Abstract
Background
Stem cell therapy has been increasingly used in the treatment of sepsis-associated acute kidney injury (AKI). Engineering stem cells, through genetic method, for optimized therapeutic outcome is a desirable strategy, which requires clear understanding of molecular mechanism underlying the interaction between stem cells and damaged kidney. The aim of this study is to evaluate the therapeutic effects of HO-1 overexpressed mesenchymal stem cells (MSCs) in AKI and investigate the role of JAK/stat3 pathway in the treatment strategy.
Method
HO-1 was overexpressed in human MSCs with transfection of the expression plasmid. Quantitative RT-PCR was used to validate HO-1 overexpression. Sepsis was induced by the Cecal ligation and puncture (CLP) in mice. Survival of the treated mice were monitored and compared to that of the untreated mice. Biochemical analysis of serum biomarkers including colony forming unit (CFU), Creatinine (Cr) and blood urea nitrogen (BUN) was acquired and acute tubular necrosis (ATN) was measured. The extent of kidney injury was assessed through H&E staining of the kidney sections. Inflammatory factors were also compared between the two groups. Western blot was used to analyze the role of JAK/stat3 signaling pathway in this treatment strategy.
Results
MSCs with HO-1 overexpression markedly improved the survival of the AKI mice, accompanied by decreasing of CFU, Cr BUN in serum and ATN scores. H&E staining validated that kidney tissue demonstrated morphology that was similar to normal kidney in HO-1 MSC treated group. Inflammatory factors were also reduced by HO-1 MSC treatment. Western blot analysis indicated an upregulation of key proteins in the JAK/stat3 pathway.
Conclusions
HO-1 overexpression enhances therapeutic effect of MSCs in AKI, which is presumably attributed to the activation of JAK/stat3 signaling pathway.
Keywords: AKI, HO-1 overexpression, MSCs, JAK/stat3 pathway, CFU
Introduction
Stem cell therapy is a promising treatment of a wide-spectrum of diseases.8,13,17 Bone marrow-derived stem cells are the most studied cell population, which mainly contain two types of stem cells, which are hematopoietic stem cells (HSC) and mesenchymal stem cells (MSCs).9 The ease of harvesting, culturing and high differential potential of MSCs make them highly interesting candidates for regenerative therapy.1 The mechanism of stem cell therapy still remains elusive as yet. It is believed that engrafted stem cells exert therapeutic effects through fusion with resident organ cells, immunomodulation, pancreatic secretion and proliferation as well as differentiation into the organ cells.5 One major obstacle of stem cell therapy is the poor survival of transplanted cells. In particular treatments of diseases that characterized by hypoxia, inflammation and pro-apoptotic factors, a highly level of cell death will occur within four days after implantation.10 A strategy to enhance the engrafted stem cell survival is in high demand.
Acute kidney injury (AKI) is a highly lethal disease and is currently untreatable beyond supportive therapy.16 Incidence of AKI in clinics is usually attributable to radiocontrast agent, drug toxicity, ischemic damage, interstitial nephritis, and so on.14 Emerging studies have focused on the renoprotective potential of MSCs for enhanced regeneration in AKI.2,19 Pancreatic actions of MSCs were shown to achieve efficient renal repair. Despite these advances, the therapeutic efficacy of MSCs in AKI is still suboptimal and efforts have been made in enhancing the reparative capacity of MSCs through genetic engineering.3,18,20 Like cardiovascular diseases, AKI is also characterized by ischemic environment that impairs the survival of stem cells. Heme oxygenase-1 (HO-1) is an antioxidant and anti-apoptotic enzyme, possessing strong cytoprotective activity in ischemia. Studies have shown that MSCs ectopically overexpressed HO-1 demonstrating enhanced survival after engraftment, thereby conferring a more potent protective effect in heart disease.18 Moreover, HO-1 can medicate the effect of inflammatory molecules such as IL-103 exerting immunosuppressive effects. Engineering MSCs with HO-1 is a promising strategy to achieve a higher therapeutic outcome for AKI stem cell therapy.
JAK/STAT pathway play roles in acute kidney injury,4,23 and studies indicated that JAK/STAT pathway is associated with the MSC-mediated tissue repair.15 Herein we hypothesize that engineering MSCs with overexpressed HO-1 can improve treatment efficacy for AKI. A CLP-induced AKI mouse model was used and the efficacy of HO-1 MSCs in ameliorating kidney injury was evaluated in terms of mouse survival, biochemical parameters of AKI and inflammatory responses. Then the role of JAK/STAT pathway was further investigated in the therapeutic effect of HO-1 MSCs therapy in AKI mice. Our research is the first study that explored the link between JAK/stat3 pathway and AKI therapy with MSCs. The data that presented in this study could provide insights of a new treatment strategy for AKI patients.
