorities, who are also disproportionately represented among the underserved, are less likely to be screened, referred to specialty care, or receive HCV treatment.20 We hypothesized that the cascade of HCV care may vary across underserved racial groups. In this study, we aimed to describe the overall birth cohort screening rates and to evaluate the influence of race on the cascade of HCV care in the DAA era, within a large, underserved, and urban birth cohort engaged in primary care.
METHODS
Patient Population
This is a retrospective review of electronic medical records of birth cohort patients engaged in care within 12 adult primary care clinics of the San Francisco Health Network (SFHN) within the San Francisco Department of Public Health.21 Patients born between January 1, 1945, to December 31, 1965, with at least one primary care visit between October 1, 2014, and October 31, 2016, were included in the study. In the SFHN, patients have access to HCV care either through insurance or the Healthy San Francisco program (a health access program available to uninsured San Francisco County residents). Patients have access to HCV medications through insurance or from pharmaceutical company patient assistance programs if uninsured or underinsured, and HCV treatment is provided in primary care clinics or by referral to liver or infectious disease clinics. This study was approved by the University of California San Francisco Committee on Human Research.
Data Extraction
Demographic data and laboratory values including alanine aminotransferase (ALT), platelets, creatinine, HIV testing (HIV antibody, viral load), hepatitis B testing (HBsAg, HBsAb), hepatitis C testing (HCV antibody, HCVRNA [Abbott Real Time PCR Assay©, lower limit of detection < 12 IU/mL], genotype), and prescription of HCV treatment were extracted. Race was categorized as White, Black, Latino, Asian/Pacific Islander (API), and other race. Records of patients who had received HCV therapy by either documentation of DAA prescription or those without documented prescription but an undetectable HCVRNA after a previously detectable level were further evaluated by individual chart review for confirmation of receipt of HCV therapy and virologic response rates until August 31, 2017. Sustained virologic response (SVR) to HCV therapy was defined as nondetectable HCV viral load (HCVRNA) 12 weeks after completion of treatment. Cirrhosis was defined as fibrosis-4 (FIB-4) score > 3.25 based on FIB − 4 = (age[years] × AST[U/L])/(PLT[109/L]x (ALT[U/L])0.5).22
Statistical Analysis
Descriptive statistics of HCV testing, diagnosis, and cascade of HCV care were used to obtain frequency (%) for categorical variables and median (interquartile range, IQR) for continuous variables. Chi-square test was used for categorical variables, while Kruskal-Wallis test was used for continuous variables to compare baseline patient characteristics, HCV status, linkage to treatment, and SVR status by race. To address the high proportion of missing values for some variables (e.g., chronic kidney disease (CKD) and HIV), multiple imputation technique23 followed by logistic regression modeling was used when assessing factors associated with HCV treatment initiation. We used the Stata “mi impute mvn” command to make 50 imputed datasets for all variables included in the model that had missing values, then used the “mi estimate” prefix when fitting the multivariable logistic regression model that included all the variables. Logistic regression modeling was used to assess factors associated with lack of confirmatory HCVRNA testing and lack of SVR testing, after adjusting for birth year category, race, and gender. For the analysis of rates of SVR and lack of SVR testing, patients who did not have documentation of HCV prescription but were identified as having received HCV therapy through chart review based on HCVRNA levels (N = 38) presented a potential for bias because the HCVRNA test used for this ascertainment was also used to determine the outcome measures (i.e., SVR or receipt of a SVR test). However, because this subset of patients had lower rates of SVR testing, they were retained in these analyses. In all analyses, statistical significance was designated at p value of < 0.05 (2-sided). Analyses were done using Stata 15 statistical software, Stata Corp LP, College Station, TX.
RESULTS
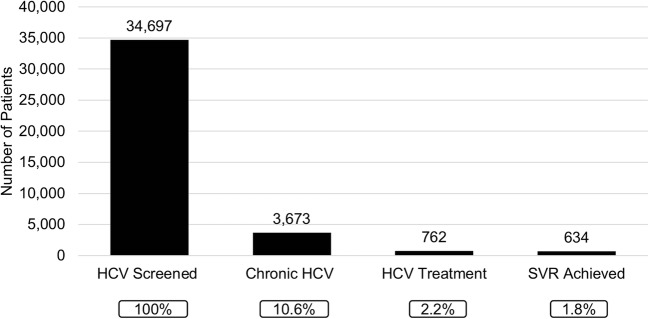
A total of 34,810 birth cohort patients were identified and included in the study, of whom 99.7% had evidence of HCV screening; 95.4% (N = 33,213) had a HCV antibody (HCVAb) test and another 4.3% (N = 1484) had a HCVRNA test without a HCVAb test. Overall, 13.8% (N = 4587) were HCVAb positive. Among HCVAb-positive patients, 61.6% (N = 2827) were viremic and 22.9% (N = 1052) did not receive confirmatory HCVRNA testing, resulting in an overall chronic HCV rate of 10.6% (N = 3673). The presence of CKD (OR 2.24, 95% CI 1.84–2.73; p < 0.001) and absence of HIV (OR 6.15, 95% CI 3.94–9.62; p < 0.001) were associated with lack of confirmatory HCVRNA testing when adjusted for birth year, race, and gender (Supplemental Table 1).
