Abstract
Introduction
Invasion of other tissues during bloodborne metastasis in part requires adhesion of cancer cells to vascular endothelium by specific fluid shear-dependent receptor–ligand interactions. This study investigates the hypothesis that the adhesion is mediated by ligands shared between endothelial E-selectin and Galectin-1 (Gal-1), both of which are upregulated during inflammation and cancer.
Methods
Flow chamber adhesion and dynamic biochemical tissue analysis (DBTA) assays were used to evaluate whether Gal-1 modulates E-selectin adhesive interactions of breast cancer cells and tissues under dynamic flow conditions, while immunocytochemistry, immunohistochemistry, western blotting, and fluorescence anisotropy were used to study molecular interactions under static conditions.
Results
Dynamic adhesion assays revealed a shear-dependent binding interaction between Gal-1hFc treated breast cancer cells and tissues and E-selectin-coated beads, causing ~ 300% binding increase of the beads compared to negative controls. Immunocyto- and immunohistochemical analyses showed that Gal-1 and E-selectin fluorescent signals colocalized on cells and tissues at ~ 75% for each assay. Immunoprecipitation and Western blotting of Mac-2BP from breast cancer cell lysates revealed that Gal-1 and E-selectin share Mac-2BP as a ligand, while fluorescence anisotropy and circulating tumor cell model systems exhibited competitive or antagonistic binding between Gal-1 and E-selectin for shared ligands, including Mac-2BP. Furthermore, Mac-2BP functional blockade inhibited the effects of Gal-1 on E-selectin binding.
Conclusions
In summary, this investigation reveals a shear-dependent interaction between E-selectin and Gal-1 that may be due to intermediation by a similar or shared ligand(s), including Mac-2BP, which may provide a rational basis for development of novel diagnostics or therapeutics for breast cancer.
Keywords: Mac-2BP, Metastasis, N-Acetyllactosamine
Introduction
The typical 5-year survival rate for a patient with breast cancer drops significantly once the cancer has created a metastatic colony, from 99% to approximately 26%, respectively.1 With over 200,000 new cases and 40,000 deaths per year, metastatic breast cancer has become the second leader in death of female cancer patients.1 Circulating tumor cells (CTCs), progenitors of metastatic colonies,7 possess mechanisms that help protect them as they travel throughout the circulatory system establishing new tumor sites and strengthening the initial colony with more aggressive capabilities. The CTCs interact with certain molecules along the endothelial cell layer or on the CTCs themselves to upregulate metastatic binding potential,7,24,31,33,34 hence the elucidation of novel therapeutic targets to reduce or eliminate CTCs and the resultant colonies could reduce mortality for breast cancer and give insight into other metastatic cancers. The two molecules investigated in this study, which are believed to interact with structurally similar ligands during the metastatic process to mediate the adhesion of CTCs to the endothelium, are E-selectin and Galectin-1 (Gal-1).
E-selectin, a type-1 transmembrane glycoprotein, is utilized by endothelial cells within the circulatory system to aid in the recruitment of immune cells to sites of inflammation.5,7,18,29,31,33,36 The E-selectin ligands expressed on immune cells mediate adhesion to E-selectin’s carbohydrate recognition domains (CRDs) that extend past the glycocalyx of endothelial cells.6 This adhesion to E-selectin is dictated by specific carbohydrate skeletons within the ligands, many of which express the carbohydrates sialyl Lewis X or sialyl Lewis A (sLex and sLea, respectively).5,7,18,29,31,33,36
CTCs express certain E-selectin ligands with these decorations, which are then recognized by the endothelial cells expressing increased amounts of E-selectin near or at the site of inflammation.7,31,33 This expression of E-selectin ligands on CTCs can be confirmed with the increased expression of sLex or sLea on CTCs and the corresponding increased expression of E-selectin in inflamed sites of the body.33 This effect is especially true for breast cancer cells in circulation, as they have been noted to have increased α2,3-sialylation, α1,3-, and α1,4-fucosylation expression,7,31 which has been confirmed with the breast cancer cell line ZR-75-1 and its expression of Mac-2BP.33
Gal-1 is a member of the S-type lectin family known as galectins, with an approximate monomer size of 14 kDa.2,6,11–13,16,17,35 Gal-1 is a secretory protein normally expressed as a homodimer in a multitude of both invertebrate and vertebrate tissue that can regulate T cell life cycle, promote cell congregation, bind β-galactoside, and has been found in abundance with higher malignancy cancers, which may cause the “immune-privileged sites” frequently observed within cancer.2,6,11–13,16,17,35 This homodimer frequently binds to glycans that have not been sialylated, with Gal-1 having affinity to bind to N-Acetyllactosamine (LacNAc), which dominates most of the ligand backbones associated with Gal-1.6,11–13,16,17,35
The distinctions between E-selectin and Gal-1 are varied, including general structure, functions within the system, and contributions to pro-inflammatory adhesion versus anti-inflammatory adhesion regulation of T cells, respectively.13,15,22,23,26,37 While these two molecules have seemingly little in common, other than being lectins, there are key points that allude to a more dynamic relationship during breast cancer metastasis. Beyond their upregulation during cancer, especially more malignant cancers,2,5,7,12,13,18,31,33,35 E-selectin and Gal-1 ligands seem to share the LacNAc backbone as a main carbohydrate skeletal feature.2,5–7,11–13,16–18,29,31,33,37 For E-selectin, LacNAc is the framework of the terminal sialylation and fucosylation moieties of its minimal ligand, sLex and sLea.25 Thus, both E-selectin and Gal-1 are related through their common general ligand structure which is further implied through the interaction that each molecule has with a protein known as Mac-2BP, also called 90 K.33,35 We have previously found that Mac-2BP is indeed a ligand for E-selectin, while Tinari et al. discovered that Mac-2BP and Gal-1 interacted even while another protein (Galectin-3) was simultaneously bound to Mac-2BP.33,35 Mac-2BP has multiple free asparagine locations within the protein that can allow for multiple scaffolding sites and, therefore, the ability to bind to multiple receptors at once.36 This evidence then poses a possible model of interaction between E-selectin and Gal-1 during breast cancer adhesion that while both are upregulated during the process, they interact with Mac-2BP or another ligand that can regulate or alter a metastatic site. Elucidating how E-selectin and Gal-1 interact with similarly structured ligands, possibly even the same ligands, during the metastatic cascade could give insight on how to therapeutically target metastasis in breast cancer patients.
Materials and Methods
Cell Culture
ZR-75-1 breast ductal carcinoma cells and THP-1 acute monocytic leukemia cells (American Type Culture Collection (ATCC), Manassas, VA) were cultured with RPMI-1640 cell culture medium (Life Technologies, Carlsbad, CA) with 10% heat inactivated fetal bovine serum (FBS) (HyClone, Logan, UT) and 1 × penicillin streptomycin (Penstrep) (HyClone), while SK-BR-3 breast adenocarcinoma cells (ATCC) were cultured with McCoy’s 5A cell culture medium (ATCC) that similarly contained 10% FBS and 1 × Penstrep. Breast cancer cell lines were harvested using a 1:100 0.5 M ethylenediaminetetraacetic acid (EDTA) (Quality Biological Inc., Gaithersburg, MD): Dulbecco’s phosphate buffered saline− (DPBS−) (HyClone) wash and lifted with a 1:3 ratio of 0.25% Trypsin (HyClone) containing EDTA: DPBS−. The suspended THP-1 cell line was harvested every 3–4 days upon confluency and harvested media was replaced with fresh media described above. One to two days prior to flow chamber assays, 60,000–80,000 ZR-75-1 cells were plated using 6.5 mm diameter flexiPERM gaskets (Greiner Bio-one, Monroe, NC) on sterile 35 × 10 mm tissue culture dishes (Corning Inc., Corning, NY) to create confluent cell monolayers for testing via flow chamber.
Antibodies and Reagents
The isotype controls, mouse IgG1, human Fc, and rabbit IgG, were obtained from BD Biosciences (San Jose, CA), Bethyl Laboratories Inc. (Montgomery, TX) and Sigma-Aldrich (St. Louis, MO), respectively. Murine E-selectin hFc chimera was obtained from R&D Systems (Minneapolis, MN), while Mac-2BP polyclonal antibody clone H300 was obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and Mac-2BP monoclonal antibody (mAb) clone SP-2 was obtained from ThermoFisher Scientific (Waltham, MA). The flow cytometry secondary antibodies, αhIgG Alexa Fluor 488, αhIgG Alexa Fluor 568, and αrabbitIgG Alexa Fluor 568, were acquired from Life Technologies, while the Western blotting secondaries, αhIgG-alkaline phosphatase (AP) and αrabbitIgG-AP, were obtained from Southern Biotech (Birmingham, AL).
Gal-1hFc Description, Isolation, and Conjugation
To mimic physiologic dimeric Gal-1, chimeric dimer Gal-1hFc that maintains its structure for extended periods of time and has the same functions as Gal-1, even under physiological conditions, was used in place of commercially available monomer Gal-1.12 This molecule is a chimeric fusion protein with a human Fc (heavy chain) fused to two mouse galectin-1 carbohydrate recognition domains.11 Gal-1hFc was isolated from transfected HEK293F cells that were generously donated by Dr. Charles Dimitroff of the Department of Dermatology, Brigham and Women’s Hospital, Harvard Medical School (Boston, MA). HEK293F cell supernatant was recovered from culture and then isolated through affinity chromatography using Sartobind® Protein A membrane adsorbers (Sartorius Corporation, Bohemia, NY).8 The isolated Gal-1hFc was quantified then conjugated with Qdot 705 fluorescent particle using the manufacturer’s protocol (Life Technologies).