Materials and Methods
Mice
8- to 12-week-old C57BL/6 male mice were obtained from the Experimental Animal Center of Bao’an Maternity and Child Health Hospital. The mice were housed at a constant room temperature with a 12-h light/dark cycle. Standard rodent chow and water were provided ad libitum. The animals were acclimated for seven days prior to initiating the experiment. The Animal Ethics Committee of Bao’an Maternity and Child Health Hospital approved all animal protocols. No human subjects research was performed.
Lentivirus Production and Infection
HEK293T cells were cultured in DMEM (GIBCO Invitrogen), 10% FCS, and 2 mM glutamine. The pLV-e-GFP or pLV-e-GFP-hHO1 vector was transfected along with t lentivirus packaging and envelope plasmids into HEK293T cells using Lipofectamine 2000 reagent (Invitrogen), the medium was replaced after 12 h. The viral supernatant was collected 48 h after transfection and concentrated by using a Centricon Plus-70 filter unit (Millipore). Human MSC cells were acquired from Lonza (USA) and infected at a low MOI to ensure 30% infection frequency such that the majority of transduced cells contained single viral integrants. The infected human MSC is selected by puromycin for 3 weeks. qPCR was used to validate the hHO-1 overexpression in MSCs. Trizol reagent was used to extract total RNA from transfected MSCs and non-transfected MSCs. cDNA synthesis was performed using PrimeScript™ One Step RT-PCR Kit (RR055A, TAKARA). The primers used for hHO-1 were 5′-AGGGAATTCTCTTGGCTGGC-3′ (forward) and 5′-GCTGCCACATTAGGGTGTCT-3′ (reverse). β-actin was used as a house-keeping gene.
Mouse Model of Cecal Ligation and Puncture
Cecal ligation and puncture (CLP) induced sepsis was generated as described previously in Ref. 6 Mice were anesthetized with 2% pentobarbital, and a 1- to 2-cm midline incision was made along the linea alba of the abdominal muscle to isolate and exteriorize the cecum. Seventy-five percent of the cecum was ligated with a 4-0 silk suture, and the cecum was punctured twice with a 21-gauge needle. A small amount (droplet) of feces was gently extruded from the penetration holes to ensure patency. The cecum was then returned to the peritoneal cavity, and the abdominal incision was closed with 4-0 silk sutures. After the operation, 1 mL pre-warmed normal saline was administered into the peritoneal cavity. In the sham group, mice underwent the same procedure but were neither ligated nor punctured.
After 3 h of CLP, mice were injected 1X106 MSCs in 0.2 mL normal saline by tail vein. As a control, the CLP mice were administered only 0.2 mL normal saline. Mice were divided into three groups: sham, CLP, CLP + MSCs and CLP+HO-1 MSC.
Real Time-PCR
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA, USA). Gene expression analysis was determined by quantitative real-time PCR using the SYBR Green Mastermix and a 7500 Real-time PCR System (Applied Biosystems). The results were analyzed using the 2-∆∆CT method with normalisation against β-actin. The following primers were used: TNF-α-F: CATCTTCTCAAAATTCGAGTGACAA, TNF-α-R: TGGGAGTAGACAAGGTACAACCC; IL-6-F: AGTTGCCTTCTTGGGACTG, IL-6-R: AGGTCTGTTGGGAGTGGTATC; IL-10-F: AGGCAGCCTTGCAGAAAAGA, IL-10-R: GCTCCACTGCCTTGCTCTTA; Ccl2-F: CCACTCACCTGCTGCTACTC, Ccl2-R: AGCTTGGTGACAAAAACTACAGC; Ccl3-F: TACAAGCAGCAGCGAGTACC; Ccl3-R: TCAGGAAAATGACACCTGGCT; Cxcl1-F: ACCGAAGTCATAGCCACACTC; Cxcl1-R: CTCCGTTACTTGGGGACACC; Cxcl2-F: GCCCAGACAGAAGTCATAGCC; Cxcl2-R: TTCTTCCGTTGAGGGACAGC; Cxcl5-F: GCCCCTTCCTCAGTCATAGC; Cxcl5-R: AGCTTTCTTTTTGTCACTGCCC.
Histochemical Analysis
Kidney tissue that freshly harvested from AKI mice were fixed in 4% PFA, being embedded in paraffin and sliced at the thickness of 0.6 µm. After de-paraffinization and antigen retrieval using standard procedures, the slides were stained with hematoxylin-eosin. The mouse PCNA antibody (2586S, Cell signaling) was used at the dilution of 1:4000. The HRP-conjugated anti-mouse antibody (8125, cell signaling) was then applied, followed by adding the DAB substrate. The staining was imaged using an inverted light scope.
