Abstract
An important step towards achieving functional diversity of biomimetic surfaces is to better understand the co-assembly of the extracellular matrix components. For this, we study type-I and type-III collagen, the two major collagen types in the extracellular matrix. By using atomic force microscopy, custom image analysis, and kinetic modeling, we study their homotypic and heterotypic assembly. We find that the growth rate and thickness of heterotypic fibrils decrease as the fraction of type-III collagen increases, but the fibril nucleation rate is maximal at an intermediate fraction of type-III. This is because the more hydrophobic type-I collagen nucleates fast and grows in both longitudinal and lateral directions, whereas more hydrophilic type-III limits lateral growth of fibrils, driving more monomers to nucleate additional fibrils. This demonstrates that subtle differences in physico-chemical properties of similar molecules can be used to fine-tune their assembly behavior.
Electronic supplementary material
The online version of this article (doi:10.1007/s12195-016-0466-3) contains supplementary material, which is available to authorized users.
Keywords: AFM, Biomimetic surface, Collagen, Extracellular matrix, Heterogeneous assembly, Macromolecular assembly
Introduction
An emerging concept regarding the extracellular matrix (ECM) in multicellular organisms is that of biological alloys, where functions are determined not by individual constituent molecules, but more collectively by their assemblies.6,12,36 In this way, structural and functional diversity of different tissue types can be achieved with a limited number of components that assemble in different proportions and suprastructures. Co-assembly of different ECM molecules also has potential in tissue engineering applications for generating matrices of tailored properties.1,11,20,22,34,51
Among the constituents of the ECM, collagen is predominant, forming about 30% of the total protein mass in the human body.44 Combining collagen with other molecules such as proteoglycans and minerals, provides enormous diversity in tissue types and properties, such as bone, tendon, blood vessels, cornea, and skin.15 Among 28 known collagen types,44 the fibrillar ones including types-I, II, III, and V are dominant, and their assembly into fibrils and fibrillar networks have been extensively studied.1,7,10,42 Collagen is also used for a wide range of biomedical and biotechnological applications, including templates for fabricating nanowires,46 as a coating material for non-biological surfaces for enhanced bio-compatibility,8,16,29 surface patterning,35,57 and for drug delivery.40
Compared to the assembly of a single collagen type, co-assembly of multiple types is far less understood. A basic question in heterotypic collagen assembly is the dependence of the fibril morphology and growth kinetics on the ratio of the component collagens, in particular, between types-I and III that are respectively the most and the second most abundant collagen types in tissues.49 Type-I collagen is found throughout many tissues including tendon, ligament, skin, bone, and cornea.15,24 Type-III collagen co-assembles with type-I and is present in soft connective tissues such as blood vessels, skin, muscle, placenta and various internal organs.14,18,26,53 A previous study suggested that type-III collagen accelerates assembly but prevents bundling of type-I fibrils.38 Also, the fibril diameter decreases with the content of type-III collagen both in vitro 1,51 and in vivo.3 Synergistic effects in fibril morphology are also present in the co-assembly of other fibrillar collagens, including types-I/V, II/III, and II/XI.2,7,42,56
To better understand the co-assembly, it is necessary to analyze the early stages of this process, for which very little is known. Although turbidimetry has been widely used to quantify fibril nucleation and growth kinetics,4,38 it does not provide morphological information at the level of individual fibrils. Besides, early stages of assembly cannot be detected by turbidimetry since the concentration of fibrils with sufficient size may not be high enough to be detected. For studying initially formed fibrils or protofibrils, atomic force microscopy (AFM) is an effective approach.8,9,23,30 AFM has been used for assembly of different collagen types including type-I,5,13,17,21,23,30,37,52 type-II,10 and type-V,42 and also to visualize mature heterotypic fibrils including type-I/III1 and type-I/V.42 However, to our knowledge, the formation of heterotypic fibrils has not been analyzed previously at the level of individual fibrils.
Here, we use AFM to study assembly of homotypic and heterotypic fibrils made of types-I and III collagen. We find that type-III fibrils are more flexible than type-I, and nucleate and grow more slowly. We further investigate both early and late stages of the co-assembly process while varying the ratio between the two collagens. AFM images of the fibrils were analyzed by using our Computer-Aided Feature Extraction (CAFE) program that can identify fibrils and measure their lengths and orientations.21 By applying a kinetic model of nucleation and growth to the measured data, we estimate fibril nucleation and growth rates as functions of the fraction of type-III collagen. While the growth rate decreases nearly monotonically as the type-III fraction increases, the nucleation rate has a maximum at an intermediate value of its fraction. During co-assembly, the higher propensity for fibril nucleation by type-I collagen and the inhibition of lateral association of collagen monomers on existing fibrils by type-III can lead to synergistic enhancement of nucleation. These results suggest that slight differences in physical properties of the collagen types may lead to synergistic effects where the resulting co-assembly process is not a simple interpolation between the homotypic assembly of individual fibrils.
Materials and Methods
Materials
Type-I collagen, acetic acid extracted from rat tail tendon, was purchased from BD Biosciences (Bedford, MA) in solubilized form at 3.54-mg/mL concentration. Aliquots were prepared by diluting the stock with 0.1% acetic acid to 1.65 mg/mL and stored at 4 °C for use up to 3 months. For the experiment, an aliquot was further diluted with a buffer consisting of 30 mM Na2HPO4, 10 mM KH2PO4 and 200 mM KCl at pH 7.30
Lyophilized type-III collagen, pepsin extracted from bovine skin (10 mg), was purchased from Millipore Corporation (Temecula, CA). It was reconstituted as 1.25 mg/mL aliquots in 80-μL volumes, with 0.5 M acetic acid (pH 2) by using a mini shaker at 4 °C for 2 h at 500 rpm until the solution appeared homogeneous. It was then stored at −20 °C and used up to 1 year. For the experiment, an aliquot was thawed on ice and diluted to 1 mg/mL by adding 0.5 M acetic acid. It was maintained in a 4 °C refrigerator up to 1 h before use. The final solution of type-III collagen for the experiment was prepared following the same protocol as for type-I collagen.
Muscovite mica (grade VI) and phlogopite mica were purchased from Ted Pella (Redding, CA) and from Axim Mica (New Hyde Park, NY), respectively.
Sample Preparation for AFM
Mica sheets were glued onto AFM metal disks and cleaved using sticky tape just prior to each experiment. A 50 μL sample aliquot was deposited on mica and incubated at room temperature in a moisture chamber to minimize evaporation. After a given incubation time, the sample was rinsed with 70 μL of deionized water in multiple directions and was left to air-dry for 15 min in a half-covered dish. Immediately after drying, the sample was imaged using AFM, as done previously.10,11,30,33,37,52
AFM Imaging
Two AFM machines were used, based on availability: a Veeco NanoScope IIIa (Figs. 1, 2, 5, and S2) and Brucker Icon (Fig. 3). Tapping mode (soft non-contact mode) topography imaging was performed at room temperature using force modulation cantilevers (Veeco Probes-FESP7 and NanoWorld Probes; 2.8 N/m force constant and 75-kHz resonance frequency in both probes). The scanning speed ranged from 0.2 to 2 Hz, depending on the scan size.
