Abstract
Introduction
Heart valve tissue engineering may provide improved treatment for valvular heart disease, yet development of a tissue engineered heart valve (TEHV) has been limited by incomplete recellularization of the valve leaflets. In this study, we compare the leaflet recellularization potential of candidate cell populations.
Methods
Four cell populations were tested: bone marrow mononuclear cells (MNC), 5 million bone marrow mesenchymal stem cells (MSC), 10 million bone marrow mesenchymal stem cells (MSC2), and 5 million valve interstitial cells (VIC). Candidate cell populations were seeded onto decellularized heart valves and underwent similar conditioning in a low-flow bioreactor for 2 weeks.
Results
MSC2 valves demonstrated the best recellularization of the interstitial leaflet tissue as well as an appropriate cell phenotype, mechanical properties, and biochemical composition. MSC valves exhibited similar leaflet repopulation, yet had decreased mechanical and biochemical properties. MNC seeding resulted in minimal recellularization of the leaflet, though an additional time point group found cells present after 3 days, which seemed to disappear at 2 weeks. VIC seeding resulted in cell clumping on the leaflet surface and poor recellularization.
Conclusions
The results of this study suggest mesenchymal stem cells are a preferred cell population for TEHV recellularization. Additionally, MSCs demonstrate the ability for repopulation of the distal valve leaflet, which will lead to more complete recellularization of future TEHVs.
Keywords: Mesenchymal stem cells, Mononuclear cells, Valve interstitial cells
Introduction
Valvular heart disease remains a significant cause of morbidity and mortality worldwide, particularly for pediatric patients with congenital valve defects.12 The current options for valve replacement, including mechanical valves, bioprosthetic valves, and cryopreserved allografts, all have associated limitations and cannot provide the necessary remodeling or growth after implantation.12 Therefore, pediatric patients requiring valve replacement often undergo multiple revision surgeries throughout their lifetime.12 The tissue engineered heart valve (TEHV) may be the ideal solution for heart valve replacement acting as a one-time intervention to create living, functional tissue capable of growth and remodeling.12
However, a number challenges remain before realizing the TEHV. Primarily is the difficulty in establishing a phenotypically appropriate cell population within the valve leaflet tissue. Native heart valves are populated by two cell phenotypes, valve endothelial cells on the luminal surfaces and valve interstitial cells (VICs) within the tissue matrix. Decellularized heart valves implanted as bioprosthetic replacements in human and animal models have shown complete re-endothelialization of the luminal surfaces and partial recellularization of the valve interstitial tissue including the conduit and leaflet base.7,19 However, despite endothelial cells, the distal free-edge of the valve leaflets exhibit an absence of VIC repopulation.7,19 Therefore, the challenge is establishing a VIC-like population within the distal leaflet interior.
Over the years, various methodologies for cell seeding using multiple cell sources have been explored.13,25 The use of endothelial cells from arterial or venous tissue, myofibroblasts, or VICs from leaflet tissue avoids cell phenotype mismatch with the native valve; however, obtaining homologous cells for clinical use is problematic.10,15,22 Bone marrow derived mesenchymal stem cells (MSCs) and mononuclear cells (MNCs) have received interest as a potentially patient-specific cell source. MSCs are an attractive option because of their differentiation potential toward a VIC lineage and their immunomodulatory capabilities.6,8,21 However, the preparation MSC populations prior to seeding can be a time consuming procedure.17 MNCs are of interest due to their potential for rapid migration and inflammation-mediated native cell recruitment through paracrine signaling.20,27
Despite the variety of cell sources for heart valve seeding, there have been very few direct comparisons between cell populations to evaluate the potential for valve recellularization. One of the only comparisons (Vincentelli et al.) utilized a direct injection technique to transplant ovine MNCs or MSCs into the annulus and arterial wall of decellularized porcine pulmonary heart valves before implantation into sheep.26 After 4 months in vivo, the MNC injected group displayed thickened leaflets, increased pressure gradients, calcification, and the presence of inflammatory cells.26 The presence of the inflammatory cells may be in line with the inflammation-mediated recellularization described previously by MNCs, although the other reported results are not encouraging and suggest a classical inflammatory response. The injection of expanded MSCs did not elicit this negative response and a limited number of α-actin positive cells were observed in the leaflet.26 However, cells cannot be clinically localized directly within the leaflet using this method due to the potential of damage to the delicate tissue.
Therefore, the purpose of this investigation is to provide a direct comparison between seeding cell populations for their TEHV recellularization potential. The recellularization criteria for determining the best cell type are repopulation of the interstitial leaflet tissue, differentiation down an appropriate VIC lineage, remodeling and production of extracellular matrix, and restoration of native leaflet biomechanics. Additionally, recruitment of host cells may also influence recellularization and therefore the production of homing cytokines should also be considered. MNCs, MSCs, and VICs, were tested for their ability to repopulate the interstitial leaflet tissue of decellularized heart valves following in vitro bioreactor conditioning. To better evaluate the MSC seeded groups, two seeding concentrations were tested, leading to a total of four tested cell populations. Although not clinically practical, VICs were included as a candidate cell population to evaluate the results of seeding an autologous cell source following the same in vitro seeding and conditioning protocol of the other candidate cell populations. It was hypothesized that valves seeded with MSCs at the higher concentration would lead to significantly higher recellularization of the leaflet tissue compared to other groups.
Methods
Tissue and Tissue Processing
Ovine aortic valves were harvested from juvenile sheep under approved IACUC protocols and in accordance with Guide for Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23). Harvested valves were cryopreserved using a procedure modeled after clinical tissue handling protocols. Valve were frozen at 1 °C min−1 using a controlled rate freezer (2100 Series, Custom BioGenics Systems) then stored at − 180 °C. Valve decellularization was performed as described previously.18 Briefly, the valves were thawed and subjected to reciprocating osmotic shock, followed by detergent (Triton X-100, sodium-lauroyl sarcosine) and enzymatic (Benzonase®) washes to remove cellular material. Extraction of organic material was performed using recirculating water and ion exchange resins. After decellularization, the valves were again cryopreserved as described and stored at − 180 °C until seeding.
