Abstract
Introduction
The neural stem cell (NSC) niche is a highly complex cellular and biochemical milieu supporting proliferating NSCs and neural progenitor cells (NPCs) with close apposition to the vasculature, primarily comprised of endothelial cells (ECs). Current in vitro models of the niche incorporate EC-derived factors, but do not reflect the physiologically relevant hemodynamic state of the ECs or the spatial resolution observed between cells within the niche.
Methods
In this work, we developed a novel in vitro model of the niche that (1) incorporates ECs cultured with fluid shear stress and (2) fosters paracrine cytokine gradients between ECs and NSCs in a spatiotemporal configuration mimicking the cytoarchitecture of the subventricular niche. A modified cone and plate viscometer was used to generate a shear stress of 10 dynes cm−2 for ECs cultured on a membrane, while statically cultured NPCs are 10 or 1000 μm below the ECs.
Results
NPCs cultured within 10 μm of dynamic ECs exhibit increased PSA-NCAM+ and OLIG2+ cells compared to progenitors in all other culture regimes and the hemodynamic EC phenotype results in distinct progeny phenotypes. This co-culture regime yields greater release of pro-neurogenic factors, suggesting a potential mechanism for the observed progenitor maturation.
Conclusions
Based on these results, models incorporating ECs exposed to shear stress allow for paracrine signaling gradients and regulate NPC lineage progression with appropriate niche spatial resolution occurring at 10 μm. This model could be used to evaluate cellular or pharmacological interactions within the healthy, diseased, or aged brain.
Electronic supplementary material
The online version of this article (10.1007/s12195-017-0516-5) contains supplementary material, which is available to authorized users.
Keywords: Neural stem cells, Vascular niche, Shear stress, Neurogenesis, Endothelial cells
Introduction
Neural stem cells (NSCs) are multipotent progenitors capable of undergoing neurogenesis within a highly-vascularized niche located within the adult central nervous system (CNS). The subventricular zone (SVZ) is one such NSC niche comprised of germinal cells that make contact with both endothelial cells (ECs) forming the microvasculature and ependymal cells lining the lateral ventricle. SVZ NSCs, also known as type B cells, are elongated cells that span and make contact with both the ependymal layer and the SVZ endothelium. They produce rapidly-dividing transit amplifying type C cells, which form the majority of the neural progenitor cell (NPC) population.13 Type C cells give rise to type A neuroblasts that migrate along the rostral migratory stream to the olfactory bulb where they differentiate into neurons.13 While both NSC and NPC populations are present, within this work we will refer to cells as NPCs, a blanket term that includes NSCs and more restricted progenitor cells. Progression through this lineage is highly regulated by the cellular and biochemical milieu, which contains localized domains within the niche. For example, epidermal growth factor (EGF) increases in concentration away from the ependymal layer toward the vasculature, while stromal derived factor 1 (SDF-1) and fibroblast growth factor 2 (FGF2) are highest near the ependymal layer and vasculature creating a u-shaped gradient with lower concentrations centralized within the niche.15,35,38 Localized gradients of cytokines released by support cells are believed to help maintain lineage progression as precursor cells move away from the ependymal wall and towards the rostral migratory stream. In the presence of SDF-1, active type B and C cells up-regulate their expression of α6 and EGFR allowing them to bind more efficiently to the laminin-rich vasculature and increase migration.35 SDF-1 sensitivity is also evident in type A neuroblasts, resulting in increased expression of the α6 integrin subunit necessary for laminin binding, helping these cells migrate out of the niche along the vasculature to the olfactory bulb.35 Similarly, NPCs increase β1 expression necessary for neurosphere formation, as well as substrate attachment in response to FGF2.62 Presumably an up-regulation of the β1 integrin subunit would also assist in migration of NPCs within the niche.
Recapitulation of these gradients in vitro would be difficult to achieve with exogenous growth factors, as there is an incomplete characterization of the biochemical composition and corresponding gradients within the niche required for lineage progression. Adding to this complexity, many growth factors, such as EGF and FGF2, have short half-lives and require stabilization to prevent degradation. Proteoglycans exist in the niche where they stabilize, sequester, and regulate receptor binding of FGF2 and EGF.4,5,8,26,32,33,45,54 NSCs and ECs have also been shown to secrete proteoglycans as free floating or matrix bound constitutes of the extracellular space in vitro and can stabilize soluble factors.27,31,52,59,64 We have previously show the mBend EC cell line can produce glycosaminoglycans, a primary constituent of proteoglycans, and that production is increased by the culturing of these cells under dynamic fluid flow.16 In this work we propose a co-culture model, wherein dynamically cultured ECs provide growth factors, as well as stabilizing proteoglycans to recapitulate complex soluble factor gradients to NSCs in vitro.
NPCs and EC have a highly interdependent relationship. In vivo, NPCs are known to preferentially proliferate approximately 10 μm from the microvessels in the SVZ.57,63 In the absence of cell–cell contact, endothelial-produced soluble factors in vitro can maintain self-renewal of both adult and embryonic NSC.19,40,56 Neuronal differentiation is promoted upon removal of the endothelial factors from embryonic NSCs56 or through direct cell–cell contact.19 Isolated vascular-derived factors, such as neurotrophin-3, have been shown to maintain NSC quiescence within the niche.11 Direct EC contact has also been shown to maintain NSC quiescence within the niche through endothelial expression of ephrinB2 and Jagged1.46 Furthermore, NSCs can modulate ECs through paracrine signaling. Li et al. demonstrated the paracrine signaling between NSCs and ECs whereby ECs release brain derived neurotrophic factor (BDNF) that stimulate NSC release of vascular endothelial growth factor (VEGF) resulting in a positive feedback loop that would naturally exist in the niche.40 To capitalize on these paracrine signaling loops, the close apposition of ECs and NSCs should be representative of the spatial resolution present within the niche,57,63 a characteristic that has been overlooked in previous in vitro NSC models.
In addition to proximity, EC source and phenotype are known to be influential on cells within a vascular niche as demonstrated by liver regeneration supported by liver sinusoidal ECs but not by other tissue-specific EC subsets.12 This would suggest that ECs from the brain may be more relevant to study EC-NSC interactions. EC phenotype can be further mediated by the application of fluid flow.2,3,6,10,37,39 Endothelium in the vascular niche is under blood flow and significant differences in soluble (growth factors, small molecules, free-floating proteoglycans) and insoluble (glycoproteins and proteoglycans) factors exist between ECs cultured under dynamic or static conditions.3,6,10,43 It is therefore expected that the novel inclusion of fluid shear stress to ECs may provide a more physiologically relevant model to recapitulate and examine the NSC niche in vitro. This is supported with previous research in which conditioned medium isolated from ECs cultured under shear stress resulted in profound differences in NPC adhesion, proliferation, survival, and differentiation compared to static EC-conditioned medium.16
In this work, a novel niche model was developed that concurrently cultures the ECs under fluid shear stress (dynamic culture), while NPCs reside in endothelial-conditioned microenvironment without fluid flow. This was achieved with a modified cone and plate viscometer that applied a shear stress of 10 dynes cm−2 to EC monolayers cultured on transwell membranes while the NPCs were cultured below at a distance of 10 or 1000 μm from the ECs. This co-culture paradigm fostered real-time paracrine signaling between the cells and allowed for NPC contact with endothelial produced matrix for NPCs within 10 μm of the ECs. We previously demonstrated that ECs stimulated by fluid flow alter cytokine production to support heterogeneous SVZ populations.16 Similarly, in this work we evaluate SVZ cell heterogeneity local to the ECs, but find less heterogeneity within NPC populations farther from EC monolayers or within static co-cultures, emphasizing the importance of a model that considers ECs exposed to fluid shear stress as well as spatiotemporal signaling between the ECs and NPCs that mimics the SVZ cytoarchitecture.