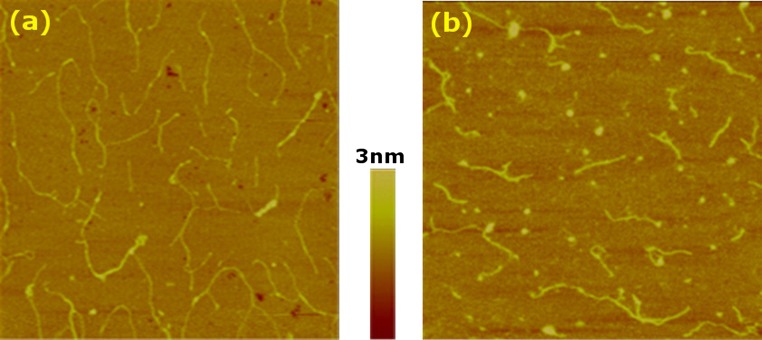
Figure 1.
Representative AFM images of collagen monomers. (a) Type-I and (b) type-III. In both cases, 0.3 μg/mL concentration and 85 s incubation time were used. Scan sizes are 2 × 2 μm and the color scale represents the vertical range.
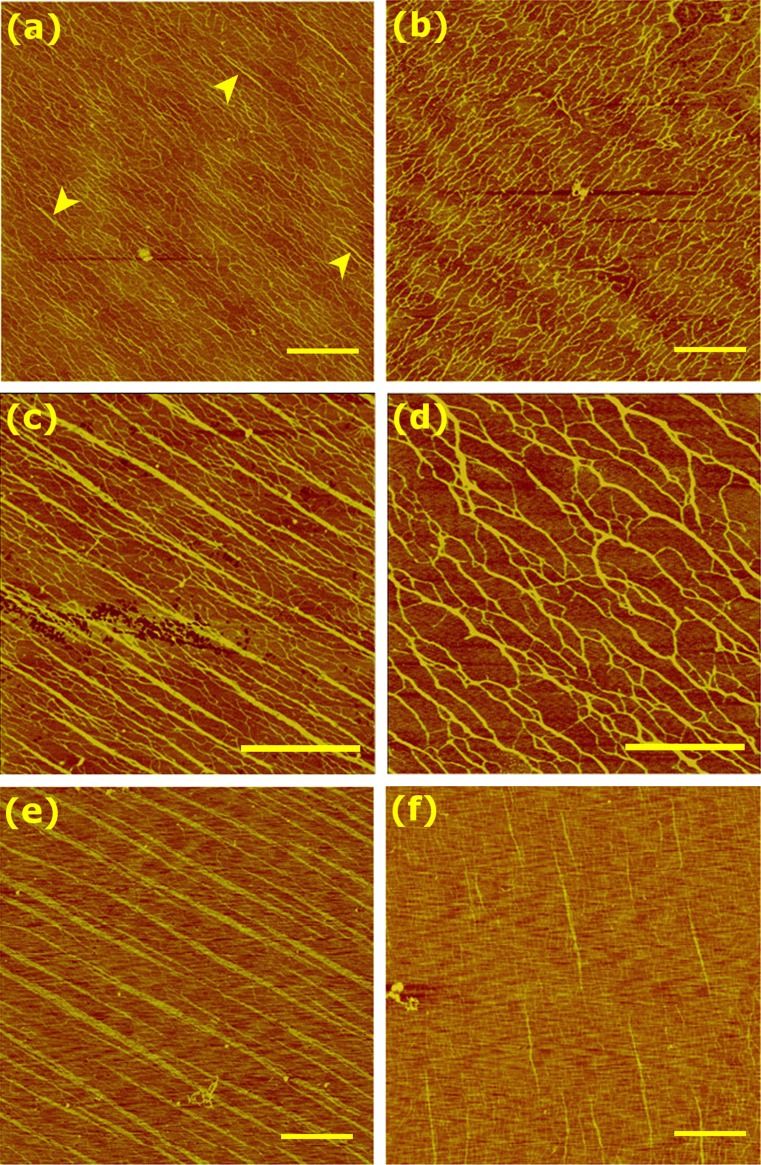
Figure 2.
Homotypic assemblies of (a, c, e) type-I and (b, d, f) type-III collagen fibrils. Muscovite mica was used, with 2 min incubation. Concentrations are: (a, b) 2 μg/mL, (c, d) 4 μg/mL, (e, f) 5 μg/mL. Color scales are (a–d) 2 nm, and (e, f) 5 nm. In (a), examples of nascent fibrils are marked by arrowheads. Scale bar: 1 μm. The apparent widths of fibrils in (c, e) are: (c) 37 ± 9 nm () and (e) 71 ± 22 nm ().
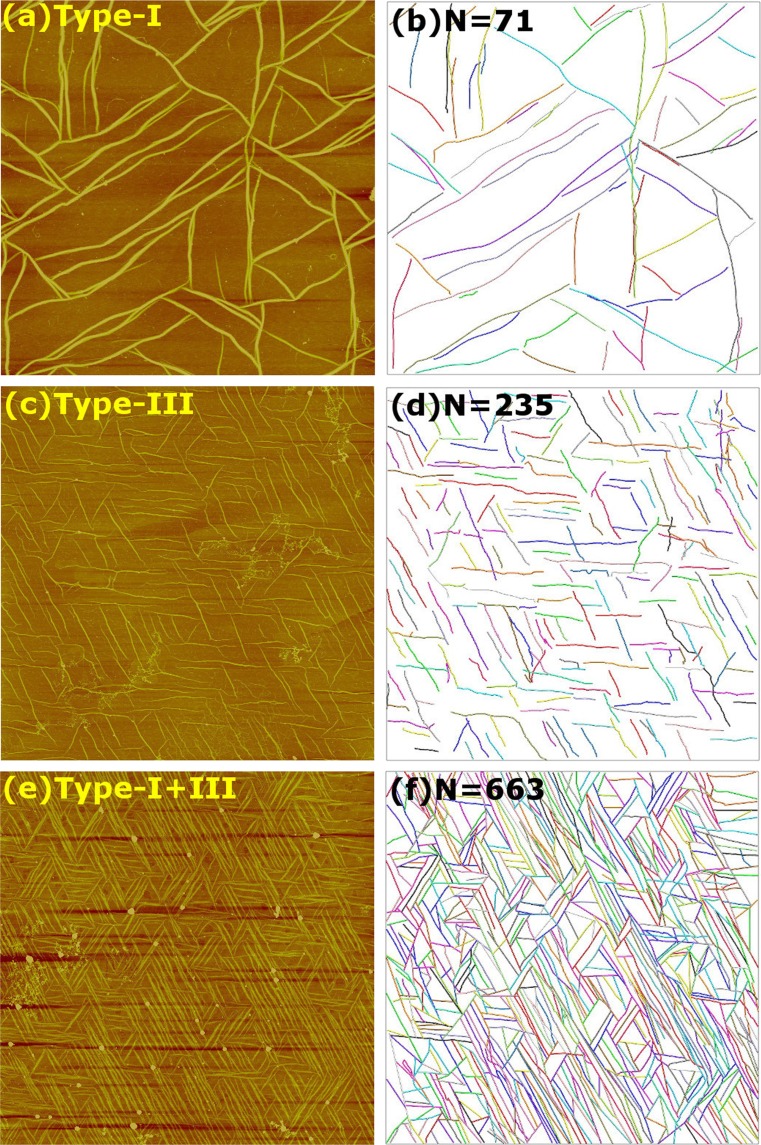
Figure 5.
Heterotypic assembly of types-I and III collagens on phlogopite mica. All images are with 5 μg/mL final concentration and 1-h incubation. (a, c, e) AFM images. Ratios of types-I:III are: (a) 1:0 (c) 0:1, and (e) 1:1. Scan sizes are 20 μm. Additional scans of the samples for (a) and (e) are in Supporting Fig. S2. Color scales correspond to (a, c) 10 nm and (e) 15 nm. (b, d, f) Corresponding networks constructed by CAFE. Number of identified filaments is given on each panel. The apparent widths of filaments are: (a) 162 ± 31 nm, (c) 45 ± 10 nm, (e) 88 ± 27 nm ( in each case).