Seeding Cell Populations
Cells populations to be used for seeding were obtained from commercial sources (Lonza) or isolated from human tissue that was obtained from a commercial tissue banking facility (LifeNet). The decellularized ovine aortic valves were randomly divided into four groups for seeding: human MNCs (MNC group), a low dose of human MSCs (MSC group), a high dose of human MSCs (MSC2 group), or human VICs (VIC group). Cell populations for MNC and MSC seeded valves were isolated from 25 mL human bone marrow aliquots obtained from a commercial source (Lonza) using a bone marrow filter system (Kaneka).14 Cell concentrations were measured using a Z-series Coulter Counter prior to valve seeding (Beckman Coulter). For MNC seeding, the entire mononuclear cell population isolated from bone marrow was used for valve seeding, which resulted in approximately 300 million cells seeded per valve. For seeding with MSC populations, mononuclear cells were filtered from bone marrow and the mesenchymal stem cell fraction was isolated through cell culture until sufficient cells were available. Valves in the low dose MSC group were seeded with 5 million cells per valve while valves in the high dose MSC2 group were seeded with 10 million cells per valve. VICs for the VIC group were isolated from human aortic valve leaflets and seeded at a 5 million cells per valve. Briefly, aortic valve leaflets were dissected, stripped of VECs through a saline wash, and then minced before being placed in a cell culture flask to allow the outgrowth of VIC cells. After sufficient proliferation, VICs were cryopreserved and stored at − 180 °C until valve seeding.
Valve Seeding and Conditioning
For each cell seeding group, the tissue engineered heart valves were seeded following established protocols.5 Decellularized valves were thawed from cryopreservation and sutured onto tissue grips. The seeding cell population for each group was then suspended in 10 mL of media and seeded into the lumen of the valve which was mounted in a static bioreactor chamber containing 200 mL valve media [DMEM F12 (Life Technologies) and 10% human serum (Sigma-Aldrich)]. After cell seeding, all groups underwent the same bioreactor conditioning protocol starting with 24 h of static culture (37 °C, 5% CO2) to allow cell adhesion. Valves were then transferred to a dynamic bioreactor chamber containing 500 mL valve media and were conditioned for 24 h under cyclic negative pressure (− 20 to 5 mmHg), followed by 24 h of cyclic low positive pressure (− 5 to 20 mmHg), and finally 2 weeks of cyclic high positive pressure (− 5 to 120 mmHg).5 A media change was performed on all valves after 1 week of high positive pressure, wherein half of the bioreactor media (250 mL) was removed and replaced with 250 mL of fresh valve media. Following bioreactor conditioning, the valves were removed and dissected for analysis, including histology, immunohistochemistry (IHC), real time PCR (rt-PCR), biomechanical testing, and biochemical analysis.
After analysis of the MNC group revealed minimal cell infiltration, an additional sub-group was added which was seeded similarly and then conditioned in the bioreactor for only 3 days (MNC short) (24 h static, 24 h negative pressure cycles, and 24 h high pressure cycles). The purpose was to elucidate temporal changes in the cell population, since previous experience has seen moderate leaflet recellularization with seeded MNCs.4 After conditioning, the MNC short term valves were analyzed by histology, IHC, and PCR. MNC short valves were not included in the mechanical or biochemical analyses since significant changes were not expected so quickly.
Histology, IHC, and PCR
Samples for histology were sectioned along the radial plane of each leaflet from the valves of each group. Hematoxylin and eosin (H&E) staining was used to evaluate repopulation of the decellularized valve leaflets, specifically by the presence of cells within the leaflet tissue and on the leaflet surface. Recellularization of the tissue engineered valves in each group (n = 9) was determined by counting the cells in a calculated area using Axiovision software. Cells were counted if they were located within the interstitial leaflet tissue, but not on the leaflet surface.
Protein expression of the seeded cells was evaluated by IHC. Unstained slides were blocked in 10% normal goat serum for 1 h before overnight incubation at 4 °C with 1:100 diluted primary antibodies. The primary antibodies (Abcam) targeted alpha smooth muscle actin (aSMA), heat shock protein 47 (HSP47), vimentin (VIM), CD90, CD68, CD271, CD34, and CD45. Fluorescent secondary antibodies (Alexa Fluor 488 or Alexa Fluor 594; Life Technologies) were then incubated for 1 h followed by nuclear counterstaining (DAPI; Life Technologies).
Gene expression in the cell populations prior to seeding and after 2 weeks of conditioning was analyzed by rt-PCR. Pre-seeding cell samples were taken immediately before seeding. Post-seeding tissue samples were isolated from each leaflet during valve dissection and immediately frozen in liquid nitrogen before being pulverized. Tissue samples from each leaflet of the valve were combined to form one sample to measure overall gene expression across the valve. RNA was isolated using the RNeasy Micro Kit (Qiagen) and then reverse transcribed to cDNA using the High Capacity cDNA Reverse Transcription Kit (Invitrogen). rt-PCR (7300 RT-PCR system, Applied Biosystems) was performed using a custom phenotyping array (TaqMan, Life Technologies). PCR data is conveyed as the relative fold change in gene expression of the cells from tissue samples compared against the matched pre-seeding cell population. Data is presented as the mean relative fold change of all valves within a group (n = 3) with standard deviation error bars.
Mechanical Testing
The mechanical behavior of the leaflet tissue from the MNC, MSC, MSC2, and VIC groups was measured by biaxial loading. MNC short seeded valves were not tested. Biaxial mechanical testing was performed in phosphate buffered saline at 37 °C using methods described previously.3 Rectangular specimens (9 × 6 mm, n = 9) were cut from the middle belly region of each leaflet and mounted onto a four motor equibiaxial loading system (LM1 TestBench, Bose ElectroForce) with the radial and circumferential directions of the sample aligned to the loading axes. Samples were then loaded to an equibiaxial tension of 30 N/m with a rise time of 10 s and the peak stretch ratios were calculated from strain data in the circumferential (λpeakC) and radial (λpeakR) directions. Areal strain was used as a measure of net extensibility and was calculated from stretch data as (λpeakC × λpeakR − 1) × 100%. Results were compared against biaxial mechanical data previously reported from cryopreserved and decellularized valves.24
Biochemical Analysis
The sulfated glycosaminoglycan (GAG), collagen, and total protein concentrations of the extracellular matrix of seeded valves was measured using colorimetric assays. GAG concentration was measured using the Blyscan Sulfated Glycosaminoglycan Assay (Biocolor). Tissue samples (approx. 15 mg; n = 9) were processed according to manufacturer protocols and the results were measured using a spectrophotometer at 656 nm. Collagen concentration was measured using the QuickZyme Total Collagen Assay (QuickZyme Biosciences). Samples (approx. 15 mg, n = 9) were prepared by overnight hydrolysis in 6 M HCl at 90 °C and the hydroxyproline concentration was measured using a UV–Vis spectrophotometer at 570 nm. Total protein concentration was measured using the QuickZyme Total Protein Assay (QuickZyme, Biosciences) and used the same hydrolysates that were prepared for collagen quantification. The GAG, collagen, and total protein concentrations are reported as µg mg−1 of wet tissue and are compared to previously reported values from cryopreserved and decellularized valves.24
The production of signaling molecules such as cytokines and chemokines from the cells of the seeded tissue engineered valves was quantified using a multiplex (Luminex) assay in accordance with manufacturer protocols. Media samples (n = 3–6) were taken at the end of bioreactor conditioning (2 weeks of high positive pressure) and frozen at − 80 °C until use. The multiplex assay identified the media concentration of FGF-2, IL-10, IL-6, MCP-1, MIP-1α, MIP-1β, TNFα and VEGF.
