Abstract
Introduction
Pre-vascularization of tissue engineered grafts is a promising strategy to facilitate their improved viability following in vivo implantation. In this process, endothelial cells (ECs) form capillary-like networks that can anastomose with host vasculature. Adipose-derived stromal cells (ASCs) are a commonly used cell population for tissue engineering and contain a subpopulation of ECs capable of assembling into robust vascular networks and anastomosing with the host. However, their initial vascular assembly is significantly impaired in hypoxic conditions (2% O2). In this study, we explored the minimum period of normoxic (20% O2) pre-treatment required to enable the formation of stable vascular networks.
Methods
ASC-derived vascular structures were allowed to preassemble in fibrin hydrogels in normoxia for 0, 2, 4, or 6 days and then transplanted into hypoxic environments for 6 days. Total vascular length, pericyte coverage, cell proliferation, apoptosis rates, and ECM production was assessed.
Results
Vascular assembly increased with time over the 6 days of culture. We found that 4 days was the minimum period of time required for stable vascular assembly. We compared the major differences in cell behavior and network structure at Days 2 and 4. Neither proliferation nor apoptosis differed, however, the Day 4 time-point was associated with a significant increase in pericyte coverage (46.1 ± 2.6%) compared to Day 2 (24.3 ± 5.3%).
Conclusions
These data suggest oxygen tension may be a mediator of EC–pericyte interactions during vascular assembly. Pre-vascularization strategies should incorporate a normoxic period of to enable successful vascular formation and development.
Electronic supplementary material
The online version of this article (10.1007/s12195-018-0539-6) contains supplementary material, which is available to authorized users.
Keywords: Oxygen tension, Hypoxia, Implantation, Endothelial cells, Pericytes, Preassembly
Introduction
Tissue engineered implants have the potential to treat a number of large volumetric musculoskeletal disorders and defects29 but are severely limited in scale by a lack of a perfused blood vessel networks.44 The inclusion of functional vascular networks within tissue engineered constructs is a promising strategy when scaling from mouse- to human-sized applications in order to provide the oxygen, nutrients, and waste removal needed by tissues.38 Neovascularization is a slow process with rates less than 1 mm/week50 and cellular constructs with thicknesses greater than 400 µm become rapidly necrotic due to the diffusion limitation of oxygen.20 Hence, the inability to provide sufficient vascularization has limited the clinical use of tissue engineered implants. Several approaches to create tissue engineered scaffolds with functional vascular networks have been reported. These include 3D-printing channels for vessel ingrowth,32 releasing angiogenic growth factors from the scaffold,6 and periods of in vitro culture to ‘pre-vascularize’ the construct before implantation.11,28
Pre-vascularization is a highly promising strategy in which endothelial cells (ECs) are stimulated to form nascent capillary-like vascular network structures that can anastomose with the host vasculature. In order to form stable networks, ECs are typically co-cultured with fibroblasts,30 mesenchymal stem cells,8,48 or pericytes,49 which act as perivascular cells and stabilize the vascular networks. Recently, adipose-derived stromal cells (ASCs) from human lipoaspirate tissues have been shown to be a suitable source of ECs and perivascular cells. The vascular potential of ASCs arises, due to a sub-population of ECs at early passages23,33 as well as their limited ability to differentiate into ECs.3,13 ASCs also include a pericyte or pericyte-like population1 and pericytes have potential to modulate the effect of angiogenic therapies.27 When cultured in fibrin hydrogels, the EC sub-population within ASCs exhibit the potential to form extensive, interconnected vascular network structures22,24 that survive in vivo implantation.22
The capacity of ECs and ASCs to assemble into vascular networks is highly oxygen-dependent. Specifically, hypoxic conditions (<5% O2) inhibit the self-assembly of vascular networks. Hypoxia (5% O2) prevented the self-assembly of vascular networks in a co-culture of human endothelial colony forming cells and multipotent stromal cells.16 Griffith and George have shown that in addition to inhibiting self-assembly, challenging constructs with hypoxia after a period of preassembly leads to the degradation of capillary networks.17 More recently, it has been shown that hypoxia inhibits de novo assembly of ASC-derived vascular networks.21 These reports run counter to the predominant narrative of hypoxia as a pro-angiogenic stimulus. In fact, while hypoxia drives new blood vessel formation via angiogenesis (and is a key mechanism underlying tumor vascularization), low oxygen tension is a known inhibitor of vascular assembly. Interestingly, while hypoxia inhibits the de novo vascular assembly of ASCs and their EC sub-populations, transferring pre-assembled ASC-vessels into hypoxic environments stimulated their growth.21 Prevascularization strategies therefore require understanding the impact of oxygen on the kinetics of assembly and stabilization.
