Abstract
Background:
Although psychological treatments for social anxiety disorder (SAD) can be highly effective, many individuals do not respond to treatment. Identifying factors associated with improved outcomes can facilitate individualized treatment choices. We investigated whether patterns of neural connectivity predicted treatment responses and whether treatment type, cognitive behavioral therapy (CBT) or acceptance and commitment therapy (ACT), moderated this effect.
Methods:
Participants with SAD (n=34) underwent fMRI prior to treatment and completed implicit and explicit emotion regulation tasks. Neural connectivity measures were estimates of amygdala-prefrontal cortex connectivity. Treatment responder status was defined using the ‘clinically significant change index’ (Loerinc et al., 2015).
Results:
Right amygdala-right ventrolateral prefrontal cortex connectivity during implicit emotion regulation was a significant predictor of treatment response (OR = 9.01, 95% CI = 1.77, 46.0, p = .008). Stronger inverse connectivity was associated with greater likelihood of treatment response. There were no significant neural moderators of treatment response to CBT versus ACT.
Limitations:
The primary limitation of this work was the small sample size which limited the power to detect significant moderation effects, and results should be interpreted as preliminary.
Conclusions:
Amygdala-vlPFC connectivity during affect labeling predicted treatment responder status following CBT or ACT for social anxiety disorder. This suggests that the functioning of neural circuitry supporting emotion regulation capacities may be a ‘gateway’ to receiving benefit from psychological treatments. Future work should aim to replicate this effect in a larger sample and consider methods for enhancing functional connectivity within this circuitry as a potential treatment adjunct.
Keywords: social anxiety disorder, fMRI, amygdala, prefrontal cortex, emotion regulation
Introduction
Although psychological treatments for social anxiety disorder (SAD) can be highly effective for some individuals, a large number of patients (as many as 55%; Loerinc et al., 2015) fail to respond to treatment, or retain residual symptoms or impairment following treatment. The ability to predict which individuals are likely to respond to which treatments not only informs individual treatment choices, but also elucidates the mechanisms of treatments themselves. Existing work in this domain has begun to identify a set of characteristics, determined by self-report, clinician assessment or task performance, that are predictive of responses to Cognitive Behavioral Therapy (CBT) for anxiety disorders (Schneider et al., 2015). Here, we extend this approach to identify neural indices that predict treatment response, an approach which can help to enhance our understanding of the effects of psychological treatment on the brain (Craske, 2014; Holmes et al., 2014).
Previous work investigating the neurobiological basis of anxiety disorders has highlighted disruptions in emotion regulation neural circuitry. The neurobiological model of emotion regulation states that reactivity to emotional stimuli in the amygdala is regulated through top-down connectivity with regions of the prefrontal cortex (PFC; Brühl et al., 2014; Goldin et al., 2009b; Ochsner and Gross, 2005; Zilverstand et al., 2016; Ziv et al., 2013). Supporting this model, previous work has demonstrated that, compared to healthy individuals, patients with SAD show: i) disrupted activation in the amygdala and regions of the prefrontal cortex (for reviews, see Berkman and Lieberman, 2009; Freitas-Ferrari et al., 2010; Kim et al., 2011) and ii) altered amygdala connectivity with vlPFC (Burklund et al., 2014a), dlPFC (Goldin et al., 2009a), vmPFC (Hahn et al., 2011; Sladky et al., 2015; Young et al., 2017) and dACC/mPFC (Demenescu et al., 2013). Emerging evidence implicates this circuitry in mechanisms of treatment response, with studies demonstrating altered connectivity following CBT between amygdala and dmPFC, mOFC and vl/dlPFC (Goldin et al., 2013; Goldin et al., 2014; Månsson et al., 2013). In addition, we previously demonstrated that SAD symptom reduction following either CBT or Acceptance and Commitment Therapy (ACT; another form of behavioral therapy) was associated with enhanced inverse connectivity between vmPFC/vlPFC and amygdala during implicit emotion regulation (Young et al., 2017).
