Abstract
Introduction
Intracellular reactive oxygen species (ROS) and nitric oxide (NO) levels are associated with vascular homeostasis and diseases. Exercise can modulate ROS and NO production through increasing frequency and magnitude of wall shear stress (WSS). However, the details of ROS and NO production in endothelial cells and their interplay under WSS induced by exercise at different intensities remain unclear.
Methods
In this study, we developed an in vitro multicomponent nonrectangular flow chamber system to simulate pulsatile WSS waveforms induced by moderate and high intensity exercise. Furthermore, the dynamic responses of ROS and NO in endothelial cells and the relationship between ROS and NO were investigated under the WSS induced by different intensity exercise.
Results
After exposing to WSS induced by moderate intensity exercise, endothelial cells produced more NO than those under high intensity exercise-induced WSS. In this process, ROS was found to play a dual role in the generation of intracellular NO. Under WSS induced by moderate intensity exercise, modest elevated ROS promoted NO production, whereas excessive ROS in endothelial cells exposed to WSS induced by high intensity exercise attenuated NO bioavailability. Interestingly, antioxidant N-acetylcysteine (NAC) could increase NO production under WSS induced by high intensity exercise.
Conclusions
Our results provide some cues for selecting appropriate exercise intensities and elevating benefits of exercise on endothelial function. Additionally, owing to the consistency of our results and some in vivo phenomena, this flow chamber system may serve as an in vitro exercise model of arterial vessel for future studies.
Keywords: Exercise, Wall shear stress (WSS), Reactive oxygen species (ROS), Nitric oxide (NO), Endothelial cells
Introduction
Wall shear stress (WSS) is the viscous force generated by flowing blood through vessels,37 which can be affected by various physiological events, such as exercise and other physical activities. The WSS modulated by these physiological events exhibits different magnitude and frequency and is constantly imposed on the endothelial cells lining the inside of the vessels. Mechanosensors in the endothelial cells can sense and distinguish these different WSS signals,13 and transduce them into biochemical signals inside the cells to modulate the release of intracellular signaling molecules and vasoactive substances, such as reactive oxygen species (ROS) and nitric oxide (NO). Therefore, WSS plays a critical role in the physiology and pathophysiology processes of endothelium.8,16,27,40
A number of studies have revealed that NO is involved in vascular function improvement, platelet activation prevention, and leukocyte adhesion to the endothelium.7,11,22 ROS participates in endothelial cells responses to WSS and acts as a signal transduction messenger.10 It is noteworthy that moderate concentration of ROS is implicated with intracellular normal biological events, such as cell survival, proliferation, and post-translational protein modifications, whereas a high level or excessive level of ROS can trigger intracellular oxidative stress and further lead to endothelial dysfunction.26,32 Therefore, more and more cardiovascular disease therapies are hence associated with NO production and ROS reduction.9,28 Previous studies have also demonstrated that ROS and NO production in endothelial cells is determined by the pattern and magnitude of WSS.18,20 Steady or non-reversing sinusoidal WSS usually produces more ROS and less NO than oscillatory WSS. Under steady WSS, intracellular ROS and NO levels are elevated with moderate increase of WSS magnitude in the physiological range.19 More importantly, the generation of intracellular ROS is related to the amount of NO production, and vice versa. Specifically, elevated ROS level results in the decrease of NO bioavailability and enhanced NO level leads to the reduction of ROS,20 thus suggesting that the interplay between ROS and NO also plays a critical role in maintaining normal endothelial function.
Appropriate exercise is an effective strategy to ameliorate endothelial function and maintain vascular homeostasis.3,14 Birk et al.5 showed that acute lower limb cycle training can improve NO-mediated vasodilation in brachial artery. Hambrecht et al.15 reported that 4 weeks of regular aerobic exercise elevates the expression and phosphorylation of endothelial nitric oxide synthase (eNOS) in the left internal mammary artery. In these in vivo studies, exercise-induced changes in WSS, including the increases in both magnitude and frequency caused by simultaneous increases of stroke volume and heart rates,36 have been found to be a primary signal in mediating the improvement of the endothelial function.4,23,24,38,39
In the vascular system, exercise not only affects WSS, it can also elevate vessel blood pressure and cyclic stretch, and affect autonomic nervous system activity. In order to exclude the influence of these factors and determine the key role of increased WSS in the responses of endothelial cells, the parallel-plate flow chamber system or the microfluidic chamber system has been commonly adopted to mimic in vivo WSS.9,19,23 By means of the parallel-plate flow chamber system, Hsieh et al.19 and Kim et al.23 investigated the intracellular ROS levels when WSS was increased. Chin et al.9 studied intracellular ROS release under the concurrent increase in frequency and magnitude of WSS using a microfluidic chamber system. However, the effects of increased frequency or/and magnitude of WSS on the dynamic response of ROS and NO and the interaction between them still remain unknown. Furthermore, in vivo studies have suggested that endothelial function largely relies on the exercise intensity.2,12 Battault et al.2 showed that continuous high-intensity exercise does not improve endothelial function primarily due to redox-dependent eNOS uncoupling, which is the cause of abundant ROS production in aortic tissue of spontaneous hypertensive rats. Therefore, the dynamic response of ROS and NO in endothelial cells subjected to WSS induced by different intensity exercise and the underlying mechanisms require further investigation.
In the abovementioned studies, the flow chamber system or the microfluidic chamber system consisted of only one key component, i.e., the cells culture chamber, which was not capable of reproducing exercise-induced WSS profiles.19,23 In our previous studies, we proposed a multicomponent flow chamber system constituted by a parallel-plate flow chamber and other accessory components including dampener, liquid controller, elastic chamber and resistance valve, which can better mimic the in vivo WSS waveform induced by exercise.36 However, the flow chamber used in our previous system had a constant rectangular cross-section, which results in one level of WSS generated in the cell culture chamber during each experiment. Therefore, this design is time-consuming when the effect of WSS magnitude on cellular responses is studied. In the present study, in order to increase experimental efficiency, a nonrectangular flow chamber aided by other peripheral components was developed for generating multiple magnitudes of WSS at one frequency. Meanwhile, different intensities of pulsatile WSS as found in the common carotid artery in resting state and during moderate and high intensity exercise were reproduced. Further, after exposing human umbilical vein endothelial cells (HUVECs) to WSS at different combinations of frequency and magnitude, intracellular ROS and NO production was detected, and the relationship between ROS and NO was also investigated. In addition, the underlying mechanism regarding to eNOS coupling/uncoupling under different WSS profiles was also illustrated.