HCV Screening by Race in the Birth Cohort
The rate of HCVAb screening was highest among API patients (98.7%), followed by Latinos (96.5%), other race (95.5%), Whites (92.8%), and Blacks (92.4%) (overall p < 0.001). The proportion of HCVAb positivity was highest among Blacks (27.8%) and Whites (22.9%) compared to other racial groups (other race 11%, Latinos 8.8%, and APIs 2%; overall p < 0.001). The rate of chronic HCV also varied by race as follows: 22.2% in Blacks, 15.9% in Whites, 6.9% in Latinos, 1.7% in APIs, and 7.4% in other race (overall p < 0.001). Table 1 describes patient characteristics overall and by HCV status.
Table 1.
Baseline Characteristics of Entire Cohort and by HCV Status
| Characteristic | Entire cohort | HCV status unknown* | HCV positive | HCV negative | p value† |
|---|---|---|---|---|---|
| N = 34,810 | N = 1165 | N = 3673 | N = 29,972 | ||
| Age (median, IQR) | 59 (55–64) | 59 (55–63) | 60 (55–64) | 59 (55–64) | < 0.001 |
| Birth year (n, %) | |||||
| 1960–1965 | 11,832 (34.0) | 380 (32.6) | 1077 (29.3) | 10,375 (34.6) | < 0.001 |
| 1955–1959 | 9748 (28.0) | 346 (29.7) | 1107 (30.1) | 8295 (27.7) | |
| 1950–1954 | 8402 (24.1) | 305 (26.2) | 1002 (27.3) | 7095 (23.7) | |
| 1945–1949 | 4828 (13.9) | 134 (11.5) | 487 (13.3) | 4207 (14.0) | |
| Race (n, %) | |||||
| White | 8869 (25.5) | 483 (41.5) | 1400 (38.1) | 6986 (23.3) | < 0.001 |
| Black | 7159 (20.6) | 453 (38.9) | 1584 (43.1) | 5122 (17.1) | |
| Latino | 6162 (17.7) | 131 (11.3) | 426 (11.6) | 5605 (18.7) | |
| Asian/PI | 11,501 (33.0) | 58 (5.0) | 190 (5.2) | 11,253 (37.6) | |
| Other | 692 (2.0) | 21 (1.8) | 51 (1.4) | 620 (2.1) | |
| Missing | 427 (1.2) | 19 (1.6) | 22 (0.6) | 386 (1.3) | |
| Gender (n, %) | |||||
| Male | 20,058 (57.6) | 864 (74.2) | 2685 (73.1) | 16,509 (55.1) | < 0.001 |
| Female | 14,752 (42.4) | 301 (25.8) | 988 (26.9) | 13,463 (44.9) | |
| English as preferred language (n, %) | |||||
| Yes | 19,510 (56.1) | 807 (69.3) | 3049 (83.0) | 15,654 (52.2) | < 0.001 |
| No | 11,349 (32.6) | 42 (3.6) | 185 (5.0) | 11,122 (37.1) | |
| Missing | 3951 (11.4) | 316 (27.1) | 439 (12.0) | 3196 (10.7) | |
| CKD (n, %) | |||||
| Present | 4575 (13.1) | 235 (20.2) | 749 (20.4) | 3591 (12.0) | < 0.001 |
| Absent | 21,018 (60.4) | 376 (32.3) | 2912 (79.3) | 17,730 (59.2) | |
| Missing | 9217 (26.5) | 554 (47.6) | 12 (0.3) | 8651 (28.9) | |
| HBV co-infection (n, %) | |||||
| Positive | 1398 (4.0) | 22 (1.9) | 53 (1.4) | 1323 (4.4) | < 0.001 |
| Negative | 30,579 (87.9) | 958 (82.2) | 3386 (92.2) | 26,235 (87.5) | |
| Missing | 2833 (8.1) | 185 (15.9) | 234 (6.4) | 2414 (8.1) | |
| HIV co-infection (n, %) | |||||
| Positive | 1202 (3.5) | 26 (2.2) | 590 (16.1) | 586 (2.0) | < 0.001 |
| Negative | 24,281 (69.8) | 762 (65.4) | 2903 (79.0) | 20,616 (68.8) | |
| Missing | 9327 (26.8) | 377 (32.4) | 180 (4.9) | 8770 (29.3) | |
*HCV status based on HCVRNA testing. Unknown defined as lack of antibody or confirmatory HCVRNA testing. †p value for HCV status, considered statistically significant if < 0.05. CKD, chronic kidney disease, defined as eGFR ≤ 60 mL/min/1.73 m2. HBV co-infection, hepatitis B virus, defined as hepatitis B surface antigen positive. HCV, hepatitis C virus. HIV co-infection, human immunodeficiency virus, defined as the presence of HIV antibody. PI, Pacific Islander
Among those with chronic HCV, a higher proportion of Blacks, APIs, and other race were born before 1950, and more Blacks had CKD than other races (Table 2). Latinos had a higher median ALT level and a higher proportion of low platelets and cirrhosis. APIs had a higher proportion of women and HBV co-infection, but a lower proportion of HIV co-infection. APIs, Latinos, and other race had lower proportions of English as their preferred language. With respect to differences in viral characteristics, the majority of Blacks had HCV genotype 1, and genotype 6 was more prevalent among APIs. Genotype distribution ranged more widely among other racial groups.
Table 2.