If, as these findings suggest, treatment for SAD works through altering connectivity within the neural circuits associated with emotion regulation, then an individual’s pre-treatment connectivity may impact their likelihood of responding to treatment. No prior studies have assessed the role of emotion regulation in predicting treatment response. Most existing studies have assessed pre-treatment measures of neural activation rather than connectivity and focused on emotional reactivity, rather than regulation. These studies have demonstrated that greater symptom reduction following CBT was associated with greater pre-treatment neural responses to emotional stimuli (emotional faces or rejecting statements) within the anterior cingulate cortex (ACC), dm/vmPFC and areas of occipital and parietal lobe (dACC and dmPFC; Burklund et al., 2017; Doehrmann et al., 2013; Klumpp et al., 2014; Klumpp et al., 2013). The role of amygdala activation in predicting treatment response remains unclear. Symptom reduction was predicted by decreased pre-treatment amygdala reactivity in one study (Klumpp et al., 2014) and increased reactivity in another (Burklund et al., 2017).
Of the two prior studies incorporating connectivity measures, one demonstrated that long-term (1 year) outcomes following internet-delivered CBT for SAD were predicted by decreased pre-treatment amygdala-dACC connectivity during a self-referential criticism task (Månsson et al., 2015). The other, using resting state functional connectivity, found that greater symptom reduction following CBT for SAD was associated with stronger pre-treatment amygdala-ACC connectivity, stronger amygdala connectivity with caudate and putamen, and reduced amygdala connectivity with central sulcus and right temporo-occipital cluster. This study additionally found that greater inferior longitudinal fasciculus (ILF) density (the white matter tract connecting amygdala with early visual areas) prior to treatment predicted greater symptom reduction following treatment (Whitfield-Gabrieli et al., 2016). In general, these findings support a role for activation and connectivity among neural circuitry involved in emotional processing in predicting treatment response, albeit with specific directions and locations of effects varying across task design.
In the current study, we build on this work by addressing two key limitations. First, we assessed neural functional connectivity during emotion regulation, a treatment-relevant process. Both CBT and ACT focus on improving emotion regulation, albeit through different approaches. CBT teaches ‘reappraisal’, the intentional re-framing of negative or unpleasant thoughts or experiences (Craske, 2010). ACT promotes ‘acceptance’, the acknowledgement that emotional experiences are fleeting and can be viewed with a sense of perspective (Hayes et al., 1999). Measuring neural connectivity during emotion regulation allows a more direct investigation of whether treatment-relevant processes predict treatment response (Young and Craske, 2018). Second, previous studies have primarily correlated responses with self-reported symptoms following treatment, or categorized ‘treatment-responders’ as those showing greatest symptom reduction. A more robust measure of treatment response can be obtained through use of a ‘clinically significant change index’ (CSCI) (Loerinc et al., 2015). This approach requires that, in order to be classified as a ‘treatment responder’, an individual must: i) demonstrate a statistically significant reduction in symptoms, and ii) move below threshold for clinical cut-offs in an independent diagnostic evaluation.
The current study aimed to investigate whether connectivity among emotion regulation neural circuitry (amygdala-prefrontal cortex) predicts whether patients with SAD are likely to respond to treatment. A secondary aim was to investigate differential predictors for treatment responses to CBT or ACT.
Methods
Participant details
Full details of the randomized controlled trial for SAD comparing CBT, ACT and a wait-list control group are described elsewhere (Craske et al., 2014). Participants were recruited from flyers, internet and newspaper advertisements and referrals. Procedures were approved by the UCLA Office for the Protection of Human Research Subjects and participants provided informed consent. Participants were aged 18–45 years, English speaking, right-handed, and had a diagnosis of SAD. Exclusion criteria were: history of bipolar disorder, substance-use disorders, suicidality, psychosis or psychiatric hospitalizations; recent modifications to psychotropic medications (within past month for benzodiazepines, past 3-months for SSRIs/SNRIs and heterocyclics); current cognitive or behavioral psychotherapy for an anxiety disorder or recent modifications to other psychotherapies (within past 6 months); and standard MRI contraindications (pregnancy, claustrophobia, non-removable metal). Data analyzed here included 34 participants, 17 who subsequently received CBT and 17 who received ACT (see Supplemental Materials for Consort diagram, full details on participant inclusion and treatment overview). Table 1 presents demographic details of participants.
Table 1.