Materials and Methods
Multicomponent Nonrectangular Parallel-Plate Flow Chamber System
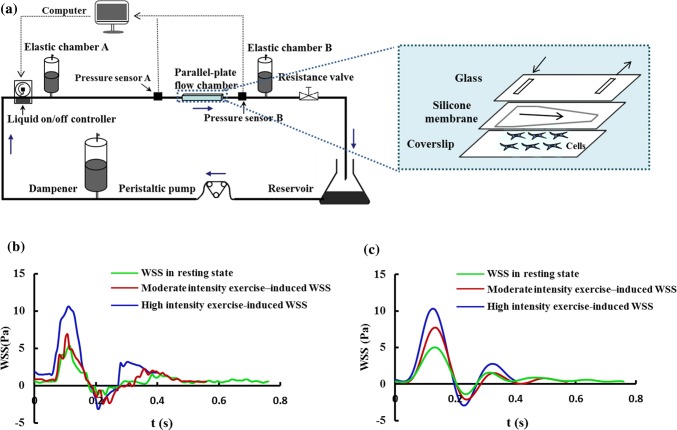
Our multicomponent nonrectangular parallel-plate flow chamber system (Fig. 1a) consists of a nonrectangular parallel-plate flow chamber, peripheral components and monitoring system, all of which are connected to the flow circuit with silicone tubes. The inside of the nonrectangular flow chamber comprises a front rectangle channel with a width of 1.53 cm and a length of 1 cm, and a subsequent trapezoid channel with a width of 1.53 cm at the beginning and gradual narrower until reaching 0.51 cm at the end across a length of 4.18 cm. This flow chamber is formed by a silicone gasket with the constant height of 0.05 cm as well as a piece of glass and a coverslip.
Figure 1.
(a) Schematic diagram of the parallel-plate flow chamber system and internal structure diagram of the parallel-plate flow chamber. (b) In vivo WSS waveforms induced by different exercise intensity. (c) In vitro WSS waveforms produced by the flow chamber system.
The flow chamber creates a uniform spatial WSS profile in the rectangle channel and a gradual increasing spatial WSS profile in the subsequent trapezoid channel. The design of the rectangle channel in the entrance is suitable for forming the uniform flow field and the trapezoid channel is convenient in finding appropriate WSS in a certain range through changing position at the bottom of the channel instead of varying the flow rate.
The peripheral components comprise a reservoir, a peristaltic pump, a dampener, a liquid on/off controller, an elastic chamber (A and B) and a resistance valve, the details of which were described in our previous work.36 The monitoring system possesses two pressure sensors (A and B) located in the upstream entrance and downstream exit of the flow chamber. The pressure difference between two sensors, namely, the pressure drop in the flow chamber, is displayed in real time on the computer.
Reproduction of WSS in the Common Carotid Artery
The hemodynamic parameters of a healthy volunteer in his resting state and immediately after moderate (55% maximal heart rate) and high (75% maximal heart rate) intensity exercise were measured by means of the methods described in the previous studies.25 These hemodynamic parameters included the inner diameter and the center-line blood flow velocity of the right common carotid artery, as well as the heart rates, brachial systolic and diastolic pressures. Subsequently, based on the above measurements, the WSS waveforms in these three distinct states were calculated with the elastic tube theoretical model (Fig. 1b).35 The study was approved by the Ethics Committee of Dalian University of Technology and was carried out in accordance with the Declaration of Helsinki (1964). Written informed consents were acquired from the volunteer before participation.
In order to simulate the above calculated WSS waveforms in vivo (Fig. 1b), the rotating rate of the peristaltic pump, the frequency of liquid on/off controller, the elastic chambers and the resistance valve were independently adjusted to produce the in vitro WSS profiles (Fig. 1c) in the flow chamber.
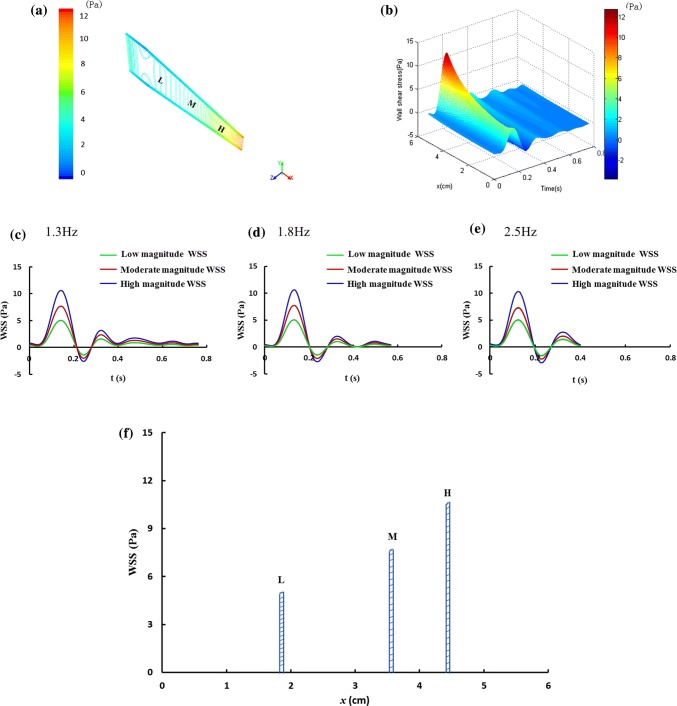
During the calculation of WSS profiles in the flow chamber, a computational fluid dynamics (CFD) simulation was first performed with the commercial software package ANSYS Fluent 14.5 (AnsysInc, 2012a). The finite element model of the flow chamber was built and its mesh was generated. The culture medium perfused in the flow chamber was modeled with the Navier–Stokes equation as an incompressible Newtonian fluid. The density and the viscosity of the perfused culture medium were set to 1 g/cm3 and 1 × 10−3 Pa s, respectively. The no-slip boundary condition was applied on the wall. The measured pressure drop profiles with different frequency and magnitude were given as the inlet boundary condition using the user-defined function and the pressure at the outlet was set to 0 Pa. Based upon the simulation results, the pressure drop profiles generated by the in vitro model were repeatedly adjusted to fit the WSS waveforms in vivo as well as possible. Finally, the WSS with different levels of frequency and magnitude, and the positions of WSS at the bottom surface of the flow chamber were found. During the experiments, we used three observation positions, i.e., x = 1.83–1.87 cm (L), 3.53–3.57 cm (M), and 4.38–4.42 cm (H), along the central axis at the bottom of the flow chamber (Fig. 2a) for detecting intracellular ROS and NO responses.
