Abstract
Introduction
Notch signaling is amongst the key intrinsic mechanisms regulating satellite cell fate, promoting the transition of activated satellite cells to highly proliferative myogenic progenitor cells and preventing their premature differentiation. Although much is known about the biochemical milieu that drives myogenic progression, less is known about the spatial cues providing spatiotemporal control of skeletal muscle repair in the context of Notch signaling.
Methods
Using a murine injury model, we quantified in vivo biophysical changes that occur within the skeletal muscle during regeneration. Employing tunable poly(ethylene glycol)—based hydrogel substrates, we modeled the measured changes in bulk stiffness in the context of Notch ligand signaling, which are present in the regenerative milieu at the time of injury.
Results
Following injury, there is a transient increase in the bulk stiffness of the tibialis anterior muscle that may be explained in part by changes in extracellular matrix deposition. When presented to primary myoblasts, Jagged-1, Jagged-2, and Dll1 in a tethered format elicited greater degrees of Notch activity compared to their soluble form. Only tethered Jagged-1 effects were tuned by substrate stiffness, with the greatest Notch activation observed on stiff hydrogels matching the stiffness of regenerating muscle. When exposed to tethered Jagged-1 on stiff hydrogels, fewer primary myoblasts expressed myogenin, and pharmacological inhibitor studies suggest this effect is Notch and RhoA dependent.
Conclusion
Our study proposes that tethered Jagged-1 presented in the context of transient tissue stiffening serves to tune Notch activity in myogenic progenitors during skeletal muscle repair and delay differentiation.
Electronic supplementary material
The online version of this article (doi:10.1007/s12195-017-0506-7) contains supplementary material, which is available to authorized users.
Keywords: Skeletal muscle, Regeneration, Biophysical cues, Biochemical cues, Spatiotemporal, Niche, Compression testing, Extracellular matrix, Hydrogel, Ligand presentation
Introduction
Muscle stem cells, also known as ‘satellite cells’, are tissue specific resident stem cells that are required for adult skeletal muscle regeneration following injury.17,23,26,38 Satellite cells in healthy, uninjured muscle are quiescent. Following injury, satellite cells are ‘activated’ and upon division can give rise to transit amplifying myogenic progenitor cells that expand and fuse to replace damaged muscle fibers or to additional satellite cells that will repopulate the niche in preparation to respond to future tissue insult. The progression of satellite cells from quiescence, to activation, to self-renewal, and the subsequent differentiation and fusion of daughter cell progeny to form contractile muscle fibers is referred to as ‘myogenesis’ and is orchestrated by myogenic regulatory transcription factors. Quiescent satellite cells are characterized by Pax7 expression. Upon damage to their surrounding microenvironment, or ‘niche’, satellite cells are activated and express Myf5 and will then exit the quiescent state and enter cell cycle to produce daughter myogenic progenitor cells that are often referred to as ‘myoblasts’. Proliferating myogenic progenitor cells are characterized by their expression of Pax7 and MyoD. Downregulation of Pax7 and MyoD and upregulation of the transcription factor myogenin marks myogenic progenitor exit from cell cycle and pushes eventual terminal differentiation and fusion with other myocytes to form new muscle fibers and restore function to the afore injured tissue.40
Amongst the key intrinsic mechanisms known to regulate this progression in satellite cell fate are classical developmental signaling pathways such as Wnt37 and Notch.3 Notch signaling promotes the transition of activated satellite cells to highly proliferative myogenic progenitor cells, while preventing differentiation to multinucleated muscle fibers. In contrast, Wnt drives myogenic progenitor differentiation, fusion, and muscle fiber formation. The mammalian Notch signaling axis is comprised of four Notch receptors (Notch 1, 2, 3, and 4) and five ligands (Jagged 1, 2, Delta-Like 1, 3, 4).3 Within hours and for days following muscle injury, there is increased expression of Notch pathway receptors and ligands (Delta-1, Jagged-1, and Notch-1) on activated satellite cells and neighboring muscle fibers.5,32,36 Receptor ligand physical attachment to the extracellular matrix is known to occur in vivo and to dramatically alter receptor activity by preventing signal dampening via internalization.16,22 Indeed, Notch ligand activity is known to be dictated by tethered presentation.1,3,35
Upon activation, the Notch receptor is cleaved to produce a Notch intracellular domain (NICD) fragment. NICD then translocates to the nucleus, where it activates transcription of target genes by binding to a DNA binding protein known as CSL (CBF1, Suppressor or Hairless, Lag-1). Enforced activation of Notch in vivo, as well as in cultured myogenic progenitors, inhibits differentiation and the formation of muscle fibers.3 Consistently, constitutive activation of Notch-1 using a retroviral approach enhances primary myoblast proliferation in vitro.5 Hence, Notch signaling plays a critical role in regulating skeletal muscle repair and understanding the mechanistic underpinnings controlling Notch signaling in the niche will support skeletal muscle regenerative medicine efforts.
Mounting evidence indicates that extrinsic cues, such as biomechanical features of the satellite cell niche, synergize with niche biochemical cues and play a critical role in directing satellite fate.27 Skeletal muscle tissue is a compliant and contractile material with an average elastic modulus of approximately 5–15 kPa.9,13 A number of culture studies show that satellite cells are able to sense and respond to the elasticity and stiffness of their microenvironment. For example, muscle stem cells cultured on highly elastic (1–2 kPa) engineered muscle fibers were shown to better sustain a quiescent and undifferentiated state.28 Muscle stem cells cultured on synthetic hydrogel culture substrates matching the softness of healthy resting skeletal muscle (12 kPa) maintained ‘stemness’ and self-renewed in culture, properties that were lost in stiff plastic petri culture dishes (~1 × 106 kPa).13 Furthermore, expanding young and aged muscle stem cells on soft hydrogel substrates in the context of p38 mitogen-activated protein kinase (MAPK) inhibition supports self-renewal expansion divisions that were not possible on stiff plastic culture.7 Other studies have shown how ECM proteins can also affect muscle stiffness and alter satellite cell behavior. A recent study demonstrated that lack of collagen VI in mice causes a decrease in muscle stiffness that was attributed to a reduction in satellite cell self-renewal capability after injury that impedes tissue repair.34 Another study showed that collapsed muscle fibers cultured ex vivo exhibit greater stiffness and cytoskeletal disorganization, which promoted proliferation of myogenic progenitor cells.31 These studies highlight the potent influence that substrate stiffness can exert on myogenic cell fate and underlie the need to better understand how biophysical aspects of the niche change during skeletal muscle repair.
Due to mechanosensitive properties of Notch receptors, it is possible that the Notch signaling axis is one mechanism by which mechanical signals are transduced into cellular responses during myogenesis.14,15,19,20,29,33,39,41 To illustrate, single-molecule studies have shown that a basic threshold of mechanical force is required to relieve receptor auto-inhibition and sensitize Notch to proteolytic activation.14 Remarkably, the mechanical loading of Notch receptors is itself sufficient to initiate receptor cleavage, independent of ligand-induced allosteric effects.29 Studies have also demonstrated that a range of physical stresses are capable of modulating Notch activity. In a murine model, the level of shear stress generated by blow flow was shown to be associated with the expression of Notch receptors and the number of ligand-receptor interactions in vascular tissue samples.19,33 Another study found that exposing vascular smooth muscle cells to cyclic stretch resulted in the nuclear translocation of NICD fragments and an increase in expression of Notch target genes.41 While the role of substrate stiffness in Notch signaling remains unclear, the expression of the h2-calponin gene was shown to be both dependent on stiffness-induced cytoskeletal tension and responsive to upstream activation or inhibition of Notch.20 A definitive demonstration of the link between stiffness and Notch activity awaits and could lend significant insight into the ability of muscle progenitor cells to interpret mechanically encoded cues.