Bead Preparation
Protein A polystyrene beads with a diameter of 9 μm (Bangs Laboratories, Fisher, IN) were prepared using Tris-buffered saline (TBS) pH 4.0, and TBS pH 8.2, 1% BSA in DPBS+. The beads were first centrifuged at 14,000 RPM for 2 min with the factory storage solution removed after centrifugation. Washes with TBS of pH 4.0 followed at the same speed and time. The 1% BSA in DPBS+ was then used as a blocking buffer with the beads incubating on a rotisserie for 30 min. The beads were then centrifuged out of the DPBS containing BSA and washed with TBS pH 8.2 twice. Once the washings were completed, the beads were ready for coating with E-selectin for flow chamber and dynamic biochemical tissue analysis (DBTA) assays.
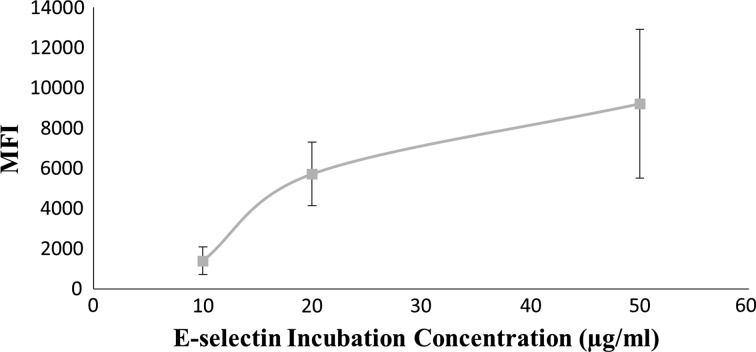
A saturation curve was generated for E-selectin on Protein A beads using incubation solutions ranging from 10 to 50 μg/mL of E-selectin on Protein A coated beads. Beads were incubated for 1 h in the primary reagent, washed with 1% BSA/DPBS+, and then incubated 30 min in 10 μg/mL of secondary on constant rotation at room temperature for both incubations. Figure 1 shows the average saturation curve for E-selectin incubation concentration on Protein A beads analyzed by flow cytometry (described below), in which the specific binding saturation point appears to be achieved at 20 μg/mL.
Figure 1.
Saturation curve of protein A polystyrene beads conjugated with E-selectin. Average mean fluorescent intensity for E-selectin chimera on Protein A polystyrene beads. Data are mean ± standard error for n = 4 independent experiments.
Flow Cytometry
Protein A polystyrene beads were prepared according to the previously stated protocol and then incubated on a rotisserie with the primary antibody for 1 h. The beads were washed with 1% BSA in DPBS+ and centrifuged at 14,000 RPM for 2 min a total of three times. The beads were then incubated in the secondary for 30 min while rotating on the rotisserie. The beads were then washed once with 1% BSA in DPBS+ then twice with DPBS+ (Life Technologies). The beads were then resuspended in DPBS+ with reagent adhesion and saturation being detected using a FACSAria Special Order Research Product flow cytometer (BD Biosciences, San Jose, CA).
For the ZR-75-1 and THP-1 cell lines, cells were harvested as stated previously, counted, and then resuspended in 0.1% BSA in DPBS+ at 107 cells/mL. They were then incubated in primary on ice for 30 min. Once the primary labeling step was completed, 3 washes with 0.1% BSA in DPBS+ were completed. The cells were then incubated with the secondary on ice, in the dark for 30 min. After the secondary labeling step, 1 wash with 0.1% BSA in DPBS+ and 2 washes with just DPBS+ were completed. The cells were resuspended in DPBS+ and analyzed using the flow cytometer.
Immunocytochemistry
Cells were harvested and washed in 0.1% BSA in DPBS+. Single stained samples were first incubated with a primary for 30 min on ice at 20 μg/mL. Single stained samples were then washed 3 times with 0.1% BSA in DPBS+ followed by secondary antibody labelling for 30 min on ice at 10 μg/mL. Dual stained samples were labelled with a primary at 20 μg/mL for 30 min on ice followed by 3 washes of 0.1% BSA in DPBS+. The secondary labelling step involved the same secondary used in the single stained samples for 30 min on ice. Finally, a tertiary labelling step with the fluorophore-conjugated reagent at 20 μg/mL incubated the cells for 30 min on ice in the dark. Samples were then resuspended in 20 μL DPBS+ and mounted on plain 25 × 75 × 1.0 mm slides using 10 μL Prolong Gold Antifade Reagent without DAPI (Life Technologies) and 2 μL of the cell solution. The samples were viewed using a Leica DMI 6000 Inverted microscope (Leica Microsystems, Wetzlar, Germany) with Nuance 3.0.2 imaging software (Caliper Life Sciences, Hopkinton, MA) and processed using colocalization thresholds and algorithms within inForm 2.0.1 (Perkin Elmer, Waltham, MA), more specifically, quantitation algorithms contained within the Nuance 3.0.2 and inForm 2.0.1 software allow for recognition and separation of distinct fluorescent signals.20 Through these program algorithms and thresholds, colocalization was determined as pixels of fluorescent signal for one fluorophore matched against the second set of signals, E-selectin and Gal-1hFc, respectively.
Tissue Preparation
De-identified, IRB-exempted human breast cancer tissue microarrays (US Biomax, Rockville, MD) were deparafinized by heating for 30 min at 60°C then subjected to three xylene washes for 5 min each, two 100% ethanol washes for 5 min each, one 95% ethanol wash for 5 min, one 70% ethanol wash for 5 min, and one final wash in distilled water for 3 min. Tissue microarrays were then submerged in a 10 mM sodium citrate 0.05% Tween 20 buffer for 30 min at 97°C to serve as an antigen retrieval method. For immunohistochemistry, tissues were dehydrated with one wash in both 70% and 95% ethanol, two washes in 100% ethanol, and three washes in xylenes all for 3 min each. This protocol was adapted from a previous procedure.10
Immunohistochemistry
The tissue samples were prepared as previously reported and an ImmEdge pen (Vector Laboratories, Burlingame, CA) was used to create hydrophobic barriers around tissue sections to ensure localized staining of the antibodies. The single stained samples were incubated with E-selectin, Gal-1hFc conjugated with QDot 705, or hFc for 1 h in the dark at room temperature at 20 μg/mL. The α-hIgG Alexa Fluor 568 secondary was then added to the E-selectin and hFc containing samples for 1 h at 10 μg/mL, while the Gal-1hFc samples incubated with 1% BSA in DPBS+. The dual stained samples underwent a similar procedure where E-selectin hFc chimera was used as the primary label, followed by the α-hIgG Alexa Fluor 568 secondary each for 1 h. A tertiary labelling step was applied to samples by incubation with the Gal-1hFc conjugate for 1 h. The slides were viewed under fluorescence using a Leica DMI 6000 Inverted microscope (Leica Microsystems) with Nuance 3.0.2 imaging software (Caliper Life Sciences), and images were processed with colocalization thresholds and algorithms of inForm 2.0.1 image software (Perkin Elmer) as described for immunocytochemistry (ICC).
Flow Chamber Assay
The ZR-75-1 cell line was cultured using 6.5 mm diameter flexiPERM gaskets (Greiner Bio-one) to create defined area monolayers for the flow chamber assays. A portion of the cell plates were stained with Gal-1hFc prior to bead perfusion, while the other portions acted as the negative controls being treated with either hFc isotype control or left untreated. Monolayers treated with Gal-1hFc 705 were imaged prior to perfusion of pre-coated beads using the Leica DMI 6000 Inverted microscope with Nuance 3.0.2 imaging software to determine relative binding. Protein A beads coated with E-selectin, hFc, or left uncoated were perfused over the monolayer using a circular Glycotech parallel plate flow chamber (Rockville, MD) with a 5.0 mm × 0.010 in flow channel and Harvard Syringe Pump (Harvard Apparatus, Holliston, MA) at varying wall shear stresses to determine binding efficiency of E-selectin.
For the assays with cells in flow, ZR-75-1 breast cancer cells and THP-1 monocytic cells were harvested as stated previously and treated with Gal-1hFc or hFc at a concentration of 20 μg/mL for 30 min on ice. E-selectin chimera and hFc reagents were incubated on sterile 35 × 10 mm tissue culture dishes (Corning Inc.) at 0.5 and 1.0 μg/mL overnight at 4°C and blocked with 1% BSA in DPBS+ for 2 h at 4°C. Plates were washed 3 × with DPBS+ before cell perfusion. ZR-75-1 and THP-1 cells were perfused over coated dishes at varying wall shear stresses (between 0.5 and 1.0 dynes/cm2) using the same Glycotech circular parallel plate flow chamber setup described previously.
Mac-2BP Antibody Blockade
Anti-Mac-2BP mAb SP-2 was used in flow chamber assays and ICC assays to act as a functional blocker of cell surface Mac-2BP on ZR-75-1 breast cancer cells. For the assays, ZR-75-1 breast cancer cells or monolayers were treated with αMac-2BP (SP-2) or mIgG1 isotype control at 5, 10, and 20 μg/mL for 30 min on ice. The assays were then completed as previously described in the ICC and flow chamber (cell monolayer) sections.
Dynamic Biochemical Tissue Analysis (DBTA)
The DBTA10 assay was used for tissue samples that had been prepared as described above, then treated with Qdot 705 conjugated Gal-1hFc or left untreated for 1 h to determine binding efficiency of E-selectin to its ligands under flow conditions. Similar to the ZR-75-1 cell monolayers in the flow chamber assays, Gal-1hFc treated and untreated control slides were imaged to determine relative binding of Gal-1hFc with the difference being that bead perfusion occurred in a rectangular flow chamber with a 60.0 mm × 10.0 mm × 0.005 in flow channel from Glycotech (Rockville, MD).