Demographic and diagnostic details of included participants (LSAS: Liebowitz Social Anxiety Scale; SIAS: Social Interaction Anxiety Scale; SPS: Social Phobia Scale)
| CBT | ACT | Full sample | |
|---|---|---|---|
| N | 17 | 17 | 34 |
| Male | 8 | 7 | 15 |
| Female | 9 | 10 | 19 |
| Age: M (SD) | 26.29 (6.20) | 26.88 (5.07) | 26.59 (5.67) |
| Responder Status | |||
| Responder | 8 | 8 | 16 |
| Non-responder | 9 | 9 | 18 |
| Race/ethnicity | |||
| White (Non-Hispanic/Latino) | 9 | 10 | 19 |
| Asian/Asian-American | 4 | 2 | 6 |
| Hispanic/Latino | 2 | 3 | 5 |
| Multiracial/Other race not specified | 2 | 2 | 4 |
| Baseline symptom scores | |||
| Symptom composite: M (SD) | −0.07 (0.62) | 0.00 (0.83) | −0.01 (0.73) |
| LSAS: M (SD) | 79.94 (17.57) | 85.41 (19.83) | 80.33 (23.15) |
| SIAS: M (SD) | 51.94 (11.51) | 51.29 (11.65) | 50.11 (14.27) |
| SPS: M (SD) | 33.38 (10.95) | 33.18 (12.40) | 32.32 (12.96) |
Assessment measures
Diagnostic evaluations were conducted using the Anxiety Disorders Interview Schedule-IV (ADIS IV; Brown et al., 1994) by trained interviewers. Included participants met DSM-IV criteria for current, principal or co-principal diagnosis of SAD, with a clinical severity rating (CSR) of 4 or higher, indicating clinically significant severity. Symptom severity was assessed using a composite of the total scores of three self-report measures: the Liebowitz Social Anxiety Scale–Self-Report Version, a 24-item measure assessing fear and avoidance of social interactions and performance situations (LSAS-SR; Fresco et al., 2001); the Social Interaction Anxiety Scale, a 20-item measure of cognitive, affective and behavioral reactions to social interaction (SIAS; Mattick and Clarke, 1998); and the Social Phobia Scale, a 20-item measure describing responses to situations or themes related to being observed by others (SPS; Mattick and Clarke, 1998). A composite score was created from the LSAS, SIAS, and SPS to generate a reliable and valid index of social anxiety symptoms (consistent with the main outcome paper for this study; Craske et al., 2014). Z-scores were calculated for each measure at pre-treatment, and standardization was based on pre-treatment means and standard deviations for each subsequent assessment (see Craske et al., 2014 for details). The composite score represented averages of the three measures. In four cases where SIAS and SPS data were missing, self-report scores were based on LSAS data.
fMRI data acquisition
Participants completed an affect labeling task (a measure of implicit emotion regulation; Burklund et al., 2014b) and an explicit emotion regulation task (measuring responses during regulation of social cues; Burklund et al., 2017). See Supplemental Materials for details on MRI acquisition and preprocessing.
Implicit emotion regulation task
In the affect labeling task, participants viewed a series of images of emotional facial expressions and geometric shapes and were asked to complete labeling and matching tasks (affect labeling, gender labeling, affect matching and shape matching). In labeling conditions, participants responded via button press to select which of two words best match the affect or gender of the face displayed (match conditions require selection of matching images rather than matching words). The current study focused on assessment of implicit emotion regulation capacity, as indexed by the contrast of affect label versus gender label. Stimuli were presented in a blocked design, with four blocks of each condition and six trials per block (30s block, 5s per trial, order counterbalanced across participants). Each block was preceded by a 10s fixation cross and a 3s instruction cue.
Explicit emotion regulation task
Participants viewed blocks of video clips from actors saying rejecting, neutral, or positive phrases. They were instructed to imagine that the people in the videos were speaking directly to them. For rejecting videos, participants were asked either to passively “watch” the video, “reappraise by trying to reduce your emotional response”, or “accept by noticing how you are responding”. The current study focused on assessment of: i) explicit ‘CBT-like’ emotion regulation (reappraisal vs. passive viewing of rejecting videos) and ii) explicit ‘ACT-like’ emotion regulation (acceptance vs. passive viewing of rejecting videos). Participants also viewed videos of positive statements and inanimate objects as other control conditions. Stimuli were presented in a blocked design, with two blocks of each condition type and six trials per block (30s block, 5s per trial, order counterbalanced across participants). Each block was preceded by a 5s instructional cue, followed by 11s rating period (during which participants provided ratings of perceived distress, not analyzed here) and a 10s fixation cross.