Survival Study and Assessment of Kidney Injury
Survival after surgery was assessed every 24 h for 7 days with 20 samples in each group. Measuring the levels of serum creatinine and blood urea nitrogen as well as calculating the changes in levels assessed kidney injury. Blood samples were collected from the vena cava at the indicated time points (10 samples in each group), and the serum was separated by centrifugation at 3000 revolutions/min for 15 min at 4 °C and then stored at − 20 °C until use.
Western Blotting Analysis
Mouse kidney homogenates (40 μg) were separated on polyacrylamide-SDS gel and electroblotted onto nitrocellulose membrane. After blocking with 5% non-fat dry milk, the membrane was incubated with antibodies at 4 °C overnight, followed by incubation with an HRP-conjugated secondary antibody and immunoreactive bands were visualized using enhanced chemiluminescence. Primary antibodies used in this study were: Caspase-3 Antibody (9662, Cell Signaling Technology); Anti-JAK1 (phospho Y1022 + Y1023) antibody (ab138005,Abcam); Phospho-Jak2 (Tyr1007/1008) Antibody (3771, Cell Signaling Technology); Phospho-Stat3 (Tyr705) (D3A7) XP® (9145,Cell Signaling Technology); Anti-STAT3 antibody (ab5073,ABCAM); JAK1 Antibody (H-106): sc-7228, Santa Cruz Biotechnology); Anti-JAK2 antibody (ab39636,ABCAM); PARP Antibody (9542,ABCAM), p53 Antibody (DO-1)(sc-126, Santa Cruz Biotechnology); c-Myc Antibody (9E10) (sc-40, Santa Cruz Biotechnology); Bax Antibody (2772,Cell Signaling Technology); Bcl-2 Antibody (C-2)(sc-7382, Santa Cruz Biotechnology); cyclin D1 Antibody (DCS-6)(sc-20044, Santa Cruz Biotechnology); Anti-Ki67 antibody (ab15580, Abcam); Anti-beta Actin antibody (ab8227, Abcam); Anti-GFP antibody (ab13970,); Anti-STAT1 antibody [SM1] (ab3987,Abcam); p-Stat1 Antibody (Tyr 701): (sc-7988, Santa Cruz Biotechnology).
Statistical Analysis
The results are expressed as mean ± SEM. Prism 5.0 (GraphPad Software Inc, La Jolla, CA, USA) was used to perform the one-way analysis of variance for the comparisons of parameters among three or more groups, or the Student’s t test between two groups.
p values < 0.05 were considered statistically significant.
Results
Construction of MSCs with HO-1 Overexpression
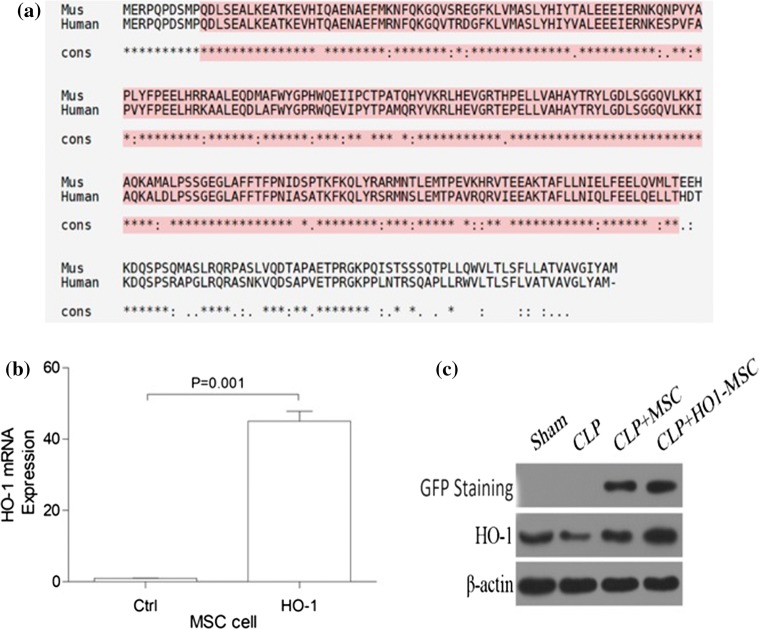
Based on the highly homology of human HO-1 and mice HO-1, and the future clinical application, we chose human HO-1 in our study. We first ectopically overexpressed HO-1 in MSCs. qPCR was used to verify the increased HO-1 levels in engineered MSCs. These engineered MSCs are abbreviated as HO-1 MSCs in the following content. As shown in Fig. 1, HO-1 MSCs demonstrated an over 40-fold overexpression of HO-1 mRNA. These MSCs were then evaluated for therapeutic efficacy in AKI mouse models (Fig. 1a). At the same time, we examined the expression of GFP and HO-1 in mice. After 48 h of MSC injection, GFP is only expressed in CLP+MSC and CLP+HO-1-MSC. Compared with the other three groups, the expression of HO-1 in CLP+HO-1-MSC group increased significantly (Figs. 1b and 1c).