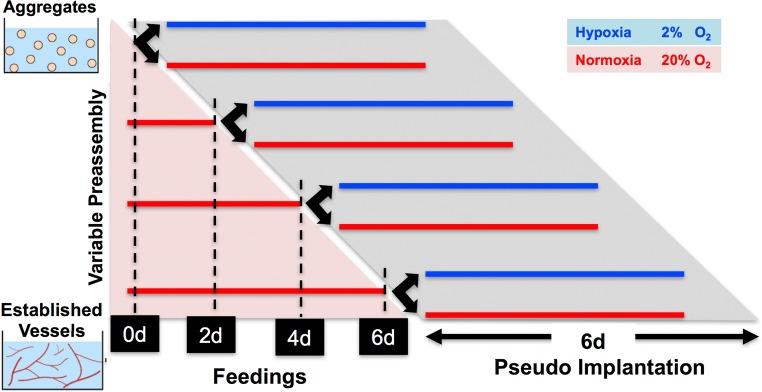
In this study, we aimed to determine the minimum amount of normoxic (20% O2) preassembly time that is needed for ASC-vascular networks to stabilize so that they when transferred to hypoxic (2% O2) conditions, the vessels would continue to elongate. To assess this, ASCs were cultured in fibrin hydrogels and allowed to pre-assemble into vascular network structures for 0, 2, 4, or 6 days. At the end of each of these pre-incubation periods, we split the cultures into two and transferred one group to continued normoxic cultures and the other group to hypoxic (2% O2) culture conditions for a further 6 days (Fig. 1). We assessed geometric and cellular properties of the networks to establish the underlying mechanism mediating the switch in behavior of ECs to hypoxic environments. These studies suggest that the interactions between ECs and pericyte-like populations are strongly oxygen- and time-dependent. Further understanding of these interactions may be critical for exploiting the vascular capacity of ASCs in pre-vascularization strategies.
Figure 1.
Schematic of the study gels of ASC aggregates were allowed to preassemble for 0, 2, 4, or 6 days in normoxia with regular feedings before moving into a pseudo-implant condition for 6 days.
Materials and Methods
ASC Isolation and Culture
Human subcutaneous adipose tissue was obtained in the form of lipoaspirate from three female Caucasian donors undergoing elective surgery and with written informed consent under the approval of the Johns Hopkins Medicine Institutional Review Board. ASCs were isolated as previously described.9,21 Briefly, tissue was digested with collagenase (1 mg/mL; Worthington Biochemical Corp.) to isolate the stromal vascular fraction of cells. These cells were plated onto tissue culture plastic and were termed “passage 0 ASC” when they reached 80–90% confluence. ASCs were used at passage 2 for all experiments. Growth medium consisted of: high glucose DMEM (Gibco) with 10% fetal bovine serum (FBS; Atlanta Biologicals), 1% penicillin/streptomycin (Gibco), and 1 ng/mL basic fibroblast growth factor (FGF-2; PeproTech).
Cell Aggregation via Suspension Culture
Cells were trypsinized and resuspended at a concentration of 250,000 cells/mL in growth medium containing 0.24% (w/v) methylcellulose (Sigma). The cell suspension was pipetted into 10-cm Petri dishes coated with 2% (w/v) agarose to minimize cellular adherence to the dish. After overnight suspension culture, cellular aggregates were collected with a pipette, and then centrifuged before encapsulation procedures.
Aggregate Encapsulation and Culture
Pre-assembly Culture
Cell aggregates were suspended in fibrinogen (8 mg/mL final; Sigma) and thrombin (2 U/mL final; Sigma) at a final cell concentration of 2 × 104 cells/μL. Fibrin gels were formed by pipetting 12 μL of gel solution into 4-mm diameter wells and incubating at 37 °C for 30 min to allow complete gelation before adding medium. Each gel sample was fed with 1 mL of culture medium containing: endothelial basal medium-2 (EBM-2, Lonza), 10% FBS, and 1% penicillin/streptomycin. The media was not supplemented with growth factors beyond those naturally present in serum. To assess the effect of preassembly on future hypoxic cultures, freshly encapsulated cells were cultured in normoxia (20% O2) for 0, 2, 4, or 6 days with the medial changed every other day to create different degrees of vascular networks.
Pseudo-implantation Culture
On the last day of preassembly, the samples were fed once more and then cultured in either normoxia or hypoxia (2% O2) for an additional 6 days with no media changes (Fig. 1). Normoxic samples were maintained in a 37 °C incubator with 5% CO2, 95% ambient air. Hypoxic samples were placed in a modular incubator chamber (Billups-Rothenberg) that was flushed every day with pre-mixed gas (2% O2/5% CO2/N2 balance) and placed in a 37 °C incubator.
Proliferation Labelling
Cells were incubated with bromodeoxyuridine (BrdU, Sigma) to detect proliferating cells. Briefly, 10 μM BrdU was pipetted into existing culture medium (i.e., medium was not changed), and samples were quickly returned to their appropriate oxygen environment (less than 5 min of normoxic exposure) for a 20-h incubation. Samples were then washed with PBS and fixed with 3.7% formaldehyde.
Whole-Mount Immunostaining
Whole-mount immunostaining of fibrin gels was performed as previously described.24 Briefly, samples were fixed with 3.7% formaldehyde for 3 h at 4 °C, washed with PBS, and blocked with 5% normal goat serum/0.2% Triton X-100/PBS for 3 h at 4 °C. Antibodies were incubated overnight at 4 °C, followed by three 1-h washes in PBS with 0.1% Tween. Primary antibodies included: mouse anti-human CD31 (4 μg/mL, Sigma), mouse anti-human collagen IV (20 µg/mL, Santa Cruz Biotech), and Cy3-conjugated mouse anti-alpha smooth muscle actin (αSMA; 7 μg/mL, Sigma). Secondary antibodies used included: DyLight 488-conjugated goat anti-mouse (3.75 µg/mL, Jackson ImmunoResearch), cy3-conjugated donkey anti-mouse (7 µg/mL, Jackson ImmunoResearch), biotin-conjugated goat-anti mouse (5.5 µg/mL, Jackson ImmunoResearch) and fluorescein-conjugated streptavidin (4.5 µg/mL, Jackson ImmunoResearch). Prior to secondary labelling for incorporated BrdU, samples were stained for all other antigens and post-fixed with 3.7% formaldehyde for 30 min to preserve the stain. Samples were then denatured with 2 N HCl/0.5% Triton X-100 for 45 min at room temperature, washed, re-blocked, and then incubated with AlexaFluor 647-conjugated mouse anti-BrdU (4 μg/mL, Invitrogen) overnight at 4 °C. Cell nuclei were counterstained with 4′-6-diamidino-2-phenylindole (DAPI; Sigma).