Methods and Materials
Isolation and Culture of NPCs
Mice were treated according to the Institutional Animal Care and Use Committee guidelines at Rensselaer Polytechnic Institute and all animal procedures were pre-approved by the Committee for collection of tissue. The SVZ was isolated from the brains of adult (5–10 weeks old) female Swiss-Webster mice (Taconic Farms, Hudson, NY). The tissue was enzymatically digested in a solution of 20 U mL−1 papain (Worthington Biochemical, Lakewood, NJ) and 12 μg mL−1 DNase (Sigma-Aldrich, St. Louis, MO) in base medium containing Dulbecco’s Modified Eagle Medium (DMEM; Cellgro, Manassas, VA) with 4.5 g L−1 glucose, 2 mM l-glutamine, 1 mM sodium pyruvate (Gibco, Grand Island, NY), and 1 mM N-Acetyle-l-cysteine (NAC; Sigma-Aldrich) for 30 min at 37 °C. Digested tissue was centrifuged at 400×g for 10 min, rinsed with DMEM, and re-centrifuged to pellet the cells.
Cells from the SVZ were re-suspended at 1.5 × 104 cells mL−1 in serum free expansion medium comprised of base medium supplemented with N2 (Gibco), B-27 (Gibco), and 20 ng mL−1 basic fibroblast growth factor (FGF2; Gibco) and epidermal growth factor (EGF; Gibco). Cells were plated in non-treated 6-well plates (Celltreat, Shirley, MA) and allowed to expand as neurospheres for 10-14 days with daily feedings at 37 °C, 5% CO2. Neurospheres were collected, centrifuged at 40×g for 2 min to remove expansion medium, and dissociated into a single cell suspension through enzymatic digestion as described above with 10U mL−1 papain solution.
Culture of ECs
Mouse brain microvascular EC line (mBend.3; ATCC, Manassas, VA) was seeded at 1.1 × 104 cells cm−2 on gelatin (Fisher, Hanover Park, IL) coated transwell culture inserts (Celltreat; 24 mm diameter inserts with 3 μm pores through the 10 μm thick membrane). This pore size was selected to permit diffusion of factors while preventing EC migration through the membrane. Cells were cultured for 4 days to develop confluent monolayers in growth medium containing DMEM with 4.5 g L−1 glucose, 2 mM l-glutamine, and 4.5 g L−1 sodium pyruvate supplemented with 50 U mL−1 penicillin–streptomycin (Mediatech, Herndon, VA) and 10% v/v fetal bovine serum (FBS; Gibco).
Dynamic Stimulation of ECs in Non-contact Co-culture with NPCs
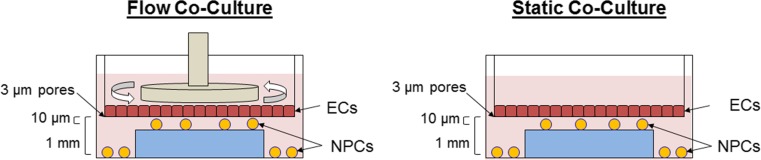
A modified cone and plate viscometer was developed that incorporates a computer interface to control shear stress based on input system parameters (medium viscosity, cone dimensions, plate dimensions) to dynamically culture ECs by exposing the cells to fluid shear stress16 on transwell inserts, while NPCs below the transwell insert remain unperturbed by fluid flow (Fig. 1). Similar models that expose ECs to fluid flow when co-cultured with vascular smooth muscle cells have been performed by others.9,28,29,65
Figure 1.
Schematic representation of non-contact EC-NPC co-culture systems. ECs are cultured on a transwell membrane (PET membrane, 3 μm pores) and stimulated at 10 dynes/cm2 (dynamic culture) or not exposed to shear stress (static culture). Shear stress in the dynamic culture is generated by the rotation of a 1° angle cone parallel to the EC monolayer. NPCs are cultured on the bottom of the well plates (on PLO coated glass) to be positioned 1 mm from the EC monolayer or on a 1 mm gasket as to be positioned ~ 10 μm (transwell membrane thickness) directly opposed to the EC monolayer.
Single cell suspensions dissociated from primary neurospheres were seeded at a density of 2.3 × 104 cells cm−2 on PLO coated, 5 mm round cover glass (Electron Microscopy Sciences, Hatfield, PA) positioned atop a 1 mm thick gasket or at the bottom of the well so as to position the glass coverslips at approximately 10 μm (proximal) or 1000 μm (far) from the EC monolayer, respectively. Alternatively for differentiation studies, NPCs were seeded on 10% Matrigel (BD Biosciences, San Jose, CA) coated, 5 mm round cover glass. NPCs were allowed to attach for 1 h before transwell inserts containing confluent EC monolayers were positioned above the NPCs and covered with 2% w/v Dextran (MW = 500 kg mol−1; Spectrum Chemical, New Brunswick, NJ) in neural progenitor base medium supplemented with N2, B-27, and 5 ng mL−1 FGF2 and EGF. Dextran was added to increase the viscosity of the culture medium to 2.1 cP to mimic blood and maintain laminar flow.
The modified cone and plate (transwell) viscometer was assembled and programmed to stimulate the ECs at a shear stress of 10 dynes cm−2 while the NPCs were protected from fluid flow and were statically cultured below the membrane. Although the exact range of appropriate shear stress within the brain microvasculature in the SVZ is unknown, 10 dynes/cm2 was chosen as it falls within the reported physiological range of 1–60 dynes/cm2 measured within small vessels from humans, canines, felines, and rodents.41,53,60 A static co-culture chamber without EC dynamic culture (flow) was also prepared. NPCs in the lower chamber were poised to receive soluble factors from the ECs above in both models, and a control without ECs was also prepared for comparison. Experimental and control cultures were stimulated for 3 days without medium replenishment, at which point the cells were chemically fixed or NPCs subjected to a differentiation regime.
Immunofluorescent Staining and Imaging
Cells were fixed with 4% w/v paraformaldehyde buffered with 4% w/v sucrose (Sigma) in a buffer contain 60 mM PIPES, 25 mM HEPES, 10 mM EGTA, 4 mM magnesium chloride hexahydrate, and sufficient potassium hydroxide to achieve pH 6.9 in diH2O. For undifferentiated NPC cultures, fixed cells were permeabilized for 20 min with 0.01% Triton-X 100 in PBS then rinsed. Samples were blocked overnight in 10% goat serum at 4 °C and stained with 1:1000 rabbit anti-GFAP (Dako, Carpenteria, CA), 1:500 rabbit anti-MASH1 (Millipore), 1:500 mouse anti-EGFR (Millipore), 1:500 rabbit anti-OLIG2 (Millipore), 1:300 rat anti-CD133 (eBiosciences, San Diego, CA), and/or 1:300 mouse anti-PSA-NCAM (Millipore). Appropriate secondary antibodies generated from a goat host were used at a concentration of 1:1000. For differentiated cultures, fixed cells were blocked overnight in 10% goat serum (Sigma-Aldrich) at 4 °C then stained with 1:4 mouse anti-O4 antibody (ATCC) followed by 1:1000 goat anti-mouse IgM secondary antibody (Invitrogen, Carlsbad, CA). Cells were permeabilized for 20 min with 0.01% Triton-X 100 in PBS, rinsed, blocked overnight in 10% goat serum at 4 °C, and stained with 1:1000 rabbit anti-GFAP and 1:500 mouse anti-NeuN (Millipore, Billerica, MA) followed by 1:1000 goat anti-rabbit IgG and goat anti-mouse IgG1 antibodies (Invitrogen). All fixed cells were stained with 1:2000 Hoechst 33,342 to visualize all cell nuclei. For visualization of the ECs, viability was assessed immediately after flow, prior to fixation, using calcein-AM and ethidium bromide. Fixed ECs on the transwell membrane were permeabilized with 0.01% Triton-X and 1:500 rhodamine-conjugated phalloidin in 5% bovine serum albumin (Sigma) was used to identify the actin cytoskeleton. The extracellular matrix was immunostained with 1:400 rabbit anti-laminin (Sigma) overnight at 4 °C in 10% goat serum followed by incubation with 1:1000 Alexafluor 488 goat anti-rabbit secondary. All samples were imaged using an Olympus IX81 inverted microscope (Olympus, Center Valley, PA) with a 20X dry objective.