Figure 1.
HO-1 overexpression in MSCs and kidney. (a) mice HO-1 Alignment and human HO-1 Alignment. (b) qPCR was used to analyze mRNA levels of HO-1 so as to verify successful transfection of HO-1 in MSCs. The expression of β-actin was used as an internal control. p value: Student’s t test. Error bar: SEM. (c) After 48 h of MSC injection, protein expression of GFP and HO-1 in mouse kidneys.
HO-1 MSCs Improve the Survival of AKI Mice
AKI mouse models were constructed through the CLP method, which causes sepsis-associated AKI within one-day after surgery. HO-1 MSCs were used to treat AKI mice. The therapeutic efficacy of HO-1 MSCs was compared with that of unmodified MSCs. Normal mice (sham) and untreated mice were used as controls. As shown in Fig. 2, while severe lethality was induced by CLP-induced AKI, both unmodified MSCs and HO-1 MSCs significantly improved the survival of AKI mice. Importantly, significantly higher survival rate can be observed in mice that treated with HO-1 MSCs, and about 80% mice remained alive in day 8 after CLP induction. This result indicates that HO-1 MSCs are able to exert potent therapeutic effects in AKI mice. This prompted us to further evaluate kidney functions assessing the observation.
Figure 2.
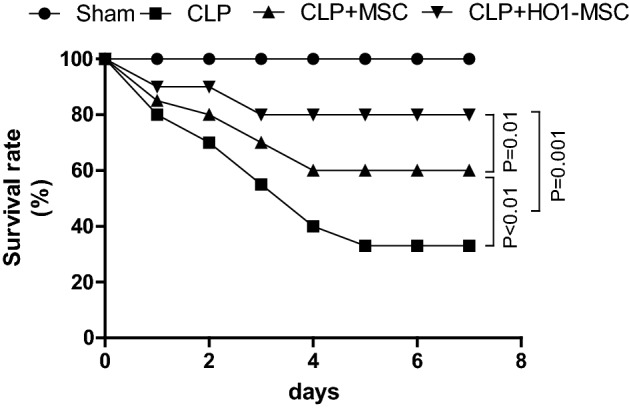
Survival analysis in AKI mice treated with MSC or MSCs with HO-1 overexpression. Normal mice (sham) and non-treated mice (CLP) were used as controls. Data were analyzed with Kaplan–Meier tests.
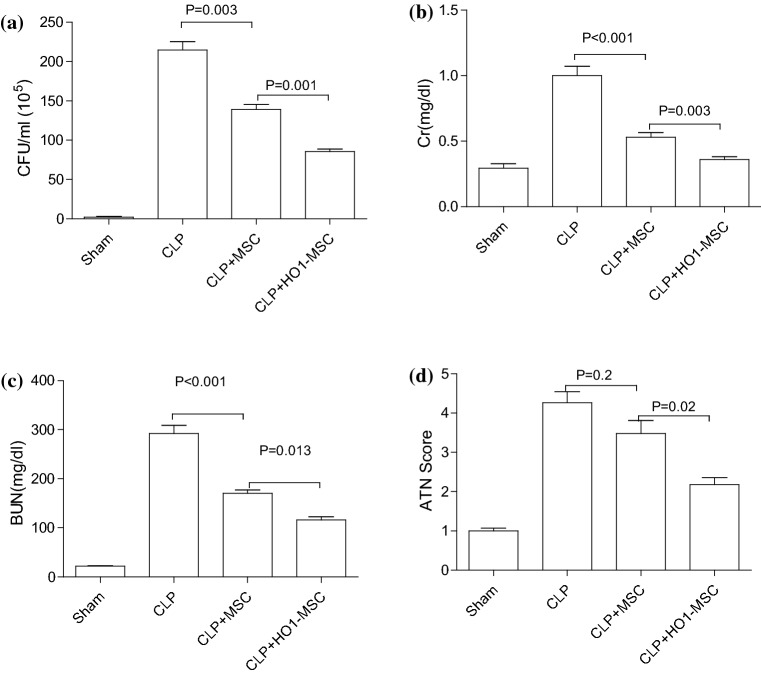
HO-1 MSCs Improve Serum Biochemical Parameters Associated with Kidney Function
CFU, Cr and BUN are important serum biochemical parameters that used in clinics to evaluate kidney functions. In the present study, we analyzed these parameters via ELISA after harvesting blood from mice in different groups after 48h of MSC treatment. As shown in Fig. 3, CLP induction dramatically increased CFU (Fig. 3a), Cr (Fig. 3b) and BUN (Fig. 3c) in mice, suggesting increased sepsis and impaired kidney function. Unmodified MSCs and HO-1 MSCs prominently decreased CFU, Cr and BUN levels, and HO-1 MSCs illustrated greater efficacy in lowering these kidney injury markers. In addition, ATN score was also significantly decreased through HO-1 MSC treatment, suggesting amelioration of the kidney functions. These results further verified the renoprotective effects of HO-1 MSCs.