Data analysis
Clinically significant change index
CSCI (Loerinc et al., 2015) was calculated based on a combination of reliable change index on the SAD symptom composite (described above) and a clinical cut-off on the ADIS-IV. Treatment responders were individuals who achieved both: i) reliable change in self-reported symptomatology on the symptom composite and ii) movement into the non-clinical range of CSRs on the ADIS-IV (a CSR of below 4). The cutoff for reliable change was calculated as the difference between the pre-treatment and post-treatment scores across the whole sample, divided by the standard error of the difference between the two scores (Jacobson and Truax, 1991). If an individual’s score exceeded the cutoff value, it was classified as a reliable change. Non-responders were defined as those who did not achieve a reliable reduction in symptom level and/or did not move below the threshold for clinically-significant CSR.
fMRI connectivity analyses
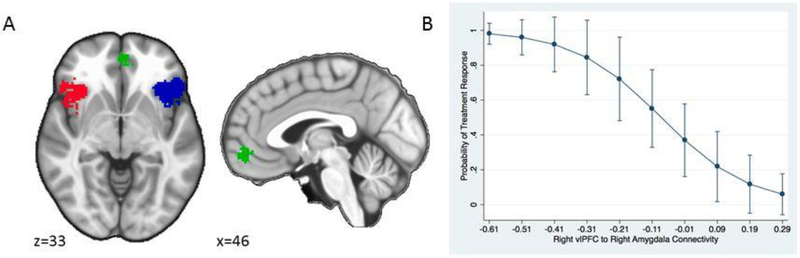
Psychophysiological interaction (PPI) analyses were conducted to assess task-related changes in functional connectivity of left and right amygdala seed regions (generalized PPI toolbox for SPM; McLaren et al., 2012). Amygdala seed regions were defined on the basis of anatomical masks (Automated Anatomical Labelling; AAL). Prefrontal cortical regions of interest (ROIs) were functionally-defined using an automated meta-analytic tool (Neurosynth; www.neurosynth.org; Yarkoni et al., 2011) using the search term ‘emotion regulation’. The resulting ‘forward inference’ map was parsed into prefrontal clusters: ventromedial PFC (vmPFC), right and left ventrolateral PFC (rvlPFC and lvlPFC; see Figure 1). Parameter estimates (β values) were extracted for each ROI using the Marsbar toolbox for SPM (Brett et al., 2002).
Figure 1.
A) Prefrontal cortex regions of interest for connectivity analyses, obtained using Neurosynth displaying right and left vlPFC (red and blue) and vmPFC (green). B) Probability of treatment response (CBT and ACT collapsed) predicted from right vlPFC-right amygdala connectivity (positive numbers indicate positive connectivity and negative numbers indicate negative connectivity)
Statistical analyses
To test amygdala-PFC connectivity as moderators (i.e., CBT vs ACT) and predictors (i.e., CBT and ACT collapsed) of treatment response, we used logistic regression. Connectivity estimate outliers (defined as greater than 2.5 standard deviations above/below the mean) were removed from analyses (n=2 for right amygdala-rvlPFC connectivity). For the moderator analysis, variables in the model were amygdala-PFC connectivity (3 PFC ROIs, 3 task conditions), treatment group, and the connectivity × treatment group interactions. We examined the significance of the interactions. If the moderator analysis was not significant, we removed the interaction from the model and examined significance of the connectivity main effect. To estimate the predictive value of the regression model for unseen data, we used leave-one-out k-fold cross validation. This approach provides an estimate of utility of the regression model for predicting treatment response among participants whose data were not used to estimate the regression model. In leave-one-out k-fold cross validation, all participants except one are used to fit the model, then the model is used to predict the out-of-sample participant’s outcome category. This is then iterated for each participant and used to predict probability of response across the full sample. Accuracy, sensitivity, and specificity can then be calculated by comparing the predicted probabilities estimated from the k-fold cross validation procedure to actual outcome (i.e. response or non-response).
Results
Treatment outcomes
Of the 34 participants included in the analyses, 16 achieved clinically significant change on the CSCI, 18 did not. Response rates were comparable across treatment conditions with eight participants in each group achieving responder status. Of the non-responders, none of the participants moved into the non-clinical diagnostic range on the ADIS (all retained a CSR ≥ 4), although 9 (3 CBT, 6 ACT) achieved significant reductions in self-reported symptomatology on the symptom composite measure.