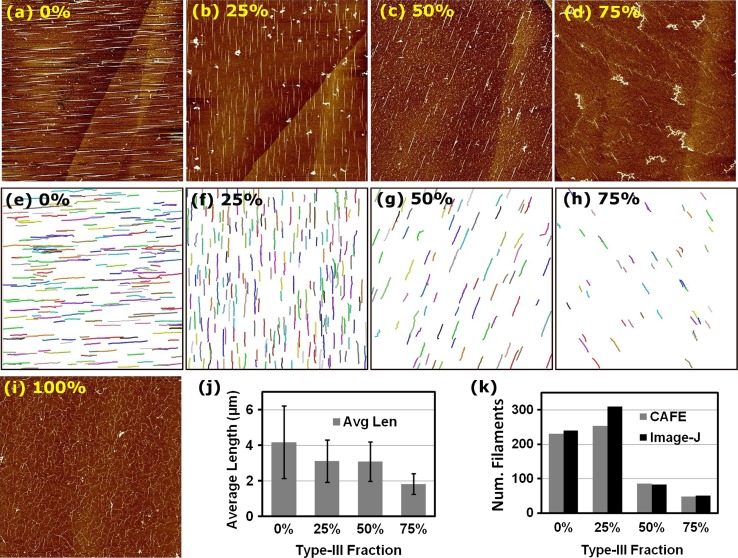
Figure 3.
Heterotypic fibril formation on muscovite mica. (a–d, i) AFM images with different fractions of type-III collagen in the type-I/III mixture (percentage of type-III is shown on each panel). All experiments were done with 0.5 μg/mL final concentration and with 30 min incubation. The color scale corresponds to a vertical range of 2 nm. Heights of fibrils (pixel intensity) decrease with increasing fraction of type-III in the mixture. Scan sizes are (a–d) 40 × 40 μm and (i) 10 × 10 μm. (e–h) Fibrils identified by the CAFE program. Fibrils are distinguished by randomly assigned colors. (j) Average length and (k) number of fibrils in (e–h). In (k), numbers of fibrils are also counted by using ImageJ, for comparison. The apparent widths of fibrils in (a–d) are: (a) 71 ± 34 nm (), (b) 33 ± 16 nm (), (c) 50 ± 16 nm (), and (d) 23 ± 7 nm ().
Image Analysis
Using the software included in the AFM machines, basic image flattening was performed. NIH ImageJ was used for measuring the apparent widths of fibrils (Figs. 2, 3, and 5; without correcting for the finite radius of curvature of the AFM tip) and counting the number of fibrils in Fig. 3. Apparent widths were measured in the middle portions of individual fibrils, and are presented as average ± standard deviation in the caption of each figure. For more automated quantitative analysis of fibrils in Figs. 3 and 5, we used our CAFE program.21
Results and Discussion
Choice of Mica Substrate
We have previously analyzed the influence of mica substrate on the assembly of collagen fibrils.30 On muscovite mica, collagen tends to assemble in parallel due to the asymmetry in the mica lattice.9,23,30,52 During early stages of assembly, the parallel arrangement helps short collagen fibrils to avoid overlapping so that their number and length can be measured (cf., Fig. 3). With long incubation times or at higher collagen concentration, fibrils on muscovite mica merge both laterally and longitudinally,9,30 making it difficult to resolve them (cf., Fig. 2). In comparison, phlogopite mica maintains surface hexagonal symmetry of the lattice27 so that collagen fibrils form triaxial networks (cf., Fig. 5). This makes it easier to resolve individual fibrils at long incubation times.13,21,30 Using both muscovite and phlogopite mica, we can thus study both early stages of assembly and later formation of more mature fibrils.
Monomers and Oligomers
To observe morphologies of collagen molecules before extensive assembly into fibrils, we used a very low concentration (0.3 μg/mL), that is well below the critical concentration for the assembly of type-I collagen fibrils in bulk (4.73 μg/mL25). We used muscovite mica and incubated the sample for about 85 s. For both types-I and III collagen, randomly adsorbed molecules without any noticeable orientational preference are seen (Fig. 1).30 For type-I, molecules are often connected in a linear fashion or form branches (Fig. 1a). Under the same experimental condition, type-III collagen did not associate as extensively (Fig. 1b). Type-I collagen has more nonpolar and less polar residues compared to type-III collagen, while their net charges are comparable (Table 1). A greater number of nonpolar residues promotes association between type-I molecules, while more polar type-III molecules experience a higher barrier to association by hydration force.43
Table 1.
Amino acid composition of type-I and type-III collagen.
| (a) | |||
|---|---|---|---|
| Gene | COL1A1 | COL1A2 | COL3A1 |
| UniProt ID | P02454 | P02466 | P04258 |
| Length (AA) | 1014 | 1023 | 1026 |
| Telopeptide | 16(N)+26(C) | – | 14(N)+9(C) |
| Nonpolar | 176+(8) | 208 | 133+(9) |
| Polar | 99+(10) | 89 | 108+(2) |
| Acidic | 78+(5) | 66 | 73+(3) |
| Basic | 87+(4) | 85 | 86+(1) |
| Pro+Hyp | 232+(4) | 203 | 248+(0) |
| (b) | ||
|---|---|---|
| Tot. count | Type-I | Type-III |
| Net charge | 37+(−2) | 39+(−6) |
| Nonpolar | 560+(16) | 399+(27) |
| Polar | 287+(20) | 324+(6) |
| Pro+Hyp | 667+(8) | 744+(0) |
Sequences are for rat (type-I) and bovine (type-III) collagen. (a) Counts for individual α chains. Type-I collagen consists of two α1 and one α2 chains, Type-III collagen is a homotrimer of α1 chains. Numbers in parentheses are counts for telopeptides. COL1A2 does not have telopeptides marked in the UniProt Database. (b) Total counts
For type-III collagen, globular structures were frequently observed (Fig. 1b), which are likely collapsed type-III molecules. Type-I collagen also takes on a globular morphology at low pH.23 When brought to pH 7 for AFM imaging, type-III collagen in the globular state may require a longer time to convert to its elongated state. Consistent with this, globular structures were not observed at longer incubation times (see below). Formation of more contacts among type-I molecules and presence of globular structures in type-III collagen thus suggest that the former takes on extended conformation and assembles more rapidly than the latter does. However, for individual extended molecules, no noticeable differences in morphology were found between types-I and III. While an earlier analysis based on the amino acid sequence, proposed that the type-III molecule is more flexible than type-I,48 as shown below, the difference becomes more pronounced at larger length scales, as they assemble into fibrils.
Homotypic Assembly
Next, we compared the emergence of homotypic type-I vs. type-III fibrils by increasing collagen concentration to 2–5 μg/mL, with 2-min incubation (Fig. 2). At 2 μg/mL, extensively connected networks form, where nascent straight fibrils can be seen in type-I but not in type-III collagen (Figs. 2a and 2b). Molecules appear slightly more parallel for type-I collagen, as evidenced by the presence of sharper peaks in the fast Fourier transform of the images (Supporting Fig. S1). For type-III collagen, beaded structures are nearly absent compared to the case above (Figs. 1b vs. 2b). In addition to the slightly longer incubation time, contacts with other molecules at higher concentration may have promoted elongation of type-III collagen molecules. Since both collagen types are stored at low pH, where molecules take on globular morphology, then brought to pH 7 for the experiment, the slower transition from a globular to an extended, polymerization-competent state in type-III collagen may contribute to its slower assembly compared to type-I.