Unlike classic tissue culture substrates, the satellite cell niche is a three-dimensional (3D) entity. Furthermore, tissue repair is a dynamic process during which stem cell fate decisions are driven in space and time by frequent changes in niche biophysical and biochemical composition. To advance regenerative therapies aimed at treating skeletal muscle related diseases, it is important to understand how the various intrinsic and extrinsic mechanisms present within the satellite cell niche synergize to orchestrate the spatiotemporal process of regeneration and myogenesis. To this end, in this study we used a murine hindlimb injury model to quantify in vivo biophysical changes that occur within the skeletal muscle during regeneration. Our results reveal that there is a transient increase in the bulk stiffness of the tibialis anterior muscle following injury. Collagen I, Fibronectin, and fibrin clot deposition increased at time-points associated with bulk tissue stiffening and returned to pre-injury levels on the fourth day of regeneration when bulk tissue apparent modulus also returned to pre-injury levels. In contrast, Collagen VI increased on the first day of regeneration, but returned to pre-injury levels on the very next day. We then used tunable poly(ethylene glycol)—based hydrogel substrates to model measured changes in bulk stiffness, in the context of Notch ligand signaling, which are present in the regenerative milieu at the time of injury.5,32,36 The hydrogel platform was also implemented to control and explore the impact of Notch ligand presentation mode (e.g., soluble, tethered) on murine primary myoblast fate. Our studies showed that increased substrate stiffness synergizes with tethered Jagged-1, but not other Notch ligands (Dll1), to tune Notch activity and inhibit primary myoblast differentiation in a Rho A dependent manner. In sum, we show that skeletal muscle biomechanical properties change during regeneration and that this alteration in the biophysical properties of the myogenic niche can synergize with tethered Jagged-1 to tune the extent of Notch activity and the timing of myogenic differentiation.
Materials and Methods
Animals
The Faculties of Medicine and Pharmacy Animal Care Committee of the Division of Comparative Medicine at the University of Toronto reviewed and approved all animal studies in this manuscript. All animal studies in this report utilized female C57BL/6N wild-type mice aged 8 weeks that were purchased from Charles River Laboratories (Canada).
Compression Testing
Compression testing was performed on tibialis anterior muscles harvested from humanely euthanized young (8-week old), uninjured wild-type mice as well as from mice that had been injured by a single 30 μL intramuscular injection of 1% BaCl2 solution 1–7 days prior.30 Care was taken to perform compression measurements within minutes of harvesting the tissue. The tibialis anterior muscle group was gently placed onto a flat platform and the dimensions of the isolated bulk TA muscle were visualized and noted using a Navitar (USA) camera. We applied a 0.5 g pre-load using a TestResources, Inc (USA) tensile machine. Five cycles of 0–20% compression was then performed, and data from the final compression cycle was collected and used in calculations for each sample. Engineering stress and strain was calculated using the TestResources software and with Microsoft Excel. Reported is the incremental apparent modulus from 10 to 13% strain.
Western Blotting
Tibialis anterior muscles were isolated from control or BaCl2 injured wild-type mice. The muscles were lysed in RIPA buffer (Sigma-Aldrich, R0278) containing 1X Halt protease inhibitor (Thermofisher, 78430). Lysate protein concentration was determined using the Pierce BCA Protein Assay kit (Thermofisher, 23227). 15 µg (Fibronectin), 25 µg (Collagen 1, Fibrin clot), or 40 µg (Collagen VI) of protein was loaded into an 8% bis–tris SDS-PAGE gel and run at 120 V for 2 h. The proteins were then transferred to a nitrocellulose membrane (VWR, CA27376-991) using the BioRad Transblot Turbo Transfer System (BioRad, 1704155). The membrane was then blocked with 5% skimmed milk (BioShop, SK1400) diluted in TBS-T (50 mM Tris–HCl, 150 mM NaCl, pH 7.6 + 0.1% Tween-20). Membranes were rocked overnight at 4 °C with the following antibodies diluted in blocking solution: rabbit-anti Fibronectin (1:1000; Abcam, ab23750), rabbit-anti Collagen 1 (1:1000; Abcam, ab21286), rabbit-anti Collagen VI (1:1000; Abcam, ab172606), anti-fibrin clot (102-10 mAb, a gift from Yasuhiro Matsumura, National Cancer Center Hospital East, Kashiwa City, Japan; 1:2000)18 or rabbit-anti Tubulin (1:1000; Abcam, ab6046). After extensive washing in blocking solution, membranes were rocked for 60 min at room temperature with the appropriate 2° antibody diluted in blocking solution: donkey-anti goat horseradish peroxidase (HRP; 1:500; Promega, V8051) or goat-anti rabbit HRP (1:500; Cell Signaling, 7074). HRP signal was visualized using SuperSignal™ West Dura Extended Duration Substrate (Thermofisher, 34075) and the DNR Bio-Imaging Systems MicroChemi 4.2 (Israel). In cases where membranes were re-probed, the Restore™ PLUS Western Blot Stripping Buffer (Thermofisher, 46428) was used to strip blots.
Immunohistochemical Analysis
Following dissection, harvested tibialis anterior muscles were flash frozen by immersing the tissue for a period of ~2 min within a metal bucket of 2-methylbutane (Sigma-Aldrich) that was chilled to near freezing with liquid nitrogen. Tissues were then either stored at −80 °C until needed or immediately sectioned using a LEICA CM3050 S cryostat to produce 10 μm thick tissue sections. Sections were then either stored at −20 °C until needed or immediately processed. Slides were first warmed to room temperature and then hydrated in PBS for 5 min followed by a 20-min incubation in a 4% paraformaldehyde solution. Sections were dipped in PBS to remove paraformaldehyde and then blocked for 2 h at room temperature in the follow blocking solution: phosphate buffered saline containing 20% normal goat serum (Gibco) and 0.3% Triton X-100 (BioShop). Blocking solution was then aspirated and replaced with 1° antibodies diluted in fresh blocking solution. The following antibodies were used: rabbit anti-Collagen 1 (1:200; Abcam, ab21286) or rat anti-Laminin (1:200; Millipore, 05-206). Slides were incubated in 1° antibodies overnight at 4 °C within a humidified chamber. The next day, slides were washed multiple times in blocking solution and then incubated for 30 min at room temperature with the appropriate 2° antibody (1:500; Life Technologies, AlexFluor series) diluted in blocking solution. Following extensive washes with blocking solution, slides were then mounted using Fluoromount (Sigma, F4680) and then epi-fluorescence snap images were acquired using an Olympus DP80 dual CCD color and monochrome camera and an Olympus IX83 inverted microscope at 10× magnification using Olympus CellSens software.