Baseline Characteristics of Chronic HCV Patients Categorized by Race
| Characteristic* | All | White | Black | Latino | Asian/PI | Other | p value† |
|---|---|---|---|---|---|---|---|
| N = 3673 | N = 1400 | N = 1584 | N = 426 | N = 190 | N = 51 | ||
| Birth year (n, %) | |||||||
| 1960–1965 | 1077 (29.3) | 471 (33.6) | 364 (23.0) | 162 (38.0) | 54 (28.4) | 19 (37.3) | < 0.001 |
| 1955–1959 | 1107 (30.1) | 434 (31.0) | 477 (30.1) | 120 (28.2) | 58 (30.5) | 9 (17.7) | |
| 1950–1954 | 1002 (27.3) | 333 (23.8) | 507 (32.0) | 100 (23.5) | 45 (23.7) | 13 (25.5) | |
| 1945–1949 | 487 (13.3) | 162 (11.6) | 236 (14.9) | 44 (10.3) | 33 (17.4) | 10 (19.6) | |
| Gender (n, %) | |||||||
| Male | 2685 (73.1) | 1035 (73.9) | 1158 (73.1) | 339 (79.6) | 96 (50.5) | 37 (72.6) | < 0.001 |
| Female | 988 (26.9) | 365 (26.1) | 426 (26.9) | 87 (20.4) | 94 (49.5) | 14 (27.5) | |
| English as preferred language (n, %) | |||||||
| Yes | 3049 (83.0) | 1210 (86.4) | 1382 (87.3) | 305 (71.6) | 105 (55.3) | 39 (76.5) | < 0.001 |
| No | 185 (5.0) | 25 (1.8) | 9 (0.6) | 76 (17.8) | 70 (36.8) | 5 (9.8) | |
| Missing | 439 (12.0) | 165 (11.8) | 193 (12.2) | 45 (10.6) | 15 (7.9) | 7 (13.7) | |
| CKD (n, %) | |||||||
| Yes | 749 (20.4) | 199 (14.2) | 446 (28.2) | 63 (14.8) | 29 (15.3) | 7 (13.7) | < 0.001 |
| No | 2912 (79.3) | 1196 (85.4) | 1132 (71.5) | 363 (85.2) | 160 (84.2) | 44 (86.3) | |
| Missing | 12 (0.3) | 5 (0.4) | 6 (0.4) | 0 (0.0) | 1 (0.5) | 0 (0.0) | |
| HBV co-infection (n, %) | |||||||
| Yes | 53 (1.4) | 15 (1.1) | 22 (1.4) | 3 (0.7) | 11 (5.8) | 1 (2.0) | < 0.001 |
| No | 3386 (92.2) | 1276 (91.1) | 1475 (93.1) | 401 (94.1) | 168 (88.4) | 47 (92.2) | |
| Missing | 234 (6.4) | 109 (7.8) | 87 (5.5) | 22 (5.2) | 11 (5.8) | 3 (5.9) | |
| HIV co-infection (n, %) | |||||||
| Yes | 590 (16.1) | 248 (17.7) | 266 (16.8) | 48 (11.3) | 15 (7.9) | 9 (17.7) | 0.001 |
| No | 2903 (79.0) | 1071 (76.5) | 1265 (79.9) | 360 (84.5) | 154 (81.1) | 36 (70.6) | |
| Missing | 180 (4.9) | 81 (5.8) | 53 (3.4) | 18 (4.2) | 21 (11.1) | 6 (11.8) | |
| HCV genotype (n, %) | |||||||
| 1 | 1891 (51.5) | 622 (44.4) | 918 (58.0) | 226 (53.1) | 95 (50.0) | 16 (31.4) | < 0.001 |
| 2 | 200 (5.5) | 101 (7.2) | 41 (2.6) | 33 (7.8) | 20 (10.5) | 4 (7.8) | |
| 3 | 241 (6.6) | 145 (10.4) | 30 (1.9) | 48 (11.3) | 11 (5.8) | 6 (11.8) | |
| 4 | 29 (0.7) | 8 (0.6) | 17 (1.1) | 1 (0.2) | 2 (1.0) | 1 (2.0) | |
| 6 | 11 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 10 (5.3) | 1 (2.0) | |
| Mixed | 3 (0.1) | 2 (0.1) | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Missing | 1298 (35.3) | 522 (37.3) | 577 (36.4) | 118 (27.7) | 52 (27.4) | 23 (45.1) | |
| Log10 HCVRNA (median, IQR) (IU/mL) | 6.06 (5.48–6.46) | 6.03 (5.40–6.46) | 6.09 (5.54–6.48) | 5.91 (5.32–6.38) | 6.13 (5.59–6.54) | 5.86 (5.07–6.30) | < 0.001 |
| ALT (median, IQR) (U/L) | 38 (24–63) | 38 (24–66) | 37 (25–57) | 42 (25–76) | 38 (21–69) | 31 (19–80) | 0.001 |