At 4 μg/mL, straight fibrils form extensively for type-I collagen, whereas type-III forms interconnected fibrils possessing branches and kinks (Figs. 2c and 2d). At 5 μg/mL, type-I collagen behaves similarly to the case of 4 μg/mL, except that fibrils are wider (Fig. 2e). This indicates that type-I fibrils can grow laterally.9,30 For type-III collagen, straight fibrils are visible (Fig. 2f), which is analogous to the nucleation of straight fibrils for type-I collagen at 2 μg/mL (Fig. 2a). Overall, these results suggest that type-III collagen has lower propensity to nucleate straight fibrils than type-I does. Since prolonged incubation leads to formation of straight type-III fibrils (cf., Fig. 5c), its lower propensity to form straight fibrils at 2-min incubation suggests that it assembles more slowly compared to type-I collagen.
As for the case of surface-adsorbed monomers (Fig. 1), the above observations are consistent with the difference in amino acid composition between type-I and type-III collagens (Table 1). The higher number of nonpolar residues in type-I collagen is likely responsible for faster assembly via increased hydrophobic effects. On the other hand, type-III collagen, possessing more polar residues, is more strongly hydrated. Instead of forming a relatively straight bundle driven by hydrophobic interactions, it will thus initially form a loosely connected and branched network, and takes longer to form a more ordered, straight fibril, which is accelerated at higher concentrations. Differences in contributions by hydrophobic and hydration effects between the two collagen types is also consistent with the general trend that type-III collagen fibrils are thinner than type-I fibrils.4,45,51,55 By comparison, previous turbidimetry experiments reported higher apparent nucleation rate for type-III than type-I collagen,4,38,49 though slower assembly of type-III collagen has also been reported.1 Turbidimetry experiments were performed with collagen concentrations in the 0.2–1.0 mg/mL range, where protofibrils of type-I collagen may form almost immediately after assembly starts. Thus, rather than nucleation of individual fibrils, those experiments may have detected bundling of fibrils or network formation. In the case of type-I collagen, the thickening of fibrils by lateral addition of monomers or bundling may continue throughout the course of measurement, while type-III collagen ceases to form a bundle after initial assembly of thinner fibrils. The formation of larger type-I bundles is consistent with their higher absorbance than for type-III fibrils after reaching plateaus in turbidimetry.1,4,38
Heterotypic Assembly
Heterotypic Assembly on Muscovite Mica
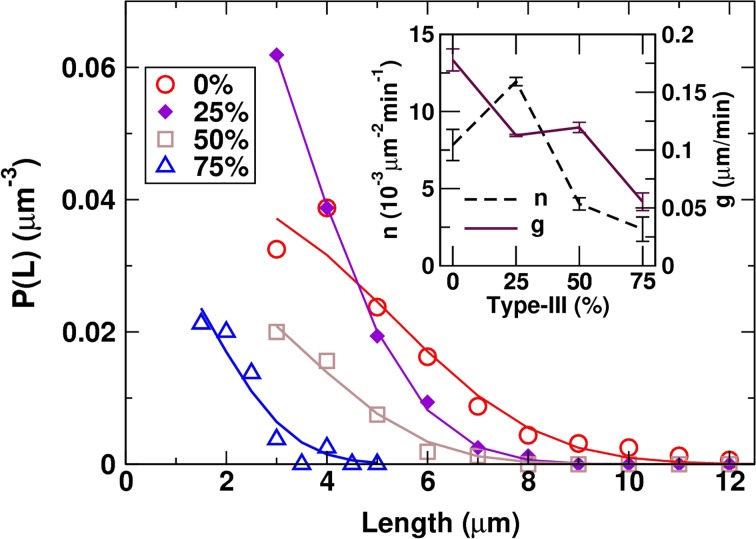
To study early stages of the co-assembly of types-I and III collagen, we used a final concentration of the collagen mixture at 0.5 μg/mL, while varying the ratio between the two types. As explained above, we used muscovite mica with short fibrils arranged in parallel, thus reducing the chance of them making contacts with each other. To minimize the influence of a variation in the collagen concentration among stock solutions, we used single stocks of types-I and III and studied the fraction of type-III at 0% (pure type-I), 25, 50, 75, and 100% (pure type-III) (Figs. 3a–3d, 3i). All samples except for the 100% case exhibited straight fibrils (Figs. 3a–3d vs. 3i). We used the CAFE program21 to measure the number and length of fibrils for the 0–75% samples (Figs. 3e–3h).
Dependence of the average length and the number of fibrils on the type-III fraction suggests corresponding differences in the fibril growth and nucleation rates. We estimated them by analyzing the filament length distribution P(L) and applying a kinetic model that we developed previously.21 We made histograms of filament lengths with 1-μm bins for Figs. 3e–3g, and 0.5-μm bins for Fig. 3h. The resulting histogram (filament count) was divided by the area of the image (40 × 40 μm2) multiplied by the bin size, which yielded P(L) in units of μm−3, so that (ΔL: bin size) is the number of filaments per unit area in the length range (L, ) (Fig. 4).
Figure 4.
Analysis of the filament length distribution P(L). Symbols: results from CAFE (Figs. 4e–4h). The unit of P(L) reflects filament length density per unit area of the substrate. Solid lines: fit using Eq. (2). Inset: Filament nucleation and growth rates estimated from the fit (error bar: standard deviation).
We assume a constant nucleation rate n (number of filaments nucleating per unit time and unit area of the substrate) and growth rate g. To validate this assumption, we estimate the fraction of collagen molecules in fibrils versus the total number of collagen molecules in the incubating buffer. A 50-μL aliquot, used for incubation on mica, contains on the order of 1010 collagen molecules at 0.5 μg/mL.21 In Fig. 3a, the total length of filaments is 965.34 μm (it is 789.32 μm for Fig. 3b, and shorter in Figs. 3c and 3d). With an apparent width of 70 nm and 2-nm thickness of a fibril, assuming each collagen molecule to be 1.5 nm in diameter and 300 nm long, 2.5 × 105 collagen molecules are incorporated in fibrils, which is less than 0.01% of the total number of collagen molecules in the aliquot. Thus, by the time fibrils in Fig. 3a form, a majority of collagen molecules are still in the incubating solution or loosely attached to the mica surface in an un-polymerized state, supporting the assumption of constant n and g. Similarly, constant rates can be assumed for Figs. 3b–3d.
The thickness (apparent width) of the fibril is not constant along its length and it is more difficult to measure accurately compared to filament length. In our model, we thus focus on n and g and analyze only P(L) without explicitly considering lateral growth. Additionally, once a fibril forms on the mica surface, it remains strongly bound and withstands multiple rounds of washing.30 Since an extended collagen molecule in a fibril will make hundreds of contacts (Table 1), unbinding will be much rarer compared to the addition of collagen molecules to a growing fibril. We thus ignore depolymerization in our model.