Predictors of treatment outcomes
Implicit emotion regulation task:
In the moderator analyses, none of the connectivity × treatment group interactions were significant (all p > .050). For the predictor analysis, right vlPFC-right amygdala connectivity predicted clinically significant treatment response such that greater inverse connectivity was associated with better treatment responding regardless of treatment condition (OR = −9.01, 95% CI = −1.77, −46.0, p = .008; see Figure 2). Based on the k-fold cross validation results, right vlPFC-right amygdala connectivity classified participants as treatment responders or not (using ≥ .5 probability as predicting treatment response and < .5 probability as non-response) with 69% accuracy, 67% sensitivity, and 71% specificity. No other connectivity measures were significant predictors of treatment response (all p > .05).
Explicit emotion regulation task:
There were no significant connectivity × treatment group interactions in moderator analyses and no significant connectivity main effects in predictor analyses on this task (all p > .05).
Discussion
Results demonstrated that stronger inverse connectivity between right amygdala and right vlPFC during implicit emotion regulation was associated with a greater likelihood of subsequent response to psychological treatment for SAD, regardless of treatment condition. In cross-validation analysis this connectivity estimate was found to predict treatment response with 69% accuracy. None of the neural connectivity estimates assessed were significant moderators of treatment response, predicting greater likelihood of response to CBT or ACT individually.
Previous work has demonstrated regions of activation or patterns of connectivity in response to emotional stimuli (i.e., emotional reactivity) that predict treatment response. Here we present the first findings, to our knowledge, that demonstrate how patterns of connectivity during emotion regulation predict treatment outcome. Given that current psychological treatments for SAD focus on emotion regulation skills, these analyses directly reflect how baseline capacity in treatment-relevant processes relates to treatment outcomes. Studies of emotional reactivity have highlighted the predictive value of activation in ACC, mPFC and occipital regions as well as connectivity between amygdala and ACC, caudate, putamen, central sulcus, and temporo-occipital regions (Burklund et al., 2017; Doehrmann et al., 2013; Klumpp et al., 2014; Klumpp et al., 2013; Månsson et al., 2015; Whitfield-Gabrieli et al., 2016). This implicates a broad network of regions in which reactivity might be related to treatment outcome. One previous study demonstrated prediction of CBT treatment response, defined solely by symptom reduction, at 81% accuracy using a combination of measures across diffusion MRI, resting state amygdala connectivity and multi-voxel pattern analysis (Whitfield-Gabrieli et al., 2016). Here we demonstrate prediction of clinically significant treatment response at 69% accuracy using a single, theoretically-driven index of connectivity in emotion regulation neural circuitry. This effect was specific to right amygdala-right vlPFC connectivity during implicit emotion regulation predicting response to treatment for social anxiety disorder (combining CBT and ACT).
Inverse prefrontal-amygdala connectivity has been highlighted as an index of emotion regulatory capacity whereby activation of prefrontal regions is thought to facilitate ‘down-regulation’ of amygdala responses (Ochsner et al., 2012). Our findings suggest that those better able to effectively engage this circuitry during implicit emotion regulation at baseline are more likely to achieve better outcomes from behavioral treatment. This effect was observed when collapsing across CBT and ACT groups and was specific to implicit emotion regulation (affect labeling). We did not observe any predictive or moderation effects of connectivity during explicit regulation (reappraisal or acceptance). While both tasks measure emotion regulation, they differ in intentionality. The goal of reappraisal or acceptance (explicit task) is to intentionally reduce emotional reactivity, while affect labeling simply involves recognizing an emotional cue, a more incidental form of regulation (Burklund et al., 2014b). We suggest that connectivity during affect labeling reflects a generalized tendency to engage regulatory circuitry when faced with emotional stimuli, whereas connectivity during explicit regulation reflects intentional engagement of this circuitry.
