Abstract
Introduction
Landomycins are a subgroup of angucycline antibiotics that are produced by Streptomyces bacteria and possess strong antineoplastic potential. Literature data suggest that enhancement of the therapeutic activity of this drug may be achieved by means of creating specific drug delivery systems. Here we propose to adopt C60 fullerene as flexible and stable nanocarrier for landomycin delivery into tumor cells.
Methods
The methods of molecular modelling, dynamic light scattering and Fourier transform infrared spectroscopy were used to study the assembly of C60 fullerene and the anticancer drug Landomycin A (LA) in aqueous solution. Cytotoxic activity of this nanocomplex was studied in vitro towards two cancer cell lines in comparison to human mesenchymal stem cells (hMSCs) using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) test and a live/dead assay. The morphology of the cells incubated with fullerene–drug nanoparticles and their uptake into target cells were studied by scanning electron microscopy and fluorescence light microscopy.
Results
The viability of primary cells (hMSCs, as a model for healthy cells) and cancer cell lines (human osteosarcoma cells, MG-63, and mouse mammary cells, 4T1, as models for cancer cells) was studied after incubation with water-soluble C60 fullerenes, LA and the mixture C60 + LA. The C60 + LA nanocomplex in contrast to LA alone showed higher toxicity towards cancer cells and lower toxicity towards normal cells, whereas the water-soluble C60 fullerenes at the same concentration were not toxic for the cells.
Conclusions
The obtained physico-chemical data indicate a complexation between the two compounds, leading to the formation of a C60 + LA nanocomposite. It was concluded that immobilization of LA on C60 fullerene enhances selectivity of action of this anticancer drug in vitro, indicating on possibility of further preclinical studies of novel C60 + LA nanocomposites on animal tumor models.
Keywords: C60 fullerene, Landomycin A, Complexation, Cytotoxicity, Membranotropic effect, Molecular modelling, Dynamic light scattering, Fourier transform infrared spectroscopy, Scanning electron microscopy, Fluorescence microscopy
Introduction
Landomycins (LAs) are a subgroup of angucycline antibiotics that are produced by Streptomyces bacteria and possess strong antineoplastic potential.12 All natural LAs share the same aglycon (landomycinone) and vary only in the number of sugar residues in their linear glycosidic chain containing only di- and trideoxysugars (β-d-olivose and α-l-rhodinose).4,12 It has been shown that LAs with longer saccharide chains have a higher activity towards tumor cell lines in vitro,19,45 and that LA with six sugar residues (LA-A) constitutes the most potent family member. Although the molecular mechanisms of the action of LAs are still poorly understood, recent studies on another family member, i.e., LA with three sugar residues (LA-E), have indicated the crucial role of specific extramitochondrial hydrogen peroxide production and early caspase-7 activation upstream of mitochondria.28
Enhancement of the therapeutic activity of anticancer drugs by means of specific drug delivery systems is considered as one of the most promising approaches in current pharmacology and medicine.15,26,47,48 Here we propose to adopt C60 fullerene as flexible and stable nanocarrier for drug delivery into tumor cells.2,10,14,27,43 This idea is based on our previous extensive studies on the bioactivity of water-soluble pristine C60 fullerenes which demonstrated a preferable targeting of tumor cells.20,32 This may be explained by their strong membranotropic properties8,29,44 as well as by a low toxicity towards normal cells and tissues of the organism.30,31,50 C60 fullerene easily forms complexes with various compounds, including the well-known anticancer drugs doxorubicin (Dox) and cisplatin (Cis) as we demonstrated earlier.37–39 Moreover, the conjugation of Dox and Cis to C60 fullerene led to a significant increase of their cytotoxic activity in vitro and a therapeutic activity towards Lewis lung carcinoma in vivo,33–35 thus confirming the role of C60 fullerene as a versatile drug delivery platform that can be widely applied in clinical oncology.
Previously, by means of such powerful physico-chemical methods as the small-angle neutron scattering and atomic force microscopy, we accomplished a pilot study of intermolecular complexation and reported the effect of binding between C60 fullerene and a novel anticancer drug candidate, i.e., LA (LA-A).40 In the present work this result was further investigated in more detail using the dynamic light scattering (DLS), Fourier transform infrared (FTIR) spectroscopy and molecular simulation techniques. The physico-chemical data were supplemented by in vitro studies of toxic effect of C60 + LA nanocomplex towards normal and tumor cells and its penetration into target cells.
Experimental
Material Preparation
A stable pristine C60 fullerene aqueous colloidal dispersion (C60FAS) at the maximum concentration of 150 µg mL−1 was prepared as described earlier,36,42 based on the original methodology which includes the transfer of C60 molecules from toluene into an aqueous phase under ultrasonication.
In all experiments, we used LA powder (99.5% purity according to the high-performance liquid chromatography data) which was extracted from culture of Streptomyces globisporus S136 strain. LA was dissolved in methanol at the maximum concentration 200 µg mL−1. Immobilization of LA on C60 fullerene (i.e., the C60 + LA mixture) was accomplished as follows40: the initial solution of C60FAS and LA (with maximum concentration) were mixed in the volume ratio 1:1. The resulting mixture was ultrasonicated for 30 min, followed by magnetic stirring for 12 h by at room temperature.
DLS and ζ-Potential Determination
The size distribution and the ζ-Potential for C60FAS and C60 + LA mixture were determined by DLS on a Zetasizer Nano-ZS90 (Malvern, Worcestershire, UK) at room temperature. The instrument was equipped with a He–Ne laser (5 mW) operating at 633 nm. The results were analyzed under the Smoluchowski approximation.
FTIR Study
FTIR spectroscopy was used to study the structural organization of pristine C60 fullerene with LA in aqueous solution. The FTIR spectra were recorded with a Perkin-Elmer BX-II spectrophotometer with a spectral resolution of 1 cm−1 in the range of 400–4000 cm−1. The samples were examined as thin film between two NaBr plates. For optimum signal-to-noise ratio, 64 scans were recorded per spectrum.