Figure 2.
WSS produced at the bottom of the flow chamber. (a) WSS equipotential lines at the bottom of the flow chamber at one time point. L represents low magnitude WSS (− 1.38 Pa to 5.02 Pa) area, M represents moderate magnitude WSS (− 2.10 Pa to 7.73 Pa) area, H represents high magnitude WSS (− 2.92 Pa to 10.31 Pa) area. (b) WSS distribution along the central axis at the bottom of flow chamber at 1.3 Hz, (c–e) WSS waveforms with different frequency and magnitude imposed on the cells. (f) WSS maximum in the three observation areas (L, M, H) along the central axis at the bottom of the flow chamber.
Cells Culture
Human umbilical vein endothelial cells (HUVECs) were purchased from ATCC. HUVECs were grown in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco, USA) and 1% (v/v) penicillin/streptomycin (PS, Gibco, USA) in a humidified atmosphere at 37 °C with 5% CO2 and 95% air. The cells at a density of around 3 × 105 cells/ml were seeded on the sterilized fibronectin-coated (Sigma, USA) coverslips.
WSS Stimulation Experiments
When cells reached approximate 80% confluency, the coverslip with cells was assembled into the flow chamber, and then connected to the flow chamber system on a super-clean worktable. Afterwards, the system was moved into the cells incubator. After adjusting and actuating the system, the cells were subjected to different patterns of WSS, including resting state WSS, WSS induced by moderate and high intensity exercise for different stimulation time periods. Before and after different time periods of WSS stimulation, intracellular ROS and NO were examined.
Intracellular ROS Detection
The generation of the intracellular ROS was detected with the Dihydroethidium (DHE, Vigorous Biotechnology, Beijing, China). Prior to WSS stimulation, the cells cultured on the coverslips were incubated in the culture medium with 50 μM fluorescent probe for 30 min. After the WSS experiments, the cells were washed with PBS for three times. Finally, the fluorescence intensity representing the concentration of intracellular ROS was determined by fluorescence microscopy (Olympus, Japan) with the excitation wavelength at 485 nm and the emission wavelength at 535 nm. For each WSS or no WSS (control group) condition, about 50 cells were examined to acquire the mean fluorescence intensity, and each condition was repeated at least three times. The intracellular fluorescence intensity was calculated after subtracting the background fluorescence using MATLAB (The Math Work R2010b, Inc.). All the experimental results were normalized by the maximal fluorescence intensity and eventually expressed as a fraction of the maximum value.
Intracellular NO Detection
NO is generated from the substrate of l-arginine under eNOS activation in HUVECs. 3-Amino,4-aminomethy1-2′,7′-difluorescein, diacetate (DAF-FM DA, Beyotime, China) was used to measure intracellular NO generation. The methods of detecting intracellular NO and calculating NO fluorescence intensity were similar to that of ROS. One difference was that the incubation concentration of DAF-FM DA was 15 μM, and the other was that the fluorescence excitation and emission wavelengths were 495 nm and 515 nm, respectively.
Statistical Analysis
All the experimental values were presented as means ±standard deviations (SD). Normal distribution of data was evaluated by the non-parametric Kolmogorov–Smirnov test. In case of normal distribution, the Student’s t test was used to analyze the statistical significance of two groups, and the analysis of variance (ANOVA) followed by Tukey post hoc tests were used for comparison of multiple groups of data. A value of p < 0.05 was considered statistically significant.
Results
The in vitro WSS Profiles at the Bottom of the Flow Chamber
The CFD simulation results provided the WSS equipotential lines at the bottom of the flow chamber at one time point (Fig. 2a) and the WSS distribution along the central axis at the bottom of flow chamber at 1.3 Hz (Fig. 2b). Based on the simulation results, the positions (L, M and H in Fig. 2a) and profiles (Figs. 2c–2e) of three magnitudes of WSS at the bottom of the flow chamber at three frequencies, i.e., 1.3 Hz, 1.8 Hz and 2.5 Hz were established. Herein, the magnitudes low WSS at the 1.3 Hz, 1.8 Hz and 2.5 Hz were approximately identical, and the magnitudes of moderate and high WSS at these three frequencies were also similar, respectively (Figs. 2c–2e). The WSS with the low magnitude (− 1.38 Pa to 5.02 Pa) and the frequency of 1.3 Hz, the moderate magnitude (− 2.10 Pa to 7.73 Pa) and the frequency of 1.8 Hz, as well as the high magnitude (− 2.92 Pa to 10.31 Pa) and the frequency of 2.5 Hz were corresponding to the resting state, moderate and high intensity exercise, respectively. In addition, as shown by Figs. 1b and 1c and Table 1, these in vitro WSS waveforms (Fig. 1c) generated by the flow chamber system were similar to those measured on the common carotid artery in vivo (Fig. 1b). Notably, the WSS maximum variation rates (Fig. 2f) across the observation areas (width, 400 μm) were small (about 1%). Therefore, the effects of these WSS gradients on the cells’ responses were not considered.
Table 1.
Characteristic values of the in vivo and in vitro WSS waveforms in resting state and immediately after moderate and high intensity exercise.