Statistical Analysis
Statistical analysis was performed by ANOVA, and post hoc comparisons were made using the Tukey test or the Kruskal–Wallis test for parametric and non-parametric data, respectively. Differences were considered statistically significant at p < 0.05.
Results
Tissue Processing
As demonstrated previously, the decellularization process resulted in complete removal of cells from the valve tissue while preserving the overall architecture of the extracellular matrix (Fig. 1j).3,24 Following cell seeding and bioreactor conditioning, the valves in all groups had a normal appearance with leaflets capable of complete coaptation.
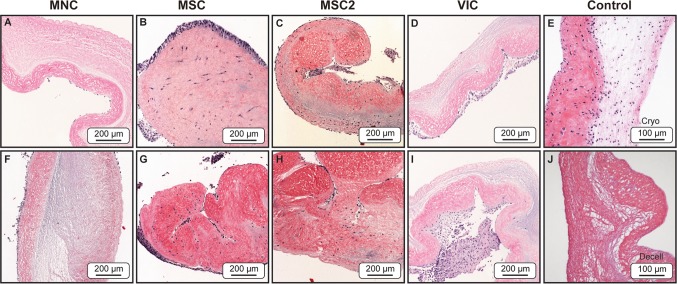
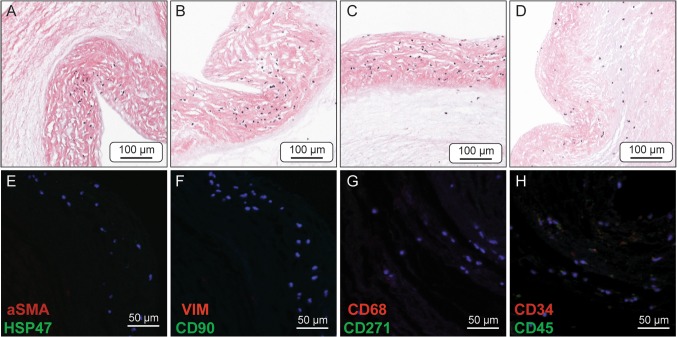
Figure 1.
Representative H&E stained sections showing various degrees of recellularization from the four groups of the tissue engineered valve leaflets, 2 weeks post seeding. MNC valves (a, f) showed no signs of interstitial recellularization and minimal cells on the leaflet surface. MSC valves (b, g) and MSC2 (c, h) valves displayed increased interstitial recellularization of the distal leaflet compared to the other tested groups. Slight cell clumping was also observed on the leaflet surface. VIC valves (d, i) showed no cell infiltration and a high degree of cell clumping along the leaflet surface. Positive and negative controls (e, j, respectively) are presented as cryopreserved (cryo) and decellularized (decell) samples. Images within the same processing group (e.g., a, f) are taken from separate valves under the same processing conditions to show reproducibility. All images are taken from the distal third region of the leaflet.
Histology and Cell Density
H&E staining of the seeded valve groups revealed differing levels of recellularization after 2 weeks of bioreactor culture (Fig. 1). MNC seeded valves had minimal recellularization with isolated cells present only on the surface of the leaflet while the interstitial matrix remained acellular (Figs. 1a and 1f). MSC seeded valves exhibited increased recellularization of the leaflet matrix compared to the other seeded groups, with a relatively high density of cells within the leaflet matrix; though it should be noted the cellular density is still low compared to native tissue (Figs. 1b and 1g). In addition to increased cells within the leaflet, multilayered clumps of cells were present on the leaflet surface. It is also noteworthy that recellularization was limited to the distal region of the leaflet, while the mid and base of the leaflet had very little cells present within the leaflet or on the surface. MSC2 seeded valves showed similar recellularization to the MSC group and interestingly appeared to have less cell clumping present on the valve surface (Figs. 1c and 1h). Again, the recellularization of the MSC2 group was primarily within the distal leaflet region. Valves seeded with VICs showed high cellularity and clumping of cells on the valve surface but very little to no cellular infiltration into the leaflet interstitium (Figs. 1d and 1i). However, it is worth noting that cellular repopulation of the leaflet interstitium following valve seeding and conditioning, regardless of cell type, remained dramatically below the cellular density observed in a cryopreserved leaflet (Fig. 1e).
The amount of recellularization within each group was quantified by measuring the cell density within the leaflet tissue (Table 1). As observed by histology, the MSC and MSC2 groups lead to significantly increased recellularization compared to the MNC and VIC groups. Additionally, all groups had dramatically lower cellular density than cryopreserved native human leaflet tissue. MNC and VIC groups had significantly lower cellular density, while the MSC and MSC2 groups were actually statistically similar to the human cryopreserved cellular density. However, that last point is simply an artifact of the statistical test due to the non-parametric nature of the data. It is apparent that the cellular density of all seeded groups was significantly lower than the cryopreserved control sample.
Table 1.