Figure 3.
HO-1 overexpressed MSCs dramatically lower serum markers of kidney injury 48 h after CLP operation. CFU (a), Cr (b), BUN (c) levels in serum were assessed by ELISA analysis in normal mice (sham), non-treated CLP mice, MSC treated CLP mice and HO-1 overexpressed MSCs treated CLP mice. (d) ATN scores were measured in each group. p value: ANOVA. Error bar: SEM.
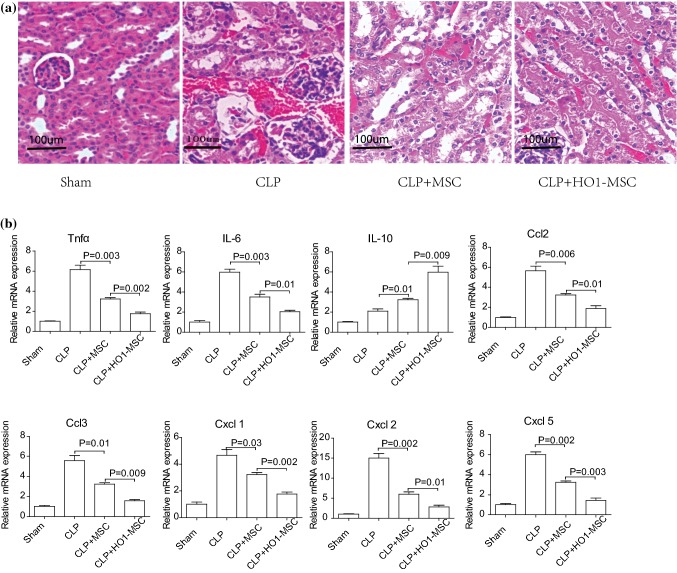
HO-1 MSCs Normalizes Kidney Tissue Morphology and Alleviated Inflammatory Responses
H&E staining was used to assess kidney injury. As shown in Fig. 4a, CLP induction led to kidney damage that evidenced by increased cellularity and loss of stroma issues, comparing to normal kidney tissues (Sham group). Consistent with the ameliorating effect of MSC treatment, dramatic decrease in cellularity was observed 72h after MSC treatment. The morphology of HO-1 MSC treated kidney is almost similar to that of normal kidney. We also evaluated the level of inflammatory factors in different groups 48 h after MSC treatment. It is clear that HO-1 MSCs most prominently downregulated proinflammatory factors, including TNF-α, IL-6, Cd2, CcB, Cxcl1, Cxcl2 and Cxcl5, while increasing anti-inflammatory factor IL-10 (Fig. 4b). Together, these evidences corroborated the superior efficacy exerted by HO-1 MSCs.
Figure 4.
Measurement of serum inflammatory factors and evaluation of kidney injury through HE staining after MSC treatment. (a) H&E staining of kidney tissue harvested from normal mice (sham), non-treated CLP mice, MSC treated CLP mice and HO-1 MSC treated CLP mice 72 h after MSC treatment. (B) Levels of inflammatory factors, including TNFa, IL-6, IL-10, Ccl2, Ccl2 Cxcl1, Cxcl2 and Cxcl5 in different groups 48h after MSC treatment. P value: Student’s t-test and ANOVA. Error bar: SEM.