During the 30-min incubation time, filaments nucleate and grow mostly without making contact. In the case of 100% type-I collagen (Fig. 3a), we estimate the probability that two aligned filaments join to form longer filaments. The average filament length is μm (Fig. 3j). Let the width of the filament be nm and the linear size of the mica μm. Without loss of generality, suppose that a filament lies horizontally and its center is at the mid-point of mica, to which we assign the coordinate origin (0,0). For another horizontal filament to overlap with this one, its center should lie within a rectangle of horizontal range () and vertical range (). The corresponding overlap probability can be calculated as the fractional area of this window, . For filaments (Fig. 3k), filament pairs can potentially overlap. Similar calculation yields the number of overlapping pairs as 8.2 (25% type-III), 1.4 (50%), and 0.12 (75%). At earlier times during which there are fewer filaments with smaller sizes (smaller and w), the number will be even lower. To a first approximation, we thus ignore formation of filaments by annealing of two shorter ones, and assume that each filament grows by addition of monomers. In this case, an expression for P(L) can be obtained in the following way. We discretize the length (we take a continuum limit later) and let L () be the degree of polymerization. New monomers () nucleate with rate n and growth occurs by addition of monomers to existing filaments with rate g. The master equation describing this process is21
| 1 |
In Eq. (1), accounts for the loss of L-mers to become -mers, and similar interpretations apply to other terms. In the continuum limit, this model has a solution involving a complementary error function21:
| 2 |
In Eq. (1), lengths are discretized in dimensionless units. The choice of units only alters the numerical values of n and g without affecting the behavior of the model. For example, if we choose 1 μm as the length unit (effective size of a ‘monomer,’ which is different from the size of the actual collagen molecule) and 1 min as the time unit, represents nucleation of a 1-μm filament per 1 μm2 area per minute, and corresponds to a growth rate of 1 μm/min (in the dimensional form, the left hand side of Eq. (1) is multiplied by ΔL). However, direct use of Eq. (1) for analysis is difficult due to the lack of data taken at multiple time points and also due to the limited accuracy of P(L). We instead use Eq. (2) to fit P(L) (with min, the incubation time), and estimate n and g (Fig. 4; fit was done using the SciPy package of Python). This indeed shows that n is the highest when the type-III fraction is 25%, and that the growth rate g decreases from 0.18 μm/min (0%) to 0.06 μm/min (75%). Linearly extrapolating n and g to 100% type-III collagen suggests that they both decline close to zero, which is consistent with the absence of fibrils in this experiment (Fig. 3i). By comparison, we previously used a stochastic simulation that is different from the model described by Eq. (1), to estimate g for triaxial networks of type-I fibrils on phlogopite.21 This yielded 0.038–0.113 μm/min, on the same order of magnitude as the values obtained above. Given that experimental conditions differ between these two studies, in general, the growth rate of collagen fibrils on mica would be on the order of 100 nm/min.
Since homotypic fibrils form more slowly for type-III than for type-I collagen (Fig. 2), for a given total collagen concentration, one may expect heterotypic fibrils to nucleate and grow more slowly as the fraction of type-III increases. In this regard, the enhanced nucleation rate at 25% is puzzling. In addition, g is slightly higher at 50% than at 25% (inset in Fig. 4). These behaviors can be seen qualitatively from the AFM images. In Fig. 3b, there are short fibrils that are greater in number than in Fig. 3a (higher n and lower g at 25% than at 0%). Also, fibrils in Fig. 3c are lower in number but their lengths are comparable to those in Fig. 3b. Though their average lengths are similar (Fig. 3j), the greater presence of shorter fibrils at 25% may have caused g to be slightly lower.
Qualitative agreement between visible features in the images and estimated values of n and g indicate that, rather than from errors in identifying fibrils by CAFE or the simplifying model assumptions, a greater source of uncertainty in n and g likely arises from variability in experimental conditions. Although analysis using Eq. (2) was performed only at a single time point (30-min incubation), it is effective in describing different assembly behaviors of systems with varying ratios of type-I and type-III collagens. Further development of the model that relaxes some of the assumptions of the present model, is subject of a future study.
In case when both n and g decrease monotonically with the type-III fraction, if g decreases more rapidly than n does, more fibrils may be observed at an intermediate range of the type-III fraction, since suppressing fibril growth by type-III collagen would mean more type-I monomers are available to nucleate new fibrils. But when the fraction of type-III collagen is too high (50% or above), the corresponding reduction in type-I collagen will make it difficult to nucleate fibrils. At 50%, the concentration of type-I collagen is only 0.25 μg/mL, which is lower than that used in Fig. 1.
Heterotypic Assembly on Phlogopite Mica
We used phlogopite mica to study longer-time heterotypic assembly of collagen. Keeping the 5 μg/mL total concentration, we set the fraction of type-III collagen to 0, 50 and 100%. After incubation of each solution on phlogopite mica for 1 h we imaged the sample by using AFM (Figs. 5a, 5c, 5e). For the 0% case (100% type-I), a sparse triaxial network of thick and long fibrils was observed (Supporting Fig. S2a shows a larger area scan of the sample for Fig. 5a), which is consistent with our previous observations.21,30 With the introduction of type-III collagen, the number of fibrils increased, with more fibrils in the 50% case than in the 100% case. This result is similar to the early-stage assembly on muscovite mica (Fig. 3). The type-III fraction that yields the largest number of fibrils may depend on factors such as the total collagen concentration, type of mica used, and the incubation time, that are different between the two experiments of Figs. 3 and 5. In contrast to the non-monotonic behavior of the number of fibrils, the thickness of fibrils decreases as the type-III collagen fraction increases (Figs. 5a, 5c, and 5e), which agrees with the results on muscovite mica (Fig. 3) and also with previous in vitro and in vivo results.1,3,45,51
More quantitative analysis of the fibril network was performed by using CAFE. After identifying individual filaments (Figs. 5b, 5d, and 5f), we measured distributions of filament orientations and lengths (Fig. 6). Compared to the 0% case, significantly more filaments were found for 50 and 100% type-III collagen (Figs. 5b vs. 5d, 5f), which suggests that the presence of type-III collagen increases the filament nucleation rate and/or reduces the growth rate, which in turn leads to nucleation of more fibrils. Compared to muscovite mica, collagen interacts less strongly with phlogopite mica, so that surface diffusion of monomers and small oligomers is enhanced.30 The sparsity of the network of type-I fibrils (Fig. 5a) indicates that monomers diffuse and incorporate into nearby fibrils, which suppresses the nucleation of new fibrils. For 100% type-III collagen, comparable or higher levels of surface diffusion are expected as more polar residues are present than in type-I collagen, leading to weaker adsorption on phlogopite mica (Table 1). The greater hydrophilicity of type-III collagen also limits the fibril diameter to a smaller value than in the case of type-I collagen due to a larger dehydration penalty in encasing type-III molecules within a fibril of a larger diameter. Suppression of lateral growth of type-III fibrils will make more monomers available to nucleate additional filaments. This effect is further enhanced on phlogopite mica since longitudinal growth of a fibril stops once it encounters other fibrils within the triaxial network. Furthermore, slower assembly of type-III collagen indicates that its initial lag time for nucleation into straight fibrils is longer. These factors are consistent with the greater number of fibrils observed in Fig. 5 compared to Fig. 3, since the former has higher overall collagen concentration, longer incubation time, and was growing on phlogopite mica, which all promote nucleation of more fibrils.
Figure 6.