Cell density measuring the recellularization of MNC, MSC, MSC2, and VIC seeded valves.
| Group | Average cell density (cells/mm2) |
|---|---|
| MNC | 1.623 ± 1.94a,b,c |
| MSC | 112.31 ± 32.04b,d |
| MSC2 | 117.03 ± 80.23c,e |
| VIC | 2.25 ± 0.82d,e,f |
| Human cryo | 1149.55 ± 401.81a,f |
Previously reported data for cryopreserved (cryo) human aortic valve leaflets is included as a positive control.24 Data is reported as mean ± standard deviation. Superscript letters of the same type indicate a significant difference between groups (p < 0.05)
Cell Phenotypes
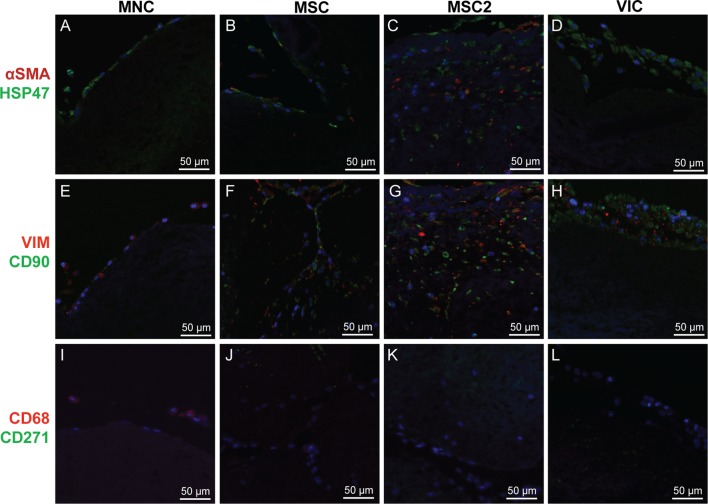
The protein expression of the seeded cells on the tissue engineered valve leaflets was evaluated by IHC (Fig. 2). The phenotype of the seeded cells was compared to native valve leaflet cells, which has been thoroughly described in the literature.2,9,16,23 Therefore, antibodies stained for the expression of markers associated with native valve cells (αSMA and VIM) and mesenchymal stem cells (CD90 and CD271), as well for matrix production (HSP47), and macrophages/mononuclear cells (CD68). Cells from the MNC group were notably negative for αSMA and CD90, but showed slight positive expression of HSP47, VIM, and CD68 (Figs. 2a, 2e, and 2i). The MSC and MSC2 groups had similar protein expression and stained positive for αSMA, HSP47, VIM, and CD90, though the MSC2 group appears to have greater protein expression (Figs. 2b, 2f, 2j, 2c, 2g, and 2k). The cells of the VIC group were positive for HSP47, CD90, and VIM while interestingly remained negative for αSMA, the classical marker pattern for activated VICs (Figs. 2d, 2h, and 2l).
Figure 2.
Immunohistochemical sections of seeded valve leaflets dual stained for the expression of: cell nuclei (blue) (all), αSMA (red) & HSP47 (green) (a–d), VIM (red) & CD90 (green) (e–h), and CD68 (red) & CD271 (green) (i–l). MNC leaflets (a, e, i) showed positive expression for HSP47, VIM, and CD68. MSC leaflets (b, f, j) and MSC2 leaflets (c, g, k) both showed positive expression for HSP47, αSMA, VIM, and CD90, although the MSC2 leaflets appear to have increased expression. VIC leaflets (d, h, l) showed positive expression for HSP47, VIM, and CD90.
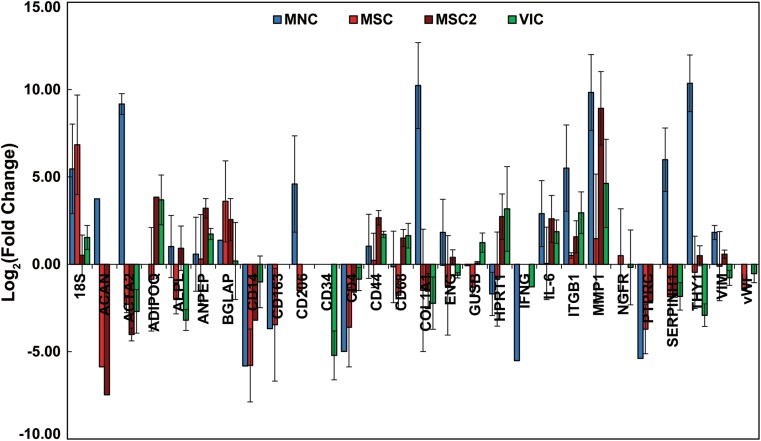
The gene expression of the cells from the seeded heart valves was measured by rt-PCR and evaluated as the relative fold change compared to the respectively matched, pre-seeded cell population (Fig. 3). Similar to the protein expression analysis, the gene expression of the seeded heart valves was compared to the phenotype of native valve cells.2,9,16,23 Gene markers were included to identify native valve cells, mesenchymal stem cells, hematopoietic stem cells, extracellular matrix production, mononuclear cells (leukocytes and macrophages), M1 and M2 immune responses, endothelial cells, and pathways of adverse stem cell differentiation. Valves from the MNC seeded group showed an up-regulation of the myofibroblast marker αSMA (ACTA2), stem cell type markers (ITGB1, SERPINH1, THY1), and markers of extracellular matrix remodeling (COL1A1, MMP1). MNC seeded valves also showed a general down-regulation of immune cell markers (CD14, CD163, CD4, IFNG, PTPRC) with the exception of CD206, a marker for M2 macrophages. The cells from the MSC group showed relatively small changes in gene expression with a general down-regulation in immune cell gene markers (CD14, CD163, CD4, PTPRC). The relative gene expression of MSC2 valves was generally similar to the MSC valves, though a greater up-regulation of MMP1 was observed. The VIC group displayed an interesting gene expression profile with slight up-regulation of MMP1 but down-regulation of ACTA2 and COL1A1.
Figure 3.
Relative fold change in the gene expression of cells from MNC, MSC, MSC2 and VIC seeded heart valves in relation to their respective pre-seeded cell populations. Changes in gene expression are displayed as the Log2 of the average relative fold change within a group. Error bars represent the standard deviation.
Mechanical Analysis
Biaxial testing of the tissue engineered leaflets revealed significant differences in the areal strain and directional stretch ratios between the cell seeding groups (Table 2). The collected data were compared to previously reported data for cryopreserved and decellularized ovine aortic valve leaflets, which act as a positive and negative control respectively.24 Interestingly, the areal strains of MNC leaflets were most similar to cryopreserved leaflets (i.e., with native cells) when compared to the other tested groups, and significantly different than decellularized (p = 0.006), MSC (p = 0.021) and VIC (p = 0.045) groups; no significant differences were found in circumferential or radial stretch ratios between MNC valves and other groups. On the other hand, MSC seeded leaflets appeared to be most mechanically similar to decellularized leaflets when compared to other groups and were found to have significantly greater areal strain (p = 0.024) and circumferential stretch (p = 0.004) than cryopreserved leaflets. Leaflets from the VIC seeded group revealed no significant differences between the cryopreserved and decellularized groups, though the average properties of the VIC leaflets was much closer to those of the decellularized leaflets. Similarly, no significant differences in mechanical properties were found between the MSC2 group and the cryopreserved or decellularized groups, however the areal strain and circumferential stretch ratio of the MSC2 group was medial to the control groups.