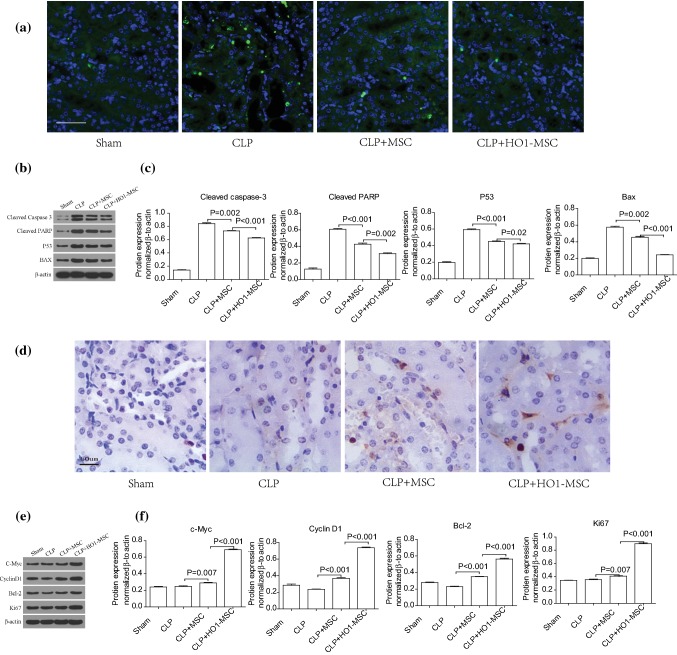
HO-1 MSCs Inhibits Cell Apoptosis and Promotes Cell Proliferation
To elucidate the mechanism of the therapeutic effects for HO-1 MSCs, we next examined whether HO-1 MSC reduces cell apoptosis and increases cell proliferation in kidney 75 h after CLP operation. As shown in Figure 5a, apoptosis was reduced by MSC therapies, and particularly by HO1 MSCs. Western blot indicated that the apoptosis biomarkers, cleaved capase3, cleaved PARP, P53 and BAX were significantly reduced by MSC and HO-1 MSC treatment (Figs. 5b and 5c). HO-1 MSC treatment exerted the highest therapeutic efficacy. On the other hand, HO-1 MSC greatly promoted cell proliferation (Figs. 5d, 5e and 5f). In sum, these data point toward the effect of HO-1 MSC in protecting the kidney cells from damage from CLP induction.
Figure 5.
HO-1 MSCs inhibits kidney apoptosis and promote cell proliferation 72 h after MSC treatment. (a) Apoptosis analysis is conducted by tunel assay. Scale bar 50 μm. (b) Western blot of cell apoptosis-related proteins. (c) Cleaved capase3, cleaved PARP, P53 and BAX were analyzed by western blot. (d) Cell proliferation assay is analyzed by PCNA staining. Scale bar: 50 μM and (e) and (f) western blot of expression of proteins involved in cell proliferation. Brown color in (d) denotes PCNA. p value: Student’s t test and ANOVA. Error bar: SEM.
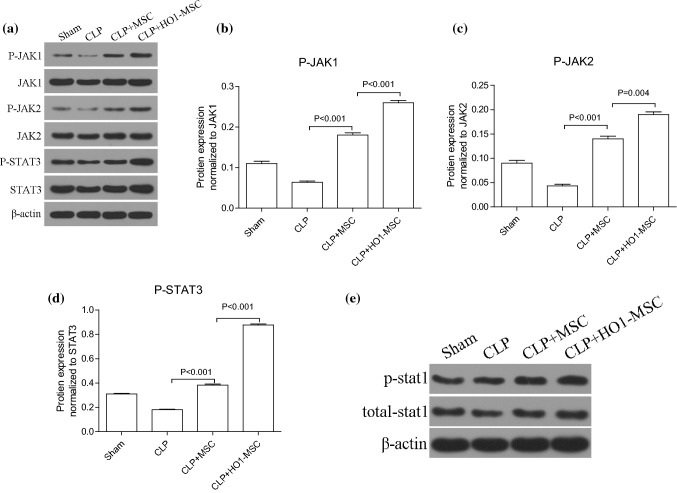
HO-1 MSCs Activates JAK/stat3 Signaling
JAK/stat3 is a putative pathway in antagonizing cell apoptosis and promoting cell proliferation. Here we evaluated whether JAK/stat3 plays a role in the regulation by HO-1 MSCs 48h after MSC treatment. Western blot analysis indicates that HO-1 MSCs evidently upregulated phosphorylated JAK1, JAK2 and STAT3 levels (Fig. 6). Therefore, the activation of JAK/stat3 signaling by HO-1 MSCs is the underlying mechanism of the therapeutic effect of HO-1 MSCs therapy in AKI mice.
Figure 6.