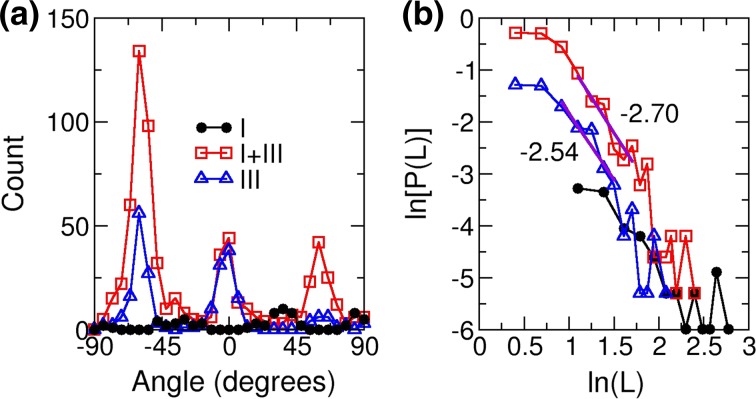
Analysis of the networks in Fig. 1 by CAFE. Fraction of type-III collagen is 0% (circle), 50% (square), and 100% (triangle). (a) Filament angle distribution (not normalized). Angle is measured relative to the horizontal direction. (b) Length distribution in log-log scale. Filament length L is in units of μm and distribution P(L) is in μm−3 (cf., Fig. 5). Thick straight lines are local fits for the intermediate range of L, with slopes as indicated.
A synergistic effect likely occurs when both collagen types are present. The stronger interaction and weaker surface diffusion associated with type-I molecules may provide stability to the initial oligomers, making it easy to nucleate a filament. Type-III collagen, being more hydrophilic, locates on the outer side of the fibril and prevents lateral growth. Localization of type-III collagen on surfaces of thin type-I-rich fibrils has also been observed in vivo,50 although it is unclear whether type-III collagen is completely excluded from the inner side. Prevention of monomers from adding to existing fibrils would in turn promote nucleation of more fibrils, which occurs faster in the presence of type-I collagen compared to the case of pure type-III collagen, as seen in the assembly of homotypic fibrils (Fig. 2). With a higher overall collagen concentration, more type-I molecules are present even when the fraction of type-III collagen is high, which may be the reason why more fibrils were observed at 50% in Fig. 5e while the maximal nucleation was observed at 25% in Fig. 3b, where the former had 10-fold higher total collagen concentration.
In addition to the overall number of fibrils, we measured the distribution of fibril orientation and length (Fig. 6). Orientational distributions mainly have three peaks separated by about 60°, reflecting the triaxial network. The type-I collagen network (0% type-III) has two major peaks at 36° and 84° and one minor peak at −30° (the distribution has a bin size of 6°), which can be more easily gleaned from a larger area scan (Supporting Fig. S2a). The case of 100% type-III collagen also has two major peaks. The presence of two major peaks is consistent with our earlier study using type-I collagen.21 On the other hand, the 50% case has a single major peak. Different levels of alignment are due to the slightly broken surface hexagonal symmetry of the phlogopite mica lattice,21,27 the buffer condition,30 and the variation in the state of the cleaved lattice domains. As for the location dependence, another scan of the 50% sample shows more symmetrically oriented fibrils, but the lower image quality made it difficult to measure the orientational distribution (Supporting Fig. S2b). Even in this case, there are more fibrils compared to the 0 and 100% cases. Thus, while various factors including the mica surface may influence the number and direction of fibrils formed, the nucleation enhancement in the mixture is more likely due to interaction among collagen molecules themselves, as also observed on muscovite mica.
On phlogopite mica, the length of a growing fibril is limited as it encounters another one at an angle, a process that we named Orientational Linear Epitaxy (OLE).21 In OLE, long filaments that started to grow early during the assembly process, randomly encounter other filaments that are sparsely distributed and do not yet form an extensive network. In contrast, shorter filaments that nucleate later encounter others that already have formed a network. These later-formed filaments thus fill progressively smaller polygonal areas in a self-similar manner. The two different regimes of behaviors in OLE lead to crossover of the filament length distribution P(L) from being exponential for long filaments to a power law for shorter ones.21 Although the number of fibrils was not large enough to see this clearly, for the 50 and 100% samples in Fig. 5, a power law can indeed be seen: with b in the 2.5–3.0 range (a universal scaling exponent in OLE,21 Fig. 6b). Therefore, while the basic architecture of the network formed by the co-assembly of types-I and III collagen on phlogopite mica obeys the physical constraint imposed by the OLE, the kinetics of nucleation and growth, and thickness of fibrils are controlled by the ratio between the two collagen types.
Conclusions
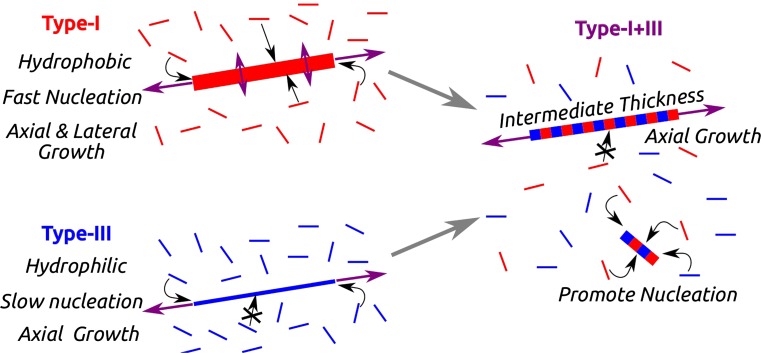
Present results suggest that type-I and type-III collagen have complementary properties which synergistically control their co-assembly behavior. The mechanism for the enhancement of nucleation in co-assembly is summarized in Fig. 7. Type-I collagen, with its greater number of nonpolar residues, nucleates and assembles fast, and the fibril can increase its diameter by lateral addition of monomers. Type-III collagen, on the other hand, is more hydrophilic so that it assembles slower and lateral growth of fibrils is limited. When the two collagens co-assemble, heterotypic fibrils become thinner as the fraction of type-III collagen increases. Since monomers can incorporate into growing fibrils mostly at the ends, a majority of monomers that are away from fibril ends increase the likelihood of nucleation of new fibrils. Thus, the nucleation rate increases with the fraction of type-III collagen and it eventually decreases as the amount of type-I collagen becomes too low. Among different ways in which the synergistic effect of the heterotypic assembly of type-I and type-III collagens manifests, e.g., in nucleation, growth, or in fibril morphology, the present study demonstrates that the fibril nucleation rate exhibits a non-monotonic dependence on the ratio between the two collagen types.
Figure 7.
Mechanism for the enhanced nucleation in the co-assembly of types-I and -III collagens. In heterotypic fibrils (represented by alternating red and blue stripes), type-III collagen (blue) impedes lateral association of monomers. This makes more collagen molecules to participate in nucleation of new fibrils, which is enhanced by the presence of type-I collagen (red).
As demonstrated by our experiments performed under different conditions, various factors affect quantitative aspects of the assembly. The fraction of type-III collagen at which the nucleation rate is maximized depends on the overall concentration of collagen, the buffer condition, and the substrate over which assembly occurs, which affects surface adsorption and diffusion (cf., Figs. 3 vs. 5). Due to the difference in the lag time for nucleation between the two collagens, it is also possible that the relative abundance of fibrils among different collagen fractions varies with the incubation time.
Of another note, since the type-I collagen that we used was acid extracted, it tends to have intact telopeptides that mediate association,51 In contrast, as type-III collagen was extracted via a protocol that involves mild pepsin treatment, the telopeptides may have been partially cleaved,19,47 which might contribute to the lower tendency of type-III collagen molecules to associate.28,51 However, this effect is present only after very extensive pepsin treatment, otherwise it only minimally affects the assembly or structure of fibrils.28 Besides, as in Table 1, telopeptides take less than 2.6% of the collagen sequence. Thus, although they might affect the initial association between collagen molecules, the more extensive interaction along the entire length of the molecule will play a dominant role in later growth of fibrils under the mechanism illustrated in Fig. 7.