Static Adhesion Assays
ZR-75-1 breast cancer cells were harvested and plated onto 96-well cell culture plates (Corning Inc.) at 1.2 × 105 cells per well. Once cells had formed confluent monolayers, they were treated with 20 μg/mL of Gal-1hFc or hFc as a negative control for 30 min on ice. Monolayers were washed 3 × in 0.1% BSA in DPBS+, then images of each well were taken before incubation with beads, using the Leica inverted microscope described previously. E-selectin, hFc, or UnRx control beads were then incubated on individual monolayers for 30 min at room temperature. Subsequently, wells were washed and re-imaged. Images were quantified according to number of bead interactions per mm2.
For the inverse assay with cells in flow interacting with substrate-coated plates, ZR-75-1 and SK-BR-3 breast cancer cells were harvested as stated previously and treated with Gal-1hFc or hFc at a concentration of 20 μg/mL for 30 min on ice. E-selectin chimera and hFc reagents were incubated on sterile 96-well cell culture plates (Corning Inc.) at 1.0 μg/mL overnight at 4°C and blocked with 1% BSA in DPBS+ for 2 h at 4°C. Plates were washed 3 × with DPBS+ before cell addition. ZR-75-1 and SK-BR-3 cells were incubated on coated dishes for 30 min at room temperature, washed, then imaged using the Leica suite described previously for quantification of cells per mm2.
Immunoprecipitation and Western Blotting
ZR-75-1 and SK-BR-3 breast cancer cells were lysed with 1% Triton X-100 in Buffer A (150 mM sodium chloride, 0.5 mM Tris-base, 20 μg/mL phenylmethanesulfonyl fluoride (PMSF), 0.02% sodium azide, and 1 × protease inhibitor cocktail without EDTA) without EDTA then incubated with αMac-2BP clone H300 at a ratio of 1 μg Ab/1.0 × 106 cell equivalent followed by Protein G microspheres at 18 μL dry beads/1.0 × 106 cell equivalent at room temperature for 2 h each. The beads were washed 3 × with 2% Triton X/1% SDS/1% BSA in Buffer A without EDTA and then 2 × with 2% Triton X/1% SDS in Buffer A without EDTA and heated at 100 °C for approximately 5 min in Laemmli 6 × reducing buffer (Biorad Laboratories, Hecules, CA) to release the Mac-2BP antigen from the antibody–microbead complex.33
Once the Mac-2BP had been recovered, the immunoprecipitation samples and whole cell lysates were loaded in duplicate into a 4-15% TGX Mini-PROTEAN gel (Biorad Laboratories) and run according to a previous SDS-PAGE protocol.33 The samples in the gel were then electrophoretically transferred to a polyvinyl difluoride (PVDF) membrane (Biorad Laboratories) via semi-dry discontinuous transfer, then blocked in heat inactivated FBS overnight at 2 °C. After blocking, the membrane was cut evenly with one side incubated with E-selectin-hFc and the other with Gal-1hFc for 90 min at room temperature. αhIgG-AP secondary was then incubated upon the blots for 1 h, with AP substrate added after three short washes in TBS. The blots were imaged using a chemiluminescence detection channel on a Biorad ChemiDoc XRS + imaging system.
Fluorescence Anisotropy
E-selectin hFc chimera was conjugated with a modified FITC fluorophore (Abcam, Cambridge, MA) to obtain a baseline for anisotropy and to measure the future sequential binding for Mac-2BP and Gal-1hFc. Immunoprecipitated Mac-2BP obtained from ZR-75-1 and SK-BR-3 breast cancer cells (described above) was added after initial E-selectin readings at 1 × 106 cell equivalent/μL. Upon obtaining anisotropy measurements for the E-selectin/Mac-2BP complex, Gal-1hFc was added at 20 μg/mL to determine binding efficiency of E-selectin and Gal-1 to Mac-2BP. Fluorescence anisotropy was measured in temperature controlled, quartz cuvettes using a Fluorolog®-3 with fluorEssence™ software (HORIBA Scientific, Edison, NJ).
Results
Gal-1hFc Treatment of ZR-75-1 Cells Significantly Increases Interactions of E-selectin Coated Beads at Low Shear Stresses
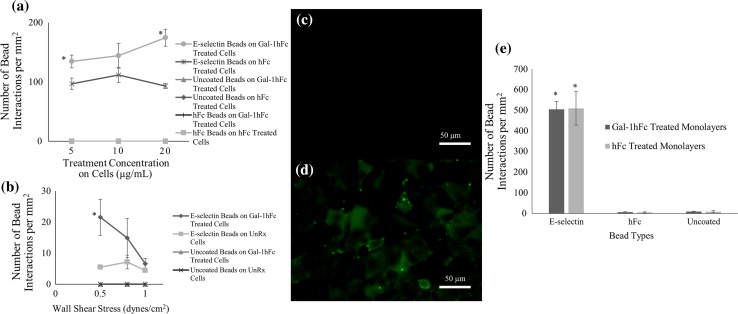
To assess the effect of Gal-1 on E-selectin bead interactions with ZR-75-1 breast cancer cell monolayers during flow, E-selectin coated, hFc coated, and uncoated beads were perfused over Gal-1hFc treated and hFc treated monolayers during flow chamber assays at varying concentrations (5, 10, and 20 μg/mL). E-selectin beads had a significant increase in binding to Gal-1hFc treated monolayers (p = 0.0005) across all concentrations, but had the greatest difference at 20 μg/mL, where it had an 88.3 ± 18.1% increase over the hFc treated monolayers (Fig. 2a). The negative controls failed to bind to any of the treated substrates. Because of the significant effects induced by Gal-1hFc treatment, further details about the dynamic relationship between Gal-1hFc and E-selectin were sought, specifically how this interaction is affected by changing shear stress.
Figure 2.
Gal-1hFc significantly increases E-selectin binding to ZR-75-1 Breast cancer cells at a low shear stress. (a) E-selectin, hFc, or uncoated beads were perfused over ZR-75-1 breast cancer monolayers treated with varying concentrations (5, 10, and 20 μg/mL) of Gal-1hFc or negative control or hFc isotype control at 0.5 dynes/cm2. Gal-1hFc treatment had a significant increase of E-selectin bead binding compared to hFc treated monolayers, especially at 20 μg/mL (p = 0.0005). Uncoated and hFc coated beads failed to bind under any condition. Data are mean ± standard error, n = 3 independent experiments. (b) Analysis of E-selectin-coated and uncoated beads’ interactions with untreated and Gal-1hFc-treated ZR-75-1 cell monolayers at various shear stresses. While the number of interactions on the monolayers at wall shear stresses of 0.8 and 1.0 dynes/cm2 are not significantly different (p > 0.05), the shear stress of 0.5 dynes/cm2 showed a significant difference (*p = 0.048) between the number of interactions on the Gal-1hFc treated monolayer, compared to the untreated monolayer. Uncoated beads failed to bind to the cell monolayers under any conditions. Data are mean ± standard error, n = 3 independent experiments. (c, d) Fluorescence microscopy confirms Gal-1hFc binding to ZR-75-1 breast cancer cell monolayers. While (c) untreated control cells had virtually no fluorescent signal, (d) Gal-1hFc (pseudocolored green) was readily detected on the treated monolayer. Images are representative of n = 3 independent experiments. (e) ZR-75-1 breast cancer cell monolayers were treated with Gal-1hFc or a negative control, then incubated with E-selectin, hFc, or uncoated beads during static adhesion assays. There was no significant difference (p > 0.05) in the number of E-selectin bead interactions between the monolayer treatments of Gal-1hFc and the negative control. Negative control beads had little to no binding to either of the treated monolayers, as expected. Data are mean ± standard error, n = 3 independent experiments.
Figure 2b displays bead interactions with ZR-75-1 monolayers across different wall shear stresses. At a wall shear stress of 0.5 dynes/cm2, Gal-1hFc significantly increased, approximately 300%, interactions of E-selectin coated beads to ZR-75-1 breast cancer monolayers. The negative control failed to bind to either untreated or Gal-1hFc treated monolayers. Fluorescent images of the untreated monolayer (Fig. 2c) and Gal-1hFc treated monolayer (Fig. 2d) verify Gal-1hFc’s absence on the untreated monolayer and its presence on the treated monolayer.
Investigation of the impact of shear forces was tested against static adhesion assays, which measured the effects of Gal-1 on interaction of E-selectin beads with cell monolayers in the absence of flow. There was no significant difference (p > 0.05) between numbers of E-selectin beads interacting on Gal-1hFc treated and the negative control treated cells (Fig. 2e). As expected, binding of negative control beads and cells were null (Fig. 2e). Collectively, these data reveal the importance of wall shear stress on Gal-1 mediated effects on breast cancer cell binding to E-selectin.