Alternatively, it is plausible that differences in cognitive load between the affect labeling and explicit regulation tasks impacted engagement of regulatory circuitry. In the affect labeling task, participants choose which of two words best describes the emotion in the face presented, a low cognitive load. In the explicit regulation task, participants are instructed to construct their own reappraisal or acceptance of negative statements, requiring a higher demand on cognitive capacities. A recent study demonstrated that regulatory neural connectivity (orbitofrontal cortex to amygdala functional connectivity) was modulated by the level of cognitive load (using a Stroop task; Minkova et al., 2017). Comparing individuals with SAD to healthy individuals, deficits in regulatory connectivity were observed only when cognitive load was low. There were no group differences observed in the high cognitive load condition, suggesting that regulatory capacity was impacted by attentional demands for all individuals. Therefore, the additional cognitive load of the explicit regulation task may reduce regulatory capacity among all individuals, masking individual differences in the tendency to engage regulatory circuitry. Future comparison of these possibilities would be of much interest, particularly given that the cognitive load of reappraisal and acceptance is likely reduced following treatment, where these skills are extensively practiced.
Nonetheless, our results suggest that only the extent to which emotion regulation neural circuitry is incidentally engaged (during low cognitive load) is predictive of subsequent treatment response. In previous work, we demonstrated that enhanced inverse connectivity within this circuitry was associated with symptom reduction following treatment, collapsing across CBT and ACT groups (Young et al., 2017). Together, this would suggest a ‘building on strengths’ model (Engebretson et al., 1989; Rude and Rehm, 1991), such that individuals with greater capacity to implicitly regulate their emotions may benefit more from psychological treatment.
Given that CBT teaches reappraisal-based emotion regulation and ACT teaches acceptance-based regulation, the explicit emotion regulation task allowed direct assessment of treatment-relevant mechanisms. It might be expected that connectivity during reappraisal would predict treatment responses to CBT and connectivity during acceptance would predict treatment response to ACT. However, this moderation effect was not significant in the current analyses. It is plausible that there are treatment-specific differences in activation or connectivity outwith the primary network of emotion regulation neural circuitry investigated here. However, it should be noted that the current sample size may be too small to sufficiently assess treatment-moderator effects and that with a larger sample, neural moderators of treatment response may be observed.
Methodological strengths
This study has a number of methodological strengths that build upon findings from previous work. First, data were collected in the context of a high-quality randomized controlled trial using manualized treatment interventions with a high level of therapist adherence to protocol and validity in assessment methods (Craske et al., 2014). Second, the use of the CSCI as a measure of treatment responder status provides a reliable and conservative index, ensuring a clinically meaningful definition of this term. It should be noted that whereas other studies have reported higher response rates to CBT for SAD, the response rate in this study (47%) is typical when a CSCI is used (Loerinc et al., 2015).
Limitations
The primary limitation of this work was the sample size, although it is consistent with prior studies in this field (Doehrmann et al., 2013; Whitfield-Gabrieli et al., 2016). We had limited power to detect significant moderators of treatment response, such as baseline capacity to engage emotion regulation neural circuitry during instructed reappraisal predicting better outcomes to CBT over ACT. In addition, we used a ‘treatment completer’ analysis, rather than an ‘intent-to-treat’ analysis, which has been criticized due to the exclusion of data from individuals who did not fully comply or did not complete treatment (Gupta, 2011).
Future directions
Although it is not currently feasible to incorporate functional MRI assessments into standard clinical care, this evidence highlights the relevance of emotion regulation neural circuitry in individual responses to treatment conditions. Future work might consider what characteristics of the non-responder group might be better targeted in future treatment. Other methods of enhancing functional connectivity within this neural circuitry might also be investigated (e.g., transcranial direct current stimulation; Kuo et al., 2014) which may be particularly effective for individuals with low baseline connectivity. In addition, future studies should employ larger samples to permit more highly powered analyses of treatment moderators.
Supplementary Material
Highlights.
Many individuals fail to respond to gold-standard psychological treatments
Identification of factors that predict outcomes can inform treatment development
This work showed that amygdala-PFC connectivity predicted treatment response
This suggests a key role for emotion regulation neural circuitry
Future work might investigate how to enhance functioning within this circuitry
Acknowledgments
The authors would like to thank the participants involved in the study as well as staff and student members of the Anxiety and Depression Research Center (ADRC) at UCLA who contributed to the running of this study.