HO-1 MSCs activates JAK/stat3 signaling pathway 48 h after MSC treatment. (a) Western blot image of key proteins in the JAK/stat3 signaling pathway, including JAK1, P-JAK2, JAK2, P-STAT3, STAT3. The expression of β-actin was used as a loading control. (b)–(d) Quantitative comparison of the expression levels of P-JAK1, P-JAK2, and P-STAT3 between different groups. (e) Western blot image of STAT1 and P-STAT1. p value: Student’s t test and ANOVA. Error bar: SEM.
Discussions
AKI is a commonly encountered condition in hospital and outpatient settings, which constitutes an important cause of morbidity and mortality. While stem cell therapy with MSCs is among the most promising approaches in the treatment of AKI, the efficacy of this method is limited by the poor survival of MSCs because engrafted cells are exposed to hypoxia, oxidative stress and repeated bouts of inflammation. To address this challenge, we constructed MSCs with HO-1 overexpression to enhance the survival of MSCs in injured kidney. As expected, the anti-apoptotic, pro-proliferative effects of HO-1 empowered MSCs to induce a marked improvement in the survival of AKI mice, accompanied by dramatic decrease of serum biochemical markers of kidney injury and ameliorated in kidney tissue integrity. These effects also coincided with the downregulation of pro-inflammatory factors and upregulation of anti-inflammatory factors, stemming from the role of MSCs as inflammation mediators. It can be speculated that the enhanced renoprotection of MSCs is a result of improved survival of engrafted therapy. Moreover, considering that the paracrine pathway is an indispensable contributor of MSCs’ therapeutic effect, we also evaluated if HO-1 overexpression in MSCs led to attenuated apoptosis and restored proliferation in kidney cells. Indeed, our histological and western blot analysis result, as shown in Fig. 5, demonstrated that there was an increased survival of kidney tissue, which indicate the protective effect of HO-1 as an anti-apoptotic and pro-survival enzyme. Our study was one of the first studies that utilize HO-1 overexpression to boost the efficacy of MSCs in AKI.
We demonstrated that JAK/stat3 signaling pathway is the underlying molecular mechanism of increased therapeutic effect of HO-1 MSCs. JAK/stat3 pathway is one of the major pathways that associated with the transduction of inflammatory signals by responding to many cytokines, chemokines, growth factors, etc. JAK/stat3 pathway is activated when the associated plasma membrane receptors bind their ligands, followed by recruitment of pairs of JAK proteins to the intracellular receptor domains and phosphorylation. The phosphorylated JAKs then activates STAT proteins, which translocate to nucleus and regulate corresponding target genes.22 Because of their close interaction with inflammatory factors, they serve as important regulators of immunological diseases, cancer, etc. In renal carcinoma, the role of JAK/stat3 pathway has been implicated in several studies,11,12,21 in which JAK/stat3 is responsible to promote the proliferation and differentiation of cancer stem cells. The correlation of regenerative therapy with MSCs and JAK/stat3 pathway has also been reported in cardiovascular diseases.15 Our research is the first study that explored the link between JAK/stat3 pathway and AKI therapy with MSCs. It is a possibility that JAK/stat3 takes an important part in the therapeutic efficacy of MSCs in other diseases as well, and development in strategies optimizing the efficacy of MSCs can hence be enabled.
Strategies of engineering MSCs are not limited to HO-1 overexpression.7 A large array of genes has been overexpressed in MSCs to improve their target homing, angiogenesis, anti-apoptosis, anti-inflammatory abilities, and so on.7 Our study revealed that HO-1 overexpression is a valuable multi-potent approach to enhance MSCs in AKI therapy. With significant advances in the tool set for genetic editing of cell, e.g., Crispr-cas9, it can be envisioned that further genetic modifications of MSCs will follow to address the challenges in clinical management of AKI.
In conclusion, here we report that HO-1 overexpressed MSCs had superior therapeutic efficacy in sepsis-associated AKI comparing with unmodified MSCs. Survival of the AKI mice were significantly improved and serum biochemical markers of kidney injury were reduced. The ameliorating effects of HO-1 MSCs were also manifested in the increased kidney tissue integrity and were decreased in inflammatory factors. Considering that there is a lack of effective treatment strategies for AKI, our study is valuable in providing a new tool for reducing morbidity and mortality of clinical AKI. Further clinical application of this approach is in need to verify the merit of HO-1 overexpressed MSCs in patients.
Acknowledgments
The authors would like to express gratitude to anonymous reviewers who provide valuable suggestions that help to improve this paper.
Funding
This study was funded by Natural Science Foundation of Shenzhen (No. JCYJ20170307161153900).
Conflict of interest
The authors (XY, XC, XH, WZ, XY, HC) declare that they have no any conflicts of interests.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This study did not involve any human subjects research.