Primary Myoblast Cell Culture
All culture studies in this report utilized murine primary myoblasts. Murine primary myoblast lines were derived exactly as previously described.2 Primary myoblasts were maintained in growth media (F12 media, 20% Fetal Bovine Serum, 1% Penicillin Streptomycin, and 0.01% basic FGF2) on Type 1 collagen coated tissue culture plates at 37 °C, 5% CO2, and 20% O2. Cell cultures were passaged using a warm solution of phosphate buffered saline when plates reached ~70% confluency. Primary myoblast cultures of passages 5–12 were used in experiments.
Hydrogel Fabrication and Culture
Flat hydrogels of varying stiffness (Young’s modulus = 4, 12, or 42 kPa) were fabricated using poly(ethylene glycol) (PEG) precursors as previously described.7,8,13 Briefly, a mixture of 10% (wt/vol) solutions of PEG-sulfhydryl (10 kDa 4-arm PEG-SH; Sunbright PTE-100SH) precursor polymer dissolved in water or PEG-vinylsulfone (10 kDa 8-arm PEG-VS; Sunbright HGEO-100VS) precursor polymer dissolved in 0.2 M triethanolamine (Sigma Aldrich) were first prepared. 10% (wt/vol) 4-arm PEG-SHsolution was vortexed with 10% (wt/vol) 8-arm PEG-VSat ratios previously established to form hydrogels with the desired mechanical properties (detailed instructions can be found in Ref. 8). The solution was then pipetted onto Sigma-coated hydrophobic glass slides containing 1 mm thick Teflon spacers on opposite sides. A 1 mm thick hydrogel ensures that cultured cells sense the stiffness of the hydrogel substrate and not the underlying plastic culture dish.10 A second Sigma-coated glass slide was then placed atop the un-polymerized hydrogel droplets and secured in place using binder clips. Following a brief incubation at 37 °C to partially polymerize the hydrogel and allow for manipulation, hydrogel surfaces were then functionalized at room temperature for a period of 45 min. All hydrogels were covalently tethered with PBS-dialyzed laminin (Roche; 0.5 mg/mL) to provide an adhesive interface. For conditions in which Notch ligand tethering was desired, Protein A (ThermoFisher Scientific; 0.5 mg/mL) was also covalently tethered. The minimal post-polymerization swelling chemistry of these hydrogels allows for equivalent laminin protein concentrations (estimated to be 7.5 ng/cm2)7,13 across the range of stiffness used in these studies. Individual hydrogels were then cut and adhered (using a 1:1 ratio of precursor solutions) to cover the surface area (2.0 cm2) of single wells within 24-well culture plates. Hydrogels were incubated with 1% antimycotic/antibiotic (LifeTechnologies) solution overnight at 4 °C prior to experimentation.
Hydrogel Culture
For the tethered protein conditions, aliquots of Fc-conjugated recombinant ligands were thawed and 500 ng was added to 1 mL of PBS and delivered to each well. Carrier-free FC conjugated recombinant Immunoglobulin G subclass 1 (IgG1), Jagged-1 (Jag1), Jagged-2 (Jag2), and Dll1 were each purchased from R&D systems. Hydrogels were incubated with 500 ng of ligand diluted in PBS for 1 h at 37 °C to allow ligand binding to Protein A in tethered conditions. The wells then underwent a series of 3 × 1 mL 30 min washes with PBS to remove untethered proteins. Prior to cell seeding, all hydrogels were equilibrated in 1 mL of growth media at 37 °C with at least three × 30-min media exchanges before use. Primary myoblast cultures were harvested from culture dishes, counted with a hemocytometer, and seeded at a density of 20,000 cells per well of a 24-well plate in 1 mL of growth media. 20 ng of recombinant protein was added to ‘soluble ligand’ conditions.
NICD Analysis
Notch activity was assessed through the visualization of Notch intracellular domain (NICD) nuclear translocation. Hydrogel cultured cells were fixed with 4% paraformaldehyde for 10 min at room temperature followed by cell permeabilization and blocking for 1 h at room temperature in blocking solution [PBS, 20% goat serum, 0.3% Triton X-100]. Cells were then incubated overnight at 4 °C in blocking solution containing anti-activated Notch 1 antibody (Abcam, 1:500). The following morning cultures were washed numerous times in blocking solution and then were incubated in secondary antibody solution (anti-rabbit Alexa Fluor 488, 1:1000) containing a nuclear counterstain (DRAQ5, 1:1000) for 30 min at room temperature. Following numerous washes with phosphate buffered saline (PBS), hydrogels containing fixed and stained cells were carefully removed from the well using a spatula and mounted upside down on glass slides for imaging. PBS droplets were maintained on the top of the hydrogel to prevent drying. Images were acquired using an Olympus IX83 inverted confocal microscope with FV-10 software and analyzed using NIH ImageJ software.
Cell Proliferation Analysis
Cell proliferation was assessed in two ways. First, entry into cell cycle was assessed using the Click-iT EdU Alexa Fluor 488 Imaging Kit (Molecular Probes). C2C12 murine myoblasts (ATCC) were plated in growth media and cultured for 24 h. Cultures were then transferred into differentiation media (DMEM, 1% Horse Serum, 1% Penicillin Streptomycin) for 48 h by performing 3 × 30 min 1 mL media exchanges. EdU (1 uM) was added to the differentiation media during the final 12 h of culture. Cells were then Click-iT labelled with Alexa Fluor 488 according to manufacturer instructions and then counterstained with Hoechst (1:1000) to visualize nuclei. Images were acquired using an Olympus IX83 microscope using an Olympus DP80 dual CCD color and monochrome camera and CellSense™ software.
In other experiments, total cell number over time was assessed to determine changes in cell population size over time. In these experiments, primary myoblasts were plated in growth media for 24 h and then exchanged into differentiation media. Differentiation media was replenished every 24 h. After 72 h in differentiation media, Hoechst nuclear stain was added to cultures, hydrogels were removed from wells, inverted onto a glass slide, and then a tiled image of the entire hydrogel was attained. Images were segmented and total cell counts obtained using ImageJ software.
Myogenin Analysis
Primary myoblasts were plated in growth media and cultured for 24 h. Cultures were then transferred into differentiation media (DMEM, 1% Horse Serum, 1% Penicillin Streptomycin) by performing 3 × 30-min 1 mL media exchanges. After 24 h in differentiation media, cultures were fixed with 4% PFA for 10 min at room temperature, followed by cell permeabilization and blocking for 1 h at room temperature in blocking solution [PBS, 20% goat serum, 0.3% Triton X-100]. Cells were then incubated overnight at 4 °C in blocking solution containing anti-myogenin (BD Pharmingen, 1:500). The following morning cultures were washed numerous times in blocking solution and then were incubated in secondary antibody solution (anti-mouse Alexa Fluor 647, 1:1000) containing a nuclear counterstain (DRAQ5, 1:1000) for 30 min at room temperature. Images were acquired using an Olympus IX83 microscope using an Olympus DP80 dual CCD color and monochrome camera and CellSense™ software. Myogenin staining that localized to the nucleus was considered positive.
Real-Time qPCR
Total RNA was extracted using the PureLink RNA Micro Kit according to the manufacturer’s protocol (ThermoFisher) from three independent hydrogel culture experiments and using three biological replicates. cDNA was reverse transcribed from 400 ng of RNA using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). For quantitative real-time PCR (qRT-PCR), MKP-1 primers were acquired from Bio-Rad and reactions were run according to manufacturer’s protocol on the Roche LightCycler 480 (Roche) using LightCycler 480 SYBR Green I Master (Roche). All results are normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical Analysis
All data are presented as mean ± standard deviation unless otherwise noted. At least three biological replicates were performed for each study. Where single cells were analyzed, a minimum of n = 50 single cells per biological replicate was assessed. Statistical comparisons were performed by a two-way ANOVA test unless otherwise stated. Values were considered to be significantly different when p < 0.05.