| State | WSS waveforms | Frequency/Hz | Maximum/Pa | Minimum/Pa |
|---|---|---|---|---|
| Resting state | In vivo WSS | 1.3 | 5.30 | − 1.25 |
| In vitro WSS | 1.3 | 5.02 | − 1.38 | |
| Moderate intensity exercise | In vivo WSS | 1.8 | 7.32 | − 2.36 |
| In vitro WSS | 1.8 | 7.73 | − 2.10 | |
| High intensity exercise | In vivo WSS | 2.5 | 10.63 | − 3.06 |
| In vitro WSS | 2.5 | 10.31 | − 2.92 |
Intracellular ROS and NO Dynamics Under WSS with Different Frequency and Magnitude
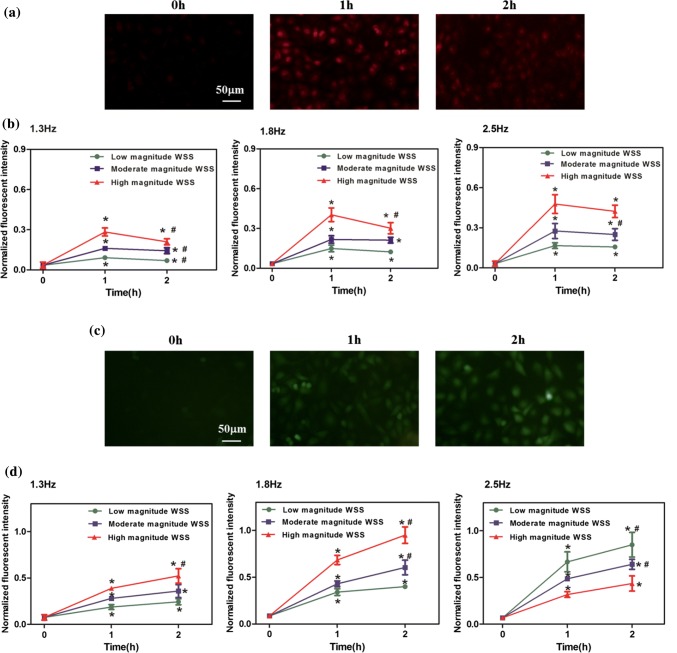
After exposing endothelial cells to nine types of WSS waveforms (Figs. 2c–2e) for different durations, we examined the intracellular ROS and NO levels, expressed as the intracellular ROS and NO fluorescence intensities. The WSS with different magnitudes and frequencies all resulted in remarkable rises in ROS and NO levels for 1 and 2 h compared with 0 h (Fig. 3). It is noted that prolonged exposure of endothelial cells to WSS from 1 h up to 2 h led to the reduction of ROS level (Figs. 3a and 3b) and the sustained increase of NO level (Figs. 3c and 3d). These findings suggest that the WSS in the present study elicits NO generation in a time-dependent manner, whereas intracellular ROS augments first and then attenuates after being exposed to WSS for an extended period of time.
Figure 3.
Intracellular ROS and NO levels subjected to WSS with different frequencies and magnitudes for 0, 1 and 2 h. Representative fluorescence images of intracellular ROS (a) and NO (c) stimulated by the WSS waveforms with 2.5 Hz and moderate magnitude for 0, 1 and 2 h. Time courses of the normalized fluorescent intensity of ROS (b) and NO (d). n = 4 per groups. *p < 0.05 vs. 0 h, #p < 0.05 vs. 1 h.
The Effects of Exercise-Induced WSS on the Production of ROS and NO
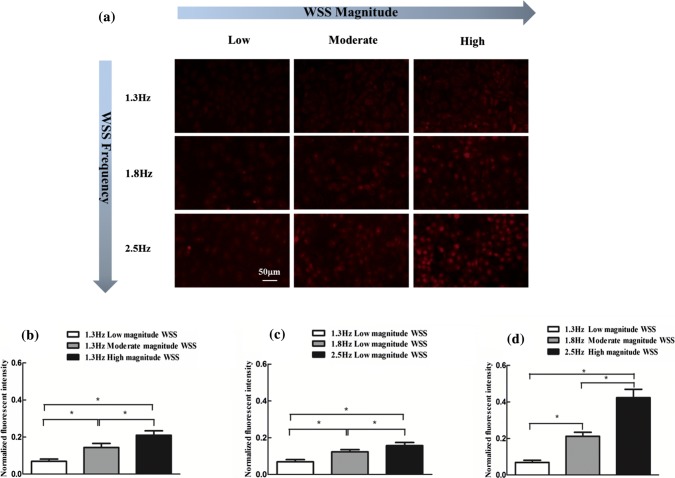
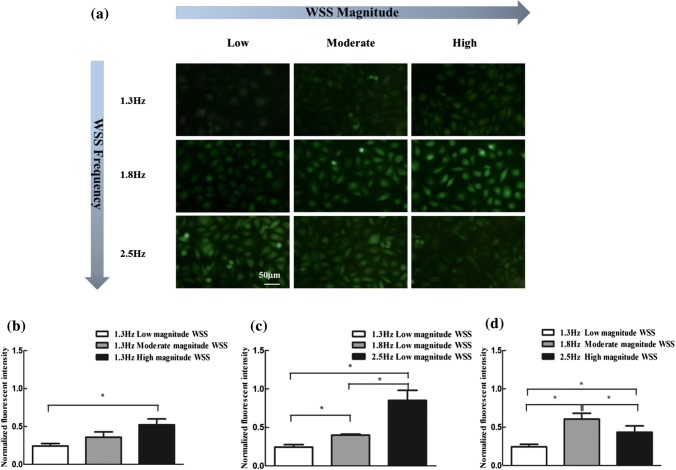
Exercise causes an increase in both magnitude and frequency of arterial WSS. To further assess the effects of increased frequency and/or magnitude of WSS on the ROS and NO generation, the intracellular fluorescence intensities of ROS and NO under the corresponding WSS conditions were examined (Figs. 4a and 5a) and calculated (Figs. 4b–4d and 5b–5d). It is clear from Figs. 4a–4c that the increase in either frequency or magnitude alone led to the conspicuous elevation of ROS production. Moreover, the increases in both frequency and magnitude of WSS, that is, the WSS induced by moderate and high intensity exercise amplified this elevation of ROS generation (Fig. 4d). Besides, the increase in WSS frequency alone at low magnitude WSS significantly augmented NO production (Figs. 5a and 5b), and the increase in WSS magnitude alone at 1.3 Hz significantly elevating ROS production only occurred in high magnitude case (Figs. 5a and 5c). The intracellular NO subjected to the WSS induced by moderate and high intensity exercise was higher than this under the WSS in resting state (Figs. 5a and 5d). However, compared with the moderate intensity exercise-induced WSS, intracellular NO level decreased after exposing the cells to WSS induced by high intensity exercise (Fig. 5d). These findings show that moderate and high intensity exercise promotes ROS generation in an intensity-dependent manner, and moderate intensity exercise is more advantageous to NO generation than high intensity exercise.
Figure 4.
ROS production respond to WSS with frequency or/and magnitude increase for 2 h. (a) Representative fluorescence images of intracellular ROS. (b) ROS production with the increase of magnitude of WSS at fixed frequency. (c) ROS production with the increase of frequency of WSS at fixed magnitude. (d) ROS production with the both increase in frequency and magnitude of WSS. n = 3 per group. *p < 0.05.
Figure 5.
NO production respond to WSS with frequency or/and magnitude increase for 2 h. (a) Representative fluorescence images of intracellular NO. (b) NO production with the increase of magnitude of WSS at fixed frequency. (c) NO production with the increase of frequency of WSS at fixed magnitude. (d) NO production with the both increase in frequency and magnitude of WSS. n = 3–4 per group. *p < 0.05.