| Low platelets (n, %) (< 150 × 103/uL) | |||||||
| Yes | 732 (19.9) | 302 (21.6) | 239 (15.1) | 129 (30.3) | 41 (21.6) | 13 (25.5) | < 0.001 |
| No | 2926 (79.7) | 1094 (78.1) | 1338 (84.5) | 295 (69.3) | 148 (77.9) | 37 (72.6) | |
| Missing | 15 (0.4) | 4 (0.3) | 7 (0.4) | 2 (0.5) | 1 (0.5) | 1 (2.0) | |
| FIB-4 score | |||||||
| < 1.45 | 1109 (30.2) | 446 (31.9) | 486 (30.7) | 109 (25.6) | 47 (24.7) | 14 (27.5) | < 0.001 |
| 1.45–3.25 | 1641 (44.7) | 595 (42.5) | 764 (48.2) | 165 (38.7) | 95 (50.0) | 18 (35.3) | |
| > 3.25 | 860 (23.4) | 332 (23.7) | 315 (19.9) | 139 (32.6) | 47 (24.7) | 16 (31.4) | |
| Missing | 63 (1.7) | 27 (1.9) | 19 (1.2) | 13 (3.1) | 1 (0.5) | 3 (5.9) | |
*Missing data: 22 for race, 4 for HCVRNA, 8 for ALT. †p value considered statistically significant if < 0.05. ALT, alanine aminotransferase. CKD, chronic kidney disease, defined as eGFR ≤ 60 mL/min/1.73 m2. FIB-4, fibrosis-4 score. HBV co-infection, hepatitis B virus, defined as hepatitis B surface antigen positive. HCV, hepatitis C virus. HIV co-infection, human immunodeficiency virus, defined as the presence of HIV antibody. PI, Pacific Islander
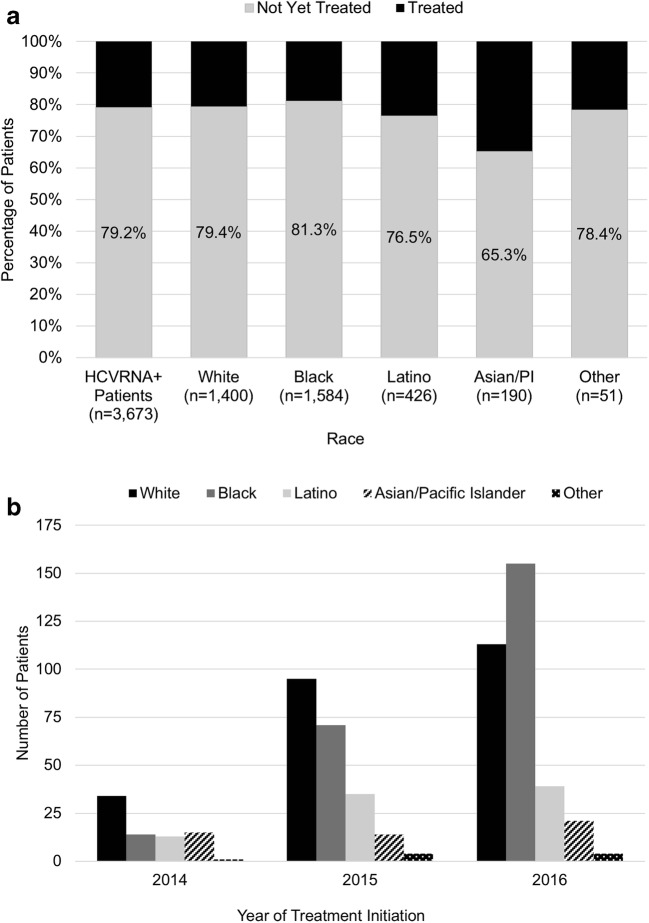
Factors Associated with HCV Treatment Initiation
HCV treatment was initiated in 20.8% (N = 762) of patients with chronic HCV by August 31, 2017. The rate of HCV treatment initiation varied by race (overall p < 0.001) (Fig. 1a). Although APIs had the highest rate of treatment initiation at 34.7% and Blacks had the lowest rate at 18.8%, there was an increase over time in the number of patients receiving therapy irrespective of race (Fig. 1b). On univariate analysis, birth years 1950–1954, API race, and HIV co-infection were associated with a greater likelihood of treatment initiation, while English as the preferred language, CKD, cirrhosis, higher ALT levels, and higher HCVRNA levels were associated with lower rates of treatment initiation (Table 3). On multivariable analysis, API race compared to White race (OR 1.20, 95% CI 1.08–1.33; p = 0.001) and the presence of HIV co-infection (OR 1.58, 95% CI 1.07–2.34; p = 0.02) were associated with higher odds of initiating HCV treatment. On the other hand, CKD (OR 0.47, 95% CI 0.37–0.60; p < 0.001), higher ALT levels (OR 0.39, 95% CI 0.35–0.44 for every doubling of ALT value; p < 0.001), and higher HCVRNA levels (OR 0.83, 95% CI 0.80–0.87 for every 10-fold increase in HCVRNA value; p < 0.001) were associated with a lower likelihood of HCV treatment initiation. Interestingly, patients with English as their preferred language were less likely to receive HCV treatment than those who preferred a non-English language (OR 0.54, 95% CI 0.36–0.79; p = 0.002).
Figure 1.
Treatment initiation among chronic HCV patients. a Percentage of patients initiated on HCV treatment categorized by race (overall p < 0.001). b Frequency of HCV treatment by race over time. HCV, hepatitis C virus.
Table 3.