NIH ImageJ toolkit (National Institute of Health, Bethesda, MD) was used to quantify total and phenotype specific cell numbers. To reduce variability in cell number due to cell seeding, cells of a given phenotype in each image are represented as a percentage of the total cells in each image. Three images were taken per sample to obtain a sample average, with a minimum of 3 sample replicates of each condition in each of the four experimental replicates of independent animal isolations (n = 4).
Cytokine Quantification
Mouse G2000 cytokine arrays (Raybiotech) were used to identify differences in soluble factor release by comparison of factors generated from EC-NPC co-cultured in dynamic or static conditions collected after 3 days of culture. Cytokine arrays were performed according to manufacturer instructions on the bulk cell conditioned medium from static or dynamic models. For cytokine array analysis, 4 experimental replicates were pooled into doublets to generate each condition.
Data and Statistical Analysis
A one-way ANOVA with post hoc Tukey multiple comparison test was performed to determine statistical significance between conditions (p value < 0.05, n = 4) for all datasets using Prism (GraphPad Software, La Jolla, CA), unless otherwise noted. All values are reported as mean ± standard deviation. Analysis of cytokine array data was performed according to manufacturer instructions to generate a ratio, or fold-change, of the signal intensity for the dynamic to static conditions. Briefly, each spot intensity was determined above background if it was ≥ 2 standard deviations above the mean negative control spot intensity and denoted with a + in Table S1, whereas spots not meeting the threshold are left blank (Table S1). All spots (+) were then normalized to the mean positive control spot intensity to eliminate any processing bias from the static or dynamic chips. The reported ratio in Table 1 was the mean average spot intensity for a given factor on the dynamic array divided by the mean average spot intensity for the same factor on the static array. In accordance with manufacturer instructions to achieve a 95% confidence interval in reporting a significant fold change, a change ≥ 1.5-fold or ≤ 0.65-fold in signal intensity are considered measurable and significantly different in expression (p < 0.05, n = 4) after complete analysis.
Table 1.
Cytokine expression is represented as a fold change for each cytokine produced in dynamic EC co-cultures over static EC co-cultures.
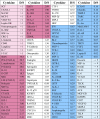
|
Eight cytokines that were absent from both the static and dynamic co-cultures were removed from the table. Numerous cytokines experienced a significantly increased (D/S > 1.5 (pink) or decreased (D/S < 0.65 (blue)) expression within the dynamic co-cultures compared to static (p < 0.05, n = 4). The fold change for factors that were not above background for the static array are represented as > 1 (light pink) and have been organized from highest to lowest intensity values within the dynamic conditioned medium. Fold change for factors absent in the dynamic medium could not be determined as they approach zero (→ 0; light blue) and have been ranked as least to most prevalent in static medium
Results
Endothelial Monolayers can be Dynamically Cultured in Co-culture with NPCs
EC (mBend.3) health was maintained following static or fluid shear stress (dynamic) culture for 3 days without exchanging the culture medium (Figure S1). Live/dead assay analysis at the end of day 3 revealed that > 99% of the monolayer was calcein+ (live cells) and exhibited elongated morphologies under dynamic culture with on average 5 × 105 cells per membrane. These data suggest that EC-NPC co-cultures can be performed for 3 days without medium exchange. Local cytokine gradients would be disrupted by regular medium exchanges. The EC monolayers were not found to be stable at longer culture durations (7 days) in the absence of a medium exchange (data not shown).
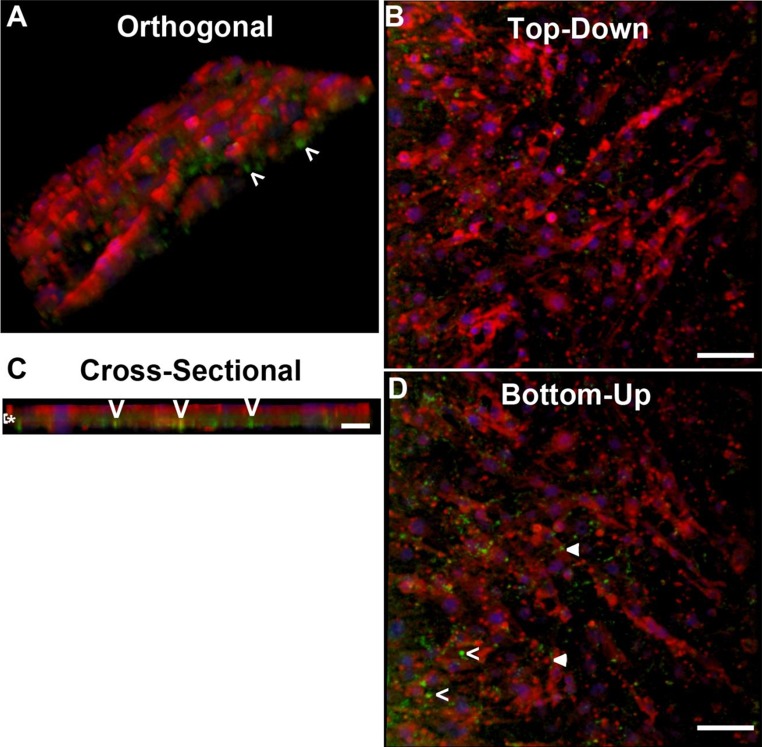
ECs increase laminin deposition in response to dynamic culture (10 dynes cm−2, Figure S2). After 3 days of dynamic culture on the transwell membrane, both the endothelial monolayer (Video S1) and EC-produced laminin were visualized (Fig. 2; Video S2). Due to imaging limitations with the transwell membrane and reduced production of laminin in the static cultures in planar cultures (Figure S2), laminin was not detected for the statically cultured EC on the transwell membrane. ECs appear to reside on top of the transwell, while the endothelial-produced laminin was localized on both sides of the membrane as well as within the transwell membrane pores (Fig. 2; Video S2), indicating laminin, but not ECs pass through the membrane. NPCs that were sufficiently close (≤ 10 μm) to the transwell membrane may contact the laminin within the extracellular matrix that may pass through the membrane as ECs can only deposit their laminin onto the membrane due to the presence of the cone above the EC monolayer. It is likely NPCs within 10 μm would have greater availability to local soluble factors that freely pass through the membrane.
Figure 2.