Interaction Between ROS and NO in HUVECs Exposed to WSS in Different States
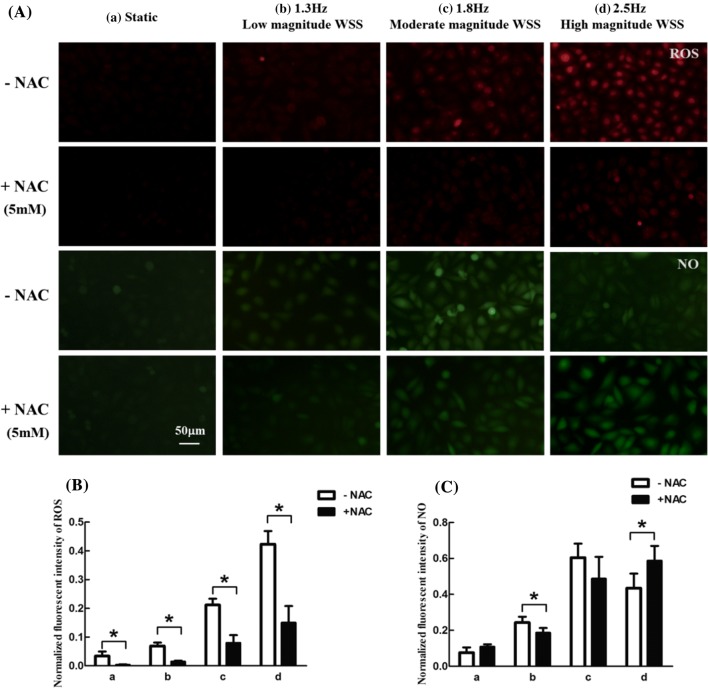
The interaction between ROS and NO generation drives pathophysiological processes of endothelial cells in response to WSS. Therefore, the correlation between ROS and NO in HUVECs exposed to different levels of WSS, corresponding to rest, moderate and high intensity exercise was investigated. To explore the role of ROS in NO production, the endothelial cells were pretreated with ROS inhibitor N-acetylcysteine (NAC, Beyotime, China) dissolved in culture medium at 5 mM for 1 h. Then, the cells were incubated with 50 μM DHE or 15 μM DAF-FM DA for 30 min. Subsequently, the cells were exposed to different levels of WSS for 2 h. As shown in Figs. 6A and 6B, intracellular ROS was significantly decreased with the treatment of NAC in all WSS conditions. Furthermore, the level of NO in cells was remarkably decreased under the frequency of 1.3 Hz and low magnitude WSS, and significantly enhanced under the frequency of 2.5 Hz and high magnitude WSS (Figs. 6A and 6C). This result implies that the amount of ROS play a critical role in the intracellular NO level under WSS induced by different intensity exercise.
Figure 6.
Effects of NAC on ROS and NO generation in endothelial cells subjected to different WSS conditions for 2 h. (A) Representative fluorescence images of intracellular ROS and NO. (B) Normalized fluorescent intensity of ROS. (C) Normalized fluorescent intensity of NO. (a) Static (without WSS stimulation), (b) 1.3 Hz and low magnitude WSS, (c) 1.8 Hz and moderate magnitude WSS, (d) 2.5 Hz and high magnitude WSS. n = 3–4 per group. *p < 0.05.
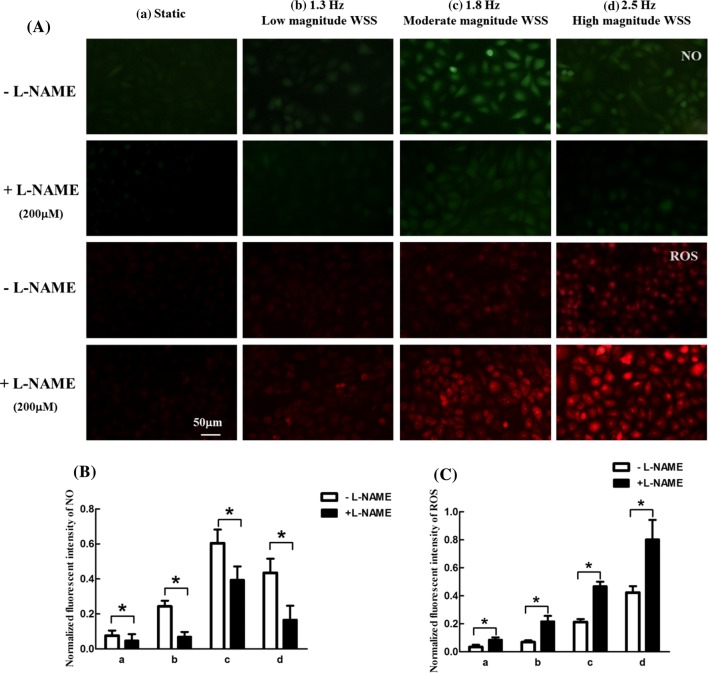
To further determine whether WSS-induced ROS upregulation was affected by NO levels, the intracellular NO and ROS levels were respectively measured by cell fluorescence images after treatment with eNOS blocker Nω-nitro-l-arginine methyl ester (L-NAME, Beyotime, China) dissolved in culture medium at 200 μM for 1 h. The results showed that intracellular NO were conspicuously decreased (Figs. 7A and 7B) and intracellular ROS level was remarkably unregulated (Figs. 7A and 7C) after L-NAME treatment in all WSS conditions, indicating that an elevated ROS level is associated with the diminished NO after exposing the cells to different WSS conditions.
Figure 7.
Effects of L-NAME on NO and ROS generation in endothelial cells exposed to different WSS conditions for 2 h. (A) Representative fluorescence images of intracellular NO and ROS.(B) Normalized fluorescent intensity of NO. (C) Normalized fluorescent intensity of ROS. (a) Static, (b) 1.3 Hz and low magnitude WSS, (c) 1.8 Hz and moderate magnitude WSS, (d) 2.5 Hz and high magnitude WSS. n = 3 per group. *p < 0.05.