Factors Associated with HCV Treatment Initiation in Univariate (Unadjusted) and Multivariable (Adjusted) Analyses (N = 762)
| Characteristic | Unadjusted OR (95% CI) |
p value† | Adjusted OR* (95% CI) |
p value† |
|---|---|---|---|---|
| Birth year | ||||
| 1960–1965 | 1.00 (Ref) | N/A | 1.00 (Ref) | N/A |
| 1955–1959 | 1.13 (0.92–1.39) | 0.25 | 1.05 (0.83–1.32) | 0.69 |
| 1950–1954 | 1.27 (1.03–1.57) | 0.03 | 1.20 (0.95–1.52) | 0.13 |
| 1945–1949 | 0.97 (0.74–1.27) | 0.80 | 0.86 (0.63–1.16) | 0.33 |
| Race | ||||
| White | 1.00 (Ref) | N/A | 1.00 (Ref) | N/A |
| Black | 0.92 (0.77–1.20) | 0.35 | 1.02 (0.92–1.13) | 0.66 |
| Latino | 1.22 (0.94–1.58) | 0.14 | 1.06 (0.95–1.17) | 0.30 |
| Asian/PI | 2.05 (1.48–2.84) | < 0.001 | 1.20 (1.08–1.33) | 0.001 |
| Other | 1.18 (0.61–2.28) | 0.62 | 0.97 (0.83–1.12) | 0.65 |
| Male | 0.87 (0.73–1.04) | 0.14 | 1.11 (0.91–1.36) | 0.32 |
| English as preferred language | 0.41 (0.30–0.56) | < 0.001 | 0.54 (0.36–0.79) | 0.002 |
| CKD | 0.58 (0.47–0.71) | < 0.001 | 0.47 (0.37–0.60) | < 0.001 |
| HBV co-infection | 0.89 (0.44–1.78) | 0.74 | 0.59 (0.27–1.29) | 0.18 |
| HIV co-infection | 1.48 (1.05–2.08) | 0.02 | 1.58 (1.07–2.34) | 0.02 |
| Cirrhosis | 0.66 (0.54–0.81) | < 0.001 | 1.10 (0.87–1.39) | 0.43 |
| Log2 ALT | 0.39 (0.35–0.43) | < 0.001 | 0.39 (0.35–0.44) | < 0.001 |
| Log10 HCVRNA | 0.83 (0.80–0.86) | < 0.001 | 0.83 (0.80–0.87) | < 0.001 |
*The adjusted OR column shows results for a single multivariable model that included all the variables listed in the table. Thus, each variable is adjusted for all the others shown. †p value considered statistically significant if < 0.05. ALT, alanine aminotransferase. CKD, chronic kidney disease. Cirrhosis, defined as FIB-4 > 3.25. HBV co-infection, hepatitis B virus, defined as hepatitis B surface antigen positive. HCV, hepatitis C virus. HIV co-infection, human immunodeficiency virus, defined as the presence of HIV antibody. PI, Pacific Islander
Factors Associated with SVR
Figure 2 shows the cascade of HCV care for those screened for HCV. By the end of the study period, 91.9% (N = 700) of those who had initiated treatment were due for SVR. Of these, 634 (90.6%) patients achieved SVR, 9 patients (1.3%) did not achieve SVR, and 57 patients (8.1%) did not have an available SVR blood test to assess SVR status. SVR rate was lowest among Latinos at 81.3% compared to other races (Blacks 90.8%, Whites 92.2%, APIs 95.2%, and other race 100%; overall p = 0.01). Among patients who had SVR blood test results (N = 643), SVR rate was 98.6% (634/643). Among the nine patients who did not achieve SVR, the median age was 63 years (IQR 58–65 years), 77.8% were male, 55.6% were Latino, and 77.8% had genotype 1 infection. Two patients were co-infected with HIV, three had cirrhosis, and five had low platelet counts prior to initiation of HCV therapy.
Figure 2.
Cascade of HCV care. Number of HCV screened patients at each step of the HCV care pathway. Chronic HCV defined as detectable HCVRNA. HCV, hepatitis C virus. SVR, sustained virologic response.
Factors Associated with Lack of SVR Testing
Among the 57 patients who did not have SVR testing done following completion of therapy, the absence of HIV co-infection was associated with lack of SVR testing (OR 2.51, 95% CI 1.11–5.64; p = 0.03) on unadjusted analysis (Table 4). On multivariable analysis, in addition to absence of HIV co-infection (OR 2.82, 95% CI 1.21–6.54; p = 0.02), birth year 1960–1965 was also independently associated (OR 3.25, 95% CI 1.04–10.1 compared to birth year 1945–1949; p = 0.04) with lack of SVR testing. The odds of not receiving SVR testing increased with later birth years. There was no statistically significant association of race with lack of SVR testing, but Latinos appeared to have higher odds of lacking SVR testing compared to Whites (OR 1.90, 95% CI 0.87–4.16; p = 0.11).
Table 4.
Multivariable Analysis of Factors Associated with Lack of SVR Testing (N = 57)
| Characteristic | Unadjusted OR (95% CI) | p value† | Adjusted OR* (95% CI) | p value† |
|---|---|---|---|---|
| Birth year | ||||
| 2003 1945–1949 | 1.00 (Ref) | N/A | 1.00 (Ref) | N/A |
| 1950–1954 | 1.56 (0.51–4.82) | 0.44 | 1.48 (0.47–4.62) | 0.50 |
| 1955–1959 | 1.52 (0.49–4.68) | 0.47 | 1.71 (0.54–5.36) | 0.36 |
| 1960–1965 | 2.69 (0.89–8.12) | 0.08 | 3.25 (1.04–10.1) | 0.04 |
| Race | ||||
| White | 1.00 (Ref) | N/A | 1.00 (Ref) | N/A |
| Black | 1.08 (0.57–2.05) | 0.80 | 1.34 (0.69–2.60) | 0.39 |
| Latino | 1.95 (0.93–4.09) | 0.08 | 1.90 (0.87–4.16) | 0.11 |
| Asian/PI | 0.63 (0.18–2.20) | 0.47 | 0.76 (0.20–2.87) | 0.68 |
| Other | 0.00 | N/A | 0.00 | N/A |
| Male | 1.26 (0.67–2.36) | 0.47 | 1.21 (0.63–2.32) | 0.58 |
| English as preferred language | 1.54 (0.54–4.39) | 0.42 | 1.93 (0.59–6.26) | 0.28 |
| CKD | 0.37 (0.13–1.04) | 0.06 | 0.44 (0.15–1.26) | 0.13 |
| HIV co-infection | ||||
| Presence | 1.00 (Ref) | N/A | 1.00 (Ref) | N/A |
| Absence | 2.51 (1.11–5.64) | 0.03 | 2.82 (1.21–6.54) | 0.02 |
| Low platelets (< 150 × 103/uL) | 1.11 (0.59–2.08) | 0.75 | 1.22 (0.63–2.36) | 0.55 |
*Adjusted for birth year, race, gender. †p value considered statistically significant if < 0.05. CKD, chronic kidney disease. HIV co-infection, human immunodeficiency virus, defined as the presence of HIV antibody. PI, Pacific Islander. SVR, sustained virologic response
DISCUSSION
This is the first study to evaluate HCV screening and the association of race on linkage to HCV DAA treatment and cure in a large underserved and ethnically diverse birth cohort engaged in primary care. In this cohort, we observed a near-universal rate of HCV screening, but nearly a quarter of HCVAb-positive patients did not receive confirmation of active HCV infection with HCVRNA testing. The rate of HCV screening and chronic HCV also varied by race. While HCV screening increased in this cohort over time, APIs in particular had the highest rates of screening and treatment initiation since the introduction of the birth cohort testing recommendation. In addition, Blacks had the highest rate of chronic HCV infection among racial groups. Despite an over 90% rate of HCV cure among treated patients (98.6% among those with SVR testing), similar to other studies, there was a significant gap in treatment initiation observed in this population.