Apoptosis Staining
The APO-BrdU TUNEL assay kit (Invitrogen) was used following manufacturer protocol before staining for other antigens. Briefly, samples were fixed in 70% ethanol at − 20 °C for 1 week, rinsed in wash buffer for 20 m twice, incubated with DNA-labelling solution for 4 h in a shaking water bath, washed with rinse buffer twice for 20 m at 4 °C, and stained with AlexaFlour 488-conjugated mouse anti-BrdU overnight at 4 °C. Samples were co-stained with DAPI.
Imaging and Analysis
Immunostained gels were mounted on glass slides and imaged using a Zeiss LSM 510 confocal microscope (×5 and ×20 objectives). Confocal z-stacks were z-projected and thresholded for quantification. AngioQuant36 software was used to quantify total vessel length (sum of the lengths of all vessel branches within a gel). Matlab (Mathworks) was used for all other image analysis. Pericyte coverage was defined as αSMA+ area within at least 5 μm of the abluminal face of vessel networks. Briefly, vessel networks were selected in the CD31 channel of thresholded image composites. Selections were enlarged by 5 μm at all edges and applied to the αSMA channel. αSMA+ area fraction within the selected area was measured and displayed as “Percent SMA Coverage”. BrdU+ nuclei were counted and counts from the whole gel indicate overall proliferation within the culture (displayed as “# BRDU/CD31 (#/mm2)”. To assess proliferation within the vessels only, CD31+ vessel area was selected and applied to the BrdU channel prior to counting within the selected area. This count was normalized to the CD31+ vessel area to account for differences in vessel density and is displayed as “#BRDU in CD31”.
Statistical Analysis
Quantitative data are expressed as mean ± standard error. Statistical analyses were performed using GraphPad Prism 5 software. Statistical significance was determined by one-way ANOVA with Tukey’s post-test and is denoted as *p < 0.05, **p < 0.005, ***p < 0.0005.
Results
Preassembly-Mediated Vascular Assembly
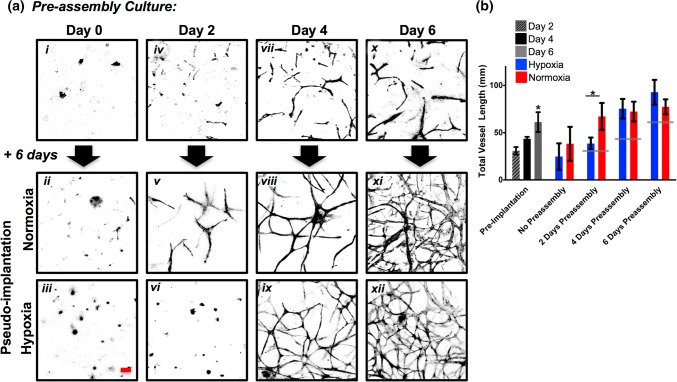
ASC aggregates were heterogenous in size. They underwent vascular morphogenesis and developed a highly-branched vascular morphology when cultured in the fibrin hydrogel for 6 days in normoxic conditions (Fig. 2a). The ASCs used in this study are isolated via plastic adherence and contain a population of 0.6–1.1% CD31+ cells,21,23 which we understand to be the building block of the vascular network, rather than ASCs differentiating into ECs. The vascular lengths at 2, 4, and 6 days were (30.9 ± 7.7), (43.6 ± 10.9), and (61.2 ± 15.3 mm), respectively (Fig. 2b). With 0 days pre-assembly, subsequent culture in either normoxia or hypoxia for 6 days without media changes (pseudo-implantation model) resulted in no visible vascular assembly, demonstrating the nutrient-starving nature of the pseudo-implant condition alone is sufficient to impair vascular assembly. However, with 2 days of preassembly, ASCs in the normoxic pseudo-implant condition assembled into preliminary branching structures with significantly greater vessel length (67.2 ± 16.8 mm) than the hypoxic pseudo-implant (38.4 ± 9.6 mm). After 4 days of preassembly, transplantation of the vascular structures into either normoxic or hypoxic pseudo-implantation conditions for 6 days resulted in continued vascular development and the formation of interconnected networks (72.3 ± 17.8 vs. 75.4 ± 18.5 mm, respectively). Similarly, with a full 6 days of preassembly ASC vascular networks continued to branch and develop into highly interconnected and dense vascular networks after pseudo-implantation in normoxia (77.4 ± 19.3 mm) and hypoxia (92.8 ± 23.2 mm). Hence, with 0 or 2 days of preassembly in a favorable, normoxic environment, transfer to hypoxic microenvironments had detrimental effects on vascular morphogenesis. However, following 4 or 6 days of pre-assembly, transfer to hypoxic microenvironments appeared to be supportive of subsequent vascular assembly. Thus, we observed a time-dependent change in the effect of hypoxia on ASC-derived vascular networks between Days 2 and 4 of pre-assembly.
Figure 2.