Results
Skeletal Muscle Regeneration is Accompanied by a Transient Increase in Bulk Stiffness
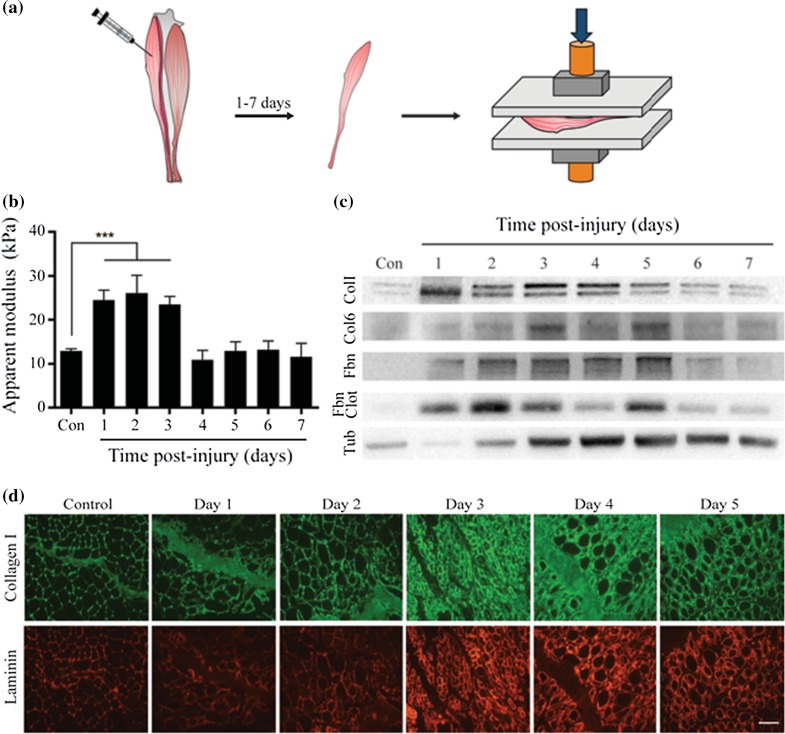
To better understand the environmental physical changes that accompany skeletal muscle repair, we used compression testing to quantify the bulk stiffness of resting and regenerating skeletal muscle tissue. Tibialis anterior muscles harvested from mice 1–7 days following a single BaCl2 intramuscular injection30 were evaluated and compared to tissue harvested from uninjured control mice (Fig. 1a and Supplemental Fig. 1a). Tibialis anterior apparent modulus measurements revealed that injured muscle undergoes a transient increase in bulk stiffness during the first three days after injury that then returns to pre-injury levels by the fourth day of repair (Fig. 1b and Supplemental Figs. 1b and 1c). Since changes in skeletal muscle tissue stiffness might arise from alterations in extracellular matrix deposition, we assessed Collagen 1, Collagen VI, Laminin, Fibronectin, and Fibrin clot formation using western blot analysis (Fig. 1c and Supplemental Fig. 2) and immunohistological methods (Fig. 1d) at time-points before and after injury. Though we observed trends towards an increase in the expression level of all matrix proteins assayed as early as one day post-injury when compared to uninjured controls and normalized to tissue cellular content (tubulin) at each time-point, only Collagen VI levels were statistically significantly different from the uninjured control (Supplemental Fig. 2). Further, protein levels of Collagen I, Fibronectin, and Fibrin clot qualitatively returned to and sustained pre-injury levels on the fourth day of regeneration mirroring changes in bulk tissue stiffness. This suggests that Collagen I, Collagen VI, Fibronectin, and Fibrin clot deposition may contribute to the bulk changes in apparent modulus that accompany the early stages of skeletal muscle repair.
Figure 1.
Transient changes in bulk tissue stiffness and ECM deposition accompany skeletal muscle regeneration. (a) Schematic of tibialis anterior (TA) muscle compression testing experiments used to quantify bulk apparent modulus of quiescent and regenerating tissues. TA muscles were harvested from mice 1–7 days following a single BaCl2 intramuscular injection or from uninjured control (Con) mice and then evaluated using (b) compression testing; (d) western blotting, and (d) immunohistochemistry. (b) Bar graph of TA muscle apparent modulus. n = 3 animals with a total of six TA muscles measured per time-point. P < 0.05 by one-way ANOVA; (c) Western blots of Collagen 1 (Col1), Collagen VI (Col6), Fibronectin (Fbn) and clotted fibrin (Fbn Clot). Bicinchoninic acid assay was used to quantify protein and ensure equal loading. Tubulin (Tub) indicates relative changes in cellular content at each time-point; (d) Immunohistochemical analysis of Collagen 1 (green; top panels) and Laminin (red; bottom panels) in transverse sections of skeletal muscle. Scale bar, 100 µm.
Tethered Jagged-1 Synergizes with Matrix Stiffness to Induce Notch Activity
The Notch signaling pathway is well accepted to modulate myogenic progression and Notch ligands including Jagged-1, Jagged-2, and DLL-1 are present in regenerating skeletal muscle tissue.5,36 In this study, we sought to determine whether tissue stiffness modulates Notch pathway activation. To this end, we implemented a tunable poly(ethylene-glycol) based hydrogel culture platform6,7,13,24,25 to propagate and study murine primary myoblasts exposed to substrates of varying stiffness and presented with soluble or tethered forms of Fc conjugated recombinant Notch ligands (Jagged-1, Jagged-2, Dll1) or an Immunoglobulin G subtype 1 (IgG1) control. Protein A, which binds the Fc portion of Immunoglobulin proteins with high affinity, was covalently tethered to the surface of hydrogels for all ‘tethered’ conditions. Laminin was covalently linked to the hydrogel surface and served as an adhesive interface for the myogenic progenitors in all conditions. 1 h after plating primary myoblasts in culture in growth media, we quantified Notch intracellular domain (NICD) nuclear translocation to assess Notch pathway activation. Consistent with prior reports, exposure to tethered Notch ligands (here, tethered to a 12 kPa hydrogel) consistently supported greater levels of NICD nuclear translocation when compared to the tethered IgG1 controls or to culture conditions where IgG1 or Notch ligands were simply added to the culture media (Supplemental Fig. 3a). Furthermore, expression of MKP-1, a p38 mitogen-activated protein kinase inhibitor that is a transcriptional target of Notch NICD,21 was induced in primary myoblasts exposed to tethered and not soluble Jagged-1 and Jagged-2 ligands, whereas exposure to Dll1 had little influence on MKP-1 expression (Supplemental Fig. 4).