Role of the Funding source
This project was funded by the National Institutes of Mental Health 1 R21 MH081299 (PIs: Craske, Lieberman and Taylor). This funding source had no role in the execution of the study, the analyses, interpretation of the data, or decision to submit results.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest
References
- Berkman ET, Lieberman MD, 2009. Using Neuroscience to Broaden Emotion Regulation: Theoretical and Methodological Considerations. Social and personality psychology compass 3, 475–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B, 2002. Region of interest analysis using an SPM toolbox 8th International Conference on Functional Mapping of the Human Brain. NeuroImage, Sendai, Japan. [Google Scholar]
- Brown TA, Barlow DH, Di Nardo PA, 1994. Anxiety Disorders Interview Schedule for DSM-IV (ADIS IV): Client Interview Schedule. Graywind Publications Incorporated. [Google Scholar]
- Brühl AB, Delsignore A, Komossa K, Weidt S, 2014. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neuroscience & Biobehavioral Reviews 47, 260–280. [DOI] [PubMed] [Google Scholar]
- Burklund LJ, Craske MG, Taylor SE, Lieberman MD, 2014a. Altered emotion regulation capacity in social phobia as a function of comorbidity. Social cognitive and affective neuroscience 10, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burklund LJ, Creswell JD, Irwin MR, Lieberman MD, 2014b. The common and distinct neural bases of affect labeling and reappraisal in healthy adults. Frontiers in psychology 5, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burklund LJ, Torre JB, Lieberman MD, Taylor SE, Craske MG, 2017. Neural responses to social threat and predictors of cognitive behavioral therapy and acceptance and commitment therapy in social anxiety disorder. Psychiatry Research: Neuroimaging 261, 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, 2010. Cognitive behavior therapy. Ametican Psychological Association, Washington, DC. [Google Scholar]
- Craske MG, 2014. Introduction to special issue: How does neuroscience inform psychological treatment? Behaviour research and therapy 62, 1. [DOI] [PubMed] [Google Scholar]
- Craske MG, Niles AN, Burklund LJ, Wolitzky-Taylor KB, Vilardaga JC, Arch JJ, Saxbe DE, Lieberman MD, 2014. Randomized controlled trial of cognitive behavioral therapy and acceptance and commitment therapy for social phobia: outcomes and moderators. J Consult Clin Psychol 82, 1034–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demenescu L, Kortekaas R, Cremers H, Renken R, van Tol M, van der Wee N, Veltman D, den Boer J, Roelofs K, Aleman A, 2013. Amygdala activation and its functional connectivity during perception of emotional faces in social phobia and panic disorder. Journal of psychiatric research 47, 1024–1031. [DOI] [PubMed] [Google Scholar]
- Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F, Keshavan A, Triantafyllou C, Saygin ZM, Whitfield-Gabrieli S, Hofmann SG, 2013. Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. JAMA psychiatry 70, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebretson TO, Matthews KA, Scheier MF, 1989. Relations between anger expression and cardiovascular reactivity: Reconciling inconsistent findings through a matching hypothesis. Journal of Personality and Social Psychology 57, 513. [DOI] [PubMed] [Google Scholar]
- Freitas-Ferrari MC, Hallak JE, Trzesniak C, Santos Filho A, Machado-de-Sousa JP, Chagas MHN, Nardi AE, Crippa JAS, 2010. Neuroimaging in social anxiety disorder: a systematic review of the literature. Progress in Neuro-Psychopharmacology and Biological Psychiatry 34, 565–580. [DOI] [PubMed] [Google Scholar]
- Fresco D, Coles M, Heimberg RG, Liebowitz M, Hami S, Stein M, Goetz D, 2001. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychological medicine 31, 1025–1035. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ, 2009a. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biological psychiatry 66, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ, 2009b. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of general psychiatry 66, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Hahn K, Heimberg R, Gross JJ, 2013. Impact of cognitive behavioral therapy for social anxiety disorder on the neural dynamics of cognitive reappraisal of negative self-beliefs: randomized clinical trial. JAMA psychiatry 70, 1048–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Ziv M, Jazaieri H, Weeks J, Heimberg RG, Gross JJ, 2014. Impact of cognitive-behavioral therapy for social anxiety disorder on the neural bases of emotional reactivity to and regulation of social evaluation. Behaviour research and therapy 62, 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, 2011. Intention-to-treat concept: a review. Perspectives in clinical research 2, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E, Kasper S, Lanzenberger R, 2011. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage 56, 881–889. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG, 1999. Acceptance and commitment therapy. Guilford Press, New York. [Google Scholar]