As the above mechanism does not require a solid substrate (it mainly influences diffusion of adsorbed molecules and direction of fibril growth), the synergistic effect is likely applicable to the case of bulk solution, although quantitative details may differ. Similar or related synergistic effect may also have a functional role in vivo. For example, expression of type-III collagen increases during the wound healing process,31,54 where enhanced nucleation of small fibrils is potentially more desirable than generating fewer and thicker fibrils that would be more difficult for cells to manipulate in tissue remodeling. Similarly, a potential role of spatiotemporally graded expression of various collagen types during development32,41 would be to control the amount and morphology of collagen fibrils and fibril networks. Interactions involved in the co-assembly of other collagen types or ECM molecules and their effects, are yet to be found.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We acknowledge using the Texas A & M Materials Characterization Facility for part of the experiments. Esma Eryilmaz was supported by the Turkish Ministry of Education fellowship.
Conflict of Interest
Esma Eryilmaz, Winfried Teizer, and Wonmuk Hwang declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
No human studies were carried out by the authors of this article. No animal studies were carried out by the authors of this article.
References
- 1.Bierbaum, S., R. Beutner, T. Hanke, D. Scharnweber, U. Hempel, and H. Worch. Modification of Ti6Al4V surfaces using collagen I, III, and fibronectin. I. Biochemical and morphological characteristics of the adsorbed matrix. J. Biomed. Mater. Res. A 67(2):421–430, 2003. [DOI] [PubMed]
- 2.Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32(3):223–237. doi: 10.1016/S0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 3.Birk DE, Mayne R. Localization of collagen types I, III and V during tendon development. changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol. 1997;72(4):352–361. [PubMed] [Google Scholar]
- 4.Birk DE, Silver FH. Collagen fibrillogenesis in vitro: comparison of types I, II, and III. Arch. Biochem. Biophys. 1984;235(1):178–185. doi: 10.1016/0003-9861(84)90266-2. [DOI] [PubMed] [Google Scholar]
- 5.Bozec L, van der Heijden G, Horton M. Collagen fibrils: nanoscale ropes. Biophys. J. 2007;92(1):70–75. doi: 10.1529/biophysj.106.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruckner P. Suprastructures of extracellular matrices: paradigms of functions controlled by aggregates rather than molecules. Cell Tissue Res. 2010;339(1):7–18. doi: 10.1007/s00441-009-0864-0. [DOI] [PubMed] [Google Scholar]
- 7.Chanut-Delalande H, Fichard A, Bernocco S, Garrone R, Hulmes DJS, Ruggiero F. Control of heterotypic fibril formation by collagen V is determined by chain stoichiometry. J. Biol. Chem. 2001;276(26):24352–24359. doi: 10.1074/jbc.M101182200. [DOI] [PubMed] [Google Scholar]
- 8.Cisneros DA, Friedrichs J, Taubenberger A, Franz CM, Muller DJ. Creating ultrathin nanoscopic collagen matrices for biological and biotechnological applications. Small. 2007;3(6):956–963. doi: 10.1002/smll.200600598. [DOI] [PubMed] [Google Scholar]
- 9.Cisneros DA, Hung C, Franz CM, Muller DJ. Observing growth steps of collagen self-assembly by time-lapse high-resolution atomic force microscopy. J. Struct. Biol. 2006;154(3):232–245. doi: 10.1016/j.jsb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Dong M, Xu S, Bünger MH, Birkedal H, Besenbacher F. Temporal assembly of collagen type II studied by atomic force microscopy. Adv. Eng. Mater. 2007;9(12):1129–1133. doi: 10.1002/adem.200700220. [DOI] [Google Scholar]
- 11.Elliott JT, Tona A, Woodward JT, Jones PL, Plant AL. Thin films of collagen affect smooth muscle cell morphology. Langmuir. 2003;19(5):1506–1514. doi: 10.1021/la026216r. [DOI] [Google Scholar]
- 12.Engel J, Chiquet M. An overview of extracellular matrix structure and function. In: Mecham RP, editor. The Extracellular Matrix: An Overview. Heidelberg: Springer; 2011. pp. 1–39. [Google Scholar]
- 13.Fang M, Goldstein EL, Matich EK, Orr BG, Banaszak Holl MM. Type I collagen self-assembly: the roles of substrate and concentration. Langmuir. 2013;29(7):2330–2338. doi: 10.1021/la3048104. [DOI] [PubMed] [Google Scholar]
- 14.Fleischmajer R, Perlish JS, Burgeson RE, Shaikh-Bahai F, Timpl R. Type I and type III collagen interactions during fibrillogenesis. Ann. N.Y. Acad. Sci. 1990;580(1):161–175. doi: 10.1111/j.1749-6632.1990.tb17927.x. [DOI] [PubMed] [Google Scholar]
- 15.Fratzl, P. Collagen: Structure and Mechanics. New York: Springer, 2008.
- 16.Friedrichs J, Taubenberger A, Franz CM, Muller DJ. Cellular remodelling of individual collagen fibrils visualized by time-lapse AFM. J. Mol. Biol. 2007;372(3):594–607. doi: 10.1016/j.jmb.2007.06.078. [DOI] [PubMed] [Google Scholar]
- 17.Gale M, Pollanen M, Markiewicz P, Goh M. Sequential assembly of collagen revealed by atomic force microscopy. Biophys. J. 1995;68(5):2124–2128. doi: 10.1016/S0006-3495(95)80393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gay, S. and E. J. Miller. Collagen in the Physiology and Pathology of Connective Tissue. Stuttgart: Fischer, 1978.