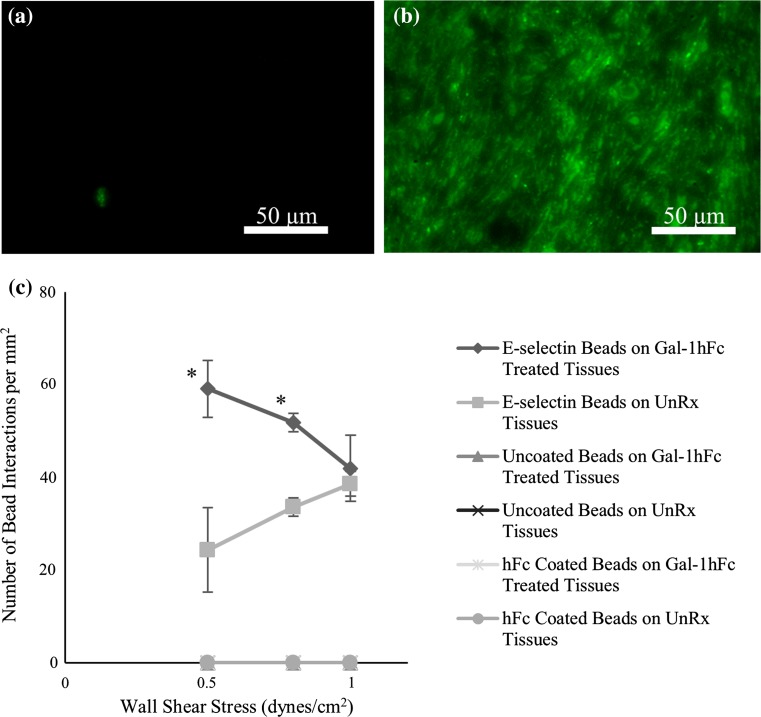
Mucinous Breast Carcinoma Tissue Microarrays Show Significant Increase in E-selectin Interaction After Gal-1hFc Treatment
To investigate this low shear stress binding increase further, and to determine if this effect was only seen in ZR-75-1 breast cancer cells, dynamic DBTA assays 10 were performed on mucinous breast carcinoma tissue microarrays under the same experimental conditions. Figures 3a and 3b show the fluorescent images of (A) untreated and (B) Gal-1hFc treated tissue arrays, again to ensure Gal-1 ligand expression in the tissues. Figure 3c shows the quantitative data of bead interactions on each of the monolayers. Similar to flow chamber assays, 0.5 dynes/cm2 shear stress showed a significant increase in E-selectin bead interactions, approximately 300%. Unlike the flow chamber cell assays though, a shear stress of 0.8 dynes/cm2 also showed a significant increase, approximately 55%, in E-selectin bead interactions. Negative control beads failed to bind on either untreated or Gal-1hFc treated tissues, as expected.
Figure 3.
Gal-1hFc treatment of mucinous breast carcinoma tissue microarrays significantly increases E-selectin bead binding at low shear stresses. (a, b) Immunohistochemistry confirms Gal-1hFc binding to mucinous breast carcinoma tissue microarrays. While the untreated control monolayer (a) showed only negligible signal, (b) Gal-1hFc (pseudocolored green) stained quite vibrantly on the treated microarray. Images are representative of n = 3 independent experiments. (c) Analysis of E-selectin-coated, hFc-coated, and uncoated beads’ interactions with untreated and Gal-1hFc treated mucinous breast carcinoma tissue microarrays. Similar to ZR-75-1 breast cancer cells, DBTA of the tissue microarrays exhibited increased binding interactions of E-selectin-coated beads at a wall shear stress of 0.5 dynes/cm2 on Gal-1hFc treated tissues compared to untreated tissues (*p = 0.0014). A significant increase in the number of E-selectin bead interactions between the Gal-1hFc treated and untreated microarrays was also achieved at a wall shear stress of 0.8 dynes/cm2 (* p = 0.0015). Uncoated and hFc-coated beads failed to bind to tissue microarrays under any conditions. Data are mean ± standard error, n = 3 independent experiments.
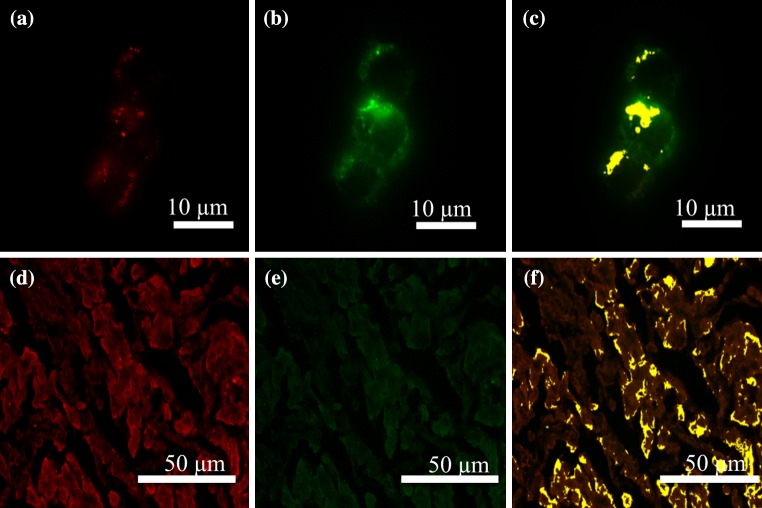
Gal-1 and E-selectin Fluorescent Signals Colocalize on ZR-75-1 Cells and Mucinous Breast Carcinoma Tissue Microarrays
Determination of whether the dynamic relationship seen at low shear stresses during flow assays was due to interaction between the same or similar ligands of Gal-1 and E-selectin, colocalization of Gal-1 and E-selectin fluorescent signals was assessed through two microscopy procedures: immunocytochemistry and immunohistochemistry. ZR-75-1 breast cancer cells were subjected to a dual staining protocol as described in the Immunocytochemistry portion of the Materials and Methods. Figures 4a–4c shows the individual fluorescent channels and the colocalization image of the dual stained ZR-75-1 cells. At a 1:1 ratio of staining concentrations, Gal-1hFc and E-selectin fluorescent signals had an average of 75 ± 6% colocalization. This shows that Gal-1hFc and E-selectin reactive carbohydrates are located in similar areas on the surface of the ZR-75-1 cell and may be the same ligand expressing multiple carbohydrate moieties.
Figure 4.
E-selectin and Gal-1 dual-stained ZR-75-1 breast cancer cells and mucinous breast carcinoma tissues exhibit co-localization of receptor-ligand signals. ZR-75-1 breast cancer cells and mucinous breast carcinoma tissue microarrays were subjected to a dual-staining protocol of murine E-selectin hFc chimera and Gal-1hFc conjugated with Qdot 705. (a) Cells were stained with 20 μg/mL of E-selectin, followed by 10 μg/mL αhIgG AlexaFluor 488 (pseudocolored red). (b) Following E-selectin treatment, Qdot 705 conjugated Gal-1hFc was incubated on the cells at 20 μg/mL (pseudocolored green). (c) Co-localization of E-selectin and Gal-1hFc reactive signals were determined through the inForm 2.0 software and are depicted in yellow. (d, f) Mucinous breast carcinoma tissue microarrays were subjected to a similar dual staining protocol with the exception of αhIgG AlexaFluor 568 used in place of the 488 secondary with E-selectin to reduce autofluorescent background noise from the tissue microarrays. Fluorescent images shown are representative of n = 3 independent experiments.
Mucinous carcinoma tissue microarrays were subjected to the same dual staining protocol as the ZR-75-1 cancer cell line through immunohistochemistry to probe whether the colocalization seen in the cell line could be seen in a tumor section. Figures 4d–4f show the individual channels of the E-selectin (pseudocolored red) and Gal-1hFc (pseudocolored green) along with the colocalization overlay (pseudocolored yellow). Mucinous tissue at a staining ratio of 1:1 Gal-1hFc to E-selectin revealed a 72 ± 5% colocalization of fluorescent signals. This average implies that Gal-1 and E-selectin are relatively close to one another on the majority of the tissue microarray, which may be the result of closely clustered similar ligands or the same shared ligand.
Mac-2BP is a Ligand Shared Between Gal-1 and E-selectin
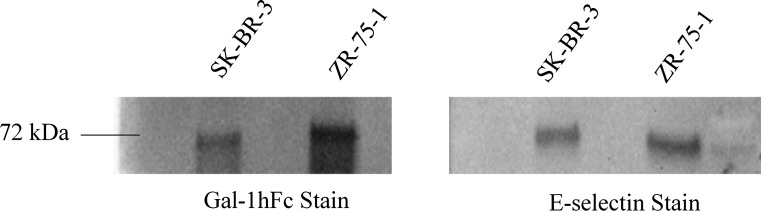
To further assess whether Gal-1 and E-selectin either bind to the same or similarly structured ligands, ZR-75-1 breast cancer cells were harvested, lysed, and subjected to immunoprecipitation of Mac-2BP, a ligand hypothesized to interact and bind with both E-selectin and Gal-1.3,27,33,35 Western blots show that both Gal-1hFc and E-selectin staining reveal bands at 72 kDa (Fig. 5), the expected molecular weight of Mac-2BP.33 This result was confirmed through a second breast cancer cell line, SK-BR-3, also seen in Fig. 5. From our previous work, it is known that Mac-2BP is an E-selectin ligand,33 however the identification of Mac-2BP expressed on breast cancer as a ligand for Gal-1 is now revealed in these results.
Figure 5.
Mac-2BP revealed to be a ligand for both Gal-1 and E-selectin in immunoprecipitation and subsequent western blotting from SK-BR-3 and ZR-75-1 breast cancer WCL. Immunoprecipitated Mac-2BP from SK-BR-3 and ZR-75-1 whole cell lysate (WCL) was loaded in duplicate into a reducing SDS-PAGE gel, run via gel electrophoresis, and transferred via semi-dry method to a PVDF membrane. The blot was cut into two distinct but identical sections and stained with either Gal-1hFc at 1 μg/mL or with recombinant murine E-selectin hFc chimera at 1 μg/mL. Both blots were incubated with αhIgG-AP secondary, then developed using an AP substrate. Both Gal-1hFc and E-selectin stained blots revealed bands at the expected Mac-2BP molecular weight of 72 kDa in SK-BR-3 and ZR-75-1 breast cancer lines. Image is representative of n = 4 independent experiments.