Structure Calculations
The procedure for structural analysis of the C60 + LA nanocomplex corresponded to those used previously to study of the same nanocomplexes,40 except that in the present work we modelled the structure with maximum coverage of the C60 fullerene surface by the ligand molecules. Briefly, the calculations of the spatial structure of C60 + LA nanocomplex were performed by molecular mechanics using the X-PLOR software with the CHARMM27 force field. Quantum-mechanical calculations of partial atomic charges on C60 fullerene and ligand atoms were performed with the Gaussian03 software within the framework of DFT (B3LYP) using a 6-31G* basis set by the Merz–Kollman method.
Cell Culture
The cytotoxic activity of the C60 + LA nanocomplex was studied in vitro on human osteosarcoma cells (MG-63), mouse mammary cells (4T1), and primary human mesenchymal stem cells (hMSCs). The cell lines were obtained from the Collection of Microorganisms and Cell Cultures of the University of Duisburg-Essen. MG-63 and 4T1 cells were cultured in Dulbecco’s modified eagle medium (DMEM), supplemented with 10% fetal calf serum (FCS), 100 U mL−1 penicillin, and 100 mg mL−1 streptomycin at 37 °C in humidified atmosphere at 5% CO2. hMSC were cultured in mesenchymal stem cell growth medium (MSCGM BulletKit™; Lonza). 12 h prior to uptake experiments, the cells were trypsinized and seeded in 48-well plates at a density of 25 × 103 cells per well in 0.25 mL DMEM with FCS for MG-63 and 4T1 cells and with MSCGM for hMSCs.
Scanning Electron Microscopy (SEM)
The attachment of free and LA-immobilized C60 fullerene to the membrane of MG-63 cells, 4T1 cells, and hMSCs was visualized by SEM. Cells were cultured for 1 h either with or without C60FAS, and rinsed twice with phosphate-buffered saline (PBS). Then the cells were fixed with 3.7% glutaraldehyde (Sigma-Aldrich, Taufkirchen, Germany) in PBS for 15 min. After a 2-fold washing with PBS, the fixed cells were dehydrated with an ascending sequence of ethanol (20, 40, 60, 80 and 96–98%). Subsequently, after evaporation of ethanol, the samples were left for 2 h at 37 °C and then analyzed by SEM. SEM was performed with an ESEM FEI Quanta 400 instrument with gold/palladium-sputtered samples.
Fluorescence Microscopy
The uptake studies of C60FAS (75 μg mL−1), of LA (100 μg mL−1), and of the C60 + LA mixture (75 + 100 μg mL−1) were carried out as follows. The cells were incubated with 5 μL dispersion/solution of the indicated compounds for 3 and 24 h, respectively. Immediately after the indicated time, the cells were washed three times with PBS to remove all dissolved compounds that were not attached to the cells. The cellular uptake was measured by transmission light microscopy and fluorescence microscopy with a Keyence Biorevo BZ-9000 instrument (Osaka, Japan), equipped with filters for 4′, 6 diamidino 2 phenylindole (DAPI, EX 360/40, DM 400, BA 460/50) at ×20 magnification. C60 + LA nanocomplexes were visible as blue fluorescing dots.
MTT and Live/Dead Assay
The cell viability was analyzed by an 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay 72 h after the incubation of MG-63 cells, 4T1 cells and hMSCs with C60FAS (75 μg mL−1), LA (100 μg mL−1) or the mixture of C60 + LA (75 + 100 μg mL−1). The following volumes of dispersion were added to the cells (total volume of 250 µL per well): 0.5, 2.5, 5 or 10 μL, which corresponds to the following concentrations: 0.15, 0.75, 1.5, 3.0 μg mL−1 of C60FAS, 0.2, 1.0, 2.0 and 4.0 μg mL−1 of LA, 0.15 + 0.2, 0.75 + 1.0, 1.5 + 2.0 and 3.0 + 4.0 μg mL−1 of C60 + LA mixture. The used ratio of concentrations in the studied mixtures, presumably, corresponds to the optimum binding efficiency of LA to C60 fullerene clusters as evidenced by structural analysis (see below).
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide; Sigma, Taufkirchen, Germany) was dissolved in PBS (5 mg mL−1) and then diluted to 1 mg mL−1 in the cell culture medium. The culture medium of the incubated cells was replaced by 300 µL of the MTT solution. The cells were incubated for 1 h at 37 °C under 5% CO2 in humidified atmosphere. 300 µL of dimethyl sulfoxide (DMSO) was added to the cells. After 30 min, a 100 µL aliquot was taken for spectrophotometric analysis with a Multiscan FC instrument (Thermo Fisher Scientific, Vantaa, Finland) at λ = 570 nm. The absorption of incubated cells was normalized to that of control (untreated) cells, thereby indicating the relative level of cell viability. Each concentration of the studied compounds was run in triplicate and normalized to blank controls, containing the equivalent volume of culture medium.
A live/dead assay was carried out according to the following protocol. 72 h after the incubation of MG-63 cells, 4T1 cells, and hMSCs with 5 μL of C60FAS (75 μg mL−1), LA (100 μg mL−1) and C60 + LA mixture (75 + 100 μg mL−1), respectively, the cells were washed with PBS and stained with a live/dead viability/cytotoxicity assay for mammalian cells (L3224, Invitrogen Co.) to evaluate the cell viability. 150 μL of the calcein AM and ethidium homodimer-1 working solution were directly added to the cells. Afterwards, the cells were subsequently incubated for 30 min at 37 °C. After incubation, the stained cells were imaged by fluorescence microscopy (Keyence). The live/dead kit determines the cell viability based on the cell membrane integrity. Living cells are stained by calcein AM which emits a green fluorescence (517 nm) when excited by blue light (494 nm), whereas dead cells are stained by EthD-1 which emits a red fluorescence (617 nm) when excited by green light (528 nm). All experiments were carried out in triplicate.
Statistics
Statistical analysis was performed by conventional methods of variation statistics. The significance of the differences between the control and experimental measurements was estimated by Student’s t test using the Origin 8.0 software (OriginLab Corporation, USA). The difference between the compared values was considered to be significant at p < 0.05.