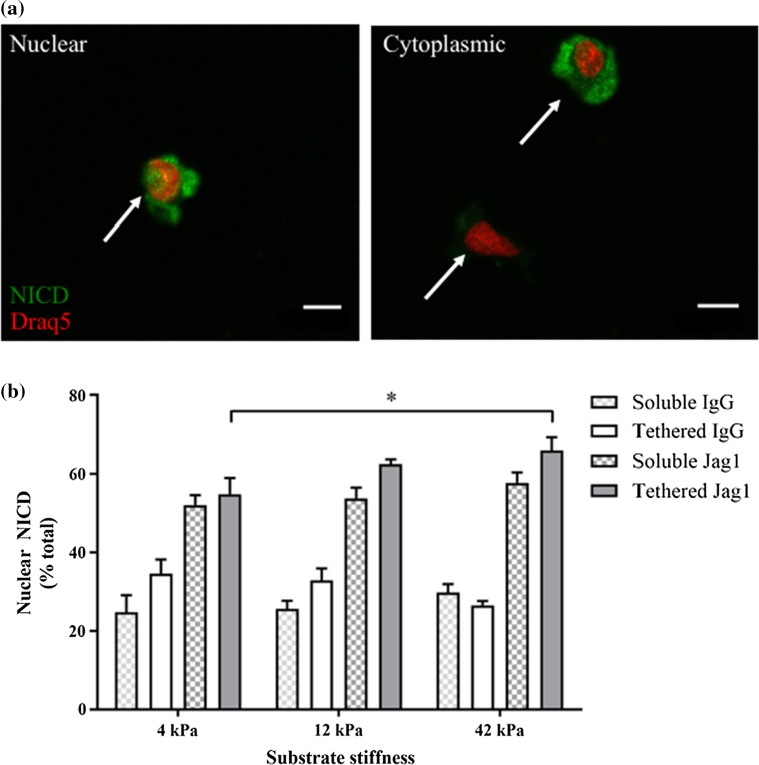
Next, we exposed primary myoblasts to tethered Notch ligands in the context of culture substrates that matched the range of stiffness we measured over the first week of skeletal muscle repair (12 and 42 kPa), as well as a softer substrate (4 kPa), and then assessed Notch pathway activation (Fig. 2a). Hydrogels were 1 mm in thickness to preclude the possibility that cultured cells might sense the stiffness of the underlying plastic dish,10 and prior studies demonstrated that ligand density does not change across the range of hydrogel stiffnesses utilized in our study.7,13 In contrast with Jagged-1 ligand treatments (Fig. 2b; all IgG1: Jagged1 student’s t test pairwise comparisons p < 0.05), hydrogel stiffness alone did not appear to influence the proportion of primary myoblasts exhibiting nuclear NICD (Fig. 2b; IgG1 ANOVA tests not significant). Intriguingly, stiff hydrogels modified to present a tethered Jagged-1 ligand induced Notch activity in a greater proportion of primary myoblasts compared with Jagged-1 tethered soft hydrogels (Fig. 2b; solid gray bars). Treatment with soluble Jagged-1 did not produce a similar effect (Fig. 2b; hatched gray bars) suggesting that hydrogel stiffness synergizes with tethered Jagged-1 ligand to elicit Notch activity in myogenic progenitors. This synergistic effect was ligand dependent as tethered Dll1-mediated Notch activation was not influenced by stiffness (Supplemental Fig. 3b).
Figure 2.
Tethered Jagged-1 synergizes with matrix stiffness to promote Notch NICD nuclear translocation. (a) Representative immunocytochemical images of primary myoblasts stained for NICD (green) and Draq5 (red). Arrows point to examples of nuclear (left) and cytoplasmic (right) NICD localization. Scale bar, 10 µm; (b) Bar graph showing the proportion of cells exhibiting nuclear NICD localization when exposed to soluble (checkered) or tethered (solid) IgG control (white) or Jagged-1 (Jag1; gray) Notch ligand in the context of 4, 12, or 42 kPa PEG hydrogels. Error bars indicate mean ± SEM. n = 3 and P < 0.05 by two-way ANOVA where indicated.
Together these studies support prior work in showing that Notch ligand presentation influences Notch activation. We further extend understanding of Notch regulation by demonstrating that tethered Jagged-1 and Jagged-2, but not tethered Dll1, induce MKP-1 gene expression and that Notch activity induced by tethered Jagged-1, but not tethered Dll1, is tuned by matrix stiffness.
Tethered Jagged-1 Synergizes with Substrate Stiffness to Influence Primary Myoblast Fate in Culture
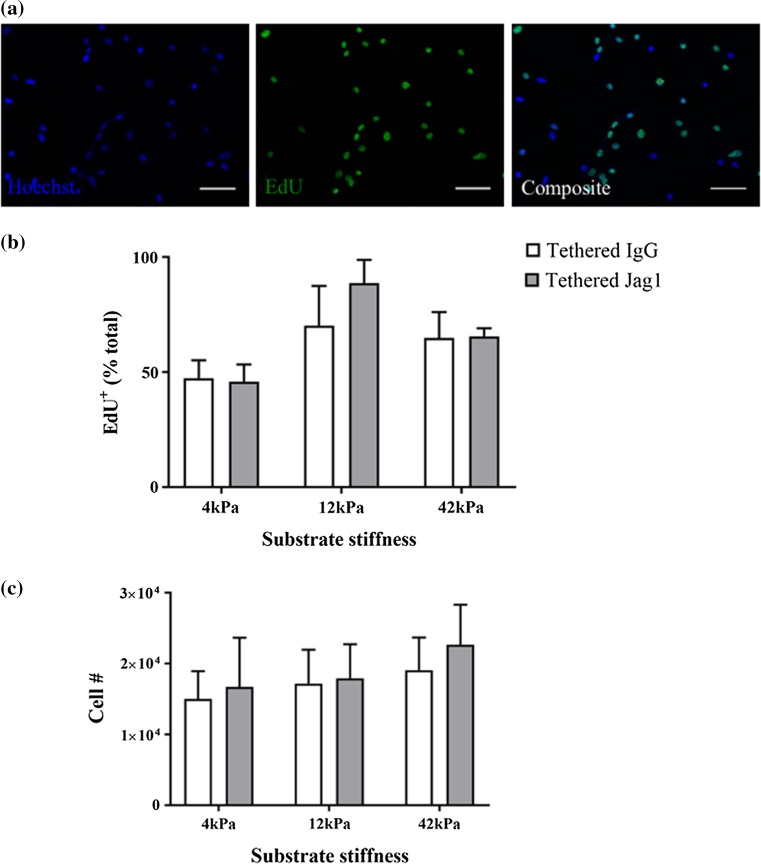
Since Notch signaling is known to support myogenic progenitor proliferation while preventing differentiation, we next explored the influence of matrix stiffness and tethered Jagged-1 on Notch-induced myoblast proliferation. We cultured myogenic progenitors on soft and stiff hydrogels tethered with Jagged-1 for one day in growth media and then transitioned cultures to conditions that support differentiation (low serum) for a period of 48 h. The thymidine analogue EdU was added to the low serum media for the final 12 h of culture. At that point, we assessed the proportion of cells that incorporated EdU during the 12-h pulse (Fig. 3a). While we observed a trend across our culture conditions (Fig. 3b) such that a greater proportion of progenitors incorporated EdU on stiffer substrates, the study did not achieve statistical significance (p = 0.06). Consistently, when we calculated the total number of primary myoblasts on each hydrogel at later time-points, we observed no differences across culture conditions (Fig. 3c).
Figure 3.