EC release of laminin during dynamic co-culture. (a–d) EC monolayers (phalloidin red, DAPI blue) dynamically stimulated for 3 days in transwell co-culture systems secrete laminin (green) into the extracellular space. Laminin penetrates through the 10 μm thick membrane as indicated by (>). Laminin is also present on the membrane as indicated by arrowheads. Z-stack height 23 μm. Scale bar 50 μm (a, b, d) or 25 μm (c).
NPCs Co-cultured with Dynamically Stimulated ECs Exhibit Different Cytokine Profiles
Previously, NPCs have been co-cultured either in direct cell–cell contact or non-contact using a transwell to separate NPCs from the statically cultured ECs.19,56 In this work, the co-culture model has been expanded to apply shear stress to the endothelial monolayer, to approach a more physiologically-relevant endothelial phenotype and evaluate differences to NSC fate.16 It is widely known that dynamic culture influencing EC production of soluble factors and ECM is dependent on shear stress magnitude, duration, type of flow, and EC source.2,10,37,49,55,60 While limited studies have evaluated protein production by the mBend.3 cell line under dynamic culture,16 mBend.3-NPC co-cultures were expected to produce a significant change in release of soluble factors with the application of shear stress. Bulk medium was collected from co-cultures and was analyzed using a cytokine array to identify differences in soluble factors as a function of EC phenotype (static or dynamically cultured). Distinct cytokine profiles were detected in the static and dynamic co-cultures (Table S1). In the dynamic co-culture, many cytokines known to promote NPC proliferation [galectin, interleukin (IL)-17B, insulin-like growth factor-1 (IGF-1), leptin, SDF-1, sonic hedgehog (SHH)], survival [granulocyte macrophage colony stimulating factor (GM-CSF), stem cell factor (SCF), SDF-1], and neurogenesis [SHH, granulocyte colony stimulating factor (G-CSF), GM-CSF, IGF-1, SDF-1], as seen in Table 1, were secreted in greater quantity in comparison to the statically-cultured control. In contrast, there was a decrease in release of NPC proliferation promoting factors [amphiregulin,17 IGF-2,39 IL-15,20 macrophage inflammatory protein-1α (MIP-1α)23], neurogenic inhibitory factors [DKK-1,1 IL-1β,36 monocyte chemoattractant protein-1 (MCP-1)23], and gliogenic promoting factors [DKK-1,1 MCP-123] in dynamically stimulated co-cultures relative to the static co-cultures (Table 1). A number of these factors, including IL-1β,14,47 IL-15,21 MCP-1,47,67 DKK-1,18 MIP-1α,34,47 GM-CSF,47 and SDF-134,47 are present on the cytokine array are inflammatory factors present after nerve injury and are more prevalent in static EC co-cultures. Undoubtedly the factors present could be released by either NPCs or ECs, however, ECs are present at a higher density (~ 100:1) and are the known variable undergoing phenotypic changes. It is likely these differences in inflammatory factors can be attributed to the ECs rather than the NPCs, due to the difference in ECs that have been previously shown to skew towards an inflammatory phenotype under aberrant flow regimes.28,65 Differences in soluble factors (Table 1) and laminin (Fig. 2) are indicative of differences in EC phenotype which can lead to differences in NPC cross-talk with the ECs,40 as well as NPC phenotype, proliferation, and differentiation.40
Increased SVZ Heterogeneity is Exhibited Proximal to Dynamic ECs
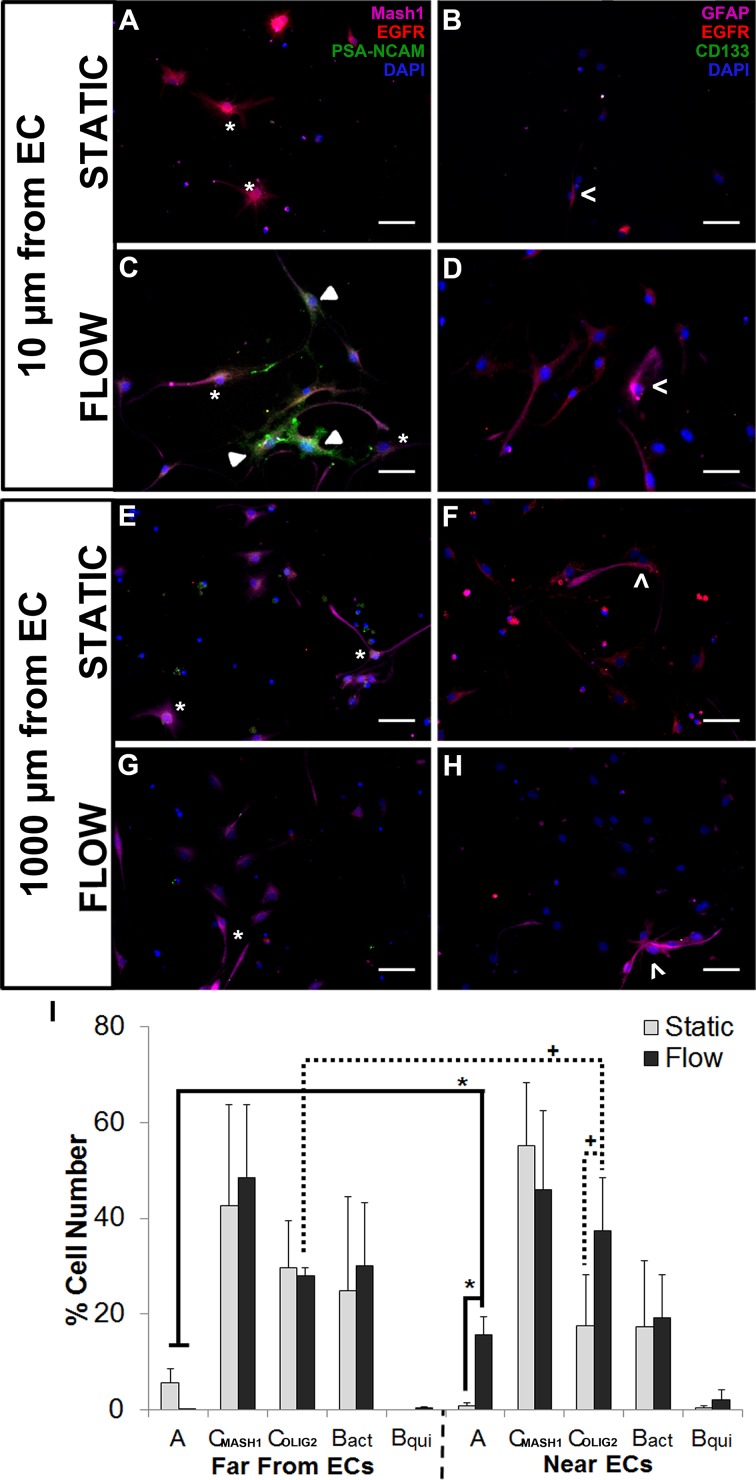
NPCs cultured at the bottom of the dish (1000 μm away from the EC monolayer) for 3 days with static- or dynamic-stimulated ECs exhibit similar morphology and phenotype expression (Fig. 3). By day 3, cells appear to be one of two subsets of transit amplifying C cells in static- (42.7 ± 21.1% EGFR+MASH1+ or 29.6 ± 9.84% EGFR+OLIG2+ progenitors) and dynamic-stimulated (48.6 ± 15.1% EGFR+MASH1+ or 27.0 ± 1.70% EGFR+OLIG2+ progenitors) ECs cultured 1000 μm from NPCs. Active B cells (GFAP+EGFR+ NSCs; Figs. 3, S2) accounted for 24.8 ± 19.7% and 31.1 ± 13.1% of cultures in static and dynamic EC cultures cultured 1000 μm from NPCs. The distribution of both type C and active B cells would be expected based on the SVZ heterogeneity reported in vivo.7,50 Neuroblasts (PSA-NCAM+) accounted for less than 5.7 ± 2.9% and 0.43 ± 0.04% of the total cell NPC populations when cultured far (1000 μm) from the static and dynamic EC monolayer, respectively (Fig. 3), or in the absence of ECs (Figure S2). It is possible that the NPCs were too far from the EC monolayer to be sensitive to differences in endothelial cytokine gradients present or for efficient paracrine signaling. A significant increase in PSA-NCAM+ neuroblasts (p < 0.05) and OLIG2+ or MASH1+ transit amplifying C cells (p < 0.1) was observed from NPCs cultured near (10 μm: 15.7 ± 3.7% PSA-NCAM+, 37.5 ± 10.9% OLIG2+, 46.1 ± 16.4% MASH1+) dynamically cultured EC compared to static ECs (0.73 ± 0.73% PSA-NCAM+, 17.5 ± 10.7% OLIG2+; Fig. 3i) or cultures devoid of ECs (0.42 ± 0.43% PSA-NCAM+, 31.8 ± 9.0% MASH1+; Figure S2). In contrast, there were no differences in MASH1+ phenotype for NPCs cultured at 10 μm (55.3 ± 13.1%) compared to 1000 μm (42.7 ± 21.1%) from the static EC culture and GFAP+EGFR+ active B cells accounting for 17.3 ± 13.8% (10 μm) and 24.8 ± 19.7% (1000 μm) of the total population in static co-cultures (Fig. 3). These results suggest that dynamically cultured ECs and close proximity of 10 μm co-cultures are both necessary to maintain SVZ phenotype heterogeneity representative of the niche.