Table 2.
Biaxial mechanical properties of MNC, MSC, MSC2, and VIC seeded ovine aortic valves.
| Cell type | Areal strain (%) | Circ. stretch ratio | Radial stretch ratio |
|---|---|---|---|
| MNC | 124.01 ± 19.06a,b,c | 1.27 ± 0.11 | 1.77 ± 0.13 |
| MSC | 153.83 ± 28.12b,d | 1.37 ± 0.11b | 1.85 ± 0.12 |
| MSC2 | 134.48 ± 8.43 | 1.30 ± 0.07 | 1.81 ± 0.06 |
| VIC | 151.99 ± 18.24c | 1.39 ± 0.05a | 1.82 ± 0.13 |
| Cryo | 124.53 ± 19.73d,e | 1.21 ± 0.06a,b,c | 1.84 ± 0.14 |
| Decell | 158.09 ± 15.40a,e | 1.36 ± 0.07c | 1.90 ± 0.11 |
Previously reported data for cryopreserved (cryo) and decellularized (decell) ovine aortic valves are included for comparison.24 Data is reported as mean ± standard deviation. Superscript letters of the same type indicate a significant difference between groups (p < 0.05)
Biochemical Analysis
The concentrations of GAG, collagen, and total protein in the seeded valve leaflets were measured and compared to previously reported data for cryopreserved and decellularized ovine aortic leaflets (Table 3).24 The previously reported data indicated a significant loss of GAG between cryopreserved and decellularized samples. However, no other significant differences were found in GAG concentration between other groups in this study. The average GAG concentration of all tissue engineered leaflets was within the bounds of the reported cryopreserved and decellularized concentrations. Conversely, the concentration of collagen was similar between cryopreserved and decellularized groups, yet all tissue engineered groups (MNC, MSC, MSC2, and VIC) had a significantly increased collagen concentration than either control group (p < 0.05). The total protein concentration in all cell seeded groups was greater than either cryopreserved or decellularized groups, although only the MSC2 group was significantly different (p < 0.05).
Table 3.
Biochemical concentrations of MNC, MSC, MSC2, and VIC seeded ovine aortic valves.
| Cell type | GAG | Collagen | Tot. protein |
|---|---|---|---|
| MNC | 0.93 ± 0.31 | 73.50 ± 8.29a,b | 203.2 ± 28.5 |
| MSC | 0.99 ± 0.32 | 68.00 ± 18.80c,d | 216.2 ± 88.4 |
| VIC | 1.04 ± 0.25 | 64.49 ± 10.18e,f | 197.2 ± 50.5 |
| 2× MSC | 0.917 ± 0.26 | 70.40 ± 12.64g,h | 248.6 ± 45.1a,b |
| Cryo | 1.62 ± 1.20a | 39.49 ± 7.11a,c,e,g | 153.5 ± 46.6a |
| Decell | 0.39 ± 0.47a | 40.25 ± 3.79b,d,f,h | 133.4 ± 64.3b |
Previously reported data for cryopreserved (cryo) and decellularized (decell) ovine aortic valves are included for comparison.24 Data is reported as mean ± standard deviation. Superscript letters of the same type indicate a significant difference between groups (p < 0.05)
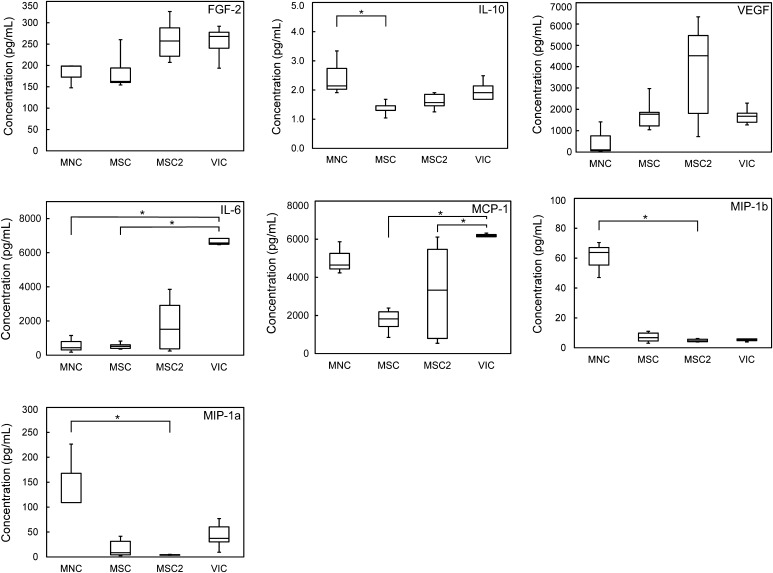
The production of cytokines by the seeded valves was measured by analyzing the culture media after the 2 week culture period using multiplex assays (Fig. 4). Cytokines were measured to estimate the potential for inflammation and/or cell recruitment. The selected cytokines represent a mix of classic inflammation signaling proteins representing an M1 macrophage response (IL-6, MIP-1α, MIP-1β, TNFα) and an assortment of tissue repair signaling proteins associated with an M2 macrophage response (FGF-2, IL-10, MCP-1, VEGF). Of the cytokines measured, IL-6, MCP-1 and VEGF were produced to the greatest concentration by all cell seeded groups. VIC seeded valves produced an exceptionally high amount of IL-6 compared to other valves, and a significantly greater amount than MNC or MSC seeded valves (p < 0.05). Similarly, VIC seeded valves produced more MCP-1 than the other seeded groups, and a significantly greater amount than MSC or MSC2 seeded valves (p < 0.05). The highest VEGF concentration was produced by MSC2 seeded valves, though there was no significant difference between any groups and there was a large variance within the MSC2 samples. FGF2 production was relatively similar across all groups. Of the classic inflammatory signaling proteins, MNC valves produced higher concentrations compared to other groups. IL-10 production was greatest in MNC valves and was significantly greater than the MSC group (p < 0.05). MIP-1α and MIP-1β was also greatest in the MNC valves and significantly greater than the MSC2 group (p < 0.05). TNFα is not included in Fig. 4 because it was below the detection limit (2.15 pg/mL) in all but one MNC sample, which had a TNFα concentration of 5.81 pg/mL.