Results and Discussion
Material Characterization
It has been established that the size of C60 fullerene particles directly correlates with their biodistribution and toxicity.21,30,31,50 Depending on the size, C60 fullerene particles can penetrate through the plasma membrane into the cell or be adsorbed on the surface of the membrane.8,9,29,44 Therefore, DLS measurements of C60FAS and C60 + LA dispersions were performed (Table 1).
Table 1.
The hydrodynamic particle diameter, the PDI value, and the ζ-Potential for C60FAS and C60 + LA aggregates at room temperature in aqueous dispersion, measured by DLS method.
| Samples | Diameter (nm) | PDI | ζ-Potential (mV) |
|---|---|---|---|
| C60FAS | 184 ± 18 | 0.209 | − 17.0 ± 2.0 |
| C60 + LA mixture | 190 ± 20 | 0.219 | − 1.0 ± 0.1 |
C60 fullerenes and C60 fullerenes with LA form particles (aggregates) in water with an average hydrodynamic size 184 and 190 nm, respectively, in good agreement with previous results.40 The obtained result means that presence of LA solution only slightly affects the distribution of C60 fullerene aggregates in aqueous solution. The value of polydispersity index (PDI, about 0.21) indicates a low polydispersity of these systems. The magnitude of ζ-Potential measured for C60 + LA mixture shifted to -1 mV from the initial value of − 17 mV measured for C60FAS, indicating an interaction between C60 fullerene and LA.
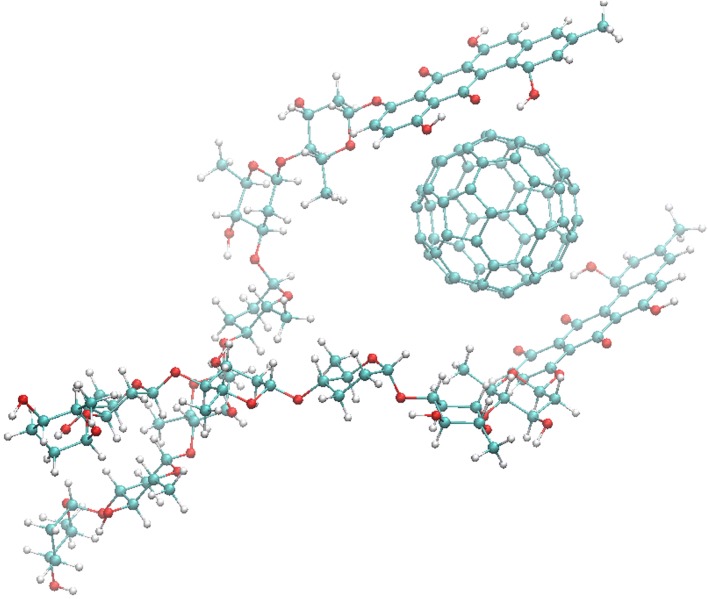
The calculated structure of the C60 + LA nanocomplex (Fig. 1) indicates the possibility of binding of up to two LA molecules to the surface of one C60 fullerene molecule, suggesting a favorable hydrophobic complexation effect. Both LA ligands are stabilized by π-stacking with the C60 molecule surface which is known as the dominant force in C60 fullerene–aromatic interactions.33–35,37–40 Importantly, the maximal binding ratio 1:2 provides rough estimate of the ratio of concentrations (1:1…1:2) to be further used in in vitro experiments and corresponding to optimal binding efficacy of LA to fullerene clusters in solution.
Figure 1.
Calculated structure of 1:2 C60 + LA nanocomplex.
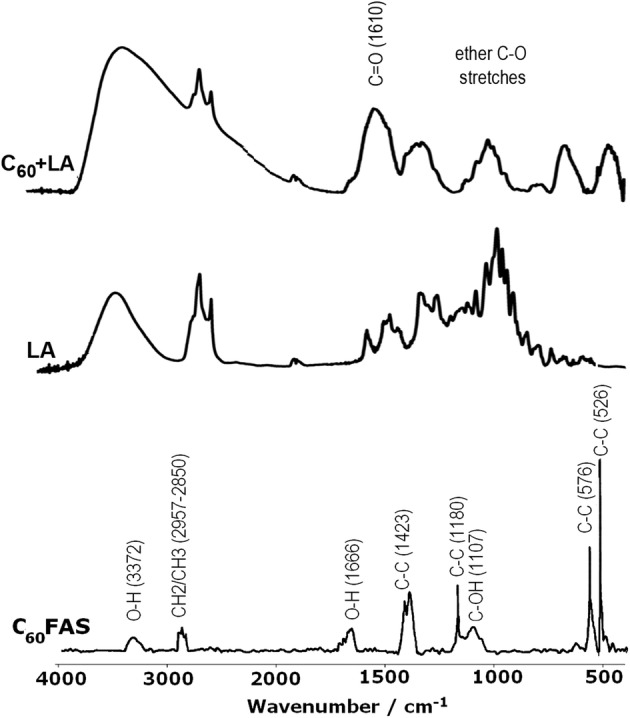
Further characterization of the C60 + LA interaction was carried out by FTIR spectroscopy. The FTIR spectrum of C60 fullerene with LA mixture is shown in Fig. 2 together with the reference spectra of C60FAS and LA. The spectrum of C60FAS exhibits four intense peaks at 1423, 1180, 576, and 526 cm−1 attributed to the C–C vibrational modes of the C60 molecule and weak bands at 3372 and 1666 cm−1, corresponding to water hydrogen-bonded O–H stretching and bending, respectively; the broad intense band near 1107 cm−1 is attributed to C–OH stretching; a weak band at 2957–2850 cm−1, corresponds to C–H stretching.18 Two strong broad bands centered at 3410 and 3373 cm−1 corresponding to hydrogen-bonded O–H stretching [ν(OH)] of the methanol and a weak band at 1734 cm−1 [δ(OH)] were observed in the FTIR spectra of the LA and C60 + LA mixture, respectively, because the measurement was performed on alcohol substance. The O–H stretching and CH3 stretch bands of the LA in the FTIR spectra of the methanol solution and C60 + LA mixture had low intensities and overlapped with bands of the solvent. Aromatic C=C stretches of LA at 1368 and 1450 cm−1 had low intensities and overlapped with bands of the C–C vibrational modes and C–O–H bending of the C60FAS system.18 The group of peaks from 1111 to 975 cm−1 centered at 1055 cm−1, corresponding to dialkyl- and alkyl–aryl ether C–O stretches of LA. Also, the characteristic strong band at 1610 cm−1 assigned to a C=O stretch conjugated with the aromatic system is found both for the LA and C60 + LA.