Tethered Jagged-1 in differentiation conditions does not modify myoblast proliferation. (a) Representative immunocytochemical images of murine myoblasts stained for Hoechst (blue; left) and EdU (green; middle) that are then merged to show a composite of the two images (right). Scale bar, 50 µm; (b) Bar graph quantifying the proportion of myoblasts that incorporate EdU in the last 12 h of a 48-h period in differentiation media when exposed to tethered IgG1 (white) or tethered Jagged-1 (Jag1; gray) in the context of 4, 12, or 42 kPa hydrogels. n = 3 and P = not significant by two-way ANOVA; (c) Bar graph indicating the total number of primary myoblasts following 72 h of incubation in differentiation media. n = 3 and P = not significant by two-way ANOVA.
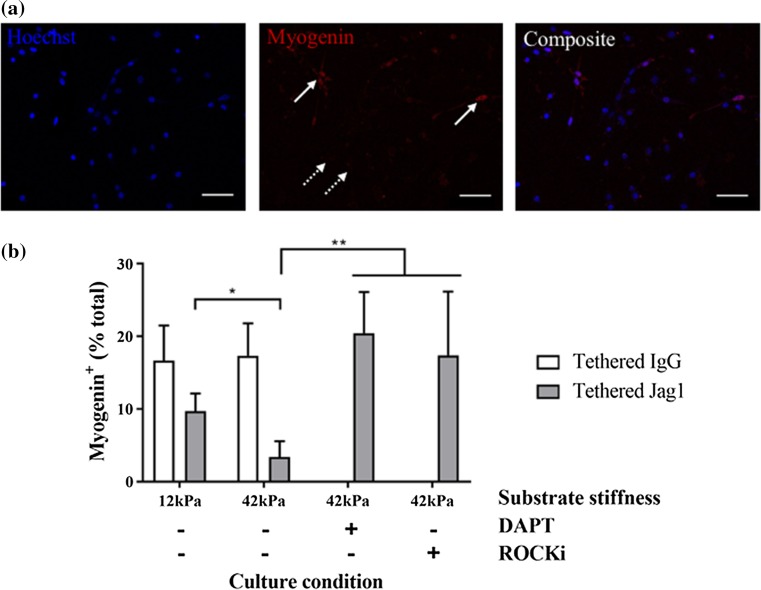
Next, we evaluated the influences of tethered Jagged-1 and culture substrate stiffness on the acquisition of myogenin expression in primary myoblasts. Myogenin is a muscle-specific transcriptional activator that turns on myogenic genes to support the transition from proliferating transit amplifying cells to differentiating muscle fibers in part by causing cell cycle exit.40 We quantified the proportion of total primary myoblasts with myogenin expression when cultured on soft or stiff culture substrates and exposed to tethered IgG1 control or Jagged-1 (Fig. 4a). As anticipated, exposure to tethered Jagged-1 resulted in a decrease in the proportion of primary myoblasts expressing nuclear myogenin (Fig. 4b). Notably, we also found that there was a statistically significant further reduction in myogenin expressing cells when cultured on Jagged-1 tethered stiff hydrogels (42 kPa) compared to soft (12 kPa) hydrogel culture (Fig. 4b).
Figure 4.
Tethered Jagged-1 synergizes with substrate stiffness to influence primary myoblast fate in culture. (a) Representative immunocytochemical images of primary myoblasts stained for Hoechst (blue; left) and Myogenin (red; middle) that are then merged to show a composite of the two images (right). Solid arrows point to examples of nuclear myogenin (positive) and dotted arrows point to examples of cells lacking myogenin staining (negative). Scale bar, 50 µm; (b) Bar graphs quantifying the proportion of primary myoblasts with nuclear myogenin staining after 24 h in differentiation media when exposed to tethered IgG1 (white) or tethered Jagged-1 (Jag1; gray) in the context of 12 or 42 kPa hydrogels and with (+) or without (−) treatment with DAPT or ROCKi. n = 3, P < 0.05 by two-way ANOVA.
Matrix stiffness induces cell contractility, which can then activate mechanosensitive signal transduction pathways. To determine whether cytoskeletal assembly contributed to stiffness-induced effects on Notch-activity, we treated primary myoblasts with a Rho-associated, coiled-coil containing protein kinase (ROCK) inhibitor in the context of Jagged-1 tethered stiff hydrogel culture. Consistently, we observed a dramatic increase in myogenin expressing cells in response to ROCK inhibition (Fig. 4b). Furthermore, by treating cultures with DAPT, a potent Notch pathway inhibitor, we also observed a dramatic increase in the proportion of myogenin expressing cells, supporting Notch pathway involvement in preventing primary myoblast differentiation in the context of hydrogel culture (Fig. 4b).
Together, these studies suggest that matrix stiffness, a biologically relevant biophysical cue, synergizes with tethered Jagged-1 Notch ligand, a biologically relevant biochemical cue, to tune myogenic progenitor fate.
Discussion
In this report, we sought to elucidate synergies between skeletal muscle biophysical and biochemical cues that may serve to provide spatiotemporal regulation of myogenesis during skeletal muscle repair. We used compression testing to quantify skeletal muscle tissue apparent modulus over the first week of repair following a chemical injury and discovered that the tissue transiently increases in stiffness in the first three days following injury and rapidly returns to pre-injury softness on the fourth day. Despite inherit challenges associated with using a compression testing device to apply homogeneous force to tissues that are heterogeneous in geometry, our measured values for uninjured skeletal muscle tissue match those attained using atomic force microscopy.9
Injured tissues exhibit edema and changes in extracellular matrix composition, organization, all of which could alter bulk tissue stiffness. While our analyses of extracellular matrix protein expression and localization over muscle repair cannot be considered exhaustive and failed to achieve statistical significance, Collagen I, Fibronectin, and Fibrin clot deposition trend with the changes in skeletal muscle bulk stiffness that we observed. When normalized to cellular content (tubulin levels), we found that Collagen I, Fibronectin, and Fibrin clot deposition increased during the first 3 days following injury and then returned to near baseline levels on Day 4 of regeneration. Collagen VI, in contrast, increased 24 h post-injury, but rapidly returned to pre-injury levels by 48 h. Since the magnitude of average bulk stiffness during the first three days of regeneration was constant, while Collagen I, Fibronectin, and Fibrin clot levels change erratically, it is likely that numerous factors contribute to the increased bulk stiffness we measured. Consistently, other studies note that tenascin-c and hyaluronic acid levels increase substantially during muscle repair.4,12 Due to its hygroscopic properties, hyaluronic acid is thought to facilitate the outpour of edematous fluid into the interstitium, which may account for some of the change in apparent modulus that we report in our study.4,12
Regardless of the causal origins, it is enticing to speculate that transient niche stiffening may play a role in the early phases of skeletal muscle repair including satellite cell activation and myogenic progenitor expansion, since stiff environments are well known to activate mechanosensitive signal transduction pathways. Furthermore, the return to pre-injury stiffness levels coincides nicely with the emergence of the first nascent muscle fibers in vivo. This is particularly intriguing in light of culture studies by others showing that myotubes mature most robustly on substrates matching the softness of resting muscle (12 kPa).9 Therefore, we elected to explore possible synergies between matrix stiffness and Notch activity in culture.