Assessing vascular length. (a) ASCs grown in fibrin hydrogels for 6 days sprouted into vascular network structures that stained positively for CD31. At 0, 2, 4, and 6 days ASCs were transferred to either normoxic or hypoxic pseudo-implantation conditions for a subsequent 6 days. (b) Measurements of total vessel length. Gray lines indicate preimplantation length. *p < 0.05, n = 4. Scale bar = 100 µm.
Endothelial Cell Proliferation
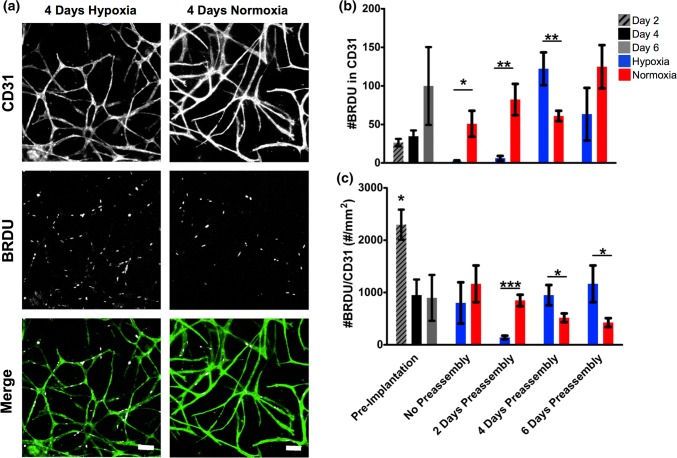
EC proliferation was assessed by monitoring the incorporation of the thymidine analog BrdU into CD31+ cells during mitosis over the final 20 h of culture (Fig. 3). Proliferation predominantly occurred within CD31+ areas. We hypothesized that ECs might be in a more proliferative state with increasing amounts of preassembly. While this hypothesis is supported by the large increase in number of BrdU+ nuclei within CD31+ regions at 6 days of preassembly, only a slight increase was observed between 2 and 4 days of preassembly (Fig. 3b, Days 2 and 4: 22.8 ± 9.2 and 32.7 ± 4.6, Day 6: 99.3 ± 49). However, this trend is not present when the number of BrdU+ nuclei was normalized to the CD31+ area (Fig. 3c). The spike in #BrdU/CD31 at 2 days of preassembly (2295.9 ± 276.2 #/mm2) is due in part to the very small area fraction of the gel that is CD31+ at that time, resulting in division by a very small number. After pseudo-implantation, proliferation is greater in normoxic than hypoxic groups with zero (hypoxic 870.0 ± 381.1, normoxic 1201.7 ± 340.1 #/mm2) and 2 days of preassembly (hypoxic 270.0.0 ± 38.8, normoxic 995.1 ± 143.1 #/mm2), and then switches to be increased in hypoxic groups with 4 (hypoxic 1038.6 ± 226.6, normoxic 594.4 ± 132.7 #/mm2) and 6 days of preassembly (hypoxic 1304.7 ± 341.6, normoxic 437.9 ± 68.0 #/mm2). These results are similar to our total vessel length analysis, indicating that cultures with four or more days of pre-assembly entering a more proliferative state after exposure to hypoxia.
Figure 3.
Proliferation analysis. (a) Total number of BrdU+ nuclei within CD31 in each gel. (b) Number of BrdU+ nuclei within CD31+ areas in each gel. (c) Density of BrdU+ nuclei within the area of CD31 in each gel. *p < 0.05, **p < 0.005, ***p < 0.0005, n = 5. Scale bar = 100 µm.
Apoptosis Analysis
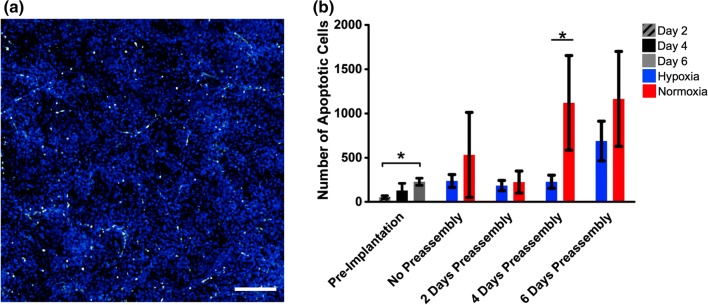
To determine if cells were undergoing apoptosis at an increased rate in hypoxia—as opposed to proliferating at an increased rate—a TUNEL assay was used to label the nicked ends of nuclear DNA as it was reduced into 200 bp fragments by the apoptotic cascade (Fig. 4). There was an increase in the number of apoptotic cells during the preassembly period (Days 2, 4, 6: 52.5 ± 4.2–129.0 ± 20.3–228.2 ± 10.1), and in the normoxic groups after the pseudo-implantation period relative to parallel hypoxic conditions (228.0 ± 68.7–1120.0 ± 333.6 with 4-day preassembly; 688.5 ± 55.9–1164.0 ± 293.9 with 6 days preassembly). Therefore, it is more likely that cells are simply not proliferating in hypoxic conditions instead of undergoing apoptosis.
Figure 4.
Quantification of apoptotic cells. (a) TUNEL staining of apoptotic cells in the fibrin hydrogel. TUNEL = green, DAPI = blue. (b) Quantification of apoptotic cells during preassembly and post-pseudo implantation. *p < 0.05, n = 4. Scale bar = 400 µm.