Figure 2.
FTIR spectra of C60FAS, LA and C60 + LA mixture.
It is known that the stacking of aromatic surfaces results in a high-frequency shift of C=O functional groups by few cm−1.7 Such shifts were initially expected for the C60 + LA interaction, which, however, they were not observable on the measured FTIR spectrum of the mixture due to strong line broadening (Fig. 2). However, the presence of C–OH vibrations in FTIR spectrum of C60FAS provides another important source of C60 + LA nanocomplex stabilization due to intermolecular hydrogen bonding to proton acceptor groups of the ligand. Note, such additional stabilization is well known for aromatic–aromatic π-stacked complexes in solution.6
In Vitro Experiments
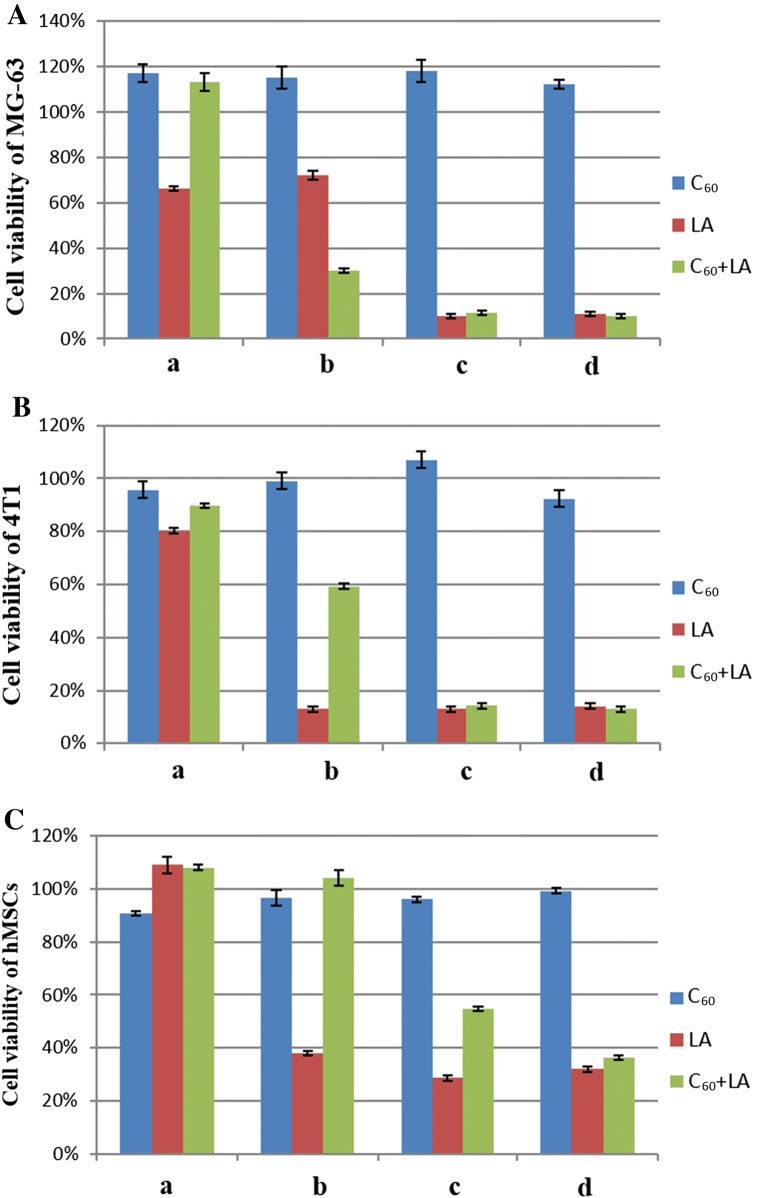
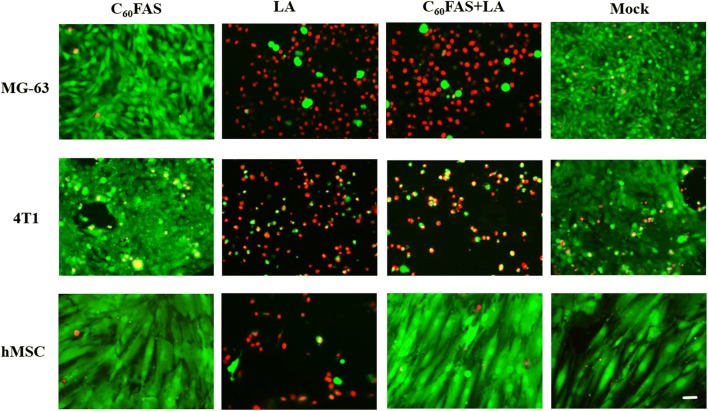
Pure LA and the C60 + LA mixture showed a clear concentration-dependent cytotoxic effect towards MG-63 cells, 4T1 cells, and hMSCs after 72 h incubation (Fig. 3). No toxic effects of C60FAS towards these cell lines were observed even at the highest concentration. The same result was observed for C60FAS in vitro against to normal cells (thymocytes) and cancer cells (Ehrlich ascitic carcinoma, leukemia L1210 and HeLa).30,31,50 LA alone was toxic for all three cell types, and its cytotoxicity increased with increasing LA concentration. In particular, IC50 values for LA were 1.81, 0.55 and 0.88 µg mL−1 for MG-63, 4T1 cells and hMSCs, respectively. Interestingly, at the increasing concentration of the C60 + LA mixture, we observed a higher toxicity for cancer cells and a weakly pronounced toxicity (up to 40%) for hMSCs. These data were also confirmed by analysis of IC50 value of C60 + LA nanocomplex: it decreased 2.5-fold for MG-63 cells (from 1.81 down to 0.74 µg mL−1), but gradually increased 2-fold for hMSCs (from 0.88 up to 1.58 µg mL−1). The live/dead assay gave the same results: C60FAS was not toxic for the cells, LA killed almost all cells and the C60 + LA mixture, in contrast to LA alone, showed high toxicity for cancer cells and low toxicity for hMSCs (Fig. 4).