Figure 4.
Box and whisker plots of cytokine concentrations from the bioreactor media of the tissue engineered valve groups, 2 weeks post seeding. The box boundaries represent the first and third quartiles, the horizontal line indicates the median, and the whiskers indicate the maximum and minimum values. *Denotes a significant difference between groups (p < 0.05).
Short Bioreactor Conditioning of MNC Valves
Following initial analysis of the MNC group, an additional sub-group was added which seeded three additional decellularized ovine aortic valves with human MNCs and cultured them in the bioreactor for 3 days (MNC short). Histology of the MNC short group revealed a cell population present within the leaflet matrix (Figs. 5a–5d). The cells were located only within the fibrosa layer of the leaflet and were not continuous, but appeared in small clusters along the length of the leaflet. IHC staining revealed the cells stained positive for CD68, CD34, or CD45 (Figs. 5e–5h). rt-PCR of the MNC short valves was similar to the MNC 2 week conditioned valves, except for a general up-regulation of classic inflammatory genes compared to the pre-seeding population, specifically CD163, CD14, and TNFα.
Figure 5.
H&E (a–d) and IHC (e–h) stained sections of MNC short heart valve leaflets, 3 days post seeding. IHC sections were stained for cell nuclei (blue) (e–h), αSMA (red) & HSP47 (green) (e), VIM (red) & CD90 (green) (f), CD68 (red) & CD271 (green) (g), and CD34 (red) & CD45 (green) (h). H&E staining revealed clusters of cell infiltration into the fibrosa along the length of the leaflet (a–d). IHC revealed positive expression of CD68, CD34, and CD45 (g, h).
Discussion
MNC Valve Seeding
Valves in the MNC group exhibited surprisingly minimal leaflet recellularization. The lack of recellularization is particularly unexpected due to the seemingly positive effect that MNC seeding had on the mechanical and biochemical properties. MNC valves exhibited mechanical properties most similar to cryopreserved valves (i.e., native control) compared to the other groups and the increased collagen in MNC valves compared to decellularized valves indicated an active cell presence during bioreactor culture. Furthermore, previous experience within our group has seen the establishment of pilot cell populations within the leaflet matrix after only 3 days when seeding with MNCs.4 Interestingly, histology of the MNC short group revealed a cell population present within the fibrosa layer of the leaflet. Protein and gene expression analysis of the MNC short cells found an up-regulation of markers associated with immunomodulatory cells, as would be expected from a mononuclear cell population.
Taken together, the MNC short and MNC valves show an evolving cell phenotype and population throughout the bioreactor culture period. Following MNC seeding, recellularization readily occurs within the fibrosa layer by macrophages, leukocytes, and hematopoietic cells. As bioreactor culture continues, those cells likely migrate or undergo cell death leading to isolated cells on the leaflet surface by 2 weeks. The cells remaining at 2 weeks were positive for CD68, HSP47, and VIM, indicating a mix of macrophage and fibroblastic phenotypes. The fibroblastic cells likely originate from the proliferation of the small population of stem cells found within bone marrow.1 The evolving cell phenotype is supported by the rt-PCR data that shows an up-regulation of the ACTA2, SERPINH1, and THY1 genes in the MNC valves compared to the pre-seeding cells, although it is worth nothing there was no positive staining for CD90 or CD271 during IHC. Despite the proliferation of the stem cell population, after 2 weeks of bioreactor culture the leaflet interstitial tissue remained acellular indicating cell proliferation was not occurring quickly enough to repopulate the leaflet. Additionally, it was found that recellularization is not a function of seeding density as the MNC group had the highest initial cell inoculant, but exhibited the least amount of recellularization compared to the other tested groups. Therefore, MNC seeding and 2 weeks of bioreactor culture is an impractical approach for in vitro TEHV recellularization as a stand-alone method.
An alternative approach may be seeding MNCs to establish a pilot cell population that can stimulate autologous recellularization in situ. Weber et al. demonstrated seeded MNCs are capable of inducing an inflammation-mediated recellularization response in polymeric heart valve scaffolds.27 Roh et al. further identified that the mononuclear cells produce the signaling cytokine MCP-1, which induces the recellularization response.20 In this study, we found the MNC valves produced moderate amounts of MCP-1. Although cytokine production was not measured for MNC short valves, it was demonstrated that MNCs can rapidly establish within the leaflet tissue and may be producing signaling cytokines much earlier than measured here. Future investigations should investigate the temporal cytokine production of MNC seeded valves and the potential for autologous cell recruitment in situ.
MSC and MSC2 Valve Seeding
Seeding with MSCs led to the most successful recellularization of the interstitial matrix of the decellularized leaflets. Surprisingly, the difference in the seeding cell concentrations, 5 million cells for the MSC group vs. 10 million cells for the MSC2 group, did not lead to greatly differing degrees of recellularization (Fig. 1). In fact, no significant differences were directly measured between the MSC or MSC2 groups by any metric in this study. However, a number of dissimilarities can be inferred based on the significant differences measured between other groups. For example, the areal strain and circumferential stretch ratio of MSC valves was similar to decellularized valves and significantly different than cryopreserved valves, indicating that cell seeding in the MSC group had little to no effect on the mechanical properties. Alternatively, the MSC2 group showed statistically similar mechanical properties to both decellularized and cryopreserved valves, indicating MSC2 seeding had a small effect on mechanical properties. The concentrations of GAGs, collagen, and total protein was also similar between MSC and MSC2 group, yet MSC2 had significantly more total protein than either decellularized or cryopreserved valves, signifying increased protein production. IHC revealed similar protein expression between the MSC and MSC2 groups, yet the staining intensity appears greater in the MSC2 group. Comparing the MSC and MSC2 groups indicate it is advantageous to seed with the higher concentration of MSCs.
Overall, these results suggest seeding with MSCs leads to better recellularization of TEHV compared to MNCs after 2 weeks of bioreactor culture. MSCs have shown great potential as a cell source for heart valve tissue engineering, due in part to their pluripotent differentiation and naturally myofibroblastic phenotype similar to native VICs.8 Additionally, MSCs possess immunomodulatory capabilities that may help reduce inflammation.6,21 However, the use of MSCs may raise some concerns since their multi-lineage potential can lead to the differentiation of adverse phenotypes, including osteoblasts, chondrocytes, and/or adipocytes.8 Before the clinical application of MSC seeded devices can be realized, the underlying factors leading to cell differentiation in valve tissues must be understood and controlled so as to avoid adverse differentiation.