- Holmes EA, Craske MG, Graybiel AM, 2014. A call for mental-health science. Nature 511, 287. [DOI] [PubMed] [Google Scholar]
- Jacobson NS, Truax P, 1991. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. Journal of consulting and clinical psychology 59, 12. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ, 2011. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural brain research 223, 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald D, Angstadt M, Post D, Phan K, 2014. Neural response during attentional control and emotion processing predicts improvement after cognitive behavioral therapy in generalized social anxiety disorder. Psychological medicine 44, 3109–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald DA, Phan K, 2013. Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry 45, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M-F, Paulus W, Nitsche MA, 2014. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. Neuroimage 85, 948–960. [DOI] [PubMed] [Google Scholar]
- Loerinc AG, Meuret AE, Twohig MP, Rosenfield D, Bluett EJ, Craske MG, 2015. Response rates for CBT for anxiety disorders: need for standardized criteria. Clinical psychology review 42, 72–82. [DOI] [PubMed] [Google Scholar]
- Månsson KN, Carlbring P, Frick A, Engman J, Olsson C-J, Bodlund O, Furmark T, Andersson G, 2013. Altered neural correlates of affective processing after internet-delivered cognitive behavior therapy for social anxiety disorder. Psychiatry Research: Neuroimaging 214, 229–237. [DOI] [PubMed] [Google Scholar]
- Månsson KN, Frick A, Boraxbekk C-J, Marquand A, Williams S, Carlbring P, Andersson G, Furmark T, 2015. Predicting long-term outcome of Internet-delivered cognitive behavior therapy for social anxiety disorder using fMRI and support vector machine learning. Translational psychiatry 5, e530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Clarke JC, 1998. Development and validation of measures of social phobia scrutiny fear and social interaction anxiety. Behaviour research and therapy 36, 455–470. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC, 2012. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 61, 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkova L, Sladky R, Kranz GS, Woletz M, Geissberger N, Kraus C, Lanzenberger R, Windischberger C, 2017. Task-dependent modulation of amygdala connectivity in social anxiety disorder. Psychiatry Research: Neuroimaging 262, 39–46. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ, 2005. The cognitive control of emotion. Trends in cognitive sciences 9, 242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT, 2012. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences 1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rude SS, Rehm LP, 1991. Response to treatments for depression: The role of initial status on targeted cognitive and behavioral skills. Clinical Psychology Review 11, 493–514. [Google Scholar]
- Schneider RL, Arch JJ, Wolitzky-Taylor KB, 2015. The state of personalized treatment for anxiety disorders: a systematic review of treatment moderators. Clinical psychology review 38, 39–54. [DOI] [PubMed] [Google Scholar]
- Sladky R, Spies M, Hoffmann A, Kranz G, Hummer A, Gryglewski G, Lanzenberger R, Windischberger C, Kasper S, 2015. (S)-citalopram influences amygdala modulation in healthy subjects: a randomized placebo-controlled double-blind fMRI study using dynamic causal modeling. NeuroImage 108, 243–250. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ghosh S, Nieto-Castanon A, Saygin Z, Doehrmann O, Chai X, Reynolds G, Hofmann S, Pollack M, Gabrieli J, 2016. Brain connectomics predict response to treatment in social anxiety disorder. Molecular psychiatry. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD, 2011. Large-scale automated synthesis of human functional neuroimaging data. Nature methods 8, 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KS, Burklund LJ, Torre JB, Saxbe D, Lieberman MD, Craske MG, 2017. Treatment for social anxiety disorder alters functional connectivity in emotion regulation neural circuitry. Psychiatry Research: Neuroimaging 261, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KS, Craske MG, 2018. The Cognitive Neuroscience of Psychological Treatment Action in Depression and Anxiety. Current Behavioral Neuroscience Reports 5, 13–25. [Google Scholar]
- Zilverstand A, Parvaz MA, Goldstein RZ, 2016. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. NeuroImage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv M, Goldin PR, Jazaieri H, Hahn KS, Gross JJ, 2013. Emotion regulation in social anxiety disorder: behavioral and neural responses to three socio-emotional tasks. Biology of mood & anxiety disorders 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.