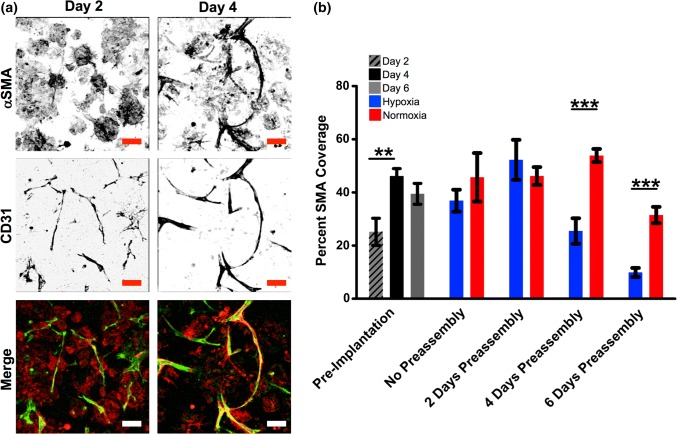
Pericyte and Collagen IV Coverage Analysis
Pericyte coverage of vessels was analyzed by staining for αSMA fibers co-localized within 5 µm of CD31+ areas (Fig. 5 and Supp. Fig. 1). Pericyte coverage was significantly increased from 2 to 4 days of preassembly (24.3 ± 5.3–46.1 ± 2.6%; p < 0.005). αSMA coverage in normoxic groups post-pseudo implantation with 4 and 6 days of preassembly was greater than parallel hypoxic groups. In fact, despite the hypoxic groups increasing total vascular length with time, they demonstrate decreasing αSMA coverage post-pseudo-implantation from 2 to 4 to 6 days of preassembly (51.9 ± 7.2–25.2 ± 4.8–9.8 ± 1.9%). This decrease in αSMA coverage might be due to pericytes migrating away from their classical position on the abluminal wall of a tubule to facilitate greater growth in hypoxia.39
Figure 5.
Analysis of pericyte coverage. (a) Constructs at Days 2 and 4 of preassembly stained for pericytes (αSMA, red) and endothelial cells (CD31, green). (b) Quantification of αSMA+ coverage of CD31+ vessels during vascular assembly of ASCs during preassembly and following transfer to pseudo-implant conditions. **p < 0.005, n = 5. Scale bar = 100 µm.
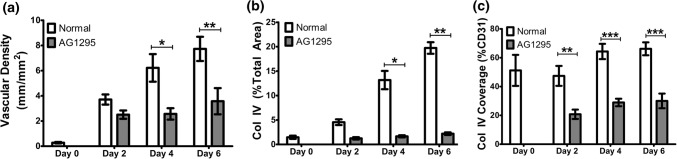
Therefore, we hypothesized that a more mature vascular structure with greater pericyte coverage was surviving and thriving in the hypoxic environment, and tested by using a pharmaceutical inhibitor of pericyte coverage. Additionally, one of the functions of the pericytes is to deposit collagen IV into a basement membrane which can provide supportive signaling to ECs during hypoxia.46 Pericytes were inhibited with AG1295 and the vascular assembly was contrasted with co-localization of collagen IV. AG1295 significantly inhibited vascular assembly at Days 4 and 6 of preassembly relative to untreated controls (Fig. 6a, Day 4: 6.22 ± 0.55–2.57 ± 0.26; Day 6: 7.73 ± 0.55–3.57 ± 0.52 mm/mm2) and almost totally eliminated collagen IV deposition throughout the construct (Fig. 6b, day 6: 20.7 ± 2.80–2.2 ± 0.13%, p < 0.05). Within CD31+ regions, AG1295 significantly reduced collagen IV deposition at all timepoints (Fig. 6c, p < 0.005).
Figure 6.
Assessment of vascular structure following AG1295 inhibition. (a) Vascular density of CD31+ networks. (b) Total area fraction of Col IV in the gel. (c) Area fraction of CD31 positive for Col IV. *p < 0.05, **p < 0.005, ***p < 0.0005, n = 3.
Discussion
The hypoxic microenvironments within volumetric tissue defects provide serious challenges to cell-based regenerative strategies. Hypoxia impairs cell survival and hypoxia vascular assembly of transplanted ECs used to rapidly vascularize engineered grafts.16,17,21 The data in the current study confirm that hypoxia is inhibitory to the earliest stages of vascular assembly. To overcome this limitation in the therapeutic application of ASCs, one solution is to preassemble the vascular networks before implantation. A preassembly period allows for a number of organizational and construction steps in vascular assembly to take place in a metabolically favorable environment. However, preassembly approaches have regulatory and manufacturing drawbacks. In this study, we modeled preassembly using a culture period with abundant nutrients and oxygen. Additionally, we investigated several parameters of vascular assembly during the preassembly period to characterize the tissue features that would support further growth in hypoxia. The data herein suggest that at minimum, 4 days of normoxic and nutrient-rich conditions are required before implantation into a hypoxic or ischemic environment. Such a period allows for a sufficient amount of pericyte and collagen IV coverage to develop, and supports the growth and proliferation of ECs in a future hypoxic and nutrient-starved environment.