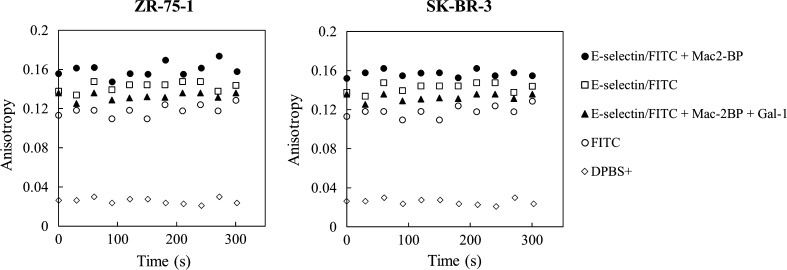
With this identification, the ability of E-selectin and Gal-1 to simultaneously bind Mac-2BP was assessed through the use of fluorescence anisotropy. Fluorescently-tagged E-selectin was combined with immunocaptured Mac-2BP and Gal-1hFc in sequential steps to measure anisotropy. Figure 6 shows the level of anisotropy over time for the sequential combinations with a baseline for the assay buffer (DPBS+) and fluorophore (modified FITC), and FITC-conjugated E-selectin (E-selectin/FITC). The E-selectin-Mac-2BP-Gal-1hFc sample had lower overall anisotropy when compared to the E-selectin and Mac-2BP complex across both cell lines tested. This decrease in anisotropy may be due to some kind of competitive or antagonistic binding for Mac-2BP between E-selectin and Gal-1 in solution. This hindering effect of Gal-1hFc towards Mac-2BP and E-selectin in solution is consistent with the immune cell model in the literature 13,23,37 and our previously proposed breast cancer Mac-2BP flow adhesion model.33
Figure 6.
Mac-2BP complexes with E-selectin and Gal-1hFc reduce anisotropy compared to Mac-2BP complexes with E-selectin. Mac-2BP from ZR-75-1 and SK-BR-3 breast cancer lysate forms binding complexes with FITC conjugated E-selectin. The binding complex decreases in anisotropy with the introduction of Gal-1hFc into the system, resulting in what can be inferred as competitive binding between Gal-1 and E-selectin for Mac-2BP. E-selectin/FITC fluorophore, DPBS+ and the FITC fluorophore alone were used as baseline controls. Data are representative of n = 3 independent experiments.
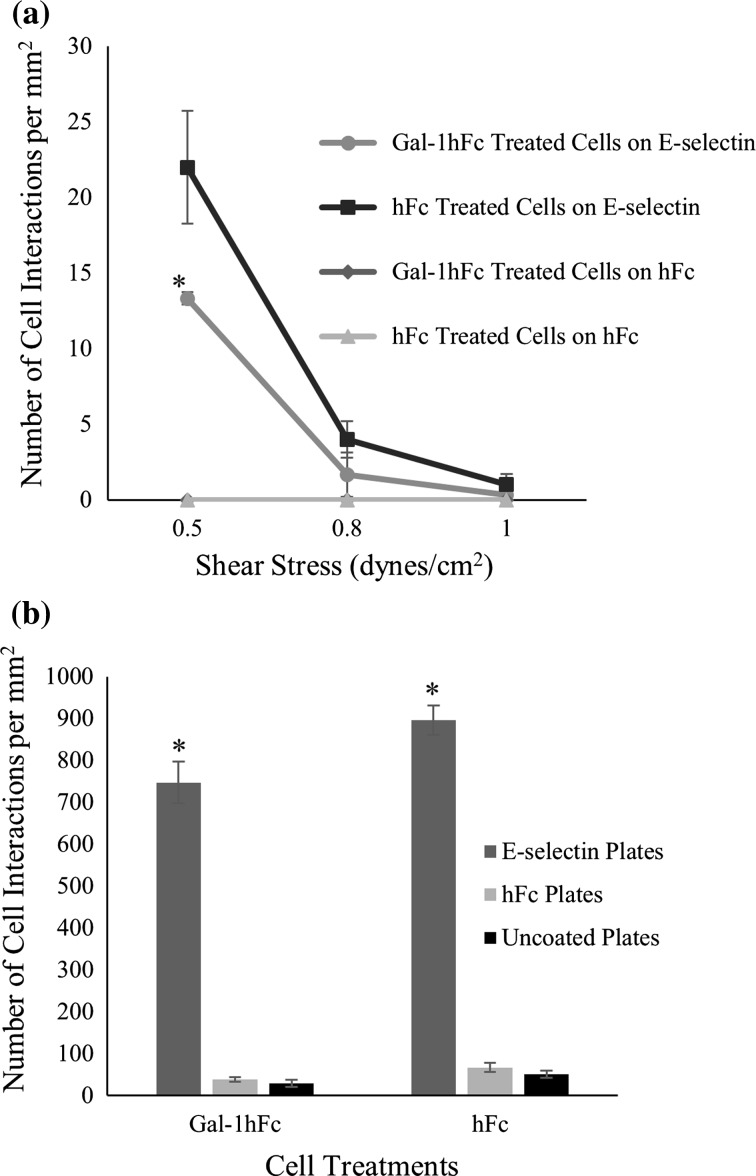
To test our proposed model, ZR-75-1 cells treated with Gal-1hFc or negative controls were perfused over E-selectin and negative control coated plates at varying wall shear stresses (Fig. 7a). The Gal-1hFc treated cell samples had significantly lower number of interactions with the E-selectin coated substrate than the negative control cells at the lower shear stress of 0.5 dynes/cm2 (p = 0.047), consistent with inhibition seen in solution anisotropy experiments, but differing from the previous cell experiments that showed increased number of interactions of E-selectin beads to Gal-1hFc treated monolayers (Figs. 2a and 2b). Static adhesion assays with ZR-75-1 breast cancer cells showed no significant difference (p > 0.05) in the number of interactions with E-selectin coated plates between the Gal-1hFc and negative control treated cells (Fig. 7b) thereby revealing the shear-dependency of Gal-1’s effects on breast cancer binding to E-selectin. Negative control plates had miniscule interactions with either Gal-1hFc or negative control cells.
Figure 7.
Gal-1hFc treatment of ZR-75-1 breast cancer cells significantly reduces E-selectin interaction with cells in flow but has no effect in static adhesion assays. (a) ZR-75-1 breast cancer cells were treated with Gal-1hFc or an hFc control, then perfused over E-selectin and hFc control plates. Gal-1hFc treatment of ZR-75-1 cells in flow significantly reduces interactions to E-selectin coated plates (*p = 0.047) when compared to hFc control treated cells. Data are mean ± standard error, n = 3 independent experiments. (b) ZR-75-1 cells were treated with Gal-1hFc or a negative control, then incubated on E-selectin or isotype control-treated cell culture wells in separate static adhesion assays. There was no significant difference (p > 0.05) in the number of cell interactions with E-selectin plates between Gal-1hFc treated cells and the negative control. E-selectin plates were significantly different (*p = 0.008) in the number of interactions with both cell lines compared to the negative controls. Data are mean ± standard error, n = 3 independent experiments.
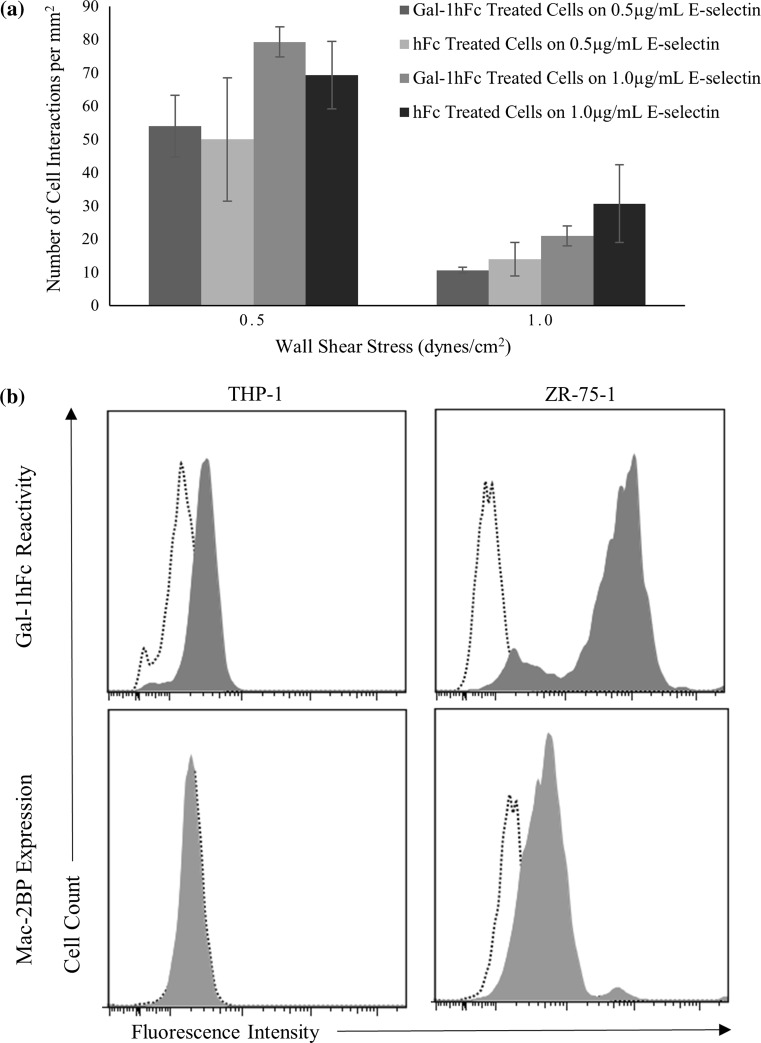
The effects of Gal-1 on the flowing cell model were then checked against a non-breast cancer cell type. Monocytic/leukemic THP-1 cells were treated with Gal-1hFc or negative control hFc and perfused over E-selectin or hFc coated plates. Figure 8a shows the interactions between the treated THP-1 cells and substrate-coated plates at two specific wall shear stresses (0.5 and 1.0 dynes/cm2) and at two specific concentrations of substrate coating (0.5 and 1.0 μg/mL). However, there were no significant differences between treatments at any concentration or shear stress (p > 0.05). Both Gal-1hFc and hFc treated cells had insignificant amounts of binding (2 or fewer events) to negative control coated plates, as expected.