Figure 3.
NPC phenotype is sensitive to EC culture (static or dynamic) and spatiotemporal placement. EC monolayers cultured on a transwell membrane were subjected to static (a, b, e, f) or dynamic (c, d, g, h) culture, while NPCs were cultured below at a distance of ~ 10 μm (a–d) or ~ 1000 μm (e–h) from the EC monolayer. NPCs were immunostained for type A neuroblasts (PSA-NCAM+—a, c, e, g: green) and transit amplifying C cells (MASH1+EGFR+—a, c, e, g: magenta and red) or for active type B cells (GFAP+EGFR+—b, d, f, h: magenta and red) and quiescent type B cells (GFAP+CD133+—b, d, f, h: magenta and green). PSA-NCAM+ (◂), MASH1+EGFR+ (*), and GFAP+EGFR+ (<) are indicated in their respective panels. (I) A significant increase in PSA-NCAM+ neuroblasts (* p < 0.05) and OLIG2+ transit amplifying C cells (+ p < 0.1) was observed near dynamic EC cultures, compared to cells cultured far from dynamically cultured ECs or at any distance from static ECs. No differences were detected in other NPC subpopulations. Cell types: PSA-NCAM+ (A), MASH1+EGFR+ (CMASH1), OLIG2+ (COLIG2), GFAP+EGFR+ (Bact), GFAP+EGFR−. Data are represented as mean ± standard deviation. 50 μm scale bar.
NPC Differentiation is Dependent on EC Phenotype and Distance from Monolayer
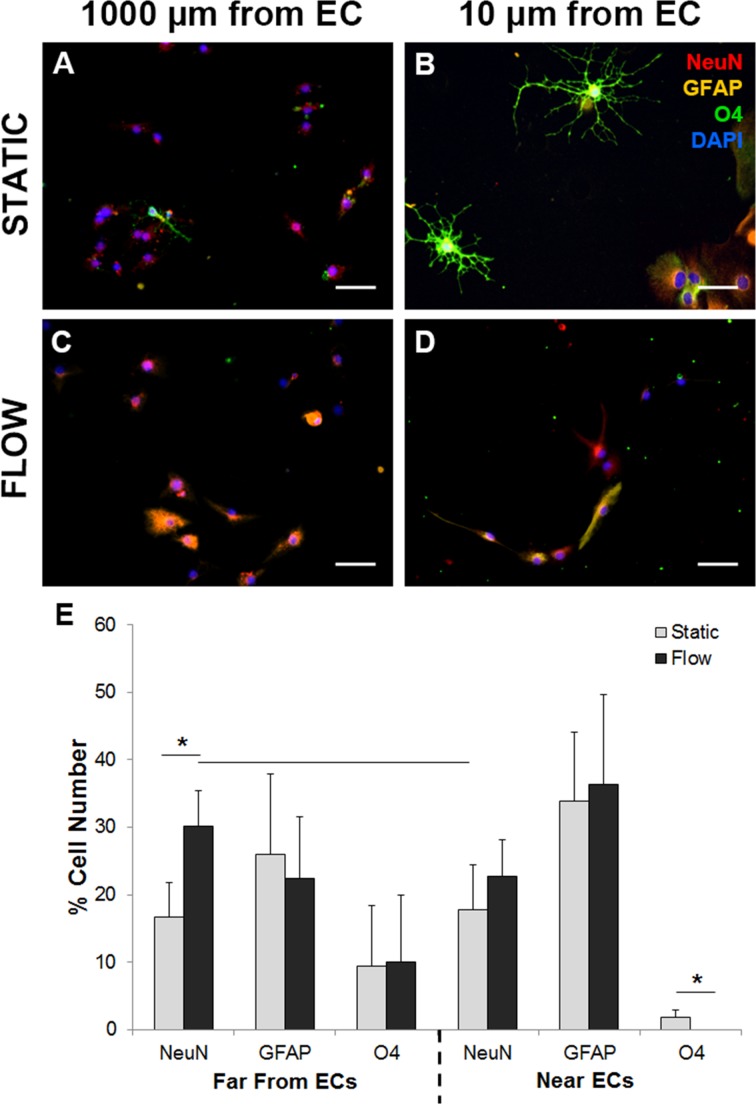
EC monolayers were cultured on transwell inserts under dynamic (10 dynes cm−2) or static conditions with NPCs cultured adjacent to (~ 10 μm) or far (~ 1000 μm) from ECs for 3 days then differentiated for 1 week and were evaluated for mature cell phenotypes with appropriate morphology (GFAP+ astrocytes, O4+ oligodendrocytes, and NeuN+ neurons). Differentiated cell populations far (~ 1000 μm) from or near (~ 10 μm) static EC populations formed all three mature cell phenotypes (Figs. 4a, 4b). Oligodendrocyte formation in cultures near statically cultured ECs (10 μm; 1.8 ± 1.1%) were greater relative to NPCs near dynamic ECs, which form populations consisting of 22.8 ± 5.3% neurons and 36.3 ± 13.4% astrocytes but are devoid of oligodendrocytes (Figs. 4d, 4e). Remaining cells may not have been terminally differentiated due to the short differentiation period (7 days) or may simply not have stained positive as markers such as NeuN are expressed by more mature neurons. NPCs cultured far (1000 μm, Fig. 4c) from the dynamically cultured EC monolayer produced significantly more neurons (30.2 ± 5.3%) than cultures within 10 μm (17.7 ± 6.8%) or 1000 μm (16.6 ± 5.23%) of static ECs (Fig. 4e). No significant differences were detected between the distribution of differentiated phenotypes between control NPCs absent of EC cultures with the experimental EC-NPC co-cultures (Figure S3). Overall, NPCs without ECs differentiate into primarily astrocytes (32.4 ± 10.1%) with some neurons (18.9 ± 7.33%) and few oligodendrocytes (3.4 ± 2.60%), while dynamic EC cultures shift more cells towards neural differentiation and static EC cultures shifted cells toward oligodendrocytes, based on the values reported above and in Fig. 4. While the hemodynamic state of the ECs in the EC-NPC co-cultures yield distinction differentiation profiles, this was found to be dependent on the proximity of the ECs to the NPCs.