Figure 3.
MTT assay for MG-63 cells (A), 4T1 cells (B) and hMSCs (C) after 72 h of incubation with C60FAS, LA and C60 + LA mixture at different concentrations: 0.15, 0.2 and 0.15 + 0.2 μg mL−1 (a), 0.75, 1.0 and 0.75 + 1.0 μg mL−1 (b), 1.5, 2.0 and 1.5 + 2.0 μg mL−1 (c), 3.0, 4.0 and 3.0 + 4.0 μg mL−1 (d). Data are given relative to the untreated control samples (p < 0.05) and represent the mean ± SD of three independent experiments.
Figure 4.
Representative live/dead staining of MG-63 cells, 4T1 cells and hMSCs after 72 h of incubation with C60FAS (1.5 μg mL−1), LA (2.0 μg mL−1) or C60 + LA mixture (1.5 + 2.0 μg mL−1) and mock (untreated cells). Scale bar 20 µm.
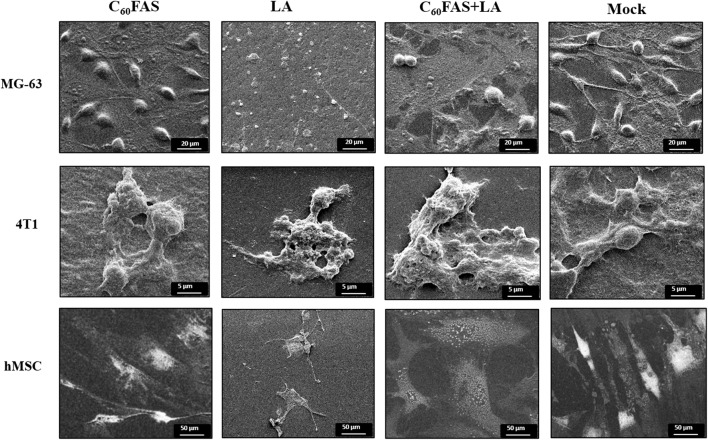
The SEM micrographs of MG-63 cells, 4T1 cells, and hMSCs, incubated with C60 fullerene, LA and C60 + LA mixture, as well as without any additions (as a control) are shown in Fig. 5. The SEM images of single cells were chosen to represent the whole population in each group as all cells in the group had the same morphology. The SEM images underline the results obtained from live/dead assay, i.e., at a LA concentration of 2 μg mL−1 both types of cancer cells as well as the primary cells were dead in contrast to the C60 + LA mixture, where we observed a high toxic effect for cancer cells, but only a low toxicity for primary cells.
Figure 5.
SEM micrographs of MG-63 cells, 4T1 cells and hMSCs after 72 h of incubation with C60FAS (1.5 μg mL−1), LA (2.0 μg mL−1) or C60 + LA mixture (1.5 + 2.0 μg mL−1) and mock (untreated cells).
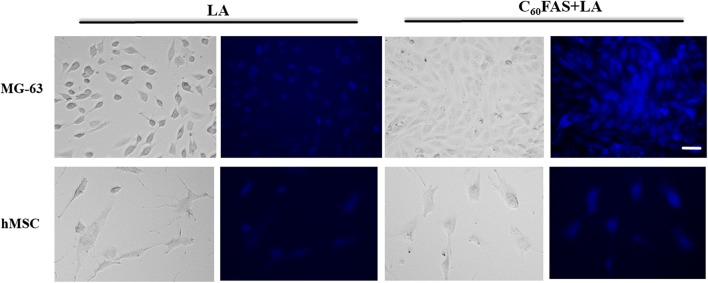
The membranotropic effect of LA and C60 + LA nanoparticles on MG-63 cells and hMSCs (as a representative model for cancer and primary cells) was studied by fluorescence microscopy. In the Fig. 6, the uptake of LA and C60 + LA nanocomplex into cells after 2 h of incubation is shown. It was revealed that LA weakly fluoresces at DAPI channel (blue) of fluorescence microscope in the hMSC and MG-63 cells. In the control (untreated cells) no blue fluorescence was detected (data not shown). Immobilization of LA on C60 fullerene leads to enhancement of LA fluorescence inside the cells in a concentration-dependent manner. Thus, C60 fullerene enhances LA’s entry inside tumor cells due to its membranotropic activity, thus improving cytotoxic activity of these nanocomplexes. Taking into consideration that similar effects of C60 fullerene were already observed by us for C60 + Dox and C60 + Cis nanocomplexes, one may conclude that enhancement of drug uptake into tumor cells is the unique feature of C60 fullerene, which may be of high importance for further pre-clinical trials of the C60 fullerene–drug nanocomplexes.25,41
Figure 6.
Transmission light microscopy and fluorescence microscopy of MG-63 and hMSC cells after 2 h of incubation with LA (2.0 μg mL−1) and C60 + LA mixture (1.5 + 2.0 μg mL−1). The blue fluorescence indicates the co-localization of LA and C60 + LA nanocomplex on the cells. Note that C60 fullerene alone is not fluorescent. Scale bar 20 µm.