VIC Valve Seeding
Due to the difficulty in obtaining a patient matched VIC population, seeding TEHVs with autologous VICs is not a clinically viable solution. Despite this, the VIC group was included in this study to evaluate the results of seeding an autologous cell source following the same in vitro seeding and conditioning protocol of the other candidate cell populations. However, the VIC group of valves showed surprisingly minimal recellularization of the leaflet interstitial tissue and cells remained clumped on the leaflet surface. Also surprising was the lack of αSMA expression by the cells in the VIC group.16
Valve interstitial cells in a healthy, native valve remain quiescent (qVIC) until damage or injury to the valve requires repair, at which point they become activated (aVICs) and are identified by their expression of αSMA.16 aVICs are responsible for remodeling the extracellular matrix and αSMA is often used as a marker for the VIC phenotype in tissue engineering applications since matrix remodeling is a basic requirement for a functioning TEHV. After repairing valve damage, aVICs should return to a quiescent state, but it is worth noting that prolonged VIC activation is linked to valve disease including fibrosis, inflammation, and ultimately calcification.16
In this study, we found VIC seeded valves did not express αSMA. We expected the seeded VICs to express an activated phenotype and begin remodeling the decellularized matrix, yet it appears the VICs remained quiescent. The apparent qVIC phenotype may explain the lack of cell infiltration in the VIC group since the cells may not have produced matrix remodeling proteins, such as MMPs, which would break down surrounding tissue matrix. The extracellular matrix of the valve leaflets appears compact and cells may be unable to infiltrate the leaflet without the expression of matrix remodeling proteins. Hof et al. similarly reported an absence of cellular infiltration after seeding VICs onto decellularized leaflet tissue due to the presence of a compact basement membrane.11 To increase the penetrability of the leaflets, Hof et al. performed laser perforation or enzymatic digestion by trypsin and found laser perforation treatment led to marginal cell infiltration while trypsin treatment led to increased infiltration.11 However, trypsin digestion led to structural alterations in the leaflet matrix, and is therefore not a clinically viable treatment option for decellularized valves.11 In addition to the clinical impracticality of seeding valves with autologous VICs, the results of this study, and others, suggest that VIC seeding leads to poor recellularization of the leaflet tissue and is likely not applicable for the TEHV.
Limitations
While this study provides fundamental information regarding candidate cell populations for heart valve seeding, it is not without limitations. For example, the in vitro nature of this study eliminates the tissue healing milieu that is expected in vivo. As such, a number of factors could not be included in this study which may change the recellularization potential of the cell populations, including paracrine and endocrine signaling, the presence of local tissue repair cells, and possible inflammatory responses. Therefore, the results of this study are simply guidelines, and set the stage for future in vivo experiments to further elucidate TEHV recellularization. Additionally, this study only investigated most groups at an arbitrary time point of 2 weeks. As evidenced by the MNC short group, recellularization likely changes throughout the culture period and future studies should investigate the time response curve of cell populations to further enhance recellularization.
Conclusion
This investigation was designed to provide a direct comparison of candidate cell populations for heart valve tissue engineering. We compared four different cell populations for their ability to repopulate the distal leaflet of decellularized heart valves after 2 weeks of in vitro bioreactor culture. We found that seeding with MSCs at a higher concentration delivered the best results as demonstrated by recellularization of the interstitial leaflet tissue, cell phenotype, mechanical properties, and biochemical analysis. Seeding with MSCs at a lower concentration exhibited similar leaflet repopulation, yet the mechanical and biochemical analysis were not as encouraging. MNC seeding led to minimal recellularization though an additional time point group demonstrated a cellular presence at only 3 days. VIC seeding resulted in cell clumping on the leaflet surface but poor recellularization of the interstitial tissue. These results confirm the hypothesis that of the tested groups, MSCs are best suited for bioreactor ex vivo TEHV recellularization.
Acknowledgments
AP acknowledges an investigator grant provided by the Institutional Development Award from the National Institute of General Medical Sciences of the NIH Award Number P20GM103638 and Umbilical Cord Matrix Project fund form the State of Kansas.
Conflict of interest
VeDepo M., Buse E., Paul A., Hopkins R., and Converse G. all declare that they have no conflicts of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Specifically, ovine aortic valves were harvested from juvenile sheep under approved IACUC protocols and in accordance with Guide for Care and Use of Laboratory Animals (National Institutes of Health Publication No. 85-23). This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Alvarez-Viejo M, Menendez-Menendez Y, Blanco-Gelaz MA, Ferrero-Gutierrez A, Fernandez-Rodriguez MA, Gala J, Otero-Hernandez J. Quantifying mesenchymal stem cells in the mononuclear cell fraction of bone marrow samples obtained for cell therapy. Transpl. Proc. 2013;45:434–439. doi: 10.1016/j.transproceed.2012.05.091. [DOI] [PubMed] [Google Scholar]
- 2.Blevins TL, Carroll JL, Raza AM, Grande-Allen KJ. Phenotypic characterization of isolated valvular interstitial cell subpopulations. J. Heart Valve Dis. 2006;15:815–822. [PubMed] [Google Scholar]
- 3.Converse GL, Armstrong M, Quinn RW, Buse EE, Cromwell ML, Moriarty SJ, Lofland GK, Hilbert SL, Hopkins RA. Effects of cryopreservation, decellularization and novel extracellular matrix conditioning on the quasi-static and time-dependent properties of the pulmonary valve leaflet. Acta Biomater. 2012;8:2722–2729. doi: 10.1016/j.actbio.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 4.Converse GL, Buse EE, Hopkins RA. Bioreactors and operating room centric protocols for clinical heart valve tissue engineering. Prog. Pediatr. Cardiol. 2013;35:95–100. doi: 10.1016/j.ppedcard.2013.09.001. [DOI] [Google Scholar]