Figure 4.
NPC differentiation is sensitive to EC phenotype (static or dynamic) and spatiotemporal placement. EC monolayers cultured on a transwell membrane were subjected to static (a, b) or dynamic (c, d) culture, while NPCs were cultured below at a distance of ~ 10 μm (a, c) or ~ 1000 μm (b, d) from the ECs. ECs were removed and NPCs were differentiated for 7 days then stained for neurons (NeuN+: green), astrocytes (GFAP+: yellow), or oligodendrocytes (O4+: red). (e) A significant increase (p < 0.1) in neurons far from dynamic EC cultures was observed compared to cells cultured in static cultures at any distance from static ECs, while cells near dynamic ECs demonstrate an increase in neurons, however, this is not significant compared to static paradigms. NPCs within 10 μm from static EC cultures exhibited a significant increase in oligodendrocytes compared to NPCs cultured in dynamic EC cultures. Data are represented as mean ± standard deviation. 50 μm scale bar.
Discussion
Development of in vitro models to study cellular niche interactions is necessary to better understand stem cell biology using simplified models that recapitulate sufficient complexity of an ill-defined niche. In previous work by Shen et al. and Gama Sosa et al., non-contact transwell co-cultures with statically cultured ECs and NSCs or NPCs have provided a platform for studying facets of the NSC niche and have demonstrated that EC-produced soluble factors promote self-renewal and neurogenesis.19,56 These previous models include real-time signaling between ECs and NSCs via cytokine gradients over long diffusion distances from ECs cultured on the transwell membrane to NPCs cultured on the bottom of the culture plate (~ 1000 μm). Although factors had to traverse large diffusion distances in previous models, there is sufficient complexity to improve self-renewal and neurogenesis of NSCs in vitro compared to NSC-only cultures and provides insight into the EC regulation of the niche. The inclusion of ECs can result in profound differences in the NPCs, and our work indicates that the EC phenotype, which can be altered by shear stress, is also an influential niche component. Previous work has demonstrated the conditioned medium collected from ECs cultured under fluid flow can shift NPC proliferation, survival, and differentiation relative to static EC conditioned medium.16 In this study, model complexity was increased through the incorporation of flow-stimulated ECs, not just soluble factors via EC-conditioned medium and further, for the first time, included the optimization of the spatial NPC placement relative to the EC monolayers to better recapitulate the adult NSC niche, as NSCs reside within 10 μm of the niche vasculature.
Hui and Bhatia developed a microfabricated chip to control hepatocyte and 3T3 cell positioning and found that the cells are sensitive to soluble factor gradients that exist on the micron level in co-culture systems.30 Given the proximity of NPCs to the vasculature in vivo, our culture paradigm sought to determine the sensitivity of NPCs to the EC phenotype but also to the proximity of the ECs. As both static and dynamic EC cultures were viable, differences in NPC phenotype can be attributed to EC phenotype and cytokine gradients based on proximity of NPCs to ECs. Over the course of 3 days, ECs deposited laminin-rich extracellular matrix that coated the membrane and entered the pores, possibly contacting NPCs. While cytokine gradients are presumed to arise based on work done by others to demonstrate cell-signaling gradients are evident in the co-culture models,30,40 they were not directly evaluated in this study. EC-deposited matrix is also expected to contribute to the formation of a local gradient by sequestering growth factors and other cytokines, increasing their local bioavailability to proximal NPCs. NPCs cultured within 10 μm would likely make contact with the matrix and the sequestered factors that pass through the pores, both of which can modulate NPC phenotype and fate.33,56 Extended culture duration that maintains gradient formation would be possible by exchanging the EC monolayers and culture medium above the EC monolayers. Alternatively, a system of tubing with an external media reservoir could be used to circulate the media above the EC monolayers, resulting in complete nutrient replenishment every 24–48 h and facilitating extended culture without disrupting the extracellular matrix or the paracrine signaling gradients below the transwell insert.
The most profound difference was an increase in PSA-NCAM+ neuroblast populations among NPCs near (~ 10 μm) the dynamically stimulated EC monolayer compared to statically cultured EC conditions or to NPCs located distal (~ 1000 μm) to the dynamic EC, a source of the cytokine gradients generating a more heterogeneous population of SVZ cell phenotypes. Within the niche, the type B stem cells (GFAP+EGFR+), transit amplifying C cells (MASH1+ or OLIG2+ NPCs) and neuroblasts (PSA-NCAM+) form a heterogeneous SVZ cell population, but this is poorly recapitulated in static co-cultures where we saw fewer of the OLIG2+ C cell subset and an absence of PSA-NCAM+ neuroblasts. By incorporating a dynamic EC phenotype, however, neuroblast populations began to appear within 10 μm of the ECs, this is consistent with the distance at which most stem and progenitor populations within the in vivo niche are located relative to the vasculature.57,63 Conversely, NPC phenotype was unaltered when cultured in bulk dynamic and static EC-conditioned medium with no discernable differences in the PSA-NCAM+ population observed with respect to the EC phenotype.16 Within the niche, transit amplifying C cells comprise the majority of the cells present (~ 70%) with fewer type B stem cells (~ 10–20%) and type A neuroblasts (10–20%)7 similar to the distribution that was evident in dynamic co-cultures with close apposition of NPCs to the EC monolayer. The generation of more heterogeneous SVZ populations representative of the in vivo distribution within 10 μm of dynamic ECs in the in vitro model developed in this work suggests that both the appropriate EC phenotype and proximity to the NPCs are important for future model development.
Dynamic ECs co-cultured with NPCs for 3 days resulted in the differential release of numerous cytokines compared to static co-cultures leading to more neuroblasts and OLIG2+ cells proximal to the ECs and more differentiated neurons farther from the ECs. The conditioned medium collected from both the dynamic and static co-cultures were analyzed using a cytokine array. It is important to note that the immunomarkers used to identify SVZ-specific progenitor phenotypes may suggest a potential differentiated cell type, however, these markers maintain the potential to differentiate into any of the three mature cell types. IGF-1 and SHH are present in the dynamic co-cultures and would be elevated closer to the EC monolayers, presuming they are the source of these factors, making them potential candidates as IGF-1 and SHH are neuroblast chemoattractants and can promote neural or oligodendrocyte differentiation in a concentration dependent manner.24,48,51,58,66,68 Given the number of soluble factors secreted into the medium, many candidates exist and are difficult to directly identify due to the interplay of the molecules in the medium, the convergence of signaling cascades, and limited research of many inflammatory factors upon neurogenesis.
Differentiation of adult SVZ NPCs in vitro primarily yields astrocyte cultures with few neurons, a fate distribution that was evident in our studies even in the presence of ECs. Interestingly, neuroblasts were more prevalent proximal to ECs in dynamic co-cultures compared to all other culture conditions yet there were no differences in the percentage of NeuN+ differentiated neurons between NPCs cultured proximal or distant to the dynamically cultured EC monolayers. Previous work has shown that dynamic EC-conditioned medium can enhance neural differentiation,16 thus achieving the same goal in the absence of nutrient replacement or diffusion distances that are present in the current study. This would suggest that EC phenotype may be more influential to NPC differentiation than the relative distance between the cells. The slightly elevated percentage of neurons in the distant compared to proximal NPCs cultures with dynamic ECs may be due to the differentiation being conducted for 1 week, as only 50–70% of the cells were positive for mature cell markers. NeuN is an indicator of more mature neurons relative to markers such as Tuj1, thus making NeuN a more conservative assessment of committed neural differentiation. Tuj1 is expressed by immature cells, such as neuroblasts in addition to neurons, which we already know are elevated in the proximal progenitor populations in the dynamic EC cultures.