The peculiarity of the biological effect of the water-soluble C60 fullerenes is their ability to selectively damage of the tumor cells, whereas they do not affect the normal cells.23,46 On the other hand, C60 fullerene, as a carrier of antitumor drug, thanks to its powerful antioxidant properties,5,51 effectively reduces the side (toxic) effects of drug relative to the normal cells.1,35
It is known that the blood vessels in most solid tumors are characterized by intense angiogenesis, high density and increased vascular permeability, defective vascular networks and defective or depressed lymphatic drainage in the intratissue space of the tumor,22 which allows nanocompounds to penetrate, accumulate and hold in tumors. In addition, due to impaired endocytosis, the tumor cells are able to accumulate more and maintain longer, than the normal cells, exogenous compounds, especially with high molecular weight.49 Therefore, it can be assumed that with an increase in the size/mass of C60 molecule due to its complexation with the chemotherapeutic drug and simultaneous association with the serum proteins, there is a passive accumulation of these nanoparticles in the tumor through the enhanced permeability and retention (EPR) effect in the tumor vasculature.3,11,13,24 In this regard, the recent study16 clearly demonstrated the feasibility of tracking and quantifying the delivery kinetics and intratumoral biodistribution of C60 fullerene-based drug delivery platform, consistent with the EPR effect on short timescales and passive transport to tumors. On the other hand, it was found that C60 fullerenes increase the sensitivity of tumor cells to the action of cytostatics due to their interaction and non-recognition by ABC-transporters.17,34
Conclusion
A complexation between pristine C60 fullerene and LA molecule in aqueous solution was characterized by molecular modelling, DLS and FTIR techniques. Cytotoxic activity of this nanocomplex was studied in vitro towards two cancer cell lines in comparison to hMSCs using MTT test and a live/dead assay. It was shown that C60 fullerene nanoparticles were non-toxic for the cells, LA at the concentration of 2 μg mL−1 killed all tested cell lines, while its nanocomplex with C60 fullerene at the same concentration of LA demonstrated high toxicity for cancer cells and much lower toxicity for mesenchymal stem cells. The same results were obtained by live/dead assay: C60 + LA mixture had shown high toxicity for cancer cells, but not for the mesenchymal stem cells. These data were supported by fluorescence microscopy studies, which have shown higher uptake of C60 + LA nanocomplex by tumor cells, and lower one by mesenchymal stem cells. The obtained results indicate on importance of using C60 fullerene as a nanoplatform for drug delivery, and, in particular, huge potential of C60 + LA nanocomplex as a novel anticancer agent.
Acknowledgments
V. Bilobrov is grateful to DAAD for financial support within the framework of the Leonhard-Euler Program. This work was partially supported by STCU Project N6256 and state support to Leading Research Group 5889.2018.3.
Conflict of interest
V. Bilobrov, V. Sokolova, S. Prylutska, R. Panchuk, O. Litsis, V. Osetskyi, M. Evstigneev, Yu. Prylutskyy, M. Epple, U. Ritter, J. Rohr declare that they have no conflicts of interest.
Ethical Approval
Neither human studies, nor animal studies were carried out by the authors for this article.
Abbreviations
- 4T1
Mouse mammary cells
- C60FAS
C60 fullerene aqueous solution
- Cis
Cisplatin
- DAPI
4′, 6 Diamidino 2 phenylindole
- DLS
Dynamic light scattering
- DMEM
Dulbecco’s modified eagle medium
- DMSO
Dimethyl sulfoxide
- Dox
Doxorubicin
- EPR
Enhanced permeability and retention
- FCS
Fetal calf serum
- FTIR
Fourier transform infrared spectroscopy
- hMSCs
Human mesenchymal stem cells
- LA
Landomycin A
- MG-63
Human osteosarcoma cells
- MTT
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
- PBS
Phosphate-buffered saline
- PDI
Polydispersity index
- SEM
Scanning electron microscopy
Authors’ Contributions
The work presented here was carried out in collaboration between all the authors. RP, JR, VO and YP created and characterized nanomaterials. VB and VS performed in vitro and fluorescence microscopy studies. OL and SP characterized nanomaterials using FTIR analysis. ME performed the computer simulations. UR synthesized and characterized C60FAS. M. Epple and YP coordinated the experimental work, analyzed the data, performed the statistical analysis, and wrote the manuscript. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
Contributor Information
M. Evstigneev, Email: max_evstigneev@mail.ru
Yu. Prylutskyy, Email: prylut@ukr.net
References
- 1.Afanasieva KS, Prylutska SV, Lozovik AV, Bogutska KI, Sivolob AV, Prylutskyy Yu I, et al. C60 fullerene prevents genotoxic effect of doxorubicin on human lymphocytes in vitro. Ukr. Biochem. J. 2015;87:91–98. doi: 10.15407/ubj87.01.091. [DOI] [PubMed] [Google Scholar]
- 2.Augustine S, Singh J, Srivastava M, Sharma M, Das A, Malhotra BD. Recent advances in carbon based nanosystems for cancer theranostics. Biomater. Sci. 2017;5:901–952. doi: 10.1039/C7BM00008A. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri P, Paraskar A, Soni S, Mashelkar RA, Sengupta S. Fullerenol cytotoxic conjugates for cancer chemotherapy. ACS Nano. 2009;3:2505–2514. doi: 10.1021/nn900318y. [DOI] [PubMed] [Google Scholar]
- 4.Elshahawi SI, Shaaban KA, Kharel MK, Thorson JS. A comprehensive review of glycosylated bacterial natural products. Chem. Soc. Rev. 2015;44:7591–7697. doi: 10.1039/C4CS00426D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eswaran SV. Water soluble nanocarbon materials: a panacea for all? Curr. Sci. 2018;114:1846–1850. [Google Scholar]