Figure 8.
THP-1 cell adhesion, Gal-1 reactivity and Mac-2BP expression differ as compared to ZR-75-1 cells. (a) THP-1 acute monocytic leukemia cells were treated with 20 μg/mL of Gal-1hFc or isotype control hFc. The cells were then perfused over E-selectin or hFc substrates at a concentration of 0.5 or 1.0 μg/mL, at wall shear stresses of 0.5 and 1.0 dynes/cm2. There were no significant differences (p > 0.05) in interactions between cells treated with Gal-1hFc or with the isotype control. Under any conditions, both Gal-1hFc and hFc cell treatment types had an insignificant amount of binding (2 or fewer events) to hFc coated plates as the negative control. Data are mean ± standard error, n = 3 independent experiments. (b) THP-1 monocytic cells and ZR-75-1 breast cancer cells were labeled with Gal-1hFc (filled) or an hFc control (dotted) (top row) and αMac-2BP pAb (filled) or a rabbitIgG control (dotted) (bottom row), followed by the appropriate secondary antibodies, then analyzed via flow cytometry. ZR-75-1 cells showed relatively high levels of fluorescent intensity for Gal-1hFc and expression levels of Mac-2BP, whereas THP-1 cells had weak to no expression levels of Gal-1hFc reactive sites and Mac-2BP expression levels. Data are representative of n = 3 (Gal-1hFc) and n = 4 (Mac-2BP) independent experiments.
Gal-1 reactivity (i.e., expression of polylactosamine Gal-1 ligands) and Mac-2BP expression levels were then probed on ZR-75-1 breast cancer cells and THP-1 monocytic cells using flow cytometry to provide a link to functional activities observed in adhesion assays. ZR-75-1 cells show relatively high levels of expression of Gal-1 ligands (Fig. 8b) and Mac-2BP (Fig. 8c) compared to THP-1 cells, which have low to no expression of Gal-1 ligands (Fig. 8b) or Mac-2BP (Fig. 8c). The low/undetectable expression of Gal-1 ligands and Mac-2BP likely explains the lack of Gal-1 inducible effects on E-selectin binding of THP-1 cells under flow conditions.
Mac-2BP Antibody Blockade Significantly Reduces Low Shear Stress Binding Interaction
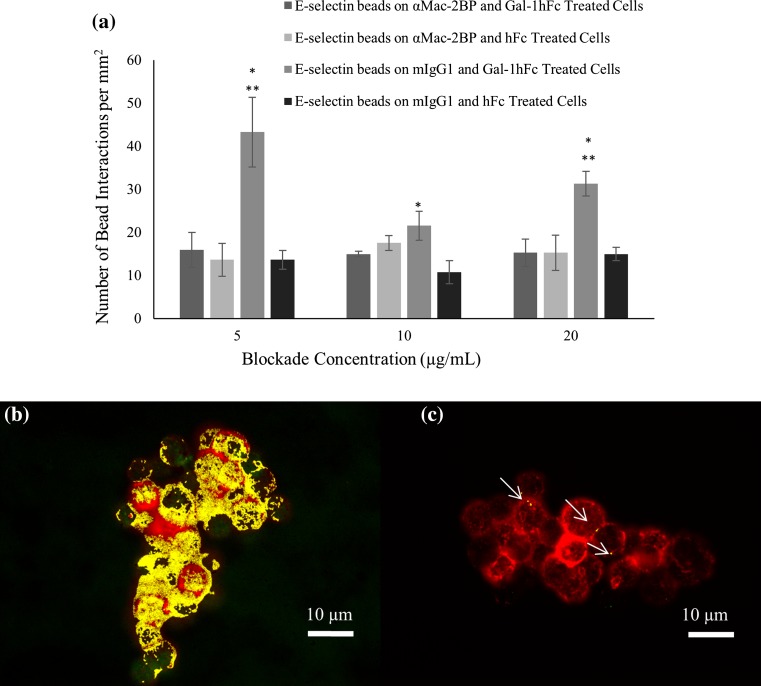
With the observation of Mac-2BP as a ligand for both Gal-1 and E-selectin, Mac-2BP functional blocking experiments in flow chamber and ICC were performed to determine if it is a mediator of the Gal-1 inducible effects on ZR-75-1’s E-selectin binding activity. When ZR-75-1 breast cancer monolayers were pre-treated with an αMac-2BP mAb before Gal-1hFc or hFc treatment, the number of interactions with E-selectin beads during perfusion at 0.5 dynes/cm2 were not statistically different (Fig. 9a), because mAb treatment ablated the significant increase induced by Gal-1hFc treatment of cells (Figs. 2a, 2b and 9a). In other words, αMac-2BP mAb inhibited the Gal-1hFc-enhanced E-selectin binding to ZR-75-1 cell monolayers at 0.5 dynes/cm2, strongly implying that Mac-2BP is a Gal-1-modulatable E-selectin ligand. The mAb seemingly blocks or antagonizes the Gal-1 binding site on Mac-2BP but not the E-selectin binding site, as mAb blockade did not significantly affect E-selectin bead binding to negative control treated cells (Fig. 9a). Negative control beads failed to bind under any treatment or condition, as expected (data not shown).
Figure 9.
Mac-2BP functional antibody blockade significantly reduces low shear stress binding interaction and colocalization of E-selectin and Gal-1 reactive signals. (a) ZR-75-1 breast cancer cell monolayers were treated with varying concentrations (5, 10, and 20 μg/mL) of αMac-2BP mAb or a negative control, followed by treatment with Gal-1hFc or an hFc negative control at 20 μg/mL, with subsequent perfusion of E-selectin beads or negative control beads over the treated monolayers. Mac-2BP antibody blockade reduced the number of E-selectin bead interactions on the Gal-1hFc treated monolayers compared to the mIgG isotype treated, Gal-1hFc treated monolayers (*significantly different from hFc treated monolayers; **significantly different from Mac-2BP antibody blockade, p < 0.04). Negative control beads failed to bind under any circumstance (data not shown). Data are mean ± standard error, n = 3 independent experiments. (b, c) Colocalization analysis of Gal-1 (pseudocolored green) and E-selectin (pseudocolored red) reactive species on ZR-75-1 breast cancer cells shows a decrease in overall colocalization in (c) Mac-2BP blocked samples compared to (b) control samples (85 ± 6 vs. 42 ± 28%), with colocalization shown in yellow or signaled with white arrows. Images are representative of n = 3 independent experiments.
Immunocytochemical and colocalization analysis of Mac-2BP functional blocking showed a decrease in the percent colocalization for Gal-1 (pseudocolored green) and E-selectin (pseudocolored red) reactive signals in mAb-blocked samples when compared to negative control samples, 85 ± 6 vs. 42 ± 28%, respectively. A representation of this colocalization (pseudocolored yellow) is shown in Figs. 9b and 9c. This loss of Gal-1hFc-enhanced binding of breast cancer cells to E-selectin and loss of colocalized Gal-1hFc and E-selectin reactive signals indicate that Mac-2BP has a functional role as the ligand intermediary between Gal-1 and E-selectin during cell adhesion.
Discussion
E-selectin or Gal-1 have individually been studied for their effect during CTC adhesion to the endothelium.3,7,19,31,33 These adhesion molecules are typically upregulated during inflammation and show preferential binding to similar sialylated moieties such as ligands bearing α2, 3-sialylation.2,4,5,7,9,11–13,18,27,31,33,35 However, to date, these two molecules have never been studied in conjunction with dynamic assays to the best of our knowledge. This investigation thus aimed to fill the knowledge gap regarding the dynamic interaction between these two molecules. It was discovered that E-selectin and Gal-1 reactive molecules colocalize on approximately 72-75% of ZR-75-1 breast cancer cells and mucinous breast carcinoma tissue microarrays. This colocalization may occur, in part, due to their shared ligand Mac-2BP, which was discovered through immunoprecipitation of ZR-75-1 and SK-BR-3 breast cancer cell lines and further confirmed through Mac-2BP functional blockade in adhesion and ICC assays. Previous research focusing singularly on E-selectin or Gal-1 supports these findings as well.3,19,33,35 Fluorescence anisotropy also found that Mac-2BP may cause competitive binding between E-selectin and Gal-1 for specific carbohydrate moiety subsets. These results, along with the discovery through dynamic assays (flow chamber and DBTA) that Gal-1 treatment of breast cancer cells and tissues significantly affects E-selectin binding interactions and Mac-2BP mAb blockade reverses the effects, aid in the assertion that Gal-1 and E-selectin do in fact interact dynamically in breast cancer and do so by binding to similar or the same ligands, including Mac-2BP.