Oligodendrocytes have not previously been obtained from adult SVZ-derived progenitors, suggesting that ex vivo expansion of NPCs alters the lineage progression seen in vivo. Lowry et al. developed an ex vivo expansion technique utilizing static ECs cultured on transwell inserts with embryonic NSCs cultured on the plate bottom (~ 1000 μm from ECs) in medium supplemented with SHH resulting in a culture paradigm that improved neuronal and oligodendrocyte differentiation rates after spinal cord injury.42 In this work, NPCs co-cultured with dynamically stimulated ECs have increased SHH release as measured by the cytokine array and when taken with the findings made by Lowry et al., it would suggest increased neuronal and oligodendrocyte differentiation profiles could be generated using the dynamic co-culture model. Only neuronal differentiation was increased in dynamic co-cultures, while oligodendrocyte differentiation increased in static co-cultures, suggesting SHH may play a role in the enhanced neurogenesis but not oligogenesis in these models. Enhanced oligodendrocyte differentiation in static EC-NPC cultures may be attributed to increased inflammatory cytokines such as MCP-1 (1.92-fold), TROY (25-fold) or the presence of Gas6 (14.3-fold) in the static cultures, as MCP-1 has been shown to promote oligodendrocyte differentiation22 while Gas6 and TROY help to maintain the OPC phenotype.25,61 Interestingly, a number of factors (amphiregulin, IL-15, DKK-1, IGF-2) that were elevated in static EC-NPC co-cultures are known inflammatory factors that can recruit progenitors and enhance proliferation and differentiation after injury.1,17,20,23,39 The limited levels of inflammatory cytokines present in the dynamic EC-NPC co-cultures suggest that this model may be a more accurate replication of a healthy niche, while static co-cultures may be more representative of injured or diseased tissue with aberrant blood flow.
In order to use adult NPCs as a cell mediated therapy, ex vivo control of stem cell fate is needed. If precursor cells committed to neural and oligodendrocyte lineages could be enriched during expansion, then the cells may be more likely to enhance tissue repair after injury by restoring beneficial neuron and oligodendrocyte populations in vivo.42 In addition to restoring lineage progression, directing NPC differentiation may allow for newly formed neurons to bridge the injury site and reconnect to distal targets and oligodendrocytes to encase the newly formed neurons in protective myelin necessary for signaling. Further population enrichment of non-astrocyte cultures could be performed by optimizing the dynamic EC-NPC co-culture system. Shear stress parameters (magnitude, duration, and type) could be modified to optimize cytokine output. Treatment with of NPCs with conditioned medium from either dynamic or static culture within the serum free medium could eliminate NPC response to the transient increase in cytokines that occurs upon induction of flow. The model may also expand our understanding of regulation within the healthy, aged, or diseased niche, as well as a platform to examine the effects of pharmacological intervention through simple modifications in the culture or shear stress parameters to control stem cell fate. Similar co-culture models have been used to model atherosclerosis-prone hemodynamic culture using smooth muscle cells and ECs to demonstrate the increase in inflammatory markers associated with the disease,28,65 suggesting the feasibility in expanding our model to evaluate neuro-vascular disease parameters that are known to impact age-associated neurodegenerative diseases.
To our knowledge, this is the first study to use dynamically cultured EC monolayers in co-culture with statically cultured neural progenitor cells and could be applied to other co-culture models where paracrine signaling with ECs are expected to be important. EC monolayers cultured under shear stress is more representative of physiological conditions sensed by the ECs and may recapitulate the dynamic EC phenotype and secretome compared to static cultures. More phenotypically relevant ECs through selection of mouse brain microvascular cells and the application of fluid flow led to profound changes within the NSC vascular niche and will likely prove to be invaluable in other EC co-culture paradigms, such as those with other stem cells, hepatocytes, islets, etc. 44 This work also highlights the distance-dependent differences that arise in paracrine signaling between ECs and the cells they support, in this case resulting in profound differences in progenitor phenotype and differentiation. Techniques developed in this work could be used to customize microenvironments of interest by incorporating tissue-specific ECs, stimulation regime (magnitude, flow type), and proximity to a secondary cell type. For example in the liver, hepatocytes reside within a vascular niche and are sensitive to the EC phenotype12 in vivo, while in vitro their proximity needs to be less than 400 μm to fibroblast feeder cells to promote survival and function.30 Using pre-established parameters, an in vitro model could be developed to study interactions between ECs and hepatocytes or to screen pharmacological agents on hepatocyte viability and functionality. Extending this model to other systems would likely provide many opportunities to evaluate EC-mediated paracrine signaling in healthy or diseased models, as well as provide a platform to test efficacy or cytotoxicity of therapeutic agents in a model niche rather than isolated cell types.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors acknowledge both the Stem Cell Biology and Microscopy Research Cores within the Center for Biotechnology and Interdisciplinary Studies at Rensselaer Polytechnic Institute. Funding was provided by the National Institutes of Health (RO1AG041861-ST), the National Science Foundation (CBET-1350240 - GD), and the New York State Department of Health NYSTEM (C026419 - DMT).
Conflict of interest
Courtney Dumont, Jennifer Piselli, Sally Temple, Guohao Dai, and Deanna Thompson declare that they have no conflicts of interest.
Ethical Approval
No human studies were carried out by the authors for this article. All animal studies were carried out in accordance with the Institutional Animal Care and Use Committee guidelines at Rensselaer Polytechnic Institute.