Notch activity is known to modulate myogenic fate, but many questions remain as to which Notch ligands activate the various Notch receptors and whether transcriptional targets and cellular outcomes downstream of differential Notch ligand-receptor combinations are distinct. We took advantage of a tractable poly(ethylene glycol) hydrogel system and used an iterative approach to first evaluate the influence of Notch ligand presentation mode (e.g. soluble, tethered) on primary myoblast Notch activity. While the activity of these ligands is known to be dictated by whether or not they are presented in an immobilized form to Notch receptors,1,3,16,22,35 synergies between Notch ligand presentation and culture substrate stiffness have not been interrogated. We cultured primary myoblasts on hydrogels matching the softness of resting skeletal muscle and exposed them to soluble or tethered IgG1, Jagged-1, or Dll1. In all cases, tethered presentation resulted in a greater proportion of cells exhibiting nuclear NICD signal compared with the IgG1 control. Notably, soluble Jagged-1 produced a statistically significant increase in the proportion of primary myoblasts with nuclear NICD signal, which was not observed in the soluble Dll1 treatment condition. Furthermore, analysis of a Notch transcriptional target, MKP-1, revealed that only tethered Jagged-1 and Jagged-2, and not the soluble treatments or Dll1, were able to induce MKP-1 gene expression. This suggests that while NICD nuclear localization is indicative of Notch activity, one must evaluate additional downstream metrics to gain an accurate vision.
Since we found that matrix stiffness transiently stiffens at early regeneration time-points, we were keen to determine whether tethered Notch ligands might synergize with matrix stiffness to modify primary myoblast fate. By evaluating myoblast NICD nuclear accumulation following culture on hydrogels across a range of physiological stiffness (4, 12, and 42 kPa) and tethered with either Jagged-1 or Dll1 and compared to a tethered IgG control, we observed a synergy between increased stiffness and tethered Jagged-1, but not Dll1. This highlights a Notch ligand dependent difference in the downstream regulation of myogenic fate. Several lines of evidence from the literature suggest that Notch activation may have a mechanical requirement14,15,19,20,29,33,39,41 and our data support the notion that mechanics may play a role in Notch activation and further suggest that only a subset of Notch ligands may contribute to the mechanical regulation of Notch activity. This is additionally supported by the increase in myogenin expressing cells in response to either Notch (DAPT) or ROCK pharmacological inhibition.
To our surprise, we did not observe Notch ligand-induced effects on myogenic progenitor proliferation. However, prior studies that observed changes in proliferative capacity elicited by Notch activity were performed under conditions that support growth (i.e. serum, bFGF) or in vivo, which contains a complicated mix of factors. Since Notch receptors were shown in other cell types to interact with fibroblast growth factors to modulate cell proliferation,11 it is possible that our studies, which were performed under conditions supporting differentiation (i.e. low serum, no bFGF) hint at a similar mechanism in myogenic cells. Alternatively, it is entirely possible that different Notch ligands elicit distinct downstream cellular effects such that Dll1, and not Jagged-1, impacts myogenic progenitor proliferation. Regardless, our study demonstrates that it is possible to separate Notch-induced effects on differentiation and proliferation.
It is notable that in the context of a regenerative biomolecule (tethered Jagged-1) that a stiff culture substrate served to prevent myogenic differentiation, while our prior studies noted that a soft culture substrate was required to maintain ‘stemness’ outside of the body.7,13 This discrepancy may be due to differential responses of stem compared to progenitor cells with respect to mechanical preferences. However, it is more likely that in the context of a regenerative milieu (biochemical cues) different outcomes are to be expected. Regardless, our results suggest that modeling the early phases of skeletal muscle repair may benefit from a dynamic hydrogel system in which stiffness and ligand presentation can be tuned over space and time.
In summary, the studies presented here contribute to a growing body of evidence that biophysical cues synergize with biochemical cues to drive cellular fate outcomes and provide a demonstration that Notch activity is sensitive to substrate stiffness.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are grateful to Dr. Yasuhiro Matsumura (National Cancer Center Hospital, Kashiwa City, Japan) for providing us with his custom antibody to analyze fibrin clot formation in our study.
Author Contributions
HS, MAB, CAS, and PMG conceived the study and designed experiments. HS, SD, RYC, AJM, and EWL performed experiments, analyzed data, performed statistical analyses, and prepared figures. HS, MAB, SD, RYC, AJM, EWL, CAS, and PMG wrote, assembled, and revised the manuscript. All authors reviewed and approved the submission.
Funding
This study was funded by the Natural Sciences and Engineering Research Council (USRA fellowship to H.S., CREATE ToEP fellowship to S.D., RGPIN 327627-06 to C.A.S., RGPIN 435724-13 and Canada Research Chair 950-231201 to P.M.G.); Toronto Musculoskeletal Centre (Graduate Scholarships to M.A.B. and R.Y.C.); Barbara and Frank Milligan Foundation (R.Y.C.); Ontario Provincial Government (OGS-visa to M.A.B., 31390 and ER15-11-073 to P.M.G.); Canada Foundation for Innovation (31390 to P.M.G.); Krembil Foundation (Scholarship to M.A.B.); Toronto Western Arthritis Program (to P.M.G.); and Canadian Institutes of Health Research (MOP-302041 to C.A.S. and ONM-137370 to P.M.G.)
Conflict of interest
All authors declared that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Abbreviations
- bFGF
Basic fibroblast growth factor
- Dll1
Delta-like 1
- DM
Differentiation media
- ECM
Extracellular matrix
- GM
Growth media
- IgG1
Immunoglobulin G subtype 1
- Jag1
Jagged-1
- Jag2
Jagged-2
- MyoG
Myogenin
- NICD
Notch intracellular domain
- PEG
Polyethylene glycol
- PBS
Phosphate buffered saline
- ROCK
Rho-associated, coiled-coil containing protein kinase
- TBST
Tris-buffered saline plus Tween-20
Footnotes
Penney Gilbert is an Assistant Professor at the University of Toronto in the Institute of Biomaterials and Biomedical Engineering. She holds cross-appointments in the Department of Biochemistry and the Donnelly Centre for Cellular and Biomolecular Research. Gilbert received her PhD from the University of Pennsylvania under the mentorship of Valerie Weaver and then pursued Postdoctoral studies with Helen Blau at Stanford University. Her research team is focused on engineering human skeletal muscle culture models and uncovering niche cues that drive muscle stem cell fate changes in vivo. She is recipient of an Ontario Early Researcher Award and a BMES-CMBE Conference ‘Rising Star’ Award. She holds a Tier II Canada Research Chair in Endogenous Repair.
This article is part of the 2017 CMBE Young Innovators special issue.