Discussion
Our designed multicomponent nonrectangular parallel-plate flow chamber system is capable of replicating different frequencies and magnitudes of pulsatile WSS waveforms in the common carotid artery under the state of resting, as well as moderate and high intensity exercise (Fig. 1). In this system, the targeted frequency of WSS was achieved through modulating the parameter of liquid on/off controller, and the desired pulsatile profile and magnitude of WSS were realized by the combined action of the peristaltic pump, the liquid on/off controller, the elastic chamber, and the resistance valve. Due to the spatial gradient in the flow chamber, a certain range of WSS magnitudes was produced at one frequency. Therefore, on the one hand, this model with gradient WSS provides more chances for finding the desired magnitude of WSS without changing the flow rate or the pressure drop within the same flow chamber; on the other hand, it offers a convenient and high-efficiency platform for investigating the role of WSS magnitude variation in endothelial cells responses. Accordingly, the experimental durations and reagent dosages can be mitigated to one-third compared with the rectangle flow chamber in the study of the responses of cells exposed to low, moderate and high magnitude WSS at one frequency.
Elevated ROS and decreased NO in endothelial cells are implicated in cardiovascular diseases, and the production of them can be modulated by WSS.6 Hence, a series of studies have been conducted to examine the relationship between the steady WSS and the production of ROS or NO.7 Hsieh et al.20 demonstrated that steady flow WSS (15, 25, or 40 dyne/cm2 for 15 min) resulted in a 0.5–1.5-fold elevation in intracellular ROS levels. Hsiai et al.18 pointed out that unidirectional and positive forward pulsatile flow WSS upregulates eNOS expression and then elevates NO production. Moreover, the generation of NO increased continuously by steady or pulsatile flow in the physiological range.20 In the present study, both intracellular ROS and NO levels were elevated after the cells were exposed to pulsatile WSS profiles with different frequency and magnitude. Notably, the production of ROS in endothelial cells decreased to a certain degree after the cells were exposed to all profiles of WSS for 2 h in comparison to this for 1 h (Figs. 3a and 3b), which is in accordance with the result of Chin et al. under the pulsatile WSS at 15 and 30 dyne/cm2.9 However, different from the ROS production, the generation of intracellular NO exhibited continuous increase during the 2 h period (Figs. 3c and 3d), similar to the previous study by Hsieh et al.20 This phenomenon may be attributed to the increase of NO which suppresses the ROS generation through eNOS/NO pathway at longer periods of time.7,20 The result that eNOS inhibitor L-NAME increased ROS production (Figs. 7A and 7C) in all WSS conditions supports this hypothesis.
Exercise triggers an increase in both the frequency and magnitude of WSS,38,39 and its impact on the endothelium is largely dependent on the characteristic parameters of WSS modulated by exercise intensity.2,21 We found that either frequency increase or magnitude increase could result in the rise of ROS and NO levels in an intensity-dependently manner. Moreover, the combined effect of simultaneous increased WSS frequency and magnitude, namely, the WSS conditions induced by moderate and high intensity exercise further augmented intracellular ROS levels, and the intracellular ROS level showed positive correlation with the frequency and magnitude of WSS induced by exercise intensity (Figs. 4a and 4d). However, as to NO production, WSS induced by high intensity exercise generated a 28% decrease compared with moderate intensity exercise (Figs. 5a and 5d). Previous studies reported that moderate intensity exercise increases NO production and ameliorates NO-mediated endothelium-dependent vasodilation.17,31 Battault et al.2 demonstrated that NO can react with O2− and produce peroxynitrite in high intensity exercise of spontaneous hypertensive rats. Goto et al.12 found that oxidative stress leads to the reduced NO bioavailability in high intensity exercise training. Hence, these in vivo results and our data may conclude that appropriate elevated ROS related with moderate intensity exercise serves as a potent cellular messenger which participates in the increased production of NO.21,33,34 However, redundant ROS associated with high intensity exercise may react with NO to form cytotoxic peroxynitrite and then attenuates NO bioavailability; furthermore, overproduction of ROS which exceeds the anti-oxidative capability of a human body will elicit oxidative stress and then diminish NO production.1,29
To further validate the aforementioned hypothesis in vitro, we pretreated the cells with ROS inhibitor NAC. As expected, the intracellular NO level exhibited significant decrease under the WSS in resting state and marked increase under the WSS induced by high intensity exercise (Figs. 6A and 6C), suggesting that modest ROS as a second messenger is important for the generation of NO. Furthermore, the NO level in cells pretreated with NAC under the WSS induced by high intensity exercise was close to the NO level with or without NAC treatment under the WSS induced by moderate intensity exercise (Fig. 6C), indicating that antioxidant can further reinforce endothelial function after high intensity exercise. This result is consistent with a previous in vivo study by Silvestro et al., who showed that the supplementation of Vitamin C increases flow-mediated dilation following maximal exercise.30 As previously described, NO level notably decreased (Figs. 7A and 7B) and intracellular ROS levels remarkably rose (Figs. 7A and 7C) after preprocessed with eNOS inhibitor L-NAME in all WSS conditions in our study, implying that intracellular NO production inhibits the generation of ROS.7 This result also demonstrated that most of eNOS are coupled in the presence of abundant ROS, i.e., eNOS does not transfer electron to O2 to further increase the ROS production and reduces NO production.34 However, Battault et al.2 showed that high intensity continuous exercise can induce eNOS uncoupling in spontaneous hypertensive rats. It seems likely that the present frequency or magnitude of WSS induced by the high intensity exercise is not large enough. Thus, the ROS level under the WSS induced by moderate and high intensity exercise insufficiently oxidizes eNOS substrates (l-arginine) or cofactors, such as tetrahydrobiopterin (BH4), flavin adenine dinucleotide (FAD) to trigger eNOS uncoupling.7 This result may also partially explain why this high intensity exercise produces less NO than moderate intensity exercise but still induces more NO production than the resting state (Fig. 5d). Nevertheless, the detailed mechanisms of intracellular ROS increase and NO decrease under the WSS induced by high intensity exercises till need to be further investigated.