- 5.Converse GL, Buse EE, Neill KR, McFall CR, Lewis HN, VeDepo MC, Quinn RW, Hopkins RA. Design and efficacy of a single-use bioreactor for heart valve tissue engineering. J. Biomed. Mater. Res. B. 2015;105:249–259. doi: 10.1002/jbm.b.33552. [DOI] [PubMed] [Google Scholar]
- 6.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 7.Dohmen PM, Hauptmann S, Terytze A, Konertz WF. In-vivo repopularization of a tissue-engineered heart valve in a human subject. J. Heart Valve Dis. 2007;16:447–449. [PubMed] [Google Scholar]
- 8.Duan B, Hockaday LA, Das S, Xu C, Butcher JT. Comparison of mesenchymal stem cell source differentiation toward human pediatric aortic valve interstitial cells within 3D engineered matrices. Tissue Eng. C. 2015;21:795–807. doi: 10.1089/ten.tec.2014.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filip DA, Radu A, Simionescu M. Interstitial cells of the heart valves possess characteristics similar to smooth muscle cells. Circ. Res. 1986;59:310–320. doi: 10.1161/01.RES.59.3.310. [DOI] [PubMed] [Google Scholar]
- 10.Hoerstrup SP, Sodian R, Daebritz S, Wang J, Bacha EA, Martin DP, Moran AM, Guleserian KJ, Sperling JS, Kaushal S, Vacanti JP, Schoen FJ, Mayer JE., Jr Functional living trileaflet heart valves grown in vitro. Circulation. 2000;102:III44–III49. doi: 10.1161/01.CIR.102.suppl_3.III-44. [DOI] [PubMed] [Google Scholar]
- 11.Hof A, Raschke S, Baier K, Nehrenheim L, Selig JI, Schomaker M, Lichtenberg A, Meyer H, Akhyari P. Challenges in developing a reseeded, tissue-engineered aortic valve prosthesis. Eur. J. Cardiothorac. Surg. 2016;50:446–455. doi: 10.1093/ejcts/ezw057. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins R. From cadaver harvested homograft valves to tissue-engineered valve conduits. Prog. Pediatr. Cardiol. 2006;21:137–152. doi: 10.1016/j.ppedcard.2005.11.002. [DOI] [Google Scholar]
- 13.Jana S, Tranquillo RT, Lerman A. Cells for tissue engineering of cardiac valves. J. Tissue Eng. Regen. Med. 2015;10:804–824. doi: 10.1002/term.2010. [DOI] [PubMed] [Google Scholar]
- 14.Kurobe H, Tara S, Maxfield MW, Rocco KA, Bagi PS, Yi T, Udelsman BV, Dean EW, Khosravi R, Powell HM, Shinoka T, Breuer CK. Comparison of the biological equivalence of two methods for isolating bone marrow mononuclear cells for fabricating tissue-engineered vascular grafts. Tissue Eng. C. 2015;21:597–604. doi: 10.1089/ten.tec.2014.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtenberg A, Tudorache I, Cebotari S, Ringes-Lichtenberg S, Sturz G, Hoeffler K, Hurscheler C, Brandes G, Hilfiker A, Haverich A. In vitro re-endothelialization of detergent decellularized heart valves under simulated physiological dynamic conditions. Biomaterials. 2006;27:4221–4229. doi: 10.1016/j.biomaterials.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 16.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am. J. Pathol. 2007;171:1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pacelli S, Basu S, Whitlow J, Chakravarti A, Acosta F, Varshney A, Modaresi S, Berkland C, Paul A. Strategies to develop endogenous stem cell-recruiting bioactive materials for tissue repair and regeneration. Adv. Drug. Deliv. Rev. 2017;120:50–70. doi: 10.1016/j.addr.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinn RW, Hilbert SL, Bert AA, Drake BW, Bustamante JA, Fenton JE, Moriarty SJ, Neighbors SL, Lofland GK, Hopkins RA. Performance and morphology of decellularized pulmonary valves implanted in juvenile sheep. Ann. Thorac. Surg. 2011;92:131–137. doi: 10.1016/j.athoracsur.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 19.Quinn R, Hilbert S, Converse G, Bert A, Buse E, Drake W, Armstrong M, Moriarty S, Lofland G, Hopkins R. Enhanced autologous re-endothelialization of decellularized and extracellular matrix conditioned allografts implanted into the right ventricular outflow tracts of juvenile sheep. Cardiovasc. Eng. Technol. 2012;3:217–227. doi: 10.1007/s13239-011-0078-y. [DOI] [Google Scholar]
- 20.Roh JD, Sawh-Martinez R, Brennan MP, Jay SM, Devine L, Rao DA, Yi T, Mirensky TL, Nalbandian A, Udelsman B, Hibino N, Shinoka T, Saltzman WM, Snyder E, Kyriakides TR, Pober JS, Breuer CK. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc. Natl. Acad. Sci. USA. 2010;107:4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 22.Steinhoff G, Stock U, Karim N, Mertsching H, Timke A, Meliss RR, Pethig K, Haverich A, Bader A. Tissue engineering of pulmonary heart valves on allogenic acellular matrix conduits: in vivo restoration of valve tissue. Circulation. 2000;102:III50–III55. doi: 10.1161/01.CIR.102.suppl_3.III-50. [DOI] [PubMed] [Google Scholar]
- 23.Taylor PM, Batten P, Brand NJ, Thomas PS, Yacoub MH. The cardiac valve interstitial cell. Int. J. Biochem. Cell. Biol. 2003;35:113–118. doi: 10.1016/S1357-2725(02)00100-0. [DOI] [PubMed] [Google Scholar]
- 24.VeDepo MC, Buse EE, Quinn RW, Williams T, Detamore MS, Hopkins RA, Converse GL. Species-specific effects of aortic valve decellularization. Acta Biomater. 2017;50:249–258. doi: 10.1016/j.actbio.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 25.VeDepo MC, Detamore M, Hopkins RA, Converse GL. Recellularization of decellularized heart valves: progress toward the tissue-engineered heart valve. J. Tissue Eng. 2017;8:2041731417726327. doi: 10.1177/2041731417726327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincentelli A, Wautot F, Juthier F, Fouquet O, Corseaux D, Marechaux S, Le Tourneau T, Fabre O, Susen S, Van Belle E, Mouquet F, Decoene C, Prat A, Jude B. In vivo autologous recellularization of a tissue-engineered heart valve: are bone marrow mesenchymal stem cells the best candidates? J. Thorac. Cardiovasc. Surg. 2007;134:424–432. doi: 10.1016/j.jtcvs.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Weber B, Scherman J, Emmert MY, Gruenenfelder J, Verbeek R, Bracher M, Black M, Kortsmit J, Franz T, Schoenauer R, Baumgartner L, Brokopp C, Agarkova I, Wolint P, Zund G, Falk V, Zilla P, Hoerstrup SP. Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur. Heart J. 2011;32:2830–2840. doi: 10.1093/eurheartj/ehr059. [DOI] [PubMed] [Google Scholar]