References
- 1.Bianco P. “Mesenchymal” stem cells. Annu. Rev. Cell Dev. Biol. 2014;30:677–704. doi: 10.1146/annurev-cellbio-100913-013132. [DOI] [PubMed] [Google Scholar]
- 2.Bussolati B, et al. Contribution of stem cells to kidney repair. Curr. Stem Cell Res. Ther. 2009;4(1):2–8. doi: 10.2174/157488809787169129. [DOI] [PubMed] [Google Scholar]
- 3.Chabannes D, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110(10):3691–3694. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 4.Erkasap S, et al. The effect of leptin and resveratrol on JAK/STAT pathways and Sirt-1 gene expression in the renal tissue of ischemia/reperfusion induced rats. Bratisl. Lek. Listy. 2017;118(8):443–448. doi: 10.4149/BLL_2017_086. [DOI] [PubMed] [Google Scholar]
- 5.Gnecchi M, et al. Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol. Biol. 2016;1416:123–146. doi: 10.1007/978-1-4939-3584-0_7. [DOI] [PubMed] [Google Scholar]
- 6.Hiramatsu M, et al. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7(4):247–253. doi: 10.1097/00024382-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Hodgkinson CP, et al. Genetic engineering of mesenchymal stem cells and its application in human disease therapy. Hum. Gene Ther. 2010;21(11):1513–1526. doi: 10.1089/hum.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jezierska-Wozniak K, et al. Stem cells as therapy for cardiac disease—a review. Folia Histochem. Cytobiol. 2011;49(1):13–25. doi: 10.5603/FHC.2011.0004. [DOI] [PubMed] [Google Scholar]
- 9.Krause DS, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105(3):369–377. doi: 10.1016/S0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 10.Lee S, et al. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy. Oxidative Med. Cell. Longev. 2015 doi: 10.1155/2015/632902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L, et al. Concomitant activation of the JAK/STAT3 and ERK1/2 signaling is involved in leptin-mediated proliferation of renal cell carcinoma Caki-2 cells. Cancer Biol. Ther. 2008;7(11):1787–1792. doi: 10.4161/cbt.7.11.6837. [DOI] [PubMed] [Google Scholar]
- 12.Li S, et al. Icaritin inhibits JAK/STAT3 signaling and growth of renal cell carcinoma. PLoS ONE. 2013;8(12):e81657. doi: 10.1371/journal.pone.0081657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat. Med. 2004;10(Suppl):S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 14.Perazella MA. Renal vulnerability to drug toxicity. Clin. J. Am. Soc. Nephrol. 2009;4(7):1275–1283. doi: 10.2215/CJN.02050309. [DOI] [PubMed] [Google Scholar]
- 15.Shabbir A, et al. Activation of host tissue trophic factors through JAK-STAT3 signaling: a mechanism of mesenchymal stem cell-mediated cardiac repair. Am. J. Physiol. Heart Circ. Physiol. 2010;299(5):H1428–H1438. doi: 10.1152/ajpheart.00488.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shum HP, et al. Septic acute kidney injury in critically ill patients—a single-center study on its incidence, clinical characteristics, and outcome predictors. Ren. Fail. 2016;38(5):706–716. doi: 10.3109/0886022X.2016.1157749. [DOI] [PubMed] [Google Scholar]
- 17.Slavin S, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91(3):756–763. [PubMed] [Google Scholar]
- 18.Tang YL, et al. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J. Am. Coll. Cardiol. 2005;46(7):1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 19.Togel F, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am. J. Physiol. Renal Physiol. 2007;292(5):F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchiya H, et al. Chondrogenesis enhanced by overexpression of sox9 gene in mouse bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2003;301(2):338–343. doi: 10.1016/S0006-291X(02)03026-7. [DOI] [PubMed] [Google Scholar]
- 21.Um HJ, et al. Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of human renal carcinoma Caki cells. Biochem. Biophys. Res. Commun. 2012;427(1):24–29. doi: 10.1016/j.bbrc.2012.08.133. [DOI] [PubMed] [Google Scholar]
- 22.Yadav, A., et al. IL-6 promotes head and neck tumor metastasis by inducing epithelial–mesenchymal transition via the JAK-STAT3-SNAIL signaling pathway. Mol. Cancer Res., 2011 [DOI] [PMC free article] [PubMed]
- 23.Yang C, et al. A novel cyclic helix B peptide inhibits dendritic cell maturation during amelioration of acute kidney graft rejection through Jak-2/STAT3/SOCS1. Cell Death Dis. 2015;6:e1993. doi: 10.1038/cddis.2015.338. [DOI] [PMC free article] [PubMed] [Google Scholar]