We found that pericyte-like cells in ASC cultures are essential stabilizers of vascular structures. Inhibition of pericyte-like cell recruitment via blocked PDGF-BB signaling closely mimics inhibition of vascular assembly in hypoxia. This suggests that hypoxia delays the contributions of pericyte-like cells during vascular assembly. Previous work identified pericyte-like ASCs as an important component of ASC-vascular assembly (via heterotypic cell assembly).23 Pericytes have well defined functions in microvascular systems controlling EC proliferation, sprouting, and stabilization43via factor signaling, regulation of the basement membrane ECM, and cell–cell contact signaling via Notch pathways.2,42 Indeed, pericyte–EC interactions are required for proper basement membrane ECM formation with fibronectin, laminin, vitronectin and collagen IV proteins, which together tune vascular tube formation via EC integrin interactions.35,46 Further, EC integrin sensing of vitronectin drives expression of the antiapoptotic protein Bcl-w and autocrine expression of vascular endothelial growth factor-A (VEGF-A).15,25
Hypoxia inhibits vascular assembly of individual ECs but promotes angiogenesis of ASC-vessels that have at least 4 days of preassembly. In general, hypoxia at physiologic (20 mmHg in bone vs. 160 mmHg in atmospheric air) or slightly lower levels causes an increased amount of EC proliferation and angiogenesis.14 However, EC-vasculature also regresses via apoptosis in response to severe hypoxia (1% or less)10,51 in contrast to the positive angiogenic effects of less severe hypoxia. The idea of a sufficient amount of preassembly is required for hypoxic function supplants the idea that exposure to hypoxia supports vascular growth, via increased expression of VEGF and increased tubule formation.40 These hypoxic vascular-boosting effects have traditionally been observed with vascular explants or other structures that have already been assembled, and not with individual ECs.
These findings suggest that direct implantation of a vascular population may not be sufficient to provide assembled vasculature to the construct before the development of anoxia and associated necrosis. However, this 4 day period of assembly might be achievable in vivo using approaches that can deliver oxygen and other nutrients during the first 4 days of implantation.12 One such approach utilizes oxygen-releasing materials in 3D-printed bone scaffolds, and can sustain delivery periods up to 48 h long.7
While the time-scale of vasculogenesis in fibrin hydrogels depends on the cell types and growth media, the assembly of a robust vascular network can commonly be observed in as little as 6 days in a number of systems.5,34,46 Chen et al. compared vascular fibrin constructs with and without 1 week of preassembly in vivo and found that preassembly accelerated anastomosis with host vasculature, increased proliferation of implanted cells, and increased production of ECM, confirming that a period of preassembly is critical to in vivo use of vascular hydrogels.5 However, it is important to limit the amount of preassembly as vessels may regress without perfusion and other physiological maturation cues.4 Additionally, there are potential benefits to limiting culture time as studies have shown that culture on tissue culture plastic could induce cell fate plasticity.18,45 Perfusion of vascular networks drives their maturation and remodeling26,47 and was not included in the current study. The increased apoptotic rate observed in normoxia might be due to natural pruning/regression as they have matured without a hypoxic or fluid shear stress signal. Remodeling is further driven by the transport demands of the surrounding tissue exceeding the ability of diffusion.14
The distance between individual ECs impacts the paracrine gradient and might have an impact on the rate of vascular assembly. Previous experience23 revealed higher seeding densities lead to a more rapid assembly of vascular networks, perhaps due to the close positioning of ECs to each other at high seeding densities. MacGabhann et al. demonstrated local gradients of VEGF have an effect on vascular assembly in skeletal muscle over distances as short as 10 µm.31 Other groups19,41 have modeled tip cell interaction by pairing computational and experimental models, but highlight that there are additional complicating factors beyond tip distance and VEGF gradients (such as Notch and EC–PC signaling) which add complexity to the system. This gradient sensing and directed vascular growth is increasingly important as systems become more complicated with anatomic geometries and physical barriers to vascular networks, avascular border regions between implant scaffold and host vasculature, and approaches where the cell density is limited.
ECs are highly sensitive to changes in oxygen levels through the hypoxia induced factor family of transcription factors, metabolism, hypoxia-regulated microRNAs, causing upregulation of VEGF and its receptors.14 VEGF is a potent mitogenic for ECs, which supports network assembly via angiogenesis or vasculogenesis.37 While we did not measure variable production of growth factors as a function of preassembly here, previous studies found VEGF-A production by ASC-vessels was only upregulated in severe (0.2% O2) hypoxia after normoxic preassembly for 6 days.21 Such induction of VEGF-A expression might be useful in large scale applications, where despite vascular preassembly, anastomosis and blood flow to interior regions might be delayed, result in network regression, and the associated VEGF-A aiding those regions in undergoing revascularization.
Conclusion
This study sought to elucidate the processes active during ASC-vascular assembly that might support the beneficial response of ASC-vasculature morphogenesis in hypoxia. It builds upon previous work that de novo assembly of ASC-vasculature is inhibited in hypoxia. In ASC-fibrin constructs with less than 4 days of preassembly, hypoxia drove disassembly of vascular structures. However, with a minimum of 4 days preassembly before exposure to hypoxia resulted in robust vasculature network formation, whereupon hypoxia drove increased network formation. The main difference observed between 2 and 4 days of preassembly was a significant amount of pericyte coverage, and inhibition of that coverage similarly inhibited vascular formation. This suggests the interactions between ECs and PCs in de novo vascular assembly is oxygen-mediated.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1 Schematic of pericyte coverage calculation. First the CD31 channel was isolated and the resulting vessels dilated 5 μm. The SMA channel was then isolated and trimmed to the area of dilated vessels, and the trimmed area of SMA relative to the dilated vessel area is reported. Scale bar = 100 μm.