- 6.Evstigneev MP. Hetero-association of aromatic molecules in aqueous solution. Int. Rev. Phys. Chem. 2014;33:229–273. doi: 10.1080/0144235X.2014.926151. [DOI] [Google Scholar]
- 7.Falk M, Gil M, Iza N. Self-association of caffeine in aqueous solution: an FTIR study. Can. J. Chem. 1990;68:1293–1299. doi: 10.1139/v90-199. [DOI] [Google Scholar]
- 8.Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, Seta P, et al. Cellular localisation of a water-soluble fullerene derivative. Biochem. Biophys. Res. Commun. 2002;294:116–119. doi: 10.1016/S0006-291X(02)00445-X. [DOI] [PubMed] [Google Scholar]
- 9.Franskevych D, Palyvoda K, Petukhov D, Prylutska S, Grynyuk I, Schuetze C, et al. Fullerene C60 penetration into leukemic cells and its photoinduced cytotoxic effects. Nanoscale Res. Lett. 2017;12:40. doi: 10.1186/s11671-016-1819-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodarzi S, Da Ros T, Conde J, Sefat F, Mozafari M. Fullerenes: biomedical engineers get to revisit an old friend. Mater. Today. 2017;20:460–480. doi: 10.1016/j.mattod.2017.03.017. [DOI] [Google Scholar]
- 11.Guo X, Ding R, Zhang Y, Ye L, Liu X, Chen C, et al. Dual role of photosensitizer and carrier material of fullerene in micelles for chemo–photodynamic therapy of cancer. J. Pharm. Sci. 2014;103:3225–3234. doi: 10.1002/jps.24124. [DOI] [PubMed] [Google Scholar]
- 12.Henkel T, Rohr J, Beale JM, Schwenen L. Landomycins, new angucycline antibiotics from Streptomyces sp. I. structural studies on landomycins A–D. J. Antibiot. 1990;43:492–503. doi: 10.7164/antibiotics.43.492. [DOI] [PubMed] [Google Scholar]
- 13.Ji Z, Sun H, Wang H, Xie Q, Liu Y, Wang Z. Biodistribution and tumor uptake of C60(OH)x in mice. J. Nanopart. Res. 2006;8:53–63. doi: 10.1007/s11051-005-9001-5. [DOI] [Google Scholar]
- 14.Joshi M, Kumar P, Kumar R, Sharma G, Singh B, Katare V, et al. Aminated carbon-based “cargo vehicles” for improved delivery of methotrexate to breast cancer cells. Mater. Sci. Eng. C. 2017;75:1376–1388. doi: 10.1016/j.msec.2017.03.057. [DOI] [PubMed] [Google Scholar]
- 15.Kumari P, Ghosh B, Biswas S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016;24:179–191. doi: 10.3109/1061186X.2015.1051049. [DOI] [PubMed] [Google Scholar]
- 16.Lapin NA, Vergara LA, Mackeyev Y, Newton JM, Dilliard SA, Wilson LJ, et al. Biotransport kinetics and intratumoral biodistribution of malonodiserinolamide-derivatized [60]fullerene in a murine model of breast adenocarcinoma. Int. J. Nanomed. 2017;12:8289–8307. doi: 10.2147/IJN.S138641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang XJ, Meng H, Wang YZ, He HY, Meng J, Lu J, et al. Metallofullerene nanoparticles circumvent tumor resistance to cisplatin by reactivating endocytosis. Proc. Natl. Acad. Sci. USA. 2010;107:7449–7454. doi: 10.1073/pnas.0909707107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu CY, Yao SD, Lin WZ, Wang WF, Lin NY, Tong YP, et al. Studies on the fullerol of C60 in aqueous solution with laser photolysis and pulse radiolysis. Radiat. Phys. Chem. 1998;53:137–143. doi: 10.1016/S0969-806X(98)00017-6. [DOI] [Google Scholar]
- 19.Luzhetskyy A, Zhu L, Gibson M, Fedoryshyn M, Dürr C, Hofmann C, et al. Generation of novel landomycins M and O through targeted gene disruption. ChemBioChem. 2005;6:675–678. doi: 10.1002/cbic.200400316. [DOI] [PubMed] [Google Scholar]
- 20.Lynchak OV, Prylutskyy Yu I, Rybalchenko VK, Kyzyma OA, Soloviov D, Kostjukov VV, et al. Comparative analysis of the antineoplastic activity of C60 fullerene with 5-fluorouracil and pyrrole derivative in vivo. Nanoscale Res. Lett. 2017;12:8. doi: 10.1186/s11671-016-1775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyon DY, Adams LK, Falkner JC, Alvarez PJ. Antibacterial activity of fullerene water suspensions: effects of preparation method and particle size. J. Environ. Sci. Technol. 2006;40:4360–4366. doi: 10.1021/es0603655. [DOI] [PubMed] [Google Scholar]
- 22.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001;41:189–207. doi: 10.1016/S0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 23.Markovic Z, Todorovic-Markovic B, Kleut D, Nikolic N, Vranjes-Djuric S, Misirkic M, et al. The mechanism of cell-damaging reactive oxygen generation by colloidal fullerenes. Biomaterials. 2007;28:5437–5448. doi: 10.1016/j.biomaterials.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent SMANCS. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 25.Misra C, Thotakura N, Kumar R, Singh B, Sharma G, Katare OP, et al. Improved cellular uptake, enhanced efficacy and promising pharmacokinetic profile of docetaxel employing glycine-tethered C60-fullerenes. Mater. Sci. Eng. C. 2017;76:501–508. doi: 10.1016/j.msec.2017.03.073. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell MJ, Jain RK, Langer R. Engineering and physical sciences in oncology: challenges and opportunities. Nat. Rev. Cancer. 2017;17:659–675. doi: 10.1038/nrc.2017.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montellano A, Da Ros T, Bianco A, Prato M. Fullerene C60 as a multifunctional system for drug and gene delivery. Nanoscale. 2011;3:4035–4041. doi: 10.1039/c1nr10783f. [DOI] [PubMed] [Google Scholar]
- 28.Panchuk RR, Lehka LV, Terenzi A, Matselyukh BP, Rohr J, Jha AK. Rapid generation of hydrogen peroxide contributes to the complex cell death induction by the angucycline antibiotic landomycin E. Free Radic. Biol. Med. 2017;106:134–147. doi: 10.1016/j.freeradbiomed.2017.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prylutska SV, Matyshevska OP, Grynyuk II, Prylutskyy YI, Ritter U, Scharff P. Biological effects of C60 fullerenes in vitro and in a model system. Mol. Cryst. Liq. Cryst. 2007;468:265–274. doi: 10.1080/15421400701230105. [DOI] [Google Scholar]