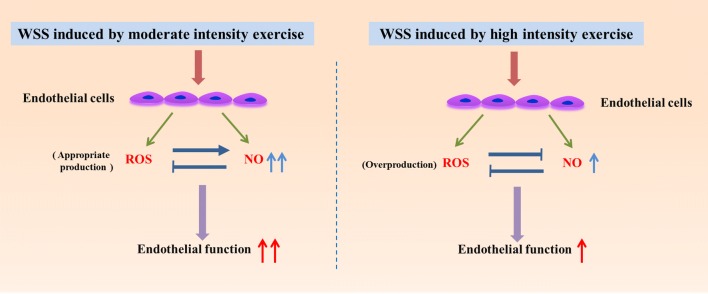
In conclusion, a multicomponent nonrectangular parallel-plate flow chamber system was developed to effectively mimic the in vivo-like pulsatile WSS induced by different intensity exercises, which may serve as an in vitro exercise WSS model of arterial vessels. The experimental results showed that WSS induced by moderate intensity exercise was associated with the significant enhancement of NO production, possibly ascribed to the appropriate increase of ROS. Nevertheless, WSS induced by high intensity produced less NO than moderate intensity exercise likely due to the abundant generation of ROS reacting with NO (Fig. 8). It is concluded that ROS plays a dual role in NO production and NO can inhibit ROS production under WSS induced by different intensity exercise. Furthermore, the supplement of antioxidant reverses the adverse effect of overproduction of ROS under the high intensity exercise. Therefore, our results provide preliminary cellular and molecular mechanisms to understand why moderate intensity exercise is better than high intensity exercise in improving endothelial function and why the supplement of antioxidants is salutary in high intensity exercise.
Figure 8.
WSS induced by moderate and high intensity exercise improves endothelial function through increasing NO production. Appropriate amount of ROS promote intracellular NO production under moderate intensity exercise induced WSS. However, as compared to moderate intensity exercise, NO production was relatively decreased under WSS induced by high intensity exercise possibly ascribed to the abundant generation of ROS.
Acknowledgments
The research described in this paper was supported in part by the National Natural Science Foundation of China (Grant Nos. 31370948, 11672065) and the Fundamental Research Funds for the Central Universities in China (Grant No. DUT18JC15).We would like to thank Prof. Wenyu Liu for kindly revising the manuscript.
Conflict of Interest
Yan-Xia Wang, Hai-Bin Liu, Peng-Song Li, Wen-Xue Yuan, Bo Liu, Shu-Tian Liu, Kai-Rong Qin declare no conflicts of interest.
Ethical Approval
All human subjects research was carried out in accordance with the Helsinki Declaration of 1975, as revised in 2000 (5) and approved by the Ethics Committee of Dalian University of Technology. No animal studies were carried out by the authors for this article.
References
- 1.Aruoma OI. Characterization of drugs as antioxidant prophylactics. Free Radic. Biol. Med. 1996;20(5):675–705. doi: 10.1016/0891-5849(95)02110-8. [DOI] [PubMed] [Google Scholar]
- 2.Battault S, Singh F, Gayrard S, Zoll J, Reboul C, Meyer G. Endothelial function does not improve with high-intensity continuous exercise training in SHR: implications of eNOS uncoupling. Hypertens. Res. 2016;39(2):70. doi: 10.1038/hr.2015.114. [DOI] [PubMed] [Google Scholar]
- 3.Beck DT, Martin JS, Casey DP, Braith RW. Exercise training improves endothelial function in resistance arteries of young prehypertensives. J. Hum. Hypertens. 2014;28(5):303. doi: 10.1038/jhh.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, Goodrich R, Mills T, Deeter L, Sargsyan A, Babu PVA, Graham TE, Symons JD. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can. J. Physiol. Pharmacol. 2014;92(7):605–612. doi: 10.1139/cjpp-2014-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birk GK, Dawson EA, Atkinson C, Haynes A, Cable NT, Thijssen DH, Green DJ. Brachial artery adaptation to lower limb exercise training: role of shear stress. J. Appl. Physiol. 2012;112(10):1653–1658. doi: 10.1152/japplphysiol.01489.2011. [DOI] [PubMed] [Google Scholar]
- 6.Blackman BR, Garcıa-Cardena G, Gimbrone MA. A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J. Biomech. Eng. 2002;124(4):397–407. doi: 10.1115/1.1486468. [DOI] [PubMed] [Google Scholar]
- 7.Chao Y, Ye P, Zhu L, Kong X, Qu X, Zhang J, Luo J, Yang H, Chen S. Low shear stress induces endothelial reactive oxygen species via the AT1R/eNOS/NO pathway. J. Cell. Physiol. 2018;233(2):1384–1395. doi: 10.1002/jcp.26016. [DOI] [PubMed] [Google Scholar]
- 8.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am. J. Physiol Heart Circ. Physiol. 2007;292(3):H1209–H1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 9.Chin LK, Yu JQ, Fu Y, Yu T, Liu AQ, Luo KQ. Production of reactive oxygen species in endothelial cells under different pulsatile shear stresses and glucose concentrations. Lab Chip. 2011;11(11):1856–1863. doi: 10.1039/c0lc00651c. [DOI] [PubMed] [Google Scholar]
- 10.Foncea R, Carvajal C, Almarza C, Leighton F. Endothelial cell oxidative stress and signal transduction. Biol. Res. 2000;33(2):89. doi: 10.4067/S0716-97602000000200008. [DOI] [PubMed] [Google Scholar]
- 11.Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459(6):923–939. doi: 10.1007/s00424-010-0808-2. [DOI] [PubMed] [Google Scholar]
- 12.Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans: role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108(5):530–535. doi: 10.1161/01.CIR.0000080893.55729.28. [DOI] [PubMed] [Google Scholar]
- 13.Gray KM, Stroka KM. Vascular endothelial cell mechanosensing: new insights gained from biomimetic microfluidic models. Semin. Cell Dev. Biol. 2017;71:106–117. doi: 10.1016/j.semcdb.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Green DJ, Eijsvogels T, Bouts YM, Maiorana AJ, Naylor LH, Scholten RR, Spaanderman MEA, Pugh CJA, Sprung VS, Schreuder T, Jones H, Cable T, Hopman MTE, Thijssen DHJ. Exercise training and artery function in humans: nonresponse and its relationship to cardiovascular risk factors. J. Appl. Physiol. 2014;117(4):345–352. doi: 10.1152/japplphysiol.00354.2014. [DOI] [PubMed] [Google Scholar]
- 15.Hambrecht R, Adams V, Erbs S, Linke A, Kränkel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107(25):3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 16.Han Y, Wang L, Yao QP, Zhang P, Liu B, Wang GL, Shen BR, Chen BC, Wang YX, Jiang ZL, Qi YX. Nuclear envelope proteins Nesprin2 and LaminA regulate proliferation and apoptosis of vascular endothelial cells in response to shear stress. Biochim. Biophys. Acta. 2015;1853(5):1165–1173. doi: 10.1016/j.bbamcr.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higashi Y, Yoshizumi M. New methods to evaluate endothelial function: method for assessing endothelial function in humans using a strain-gauge plethysmography: nitric oxide-dependent and-independent vasodilation. J. Pharmacol. Sci. 2003;93(4):399–404. doi: 10.1254/jphs.93.399. [DOI] [PubMed] [Google Scholar]