To evaluate the dynamic interactions through ligands between E-selectin and Gal-1, E-selectin-coated beads along with hFc-coated beads, were separately perfused over ZR-75-1 monolayers and mucinous breast carcinoma microarrays that were either treated with Gal-1hFc or left untreated at varying wall shear stresses. Gal-1hFc treatment significantly increased (p = 0.0005) E-selectin bead interactions at all concentrations tested compared to the hFc treated control, and in both the flow chamber assays and the DBTA assays, Gal-1hFc treatment significantly increased (p = 0.048 and p = 0.0015, respectively) E-selectin bead interactions at 0.5 dynes/cm2 in both assays and increased bead interactions at 0.8 dynes/cm2 in DBTA (Figs. 2 and 3). One possible explanation for these results is that Gal-1 is able to bind with greater efficacy to multiple carbohydrate moieties rather than to a single lactosamine unit,28 which dovetails with the phenomena of Gal-1 clustering E-selectin ligands together into specific domains.14,21,23,28,30 However, this phenomena may not be seen in formaldehyde fixed samples due to decreased mobility of cellular components. In either scenario, increased E-selectin adhesion efficacy induced by Gal-1 cell treatment may be able to overcome shear-disruptive effects of lower wall shear stresses, but the effect is lost at increasing wall shear stresses and during static adhesion assays.21,23,28,30 In contrast there was significant decrease in breast cancer cell binding to E-selectin when the ZR-75-1 cell line was treated with Gal-1hFc and perfused over an E-selectin-coated plate (Fig. 7a). This CTC-like model more closely follows what has previously been seen with Gal-1 and its modulatory effects on cell adhesion to T-cells and other immune cells.13,23 Gal-1hFc treatment had no significant effect on E-selectin interactions of THP-1 cells (Fig. 8a). This implies that there is something specific to breast cancer that is not seen in the monocytic/leukemic cell lines, attributed herein to the presence of Gal-1 ligands on the ZR-75-1 cells compared to the THP-1 cells (Fig. 8b). From these data, immunocyto- and immunohistochemical analysis of breast cancer cells and tissues were performed to attempt to elucidate a mechanism for this dynamic, shear-dependent, and spatially-oriented ligand intermediation between Gal-1 and E-selectin.
ZR-75-1 breast cancer cells and mucinous breast carcinoma showed a high level of colocalization of E-selectin and Gal-1hFc fluorescent signals (Fig. 4). This colocalization may be due to one of two occurrences: (i) Gal-1 and E-selectin are binding to the same ligand, which is alluded to by immunoprecipitation and western blotting data that reveal Mac-2BP is a ligand for both molecules (Fig. 5), the Mac-2BP blocking assays which showed reduced colocalization of E-selectin and Gal-1 reactive signals as well as abrogation of Gal-1 enhanced adhesion to E-selectin at 0.5 dynes/cm2 in flow chamber assays (Fig. 9a), and that Mac-2BP has multiple N-glycosylation sites for E-selectin and Gal-1 reactive carbohydrates to stem from and interact with simultaneously 35,36; or (ii) Gal-1 and E-selectin are binding to similar but distinct ligand molecules that are congregated in the same area, supported by E-selectin’s preference for α2,3-sialylation and α1,3- and α1,4-fucosylation on ligands and Gal-1’s affinity for α2,3-sialylated and α1,2-fucosylated ligands.3,7,19,31,33 While the results of the immunoprecipitation and western blotting support the first explanation, fluorescence anisotropy (Fig. 6) seems to support the second with competitive or antagonistic binding for Mac-2BP by Gal-1 and E-selectin, forcing E-selectin to find a different form of Mac-2BP with higher carbohydrate moiety affinities or an entirely different ligand within the same relative location. This proposition is supported again through the clustering of ligands into specific domains,14,21,23,28,30 where multiple forms of Mac-2BP can reside and bind to competitively between Gal-1 and E-selectin while maintaining apparent colocalization.
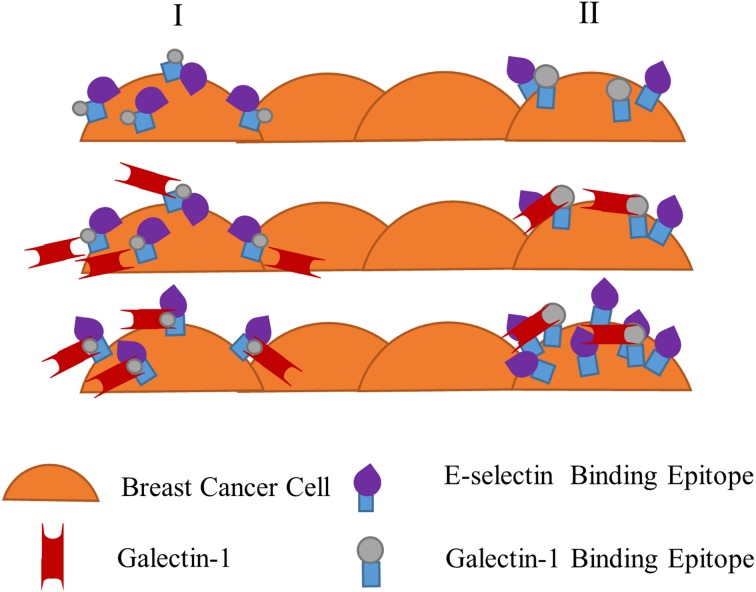
While the Gal-1-CTC-E-selectin model observations are consistent with our prior work characterizing adhesion mechanisms of metastasizing CTCs,33 the Gal-1-breast cancer monolayer-E-selectin model is more diagnostically/prognostically relevant than physiologically relevant, per se. That is, the monolayer model could yield diagnostic or prognostic information from sectioned tissue or ex vivo cultured patient tissue samples, in which changes of disease-associated Gal-1 or E-selectin responses can be assessed. Two main hypotheses were created in an attempt to explain the observed monolayer phenomena (Fig. 10): (i) Gal-1 binds to a shared ligand thereby causing a conformational change in the shared ligand and exposing the E-selectin preferred site to bind in monolayer adhesion; or (ii) Gal-1 binds to similar yet distinct ligand sets that would cause a signal cascade increase of separate E-selectin ligands within a domain to cluster or activate, thereby aiding in increased adhesion events. Possible ligand targets can be glycolipids,31 MUC1,22 or CD44/HCELL 32 however the exact effect of Gal-1 on these ligands would need to be identified.
Figure 10.
Hypothesized effects of Gal-1 on E-selectin receptor-ligand binding interaction with a monolayer of breast cancer cells. Two hypothesized pathways, column I and column II, of Gal-1’s effect on E-selectin receptor-epitope interaction. Column I proposes E-selectin binding epitopes (sialofucosylated moieties such as sialyl Lewis X and sialyl Lewis A) are hidden or the entire E-selectin ligand is in a less active conformation on breast cancer cells until Gal-1 binds to N-Acetyllactosamine (minimal Gal-1 binding epitope) on the ligand. In this shared ligand model, Gal-1 binding leads to a conformational change, exposing E-selectin-reactive portions on the same ligand. Breast cancer cells are then able to bind more effectively to E-selectin at the lower range of physiologically-relevant fluid shear stress. Column II alternatively proposes that Gal-1 and E-selectin epitopes are expressed on distinct but similarly structured ligands. Gal-1 binding to its recognition epitope on one type of ligand induces a signal in the breast cancer cell to express more reactive E-selectin epitopes on another distinct ligand. Once again, enhanced binding of breast cancer cells to E-selectin occurs, particularly at low shear stress.
The first hypothesis of Gal-1-induced conformational change of a ligand shared with E-selectin is supported by experiments using tissue sections from formalin fixed specimens (Fig. 3), since signaling-dependent ligand clustering (second hypothesis) is impossible in non-viable samples. Gal-1-induced conformational changes in ligands are still feasible, since carbohydrate binding epitopes of ligands for Gal-1 and E-selectin are largely unaffected by standard fixatives that crosslink or denature proteins. The first hypothesis of conformational change (with or without cell signaling involvement) or the second hypothesis of clustering are both possible in viable cells. To the latter, multiple sources state that Gal-1 can both cluster ligands into specific domains, as well as increase ligand residence time on the cell surface.19,21,28 This is especially relevant considering that Mac-2BP is a Gal-1 ligand that also acts as an E-selectin ligand. Having the ability to not only change the location of ligands that Gal-1 and E-selectin share but to maintain their presence as well would be a powerful mechanism to regulate cancer cell migration and adhesion. However, investigation is still necessary to elucidate the true mechanism of action for this dynamic system and ultimately clinical utility.
In summation, this investigation has elucidated a shear and spatially dependent dynamic interaction between E-selectin and Gal-1 that are in part the result of intermediation of Mac-2BP, a shared ligand of both proteins. The specific role that Mac-2BP plays in the molecular pathways of Gal-1 and E-selectin monolayer interaction still needs to be elucidated, and while this adhesion model could be classified as noncanonical, there is merit in its use, especially within the diagnostic setting. These adhesion assays allow for in vitro characterization of primary tumors and cancer cells, a diagnostic field of growing importance.10 Therefore, the results discovered herein may aid in the discovery of new diagnostics, prognostics, or therapeutics for breast cancer through the identification of new galectin/selectin molecular pathways in breast cancer adhesion.
Acknowledgments
The authors would like to thank Mr. Alexander Ostermann, Mr. Yinan Huang, and Dr. Grady Carlson (Department of Chemical and Biomolecular Engineering, Ohio University) for their insight and technical expertise. This research was funded in part by National Institutes of Health R15CA161830 (to M.M.B.), National Science Foundation Grant 1039869 (to M.M.B.), and an Ohio University Foundation Board of Trustees 1804 Fund Award (to A.M.F.).
Conflict of interest
Monica Burdick has received research grant R15CA161830 from the National Institutes of Health and Major Research Instrumentation grant 1039869 from the National Science Foundation. Amir Farnoud has received a research grant 1804 Fund Award from the Ohio University Board of Trustees. Nathan Reynolds, Amina Mohammadalipour, Claire Hall, and Ali Asghari Adib declare that they have no conflicts of interest.
Human Studies/Informed Consent
No human studies were carried out by the authors for this article.
Animal Studies
No animal studies were carried out by the authors for this article.