In our study, nearly 100% of birth cohort patients engaged in care were screened with antibody testing for HCV, a screening rate significantly higher than the 21–64% reported in other populations also engaged in care.24–26 Differences in practice setting and their established screening protocols (e.g., jail, primary care clinics, integrated care systems), as well as the higher rates of HCV risk factors observed in the underserved that may translate into heightened awareness of HCV among patients and providers,27–29 may have contributed to the wide range of reported screening rates. Interestingly, with the introduction of the birth cohort recommendations, we observed the greatest rise in HCV screening and treatment among APIs, which may be attributed to lower rates of traditional HCV risk factors observed in this population. Indeed, in a study by Kin et al., 68.5% of HCV-infected APIs lacked traditional risk factors and instead other factors such as acupuncture were independently associated with HCV infection.30 Thus, the implementation of birth cohort testing appears to have improved HCV identification and potential linkage to therapy among populations who may have otherwise been excluded by risk-based HCV screening.
The rate of chronic HCV (11%) in our cohort was similar to rates reported in other healthcare settings,31,32 and the proportion initiated on therapy (21%) was slightly higher than the 18% reported from large integrated healthcare organizations caring for patients with predominantly private insurance (62%).33 Thus, despite the challenges of caring for underserved patients who face higher rates of mental illness34 and substance use,27 we achieved comparable linkage to treatment, suggesting the feasibility of treatment uptake in this population. This may reflect expanded access to HCV medications through Medicaid or drug companies’ compassionate use programs, and the expansion of HCV treatment to primary care settings. Moreover, integrated patient-centered interventions such as direct access to formal HCV education classes through the liver clinic in our system has already been shown to improve patient knowledge and HCV care coordination.35,36 Importantly, patient factors also influenced treatment initiation. Although Blacks had the lowest rate of treatment initiation, Black race was not independently associated with therapy. On the other hand, API race and HIV co-infection were predictive of linkage to HCV therapy. The reason for increased uptake of therapy among APIs is unclear, but may be related to higher rates of testing that specifically occurred in this cohort during the study period. Furthermore, English language was associated with decreased treatment. The impact of language on access to and initiation of HCV treatment is unknown. Our finding may be related to unmeasured factors or potential for ascertainment bias inherent to medical record data collection. In a smaller Canadian study of 725 Canada-born and 185 immigrant patients with universal access to healthcare, language barrier was not associated with HCV treatment initiation.37 Additional studies are needed to explore the relationship between language and HCV treatment uptake.
Overall, about 91% of our treated patients had documented SVR, a rate consistent with real-world data showing SVR rates of greater than 95% with use of DAA therapies.28,38 Latinos however had the lowest SVR rate among racial groups and more than half of the nine patients who did not achieve SVR were Latino. A real-world study of veterans has also shown that Latinos had increased rates of treatment failure in the DAA era.39 Although in our cohort correlations of treatment failure could not be assessed due to a low number of events, a higher proportion of Latinos had cirrhosis, which may have influenced treatment response.
The proportion of patients without documented SVR testing to confirm cure following treatment was relatively low, but we identified the absence of HIV co-infection and younger age as factors associated with lack of SVR testing. This may be related to a higher rate of engagement in primary care among HIV/HCV co-infected patients40 and older patients. Therefore, ongoing engagement in medical care along with emphasis on the importance of confirmatory SVR testing especially in the younger birth cohort patients will be critical to enhancing documentation of HCV treatment response in this population.
While this study was limited by its retrospective design and the risk of misclassification and ascertainment bias inherent to self-reported race and use of medical record data to document language and treatment initiation, our large cohort size and racial diversity enabled us to study the role of race on the HCV care continuum in a traditionally underscreened and undertreated population at risk for HCV health disparities. Since the population accessed was engaged in primary care, our results are not generalizable to all underserved populations. Specifically, we were unable to evaluate those not already engaged in care, who remain an important group at risk for lacking linkage to HCV care, limiting our ability to make recommendations regarding interventions to address the unique needs of that population. To minimize misclassification, all data related to the receipt of HCV therapy was assessed by individual chart review to confirm HCV treatment initiation and response in the viremic cohort.