- 18.Hsiai TK, Hwang J, Barr ML, Correa A, Hamilton R, Alavi M, Rouhanizadeh M, Hazen SL. Hemodynamics influences vascular peroxynitrite formation: Implication for low-density lipoprotein apo-B-100 nitration. Free Radic Biol. Med. 2007;42(4):519–529. doi: 10.1016/j.freeradbiomed.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh HJ, Cheng CC, Wu ST, Chiu JJ, Wung BS, Wang DL. Increase of reactive oxygen species (ROS) in endothelial cells by shear flow and involvement of ROS in shear-induced c-fos expression. J. Cell. Physiol. 1998;175(2):156–162. doi: 10.1002/(SICI)1097-4652(199805)175:2<156::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh HJ, Liu CA, Huang B, Tseng AH, Wang DL. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 2014;21(1):3. doi: 10.1186/1423-0127-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson BD, Padilla J, Wallace JP. The exercise dose affects oxidative stress and brachial artery flow-mediated dilation in trained men. Eur. J. Appl. Physiol. 2012;112(1):33–42. doi: 10.1007/s00421-011-1946-8. [DOI] [PubMed] [Google Scholar]
- 22.Kang H, Fan Y, Deng X. Vascular smooth muscle cell glycocalyx modulates shear-induced proliferation, migration, and NO production responses. Am. J. Physiol. Heart Circ. Physiol. 2010;300(1):H76–H83. doi: 10.1152/ajpheart.00905.2010. [DOI] [PubMed] [Google Scholar]
- 23.Kim B, Lee H, Kawata K, Park JY. Exercise-mediated wall shear stress increases mitochondrial biogenesis in vascular endothelium. PLoS ONE. 2014;9(11):e111409. doi: 10.1371/journal.pone.0111409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laughlin MH, McAllister RM. Exercise training-induced coronary vascular adaptation. J. Appl. Physiol. 1992;73(6):2209–2225. doi: 10.1152/jappl.1992.73.6.2209. [DOI] [PubMed] [Google Scholar]
- 25.Liu HB, Yuan WX, Qin KR, Hou J. Acute effect of cycling intervention on carotid arterial hemodynamics: basketball athletes versus sedentary controls. Biomed. Eng. Online. 2015;14(1):S17. doi: 10.1186/1475-925X-14-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol Cell Physiol. 2001;280(4):C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 27.Pan S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid. Redox Signal. 2009;11(7):1669–1682. doi: 10.1089/ars.2009.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schirmer SH, Degen A, Baumhäkel M, Custodis F, Schuh L, Kohlhaas M, Friedrich E, Bahlmann F, Kappl R, Maack C, Böhm M. Heart-rate reduction by If-channel inhibition with ivabradine restores collateral artery growth in hypercholesterolemic atherosclerosis. Eur. Heart J. 2011;33(10):1223–1231. doi: 10.1093/eurheartj/ehr255. [DOI] [PubMed] [Google Scholar]
- 29.Shafique E, Torina A, Liu Y, Feng J, Benjamin L, Harrington E, Sellke F, Abid R. Oxidant-induced endothelial dysfunction is a failure of the mitochondria to process cytosolic ROS. Eur. J. Pharmacol. 2003;480(1–3):43–50. [Google Scholar]
- 30.Silvestro A, Scopacasa F, Oliva G, De CT, Iuliano L, Brevetti G. Vitamin C prevents endothelial dysfunction induced by acute exercise in patients with intermittent claudication. Atherosclerosis. 2002;165(2):277–283. doi: 10.1016/S0021-9150(02)00235-6. [DOI] [PubMed] [Google Scholar]
- 31.Sun MW, Zhong MF, Gu J, Qian FL, Gu JZ, Chen H. Effects of different levels of exercise volume on endothelium-dependent vasodilation: roles of nitric oxide synthase and heme oxygenase. Hypertens. Res. 2008;31(4):805. doi: 10.1291/hypres.31.805. [DOI] [PubMed] [Google Scholar]
- 32.Takabe W, Jen N, Ai L, Hamilton R, Wang S, Holmes K, Dharbandi F, Khalsa B, Bressler S, Barr ML. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxid. Redox Signal. 2011;15(5):1379. doi: 10.1089/ars.2010.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka LY, Bechara LRG, dos Santos AM, Jordão CP, de Sousa LGO, Bartholomeu T, Ventura LI, Laurindo FRM, Ramires PR. Exercise improves endothelial function: a local analysis of production of nitric oxide and reactive oxygen species. Nitric Oxide. 2015;45:7–14. doi: 10.1016/j.niox.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Thomas S, Kotamraju S, Zielonka J, Harder DR, Kalyanaraman B. Hydrogen peroxide induces nitric oxide and proteosome activity in endothelial cells: a bell-shaped signaling response. Free Radic. Biol. Med. 2007;42(7):1049–1061. doi: 10.1016/j.freeradbiomed.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang YX, Wang Y, Li SQ, Aziz URA, Liu ST, Qin KR. The analysis of wall shear stress modulated by acute exercise in the human common carotid artery with an elastic tube model. Comput. Model. Eng. 2018;116(2):127–147. [Google Scholar]
- 36.Wang YX, Xiang C, Liu B, Zhu Y, Luan Y, Liu ST, Qin KR. A multi-component parallel-plate flow chamber system for studying the effect of exercise-induced wall shear stress on endothelial cells. Biomed. Eng. Online. 2016;15(2):154. doi: 10.1186/s12938-016-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto K, Ando J. Endothelial cell and model membranes respond to shear stress by rapidly decreasing the order of their lipid phases. J. Cell Sci. 2013;126(5):1227–1234. doi: 10.1242/jcs.119628. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Friedman MH. Adaptive response of vascular endothelial cells to an acute increase in shear stress magnitude. Am. J. Physiol. Heart Circ. Physiol. 2012;302(4):H983. doi: 10.1152/ajpheart.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Friedman MH. Adaptive response of vascular endothelial cells to an acute increase in shear stress frequency. Am. J. Physiol. Heart Circ. Physiol. 2013;305(6):H894–H902. doi: 10.1152/ajpheart.00174.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Li YS, Chien S. Shear stress-initiated signaling and its regulation of endothelial function significance. Arterioscler. Thromb. Vasc. Biol. 2014;34(10):2191–2198. doi: 10.1161/ATVBAHA.114.303422. [DOI] [PMC free article] [PubMed] [Google Scholar]