References
- 1.Bjornson CRR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30:232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blau HM, Webster C. Isolation and characterization of human muscle cells. Proc. Natl. Acad. Sci. USA. 1981;78:5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buas MF, Kadesch T. Regulation of skeletal myogenesis by Notch. Exp. Cell Res. 2010;316:3028–3033. doi: 10.1016/j.yexcr.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calve S, Isaac J, Gumucio JP, Mendias CL. Hyaluronic acid, HAS1, and HAS2 are significantly upregulated during muscle hypertrophy. Am. J. Physiol. Cell Physiol. 2012;303:C577–C588. doi: 10.1152/ajpcell.00057.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev. Cell. 2002;3:397–409. doi: 10.1016/S1534-5807(02)00254-X. [DOI] [PubMed] [Google Scholar]
- 6.Cordey M, Limacher M, Kobel S, Taylor V, Lutolf MP. Enhancing the reliability and throughput of neurosphere culture on hydrogel microwell arrays. Stem Cells. 2008;26:2586–2594. doi: 10.1634/stemcells.2008-0498. [DOI] [PubMed] [Google Scholar]
- 7.Cosgrove BD, Gilbert PM, Porpiglia E, Mourkioti F, Lee SP, Corbel SY, Llewellyn ME, Delp SL, Blau HM. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat. Med. 2014;20:255–264. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davoudi S, Gilbert PM. Optimization of satellite cell culture through biomaterials. Methods. Mol. Biol. 2017;1556:329–341. doi: 10.1007/978-1-4939-6771-1_18. [DOI] [PubMed] [Google Scholar]
- 9.Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 11.Faux CH, Turnley AM, Epa R, Cappai R, Bartlett PF. Interactions between growth factors and Notch regulate neuronal differentiation. J Neurosci. 2001;21:5587–5596. doi: 10.1523/JNEUROSCI.21-15-05587.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerdin B, Hällgren R. Dynamic role of hyaluronan (HYA) in connective tissue activation and inflammation. J. Intern. Med. 1997;242:49–55. doi: 10.1046/j.1365-2796.1997.00173.x. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nat Struct Mol Biol. 2007;14:295–300. doi: 10.1038/nsmb1227. [DOI] [PubMed] [Google Scholar]
- 15.Gordon WR, Zimmerman B, He L, Miles LJ, Huang J, Tiyanont K, McArthur DG, Aster JC, Perrimon N, Loparo JJ, Blacklow SC. Mechanical allostery: evidence for a force requirement in the proteolytic activation of Notch. Dev. Cell. 2015;33:729–736. doi: 10.1016/j.devcel.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 17.Günther S, Kim J, Kostin S, Lepper C, Fan CM, Braun T. Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell. 2013;13:590–601. doi: 10.1016/j.stem.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hisada Y, Yasunaga M, Hanaoka S, Saijou S, Sugino T, Tsuji A, Saga T, Tsumoto K, Manabe S, Kuroda J, Kuratsu J, Matsumura Y. Discovery of an uncovered region in fibrin clots and its clinical significance. Sci. Rep. 2013;3:2604. doi: 10.1038/srep02604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jahnsen ED, Trindade A, Zaun HC, Lehoux S, Duarte A, Jones EAV. Notch1 is pan-endothelial at the onset of flow and regulated by flow. PLoS ONE. 2015;10:1–14. doi: 10.1371/journal.pone.0122622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang WR, Cady G, Hossain MM, Huang QQ, Wang X, Jin JP. Mechanoregulation of h2-calponin gene expression and the role of notch signaling. J. Biol. Chem. 2014;289:1617–1628. doi: 10.1074/jbc.M113.498147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondoh K, Sunadome K, Nishida E. Notch signaling suppresses p38 MAPK activity via induction of MKP-1 in myogenesis. J. Biol. Chem. 2007;282:3058–3065. doi: 10.1074/jbc.M607630200. [DOI] [PubMed] [Google Scholar]
- 22.Kuhl PR, Griffith-Cima LG. Tethered epidermal growth factor as a paradigm for growth factor–induced stimulation from the solid phase. Nat. Med. 1996;2:1022–1027. doi: 10.1038/nm0996-1022. [DOI] [PubMed] [Google Scholar]
- 23.Lepper C, Conway SJ, Fan C-M. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutolf MP, Doyonnas R, Havenstrite K, Koleckar K, Blau HM. Perturbation of single hematopoietic stem cell fates in artificial niches. Integr Biol. 2009;1:59–69. doi: 10.1039/B815718A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutolf MP, Hubbell JA. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4:713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- 26.Mauro A, Adams WR. The structure of the sarcolemma of the frog skeletal muscle fiber. J. Biophys. Biochem. Cytol. 1961;10:177–185. doi: 10.1083/jcb.10.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrissey JB, Cheng RY, Davoudi S, Gilbert PM. Biomechanical origins of muscle stem cell signal transduction. J. Mol. Biol. 2015;428:1441–1454. doi: 10.1016/j.jmb.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Quarta M, Brett JO, DiMarco R, De Morree A, Boutet SC, Chacon R, Gibbons MC, Garcia VA, Su J, Shrager JB, Heilshorn S, Rando TA. An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat. Biotechnol. 2016;34:752–759. doi: 10.1038/nbt.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo D, Southard KM, Kim J, Cheon J, Gartner ZJ, Jun Y, Seo D, Southard KM, Kim J, Lee HJ, Farlow J, Lee J. A mechanogenetic toolkit for interrogating cell signaling in space and time. Cell. 2016;165:1507–1518. doi: 10.1016/j.cell.2016.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tierney MT, Sacco A. Methods in Molecular Biology. Clifton: Springer; 2016. Inducing and Evaluating Skeletal Muscle Injury by Notexin and Barium Chloride; pp. 53–60. [DOI] [PubMed] [Google Scholar]
- 31.Trensz F, Lucien F, Couture V, Söllrald T, Drouin G, Rouleau A-J, Grandbois M, Lacraz G, Grenier G. Increased microenvironment stiffness in damaged myofibers promotes myogenic progenitor cell proliferation. Skelet. Muscle. 2015;5:5. doi: 10.1186/s13395-015-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsivitse S. Notch and Wnt signaling, physiological stimuli and postnatal myogenesis. Int. J. Biol. Sci. 2010;6:268–281. doi: 10.7150/ijbs.6.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu J, Li Y, Hu Z. Notch1 and 4 signaling responds to an increasing vascular wall shear stress in a rat model of arteriovenous malformations. Biomed. Res. Int. 2014 doi: 10.1155/2014/368082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urciuolo A, Quarta M, Morbidoni V, Gattazzo F, Molon S, Grumati P, Montemurro F, Tedesco FS, Blaauw B, Cossu G, Vozzi G, Rando TA, Bonaldo P. Collagen VI regulates satellite cell self-renewal and muscle regeneration. Nat. Commun. 2013;4:1964. doi: 10.1038/ncomms2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varnum-Finney B, Wu L, Yu M, Brashem-Stein C, Staats S, Flowers D, Griffin JD, Bernstein ID. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J. Cell Sci. 2000;113(Pt 23):4313–4318. doi: 10.1242/jcs.113.23.4313. [DOI] [PubMed] [Google Scholar]
- 36.Vieira NM, Elvers I, Alexander MS, Moreira YB, Eran A, Gomes JP, Marshall JL, Karlsson EK, Verjovski-Almeida S, Lindblad-Toh K, Kunkel LM, Zatz M. Jagged 1 rescues the duchenne muscular dystrophy phenotype. Cell. 2015;163:1204–1213. doi: 10.1016/j.cell.2015.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA. Wnt signaling in myogenesis. Trends Cell Biol. 2012;22:602–609. doi: 10.1016/j.tcb.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc. Natl. Acad. Sci. USA. 2013;110:16474–16479. doi: 10.1073/pnas.1307680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Ha T. Defining single molecular forces required to activate integrin and notch signaling. Science. 2013;340:991–994. doi: 10.1126/science.1231041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu J-H, Chen C-L, Flavahan S, Harr J, Su B, Flavahan NA. Cyclic stretch stimulates vascular smooth muscle cell alignment by redox-dependent activation of Notch3. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H1770–H1780. doi: 10.1152/ajpheart.00535.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.