- 19.Helseth DL, Veis A. Collagen self-assembly in vitro. Differentiating specific telopeptide-dependent interactions using selective enzyme modification and the addition of free amino telopeptide. J. Biol. Chem. 1981;256(14):7118–7128. [PubMed] [Google Scholar]
- 20.Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, Rofail F, Smith H, Wu BM, Shemin R, Beygui RE, MacLellan WR. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials. 2008;29(19):2907–2914. doi: 10.1016/j.biomaterials.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang W, Eryilmaz E. Kinetic signature of fractal-like filament networks formed by orientational linear epitaxy. Phys. Rev. Lett. 2014;113(2):025502. doi: 10.1103/PhysRevLett.113.025502. [DOI] [PubMed] [Google Scholar]
- 22.Javid N, Roy S, Zelzer M, Yang Z, Sefcik J, Ulijn RV. Cooperative self-assembly of peptide gelators and proteins. Biomacromolecules. 2013;14(12):4368–4376. doi: 10.1021/bm401319c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang F, Hörber H, Howard J, Müller DJ. Assembly of collagen into microribbons: effects of pH and electrolytes. J. Struct. Biol. 2004;148(3):268–278. doi: 10.1016/j.jsb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J. Cell Sci. 2007;120(12):1955–1958. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- 25.Kadler KE, Hojima Y, Prockop DJ. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase. Assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J. Biol. Chem. 1987;262(32):15696–15701. [PubMed] [Google Scholar]
- 26.Keene DR, Sakai LY, Bächinger HP, Burgeson RE. Type III collagen can be present on banded collagen fibrils regardless of fibril diameter. J. Cell Biol. 1987;105(5):2393–2402. doi: 10.1083/jcb.105.5.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuwahara Y. Comparison of the surface structure of the tetrahedral sheets of muscovite and phlogopite by AFM. Phys. Chem. Miner. 2001;28(1):1–8. doi: 10.1007/s002690000126. [DOI] [Google Scholar]
- 28.Kuznetsova N, Leikin S. Does the triple helical domain of type I collagen encode molecular recognition and fiber assembly while telopeptides serve as catalytic domains? Effect of proteolytic cleavage on fibrillogenesis and on collagen–collagen interaction in fibers. J. Biol. Chem. 1999;274(51):36083–36088. doi: 10.1074/jbc.274.51.36083. [DOI] [PubMed] [Google Scholar]
- 29.Lee CH, Singla A, Lee Y. Biomedical applications of collagen. Int. J. Pharm. 2001;221(1):1–22. doi: 10.1016/S0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 30.Leow WW, Hwang W. Epitaxially guided assembly of collagen layers on mica surfaces. Langmuir. 2011;27(17):10907–10913. doi: 10.1021/la2018055. [DOI] [PubMed] [Google Scholar]
- 31.Liu SH, Yang R-S, Al-Shaikh R, Lane JM. Collagen in tendon, ligament, and bone healing: a current review. Clin. Orthop. Relat. Res. 1995;318:265–278. [PubMed] [Google Scholar]
- 32.Liu X, Wu H, Byrne M, Krane S, Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl Acad. Sci. U.S.A. 1997;94(5):1852–1856. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loo RW, Goh MC. Potassium ion mediated collagen microfibril assembly on mica. Langmuir. 2008;24(23):13276–13278. doi: 10.1021/la803041v. [DOI] [PubMed] [Google Scholar]
- 34.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 35.Monroe MR, Li Y, Ajinkya SB, Gower LB, Douglas EP. Directed collagen patterning on gold-coated silicon substrates via micro-contact printing. Mater. Sci. Eng. C. 2009;29(8):2365–2369. doi: 10.1016/j.msec.2009.06.007. [DOI] [Google Scholar]
- 36.Mouw JK, Ou G, Weaver VM. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014;15(12):771–785. doi: 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narayanan B, Gilmer GH, Tao J, De Yoreo JJ, Ciobanu CV. Self-assembly of collagen on flat surfaces: the interplay of collagen–collagen and collagen–substrate interactions. Langmuir. 2014;30(5):1343–1350. doi: 10.1021/la4043364. [DOI] [PubMed] [Google Scholar]
- 38.Notbohm H, Mosler S, Müller PK, Brinckmann J. In vitro formation and aggregation of heterotypic collagen I and III fibrils. Int. J. Biol. Macromol. 1993;15(5):299–304. doi: 10.1016/0141-8130(93)90030-P. [DOI] [PubMed] [Google Scholar]
- 39.Ostendorf F, Schmitz C, Hirth S, Kuhnle A, Kolodziej JJ, Reichling M. How flat is an air-cleaved mica surface? Nanotechnology. 2008;19:305705–305711. doi: 10.1088/0957-4484/19/30/305705. [DOI] [PubMed] [Google Scholar]
- 40.Papi M, Palmieri V, Maulucci G, Arcovito G, Greco E, Quintiliani G, Fraziano M, De Spirito M. Controlled self assembly of collagen nanoparticle. J. Nanopart. Res. 2011;13(11):6141–6147. doi: 10.1007/s11051-011-0327-x. [DOI] [Google Scholar]
- 41.Peacock JD, Lu Y, Koch M, Kadler KE, Lincoln J. Temporal and spatial expression of collagens during murine atrioventricular heart valve development and maintenance. Dev. Dyn. 2008;237(10):3051–3058. doi: 10.1002/dvdy.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piechocka IK, van Oosten ASG, Breuls RGM, Koenderink GH. Rheology of heterotypic collagen networks. Biomacromolecules. 2011;12(7):2797–2805. doi: 10.1021/bm200553x. [DOI] [PubMed] [Google Scholar]
- 43.Ravikumar KM, Hwang W. Role of hydration force in the self-assembly of collagens and amyloid steric zipper filaments. J. Am. Chem. Soc. 2011;133(30):11766–11773. doi: 10.1021/ja204377y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ricard-Blum S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011;3(1):a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romanic AM, Adachi E, Kadler KE, Hojima Y, Prockop DJ. Copolymerization of pNcollagen III and collagen I. pNcollagen III decreases the rate of incorporation of collagen I into fibrils, the amount of collagen I incorporated, and the diameter of the fibrils formed. J. Biol. Chem. 1991;266(19):12703–12709. [PubMed] [Google Scholar]
- 46.Salhi B, Vaurette F, Grandidier B, Stiévenard D, Melnyk O, Coffinier Y, Boukherroub R. The collagen assisted self-assembly of silicon nanowires. Nanotechnology. 2009;20(23):235601. doi: 10.1088/0957-4484/20/23/235601. [DOI] [PubMed] [Google Scholar]
- 47.Silver FH. A two step model for lateral growth of collagen fibrils. Collagen Relat. Res. 1983;3(3):167–179. doi: 10.1016/S0174-173X(83)80001-6. [DOI] [PubMed] [Google Scholar]
- 48.Silver FH, Horvath I, Foran DJ. Mechanical implications of the domain structure of fiber-forming collagens: comparison of the molecular and fibrillar flexibilities of the α1-chains found in types I-III collagen. J. Theor. Biol. 2002;216(2):243–254. doi: 10.1006/jtbi.2002.2542. [DOI] [PubMed] [Google Scholar]
- 49.Speranza ML, Valentini G, Calligaro A. Influence of fibronectin on the fibrillogenesis of type I and type III collagen. Collagen Relat. Res. 1987;7(2):115–123. doi: 10.1016/S0174-173X(87)80003-1. [DOI] [PubMed] [Google Scholar]
- 50.Spiess K, Zorn TMT. Collagen types I, III, and V constitute the thick collagen fibrils of the mouse decidua. Microsc. Res. Tech. 2007;70(1):18–25. doi: 10.1002/jemt.20381. [DOI] [PubMed] [Google Scholar]
- 51.Stuart K, Panitch A. Characterization of gels composed of blends of collagen I, collagen III, and chondroitin sulfate. Biomacromolecules. 2009;10(1):25–31. doi: 10.1021/bm800888u. [DOI] [PubMed] [Google Scholar]
- 52.Sun, M., A. Stetco, and Merschrod S, E. F. Surface-templated formation of protein microfibril arrays. Langmuir 24(10):5418–5421, 2008. [DOI] [PubMed]
- 53.Teodoro WR, Witzel SS, Velosa APP, Shimokomaki M, Abrahamsohn PA, Zorn TMT. Increase of interstitial collagen in the mouse endometrium during decidualization. Connect. Tissue Res. 2003;44(2):96–103. doi: 10.1080/03008200390200238. [DOI] [PubMed] [Google Scholar]
- 54.Volk SW, Wang Y, Mauldin EA, Liechty KW, Adams SL. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs. 2011;194(1):25–37. doi: 10.1159/000322399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wess TJ. Collagen fibril form and function. Adv. Protein Chem. 2005;70:341–374. doi: 10.1016/S0065-3233(05)70010-3. [DOI] [PubMed] [Google Scholar]
- 56.Wu JJ, Weis MA, Kim LS, Eyre DR. Type III collagen, a fibril network modifier in articular cartilage. J. Biol. Chem. 2010;285(24):18537–18544. doi: 10.1074/jbc.M110.112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu B, Lu Q, Yin J, Hu J, Wang Z. Alignment of osteoblast-like cells and cell-produced collagen matrix induced by nanogrooves. Tissue Eng. 2005;11(5–6):825–834. doi: 10.1089/ten.2005.11.825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.