In conclusion, the introduction of the HCV birth cohort testing recommendation appears to have impacted rates of HCV testing in the underserved, with higher rates of testing and treatment initiation in APIs compared to other races. However, despite near universal HCVAb testing and high rates of cure with therapy in this large racially diverse underserved birth cohort, significant gaps in confirmation of active HCV infection with viral load testing and linkage to therapy were identified. Thus, HCV testing and awareness alone are insufficient to address the burden of HCV disease in this population. Consequently, as opportunities for HCV treatment expand to primary care settings, prioritizing interventions to address these gaps in care are critical to eradicating HCV in the underserved population. Potential clinical care recommendations include the following: (1) establishing culturally appropriate patient education with emphasis on HCV confirmation, treatment, and documentation of cure; (2) broad dissemination of HCV clinical guidelines and cultivating positive attitudes towards HCV care among providers and interprofessional teams (e.g., social workers, health educators) caring for the underserved; (3) enhanced use and integration of existing safety-net resources to address potential barriers to receipt and completion of HCV testing and therapy (e.g., use of health navigators, mental health and substance use services, transportation services, access to interpreters, access to insurance and patient assistance programs); (4) increasing HCV treaters; and (5) establishing practice-based quality metrics for the HCV care continuum.
Electronic supplementary material
(DOCX 15 kb)
Acknowledgments
Prior Presentations
A poster based on an earlier version of this paper was previously presented at Digestive Disease Week in May 2017.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ALT
Alanine aminotransferase
- API
Asian/Pacific Islander
- CKD
Chronic kidney disease
- DAA
Direct-acting antivirals
- HBV
Hepatitis B virus
- HBsAg
Hepatitis B surface antigen
- HBsAb
Hepatitis B surface antibody
- HCV
Hepatitis C virus
- HCVAb
Hepatitis C antibody
- HIV
Human immunodeficiency virus
- IDSA
Infectious Diseases Society of America
- IQR
Interquartile range
- SVR
Sustained virologic response
- ZSFG
Zuckerberg San Francisco General
Funders
This work was supported by National Institutes of Health, Grant Number K24AA022523 (to M.K.).
Compliance with Ethical Standards
This study was approved by the University of California San Francisco Committee on Human Research.
Conflict of Interest
The authors do not have any conflicts of interest to report in connection with this manuscript.
Dr. Khalili’s full disclosure includes research grant funding (to her institution) from Gilead Sciences Inc., Intercept Pharmaceuticals, and Abbvie, and she has served on the scientific advisory boards of Gilead Sciences Inc., and Intercept Pharmaceuticals.
References
- 1.Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising mortality associated with hepatitis C virus in the United States, 2003-2013. Clin Infect Dis. 2016;62:1287–8. doi: 10.1093/cid/ciw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Khoury AC, Klimack WK, Wallace C, Razavi H. Economic burden of hepatitis C-associated diseases in the United States. J Viral Hepat. 2012;19:153–60. doi: 10.1111/j.1365-2893.2011.01563.x. [DOI] [PubMed] [Google Scholar]
- 3.van der Meer AJ. Achieving sustained virological response: what’s the impact on further hepatitis C virus-related disease? Expert Rev Gastroenterol Hepatol. 2015;9:559–66. doi: 10.1586/17474124.2015.1001366. [DOI] [PubMed] [Google Scholar]
- 4.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 5.Younossi ZM, Stepanova M, Henry L, et al. Effects of sofosbuvir-based treatment, with and without interferon, on outcome and productivity of patients with chronic hepatitis C. Clin Gastroenterol Hepatol. 2014;12:1349–59. doi: 10.1016/j.cgh.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 6.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 7.Reilley B, Leston J, Hariri S, et al. Birth cohort testing for hepatitis C virus - Indian health service 2012-2015. MMWR Morb Mortal Wkly Rep. 2016;65:467–9. doi: 10.15585/mmwr.mm6518a3. [DOI] [PubMed] [Google Scholar]
- 8.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–37. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Testing Recommendations for Hepatitis C Virus Infection. Centers for Disease Control, 2012. Accessed October 11, 2017, at https://www.cdc.gov/hepatitis/hcv/guidelinesc.htm
- 10.Final Update Summary: Hepatitis C: Screening. U.S. Preventive Services Task Force, 2013. Accessed October 11, 2017, at https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening.
- 11.Asrani SK, Davis GL. Impact of birth cohort screening for hepatitis C. Curr Gastroenterol Rep. 2014;16:381. doi: 10.1007/s11894-014-0381-5. [DOI] [PubMed] [Google Scholar]
- 12.McEwan P, Ward T, Yuan Y, Kim R, L’Italien G. The impact of timing and prioritization on the cost-effectiveness of birth cohort testing and treatment for hepatitis C virus in the United States. Hepatology. 2013;58:54–64. doi: 10.1002/hep.26304. [DOI] [PubMed] [Google Scholar]
- 13.Rein DB, Smith BD, Wittenborn JS, et al. The cost-effectiveness of birth-cohort screening for hepatitis C antibody in U.S. primary care settings. Ann Intern Med. 2012;156:263–70. doi: 10.7326/0003-4819-156-4-201202210-00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kattakuzhy S, Gross C, Emmanuel B, et al. Expansion of Treatment for Hepatitis C Virus Infection by Task Shifting to Community-Based Nonspecialist Providers: A Nonrandomized Clinical Trial. Ann Intern Med. 2017;167:311–8. doi: 10.7326/M17-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muir AJ, Naggie S. Hepatitis C virus treatment: is it possible to cure all hepatitis c virus patients? Clin Gastroenterol Hepatol. 2015;13:2166–72. doi: 10.1016/j.cgh.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C.