- 30.Prylutska SV, Matyshevska OP, Golub AA, Prylutskyy YI, Potebnya GP, Ritter U, et al. Study of C60 fullerenes and C60-containing composites cytotoxicity in vitro. Mater. Sci. Eng. C. 2007;27:1121–1124. doi: 10.1016/j.msec.2006.07.009. [DOI] [Google Scholar]
- 31.Prylutska SV, Grynyuk II, Grebinyk SM, Matyshevska OP, Prylutskyy YI, Ritter U, et al. Comparative study of biological action of fullerenes C60 and carbon nanotubes in thymus cells. Mater. Wiss. Werkst. 2009;40:238–241. doi: 10.1002/mawe.200900433. [DOI] [Google Scholar]
- 32.Prylutska S, Grynyuk I, Matyshevska O, Prylutskyy Yu, Evstigneev M, Scharff P, et al. C60 fullerene as synergistic agent in tumor-inhibitory doxorubicin treatment. Drugs R&D. 2014;14:333–340. doi: 10.1007/s40268-014-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prylutska S, Skivka L, Didenko G, Prylutskyy Yu, Evstigneev M, Potebnya G, et al. Complex of C60 fullerene with doxorubicin as a promising agent in antitumor therapy. Nanoscale Res. Lett. 2015;10:499. doi: 10.1186/s11671-015-1206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prylutska S, Panchuk R, Gołuński G, Skivka L, Prylutskyy Yu, Hurmach V, et al. C60 fullerene enhances cisplatin anticancer activity and overcomes tumor cells drug resistance. Nano Res. 2017;10:652–671. doi: 10.1007/s12274-016-1324-2. [DOI] [Google Scholar]
- 35.Prylutska SV, Politenkova SV, Afanasieva KS, Korolovych VF, Bogutska KI, Sivolob AV, et al. A nanocomplex of C60 fullerene with cisplatin: design, characterization and toxicity. Beilstein J. Nanotechnol. 2017;8:1494–1501. doi: 10.3762/bjnano.8.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prylutskyy Yu I, Yashchuk VM, Kushnir KM, Golub AA, Kudrenko VA, Prylutska SV, et al. Biophysical studies of fullerene-based composite for bio-nanotechnology. Mater. Sci. Eng. C. 2003;23:109–111. doi: 10.1016/S0928-4931(02)00244-8. [DOI] [Google Scholar]
- 37.Prylutskyy Yu I, Evstigneev MP, Pashkova IS, Wyrzykowski D, Woziwodzka A, Gołuński G, et al. Characterization of C60 fullerene complexation with antibiotic doxorubicin. Phys. Chem. Chem. Phys. 2014;16:23164–23172. doi: 10.1039/C4CP03367A. [DOI] [PubMed] [Google Scholar]
- 38.Prylutskyy Yu I, Evstigneev MP, Cherepanov VV, Kyzyma OA, Bulavin LA, Davidenko NA, et al. Structural organization of C60 fullerene, doxorubicin and their complex in physiological solution as promising antitumor agents. J. Nanopart. Res. 2015;17:45. doi: 10.1007/s11051-015-2867-y. [DOI] [Google Scholar]
- 39.Prylutskyy Yu I, Cherepanov VV, Evstigneev MP, Kyzyma OA, Petrenko VI, Styopkin VI, et al. Structural self-organization of C60 and cisplatin in physiological solution. Phys. Chem. Chem. Phys. 2015;17:26084–26092. doi: 10.1039/C5CP02688A. [DOI] [PubMed] [Google Scholar]
- 40.Prylutskyy YI, Cherepanov VV, Kostjukov VV, Evstigneev MP, Kyzyma OA, Bulavin LA, et al. Study of the complexation between Landomycin A and C60 fullerene in aqueous solution. RSC Adv. 2016;6:81231–81236. doi: 10.1039/C6RA18807A. [DOI] [Google Scholar]
- 41.Prylutskyy Y, Bychko A, Sokolova V, Prylutska S, Evstigneev M, Rybalchenko V, et al. Interaction of C60 fullerene complexed to doxorubicin with model bilipid membranes and its uptake by HeLa cells. Mater. Sci. Eng. C. 2016;59:398–403. doi: 10.1016/j.msec.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 42.Ritter U, Prylutskyy YI, Evstigneev MP, Davidenko NA, Cherepanov VV, Senenko AI, et al. Structural features of highly stable reproducible C60 fullerene aqueous colloid solution probed by various techniques. Fuller. Nanotubes Carbon Nanostruct. 2015;23:530–534. doi: 10.1080/1536383X.2013.870900. [DOI] [Google Scholar]
- 43.Samanta PN, Das KK. Noncovalent interaction assisted fullerene for the transportation of some brain anticancer drugs: a theoretical study. J. Mol. Graph. Model. 2017;72:187–200. doi: 10.1016/j.jmgm.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Schuetze C, Ritter U, Scharff P, Bychko A, Prylutska S, Rybalchenko V, et al. Interaction of N-fluorescein-5-isothiocyanate pyrrolidine–C60 compound with a model bimolecular lipid membrane. Mater. Sci. Eng. C. 2011;31:1148–1150. doi: 10.1016/j.msec.2011.02.026. [DOI] [Google Scholar]
- 45.Shaaban KA, Srinivasan S, Kumar R, Damodaran C, Rohr J. Landomycins P–W, cytotoxic angucyclines from Streptomyces cyanogenus S-136. J. Nat. Prod. 2011;74:2–11. doi: 10.1021/np100469y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimizu K, Kubota R, Kobayashi N, Tahara M, Sugimoto N, Nishimura T, et al. Cytotoxic effects of hydroxylated fullerenes in three types of liver cells. Materials. 2013;6:2713–2722. doi: 10.3390/ma6072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh R, Lillard JW., Jr Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009;86:215–223. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur. J. Pharm. Sci. 2013;48:416–427. doi: 10.1016/j.ejps.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tabata Y, Murakami Y, Ikada Y. Photodynamic effect of polyethylene glycol-modified fullerene on tumor. Jpn. J. Cancer Res. 1997;88:1108–1116. doi: 10.1111/j.1349-7006.1997.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tolkachov M, Sokolova V, Korolovych V, Prylutskyy Y, Epple M, Ritter U, et al. Study of biocompatibility effect of nanocarbon particles on various cell types in vitro. Mater. Wiss. Werkst. 2016;47:216–221. doi: 10.1002/mawe.201600486. [DOI] [Google Scholar]
- 51.Vereshchaka IV, Bulgakova NV, Maznychenko AV, Gonchar OO, Prylutskyy Yu I, Ritter U, et al. C60 fullerenes diminish the muscle fatigue in rats comparable to N-acetylcysteine or β-alanine. Front. Physiol. 2018;9:517. doi: 10.3389/fphys.2018.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]