Acknowledgments
The authors thank The Wilmer Imaging and Microscopy Core Grant (P30-EY001765) for use of the Zeiss LSM 510 confocal microscope.
Funding
This Project was funded by the Maryland Stem Cell Research Fund (2014-MSCRFI-0699), NSF CAREER Award (CBET 1350554) awarded to WLG. ELN was supported by an NIH Training Grant.
Conflict of interest
Ethan Nyberg and Warren Grayson declare no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional Research Committee and with the 1964 Helsinki Declaration and its later amendments. No animal research was performed in this study.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Amos PJ, Shang H, Bailey AM, Taylor A, Katz AJ, Peirce SM. IFATS collection: the role of human adipose-derived stromal cells in inflammatory microvascular remodeling and evidence of a perivascular phenotype. Stem Cells. 2008;26:2682–2690. doi: 10.1634/stemcells.2008-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neurooncology. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boquest AC, Noer A, Sørensen AL, Vekterud K, Collas P. CpG methylation profiles of endothelial cell-specific gene promoter regions in adipose tissue stem cells suggest limited differentiation potential toward the endothelial cell lineage. Stem Cells. 2007;25:852–861. doi: 10.1634/stemcells.2006-0428. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CCW, George SC. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng. A. 2009;15:1363–1371. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen RR, Silva EA, Yuen WW, Mooney DJ. Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm. Res. 2007;24:258–264. doi: 10.1007/s11095-006-9173-4. [DOI] [PubMed] [Google Scholar]
- 7.Cook CA, Hahn KC, Morrissette-McAlmon JBF, Grayson WL. Oxygen delivery from hyperbarically loaded microtanks extends cell viability in anoxic environments. Biomaterials. 2015;52:376–384. doi: 10.1016/j.biomaterials.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correia C, Grayson WL, Park M, Hutton D, Zhou B, Guo XE, Niklason L, Sousa RA, Reis RL, Vunjak-Novakovic G. In vitro model of vascularized bone: synergizing vascular development and osteogenesis. PLoS ONE. 2011;6:1–9. doi: 10.1371/journal.pone.0028352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois, S. G., E. Z. Floyd, S. Zvonic, G. Kilroy, X. Wu, S. Carling, Y. D. C. Halvorsen, E. Ravussin, and J. M. Gimble. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. In: Methods in Molecular Biology (Clifton, N.J.). 2008, pp. 69–79. 10.1007/978-1-60327-169-1_5. [DOI] [PubMed]
- 10.Ezhilarasan R, Mohanam I, Govindarajan K, Mohanam S. Glioma cells suppress hypoxia-induced endothelial cell apoptosis and promote the angiogenic process. Int. J. Oncol. 2007;30:701–707. [PMC free article] [PubMed] [Google Scholar]
- 11.Fan H, Zeng X, Wang X, Zhu R, Pei G. Efficacy of prevascularization for segmental bone defect repair using beta-tricalcium phosphate scaffold in rhesus monkey. Biomaterials. 2014;35:7407–7415. doi: 10.1016/j.biomaterials.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 12.Farris A, Rindone A, Grayson W. Oxygen delivering biomaterials for tissue engineering. J. Mater. Chem. B. 2016 doi: 10.1039/C5TB02635K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer LJ, McIlhenny S, Tulenko T, Golesorkhi N, Zhang P, Larson R, Lombardi J, Shapiro I, DiMuzio PJ. Endothelial differentiation of adipose-derived stem cells: effects of endothelial cell growth supplement and shear force. J. Surg. Res. 2009;152:157–166. doi: 10.1016/j.jss.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev. Cell. 2009;16:167–179. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K, Dc W. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2012;118:2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gawlitta D, Fledderus JO, van Rijen MHPP, Dokter I, Alblas J, Verhaar MC, Dhert WJAA. Hypoxia impedes vasculogenesis of in vitro engineered bone. Tissue Eng. A. 2012;18:208–218. doi: 10.1089/ten.tea.2010.0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffith CK, George SC. The effect of hypoxia on in vitro prevascularization of a thick soft tissue. Tissue Eng. A. 2009;15:2423–2434. doi: 10.1089/ten.tea.2008.0267. [DOI] [PubMed] [Google Scholar]
- 18.Guimarães-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, Rockenstein E, Masliah E, Peterson KL, Stallcup WB, Chen J, Evans SM. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017 doi: 10.1016/j.stem.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashambhoy YL, Chappell JC, Peirce SM, Bautch VL, MacGabhann F. Computational modeling of interacting VEGF and soluble VEGF receptor concentration gradients. Front. Physiol. 2011;2:62. doi: 10.3389/fphys.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helmlinger G, Yuan F, Dellian M, Jain Rakesh. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 21.Hutton DL, Grayson WL. Hypoxia inhibits de novo vascular assembly of adipose-derived stromal/stem cell populations but promotes growth of pre-formed vessels. Tissue Eng. A. 2015;22:1–26. doi: 10.1089/ten.tea.2015.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutton DL, Kondragunta R, Moore EM, Hung BP, Jia X, Grayson WL. Tumor necrosis factor improves vascularization in osteogenic grafts engineered with human adipose-derived stem/stromal cells. PLoS ONE. 2014;9:1–9. doi: 10.1371/journal.pone.0107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutton DLD, Logsdon EEA, Moore EME, MacGabhann F, Gimble JM, Grayson WL. Vascular morphogenesis of adipose-derived stem cells is mediated by heterotypic cell–cell interactions. Tissue Eng. A. 2012;18:1729–1740. doi: 10.1089/ten.tea.2011.