- 17.Spradling PR, Xing J, Rupp LB, et al. Uptake of and factors associated with direct-acting antiviral therapy among patients in the chronic hepatitis cohort study, 2014 to 2015. J Clin Gastroenterol. 2017. [DOI] [PMC free article] [PubMed]
- 18.Backus LI, Belperio PS, Loomis TP, Mole LA. Impact of race/ethnicity and gender on HCV screening and prevalence among U.S. veterans in Department of Veterans Affairs Care. Am J Public Health. 2014;104(Suppl 4):S555–61. doi: 10.2105/AJPH.2014.302090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vutien P, Hoang J, Brooks L, Jr, Nguyen NH, Nguyen MH. Racial disparities in treatment rates for chronic hepatitis c: analysis of a population-based cohort of 73,665 patients in the United States. Medicine (Baltimore) 2016;95:e3719. doi: 10.1097/MD.0000000000003719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falade-Nwulia O, Mehta SH, Lasola J, et al. Public health clinic-based hepatitis C testing and linkage to care in Baltimore. J Viral Hepat. 2016;23:366–74. doi: 10.1111/jvh.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrlich S. Zuckerberg San Francisco General FY1516 Annual Report2016.
- 22.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 23.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourgi K, Brar I, Baker-Genaw K. Health disparities in hepatitis c screening and linkage to care at an integrated health system in Southeast Michigan. PLoS One. 2016;11:e0161241. doi: 10.1371/journal.pone.0161241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geboy AG, Mahajan S, Daly AP, et al. High hepatitis C infection rate among baby boomers in an urban primary care clinic: results from the HepTLC initiative. Public Health Rep. 2016;131(Suppl 2):49–56. doi: 10.1177/00333549161310S209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akiyama MJ, Kaba F, Rosner Z, Alper H, Holzman RS, MacDonald R. Hepatitis C screening of the “birth cohort” (Born 1945-1965) and younger inmates of new York City jails. Am J Public Health. 2016;106:1276–7. doi: 10.2105/AJPH.2016.303163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kowalchuk AA, Gonzalez SJ, Zoorob RJ. Substance use issues among the underserved: United States and International Perspectives. Prim Care. 2017;44:113–25. doi: 10.1016/j.pop.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Beck KR, Kim N, Khalili M. Sofosbuvir-containing regimens for chronic hepatitis C are successful in the safety-net population: a real-world experience. Dig Dis Sci. 2016;61:3602–8. doi: 10.1007/s10620-016-4340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sulkowski MS, Mast EE, Seeff LB, Thomas DL. Hepatitis C virus infection as an opportunistic disease in persons infected with human immunodeficiency virus. Clin Infect Dis. 2000;30(Suppl 1):S77–84. doi: 10.1086/313842. [DOI] [PubMed] [Google Scholar]
- 30.Kin KC, Lin B, Chaung KT, et al. Less-established risk factors are common in Asian Americans with hepatitis C virus: a case-controlled study. Dig Dis Sci. 2013;58:3342–7. doi: 10.1007/s10620-013-2884-6. [DOI] [PubMed] [Google Scholar]
- 31.Patel RC, Vellozzi C, Smith BD. Results of hepatitis C birth-cohort testing and linkage to care in selected U.S. sites, 2012–2014. Public Health Rep. 2016;131(Suppl 2):12–9. doi: 10.1177/00333549161310S203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong RJ, Campbell B, Liu B, Baden R, Bhuket T. Sub-optimal testing and awareness of HCV and HBV among high risk individuals at an underserved safety-net hospital. J Community Health 2017. [DOI] [PubMed]
- 33.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–61. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devine M, DeCaporale-Ryan L, Lim M, Berenyi J. Psychological issues in medically underserved patients. Prim Care. 2017;44:99–112. doi: 10.1016/j.pop.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Lubega S, Agbim U, Surjadi M, Mahoney M, Khalili M. Formal hepatitis C education enhances HCV care coordination, expedites HCV treatment and improves antiviral response. Liver Int. 2013;33:999–1007. doi: 10.1111/liv.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surjadi M, Torruellas C, Ayala C, Yee HF, Jr, Khalili M. Formal patient education improves patient knowledge of hepatitis C in vulnerable populations. Dig Dis Sci. 2011;56:213–9. doi: 10.1007/s10620-010-1455-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giordano C, Druyts EF, Garber G, Cooper C. Evaluation of immigration status, race and language barriers on chronic hepatitis C virus infection management and treatment outcomes. Eur J Gastroenterol Hepatol. 2009;21:963–8. doi: 10.1097/MEG.0b013e328326f598. [DOI] [PubMed] [Google Scholar]
- 38.Norton BL, Fleming J, Bachhuber MA, et al. High HCV cure rates for people who use drugs treated with direct acting antiviral therapy at an urban primary care clinic. Int J Drug Policy. 2017;47:196–201. doi: 10.1016/j.drugpo.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su F, Green PK, Berry K, Ioannou GN. The association between race/ethnicity and the effectiveness of direct antiviral agents for hepatitis C virus infection. Hepatology. 2017;65:426–38. doi: 10.1002/hep.28901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberson JL, Lagasca AM, Kan VL. Comparison of the hepatitis C continua of care between HCV/HIV co-infected and HCV mono-infected patients in two treatment eras during 2008-2015. AIDS Res Hum Retrovir. 2017. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 15 kb)