References
- 1.American Cancer Society . Cancer Facts and Figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Astorgues-Xerri L, Riveiro ME, Tijeras-Raballand A, Serova M, Neuzillet C, Albert S, Raymond E, Faivre S. Unraveling galectin-1 as a novel therapeutic target for cancer. Cancer Treat. Rev. 2014;40:307–319. doi: 10.1016/j.ctrv.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Auvynet C, Moreno S, Melchey E, Coronado-Martinez I, Montiel JL, Aguilar-Delfin I, Rosenstein Y. Galectin-1 promotes human neutrophil migration. Glycobiology. 2013;23:32–42. doi: 10.1093/glycob/cws128. [DOI] [PubMed] [Google Scholar]
- 4.Banh A, Zhang J, Cao H, Bouley DM, Kwok S, Kong C, Giaccia AJ, Koong AC, Le QT. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer Res. 2011;71:4423–4431. doi: 10.1158/0008-5472.CAN-10-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11:1473–1491. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum LG, Pang M, Perillo NL, Wu T, Delegeane A, Uittenbogaart CH, Fukuda M, Seilhamer JJ. Human thymic epithelial cells express an endogenous lectin, galectin-1, which binds to core 2 o-glycans on thymocytes and T lymphoblastoid cells. J. Exp. Med. 1995;181:877–887. doi: 10.1084/jem.181.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burdick MM, Henson KA, Delgadillo LF, Choi YE, Goetz DJ, Tees DFJ, Benencia F. Expression of E-selectin ligands on circulating tumor cells: cross regulation with cancer stem cell regulatory pathways? Front Oncol. 2012;2:1–11. doi: 10.3389/fonc.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdick MM, Reynolds NM, Martin EW, Hawes JV, Carlson GE, Cuckler CM, Bates MC, Barthel SR, Dimitroff CJ. Isolation and characterization of chimeric human Fc-expressing proteins using protein a membrane adsorbers and a streamlined workflow. J. Vis. Exp. 2014;83:e51023. doi: 10.3791/51023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- 10.Carlson GE, Martin EW, Shirure VS, Malgor R, Resto VA, Goetz DJ, Burdick MM. Dynamic biochemical tissue analysis detects functional L-selectin ligands on colon cancer tissues. PLoS ONE. 2017;12(3):173737. doi: 10.1371/journal.pone.0173747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cedeno-Laurent F, Barthel SR, Opperman MJ, Lee DM, Clark RA, Dimitroff CJ. Development of a nascent galectin-1 chimeric molecule for studying the role of leukocyte galectin-1 ligands and immune disease modulation. J. Immunol. 2010;185:4659–4672. doi: 10.4049/jimmunol.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cedeno-Laurent F, Dimitroff CJ. Galectins and their ligands: negative regulators of anti-tumor immunity. Glycoconj. J. 2012;29:619–625. doi: 10.1007/s10719-012-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cedeno-Laurent F, Dimitroff CJ. Galectin-1 research in T-cell immunity: past, present and future. Clin Immunol. 2012;142:107–116. doi: 10.1016/j.clim.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chase SD, Magnani JL, Simon SI. E-selectin ligands as mechanosensitive receptors on neutrophils in health and disease. Ann. Biomed. Eng. 2012;40:849–859. doi: 10.1007/s10439-011-0507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen XL, Tummala PE, Olliff L, Medford RM. E-selectin gene expression in vascular smooth muscle cells: evidence for a tissue-specific repressor protein. Circ. Res. 1997;80:305–311. doi: 10.1161/01.RES.80.3.305. [DOI] [PubMed] [Google Scholar]
- 16.Cho M, Cummings RD. Galectin-1, a β-galactoside-binding lectin in Chinese hamster ovary cells: physical and chemical characterization. J. Biol. Chem. 1995;270:5198–5206. doi: 10.1074/jbc.270.10.5198. [DOI] [PubMed] [Google Scholar]
- 17.Cummings RD. S-type lectins (galectins) In: Consortium of Glycobiology Editors, editor. Essentials of Glycobiology. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- 18.Cummings RD, Lowe JB. Selectins. In: Consortium of Glycobiology Editors, editor. Essentials of Glycobiology. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- 19.Elola MT, Chiesa ME, Alberti AF, Mordoh J, Fink NE. Galectin-1 receptors in different cell types. J. Biomed. Sci. 2005;12:13–29. doi: 10.1007/s11373-004-8169-5. [DOI] [PubMed] [Google Scholar]
- 20.Esbona K, Inman D, Saha S, Jeffery J, Schedin P, Wilke L, Keely P. COX-2 modulates mammary tumor progression in response to collagen density. Breast Cancer Res. 2015;18:35. doi: 10.1186/s13058-016-0695-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garner OB, Baum LG. Galectin-glycan lattices regulate cell-surface glycoprotein organization and signaling. Biochem. Soc. Trans. 2010;36:1472–1477. doi: 10.1042/BST0361472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geng Y, Takatani T, Yeh K, Hsu JW, King MR. Targeting unglycosylated MUC1 for the selective capture of highly metastatic breast cancer cells under flow. Cell. Mol. Bioeng. 2013;6:148–159. doi: 10.1007/s12195-013-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He J, Baum LG. Endothelial cell expression of galectin-1 induced by prostate cancer cells inhibits T-cell transendothelial migration. Lab. Invest. 2006;86:578–590. doi: 10.1038/labinvest.3700420. [DOI] [PubMed] [Google Scholar]
- 24.Hudelist G, Singer CF, Pischinger KID, Kaserer K, Manavi M, Kubusta E, Czerwenka KF. Proteomic analysis in human breast cancer: identification of a characteristic protein expression profile of malignant breast epithelium. Proteomics. 2006;6:1989–2002. doi: 10.1002/pmic.200500129. [DOI] [PubMed] [Google Scholar]
- 25.Ichikawa Y, Lin YC, Dumas DP, Shen GJ, Garcia-Junceda E, Williams MA, Bayer R, Ketcham C, Walker LE, Paulson JC, Wong CH. Chemical-enzymatic synthesis and conformational analysis of sialyl Lewis X and derivatives. J. Am. Chem. Soc. 1992;114:9283–9298. doi: 10.1021/ja00050a007. [DOI] [Google Scholar]
- 26.Konnecke M, Boscke R, Waldmann A, Bruchhage KL, Linke R, Pries R, Wollenberg B. Immune imbalance in nasal polyps of Caucasian chronic rhinosinusitis patients is associated with a downregulation of E-selectin. J. Immunol. Res. 2014;1–8:2014. doi: 10.1155/2014/959854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leppanen A, Stowell S, Blixt O, Cummings RD. Dimeric galectin-1 binds with high affinity to α2,3-sialylated and non-sialylated terminal n-acetyllactosamine units on surface-bound extended glycans. J. Biol. Chem. 2004;280:5549–5562. doi: 10.1074/jbc.M412019200. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Bosch N, Navarro P. Glycans and galectins: sweet new approaches in pancreatic cancer diagnosis and treatment. In: Srivastava SK, editor. Pancreatic Cancer—Molecular Mechanism and Targets. Rijeka: InTech Europe; 2012. pp. 305–328. [Google Scholar]
- 29.Nimrichter L, Burdick MM, Aoki K, Laroy W, Fierro MA, Hudson SA, Von Seggern CE, Cotter RJ, Bockner BS, Tiemeyer M, Konstantopoulos K, Schnaar RL. E-selectin receptors on human leukocytes. Blood. 2008;112:3744–3752. doi: 10.1182/blood-2008-04-149641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seelenmeyer C, Wegehingel S, Tews I, Künzler M, Aebi M, Nickel W. Cell surface counter receptors are essential components of the unconventional export machinery of galectin-1. J. Cell Biol. 2005;171:373–381. doi: 10.1083/jcb.200506026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirure VS, Henson KA, Schnaar RL, Nimrichter L, Burdick MM. Gangliosides expressed on breast cancer cells are E-selectin ligands. Biochem. Biophys. Res. Commun. 2011;406:423–429. doi: 10.1016/j.bbrc.2011.02.061. [DOI] [PubMed] [Google Scholar]
- 32.Shirure VS, Liu T, Delgadillo LF, Cuckler CM, Tees DFJ, Benencia F, Goetz DJ, Burdick MM. CD44 variant isoforms expressed by breast cancer cells are functional E-selectin ligands under flow conditions. Am. J. Physiol. Cell Physiol. 2015;308(1):C68–C78. doi: 10.1152/ajpcell.00094.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirure VS, Reynolds NM, Burdick MM. Mac-2 binding protein is a novel E-selectin ligand expressed by breast cancer cells. PLoS ONE. 2012;7:1–12. doi: 10.1371/journal.pone.0044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song CX, Fu G, Yang X, Jiang Z, Wang Y, Zhou GW. Protein expression profiling of breast cancer cells by dissociable antibody microarray (DAMA) staining. Mol. Cell. Proteomics. 2008;7:163–169. doi: 10.1074/mcp.M700115-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Tinari N, Kuwabara I, Huflejt ME, Shen PF, Iacobelli S, Liu FT. Glycoprotein 90 K/MAC-2BP interacts with galectin-1 and mediates galectin-1-induced cell aggregation. Int. J. Cancer. 2001;91:167–172. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1022>3.3.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 36.Ullrich A, Sures I, D’Egidio M, Jallal B, Powell TJ, Herbst R, Dreps A, Azam M, Rubinstein M, Natoli C, Shawver LK, Schlessinger J, Iacobelli S. The secreted tumor-associated antigen 90 K is a potent immune stimulator. J. Biol. Chem. 1994;269:18401–18407. [PubMed] [Google Scholar]
- 37.Varki A. Selectin ligands: will the real ones please stand up? J. Clin Invest. 1997;99:158–162. doi: 10.1172/JCI119142. [DOI] [PMC free article] [PubMed] [Google Scholar]