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutton DL, Moore EM, Gimble JM, Grayson WL. Platelet-derived growth factor and spatiotemporal cues induce development of vascularized bone tissue by adipose-derived stem cells. Tissue Eng. A. 2013;19:2076–2086. doi: 10.1089/ten.tea.2012.0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isik FF, Gibran NS, Jang YC, Sandell L, Schwartz SM. Vitronectin decreases microvascular endothelial cell apoptosis. J. Cell. Physiol. 1998;175:149–155. doi: 10.1002/(SICI)1097-4652(199805)175:2<149::AID-JCP4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 26.Jain RK. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 27.Kelly-Goss MR, Sweat RS, Stapor PC, Peirce SM, Murfee WL. Targeting pericytes for angiogenic therapies. Microcirculation. 2014;21:345–357. doi: 10.1111/micc.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laschke MW, Menger MD. Prevascularization in tissue engineering: current concepts and future directions. Biotechnol. Adv. 2016;34:112–121. doi: 10.1016/j.biotechadv.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Laurencin CT, Ambrosio AMA, Borden MD, Cooper JA. Tissue engineering: orthopedic applications. Annu. Rev. Biomed. Eng. 1999;1:19–46. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 30.Lesman A, Koffler J, Atlas R, Blinder YJ, Kam Z, Levenberg S. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials. 2011;32:7856–7869. doi: 10.1016/j.biomaterials.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 31.MacGabhann F, Ji JW, Popel AS. VEGF gradients, receptor activation, and sprout guidance in resting and exercising skeletal muscle. J. Appl. Physiol. 2007;102:722–734. doi: 10.1152/japplphysiol.00800.2006. [DOI] [PubMed] [Google Scholar]
- 32.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, Toro E, Chen AA, Galie PA, Yu X, Chaturvedi R, Bhatia SN, Chen CS. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012;11:768–774. doi: 10.1038/nmat3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 34.Nakatsu MN, Sainson RCA, Aoto JN, Taylor KL, Aitkenhead M, Pérez-del-Pulgar S, Carpenter PM, Hughes CCW. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvasc. Res. 2003;66:102–112. doi: 10.1016/S0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 35.Newman AC, Nakatsu MN, Chou W, Gershon PD, Hughes CCW. The requirement for fibroblasts in angiogenesis: fibroblast-derived matrix proteins are essential for endothelial cell lumen formation. Mol. Biol. Cell. 2011;22:3791–3800. doi: 10.1091/mbc.e11-05-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niemistö A, Dunmire V, Yli-Harja O, Zhang W, Shmulevich I. Robust quantification of in vitro angiogenesis through image analysis. IEEE Trans. Med. Imaging. 2005;24:549–553. doi: 10.1109/TMI.2004.837339. [DOI] [PubMed] [Google Scholar]
- 37.Nör JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am. J. Pathol. 1999;154:375–384. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv. Drug Deliv. Rev. 2011;63:300–311. doi: 10.1016/j.addr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6:241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parenti A, Brogelli L, Filippi S, Donnini S, Ledda F. Effect of hypoxia and endothelial loss on vascular smooth muscle cell responsiveness to VEGF-A: role of flt-1/VEGF-receptor-1. Cardiovasc. Res. 2002;55:201–212. doi: 10.1016/S0008-6363(02)00326-7. [DOI] [PubMed] [Google Scholar]
- 41.Peirce SM, MacGabhann F, Bautch VL. Integration of experimental and computational approaches to sprouting angiogenesis. Curr. Opin. Hematol. 2012;19:184–191. doi: 10.1097/MOH.0b013e3283523ea6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phng L-K, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev. Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int. J. Dev. Biol. 2011;55:261–268. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 44.Rouwkema J, Khademhosseini A. Vascularization and angiogenesis in tissue engineering: beyond creating static networks. Trends Biotechnol. 2016;34:733–745. doi: 10.1016/j.tibtech.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Snippert HJ, Clevers H. Tracking adult stem cells. EMBO Rep. 2011;12:113–122. doi: 10.1038/embor.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Topper JN, Gimbrone MA. Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol. Med. Today. 1999;5:40–46. doi: 10.1016/S1357-4310(98)01372-0. [DOI] [PubMed] [Google Scholar]
- 48.Tsigkou O, Pomerantseva I, Spencer JA, Redondo PA, Hart AR, O’Doherty E, Lin Y, Friedrich CC, Daheron L, Lin CP, Sundback CA, Vacanti JP, Neville C. Engineered vascularized bone grafts. Proc. Natl. Acad. Sci. USA. 2010;107:3311–3316. doi: 10.1073/pnas.0905445107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waters JP, Kluger MS, Graham M, Chang WG, Bradley JR, Pober JS. In vitro self-assembly of human pericyte-supported endothelial microvessels in three-dimensional coculture: a simple model for interrogating endothelial–pericyte interactions. J. Vasc. Res. 2013;50:324–331. doi: 10.1159/000353303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zawicki DF, Jain RK, Schmid-Schoenbein GW, Chien S. Dynamics of neovascularization in normal tissue. Microvasc. Res. 1981;21:27–47. doi: 10.1016/0026-2862(81)90003-0. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Tan Z, Tran ND. Chemical hypoxia-ischemia induces apoptosis in cerebromicrovascular endothelial cells. Brain Res. 2000;877:134–140. doi: 10.1016/S0006-8993(00)02666-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.