References
- 1.Ahn SM, Byun K, Kim D, Lee K, Yoo JS, Kim SU, et al. Olig2-induced neural stem cell differentiation involves downregulation of Wnt signaling and induction of Dickkopf-1 expression. PLoS ONE. 2008;3:e3917. doi: 10.1371/journal.pone.0003917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando J, Yamamoto K. Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ. J. 2009;73:1983–1992. doi: 10.1253/circj.CJ-09-0583. [DOI] [PubMed] [Google Scholar]
- 3.Arisaka T, Mitsumata M, Kawasumi M, Tohjima T, Hirose S, Yoshida Y. Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann. N Y Acad. Sci. 1995;748:543–554. doi: 10.1111/j.1749-6632.1994.tb17359.x. [DOI] [PubMed] [Google Scholar]
- 4.Aviezer D, Levy E, Safran M, Svahn C, Buddecke E, Schmidt A, et al. Differential structural requirements of heparin and heparan sulfate proteoglycans that promote binding of basic fibroblast growth factor to its receptor. J. Biol. Chem. 1994;269:114–121. [PubMed] [Google Scholar]
- 5.Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol. Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- 6.Barakat A, Lieu D. Differential responsiveness of vascular endothelial cells to different types of fluid mechanical shear stress. Cell Biochem. Biophys. 2003;38:323–343. doi: 10.1385/CBB:38:3:323. [DOI] [PubMed] [Google Scholar]
- 7.Capela A, Temple S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 2002;35:865–875. doi: 10.1016/S0896-6273(02)00835-8. [DOI] [PubMed] [Google Scholar]
- 8.Chang Z, Meyer K, Rapraeger AC, Friedl A. Differential ability of heparan sulfate proteoglycans to assemble the fibroblast growth factor receptor complex in situ. FASEB J. 2000;14:137–144. doi: 10.1096/fasebj.14.1.137. [DOI] [PubMed] [Google Scholar]
- 9.Chiu JJ, Chen LJ, Chen CN, Lee PL, Lee CI. A model for studying the effect of shear stress on interactions between vascular endothelial cells and smooth muscle cells. J. Biomech. 2004;37:531–539. doi: 10.1016/j.jbiomech.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado AC, Ferron SR, Vicente D, Porlan E, Perez-Villalba A, Trujillo CM, et al. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron. 2014;83:572–585. doi: 10.1016/j.neuron.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doetsch F. A niche for adult neural stem cells. Curr. Opin. Genet. Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douet V, Kerever A, Arikawa-Hirasawa E, Mercier F. Fractone-heparan sulphates mediate FGF-2 stimulation of cell proliferation in the adult subventricular zone. Cell Prolif. 2013;46:137–145. doi: 10.1111/cpr.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumont CM, Piselli JM, Kazi N, Bowman E, Li G, Linhardt RJ, et al. Factors released from endothelial cells exposed to flow impact adhesion, proliferation, and fate choice in the adult neural stem cell lineage. Stem Cells Dev. 2017;26:1–15. doi: 10.1089/scd.2016.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falk A, Frisen J. Amphiregulin is a mitogen for adult neural stem cells. J. Neurosci. Res. 2002;69:757–762. doi: 10.1002/jnr.10410. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, Maqueda A, Arenas E, Rodriguez FJ. Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS ONE. 2011;6:e27000. doi: 10.1371/journal.pone.0027000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gama Sosa MA, De Gasperi R, Rocher AB, Perez GM, Simons K, Cruz DE, et al. Interactions of primary neuroepithelial progenitor and brain endothelial cells: distinct effect on neural progenitor maintenance and differentiation by soluble factors and direct contact. Cell Res. 2007;17:619–626. doi: 10.1038/cr.2007.53. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Nicola D, Valle-Argos B, Pallas-Bazarra N, Nieto-Sampedro M. Interleukin-15 regulates proliferation and self-renewal of adult neural stem cells. Mol. Biol. Cell. 2011;22:1960–1970. doi: 10.1091/mbc.E11-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Nicola D, Valle-Argos B, Suardiaz M, Taylor JS, Nieto-Sampedro M. Role of IL-15 in spinal cord and sciatic nerve after chronic constriction injury: regulation of macrophage and T-cell infiltration. J. Neurochem. 2008;107:1741–1752. doi: 10.1111/j.1471-4159.2008.05746.x. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Perez O, Gutierrez-Fernandez F, Lopez-Virgen V, Collas-Aguilar J, Quinones-Hinojosa A, Garcia-Verdugo JM. Immunological regulation of neurogenic niches in the adult brain. Neuroscience. 2012;226:270–281. doi: 10.1016/j.neuroscience.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon RJ, Mehrabi NF, Maucksch C, Connor B. Chemokines influence the migration and fate of neural precursor cells from the young adult and middle-aged rat subventricular zone. Exp. Neurol. 2012;233:587–594. doi: 10.1016/j.expneurol.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Gritli-Linde A, Lewis P, McMahon AP, Linde A. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev. Biol. 2001;236:364–386. doi: 10.1006/dbio.2001.0336. [DOI] [PubMed] [Google Scholar]
- 25.Gruber RC, Ray AK, Johndrow CT, Guzik H, Burek D, de Frutos PG, et al. Targeted GAS6 delivery to the CNS protects axons from damage during experimental autoimmune encephalomyelitis. J. Neurosci. 2014;34:16320–16335. doi: 10.1523/JNEUROSCI.2449-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagihara K, Watanabe K, Chun J, Yamaguchi Y. Glypican-4 is an FGF2-binding heparan sulfate proteoglycan expressed in neural precursor cells. Dev. Dyn. 2000;219:353–367. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1059>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Han J, Zhang F, Xie J, Linhardt RJ, Hiebert LM. Changes in cultured endothelial cell glycosaminoglycans under hyperglycemic conditions and the effect of insulin and heparin. Cardiovasc. Diabetol. 2009;8:46. doi: 10.1186/1475-2840-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am. J. Physiol. Cell. Physiol. 2007;293:C1824–C1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- 29.Heydarkhan-Hagvall S, Chien S, Nelander S, Li YC, Yuan S, Lao J, et al. DNA microarray study on gene expression profiles in co-cultured endothelial and smooth muscle cells in response to 4- and 24-h shear stress. Mol. Cell. Biochem. 2006;281:1–15. doi: 10.1007/s11010-006-0168-6. [DOI] [PubMed] [Google Scholar]
- 30.Hui EE, Bhatia SN. Micromechanical control of cell-cell interactions. Proc. Natl. Acad. Sci. USA. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ida M, Shuo T, Hirano K, Tokita Y, Nakanishi K, Matsui F, et al. Identification and functions of chondroitin sulfate in the milieu of neural stem cells. J. Biol. Chem. 2006;281:5982–5991. doi: 10.1074/jbc.M507130200. [DOI] [PubMed] [Google Scholar]
- 32.Kato T, Sasaki H, Katagiri T, Sasaki H, Koiwai K, Youki H, et al. The binding of basic fibroblast growth factor to Alzheimer’s neurofibrillary tangles and senile plaques. Neurosci. Lett. 1991;122:33–36. doi: 10.1016/0304-3940(91)90186-W. [DOI] [PubMed] [Google Scholar]
- 33.Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, et al. Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells. 2007;25:2146–2157. doi: 10.1634/stemcells.2007-0082. [DOI] [PubMed] [Google Scholar]
- 34.Knerlich-Lukoschus F, von der Ropp-Brenner B, Lucius R, Mehdorn HM, Held-Feindt J. Spatiotemporal CCR1, CCL3(MIP-1alpha), CXCR4, CXCL12(SDF-1alpha) expression patterns in a rat spinal cord injury model of posttraumatic neuropathic pain. J. Neurosurg. Spine. 2011;14:583–597. doi: 10.3171/2010.12.SPINE10480. [DOI] [PubMed] [Google Scholar]
- 35.Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, et al. Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell. 2010;7:163–173. doi: 10.1016/j.stem.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, et al. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010;64:721–728. doi: 10.1002/syn.20768. [DOI] [PubMed] [Google Scholar]
- 37.LaMack JA, Friedman MH. Individual and combined effects of shear stress magnitude and spatial gradient on endothelial cell gene expression. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H2853–H2859. doi: 10.1152/ajpheart.00244.2007. [DOI] [PubMed] [Google Scholar]
- 38.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 39.Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J. Neurosci. Res. 2006;84:1656–1668. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 41.Lipowsky HH. Microvascular rheology and hemodynamics. Microcirculation. 2005;12:5–15. doi: 10.1080/10739680590894966. [DOI] [PubMed] [Google Scholar]
- 42.Lowry N, Goderie SK, Adamo M, Lederman P, Charniga C, Gill J, et al. Multipotent embryonic spinal cord stem cells expanded by endothelial factors and Shh/RA promote functional recovery after spinal cord injury. Exp. Neurol. 2008;209:510–522. doi: 10.1016/j.expneurol.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 43.Morita T, Yoshizumi M, Kurihara H, Maemura K, Nagai R, Yazaki Y. Shear stress increases heparin-binding epidermal growth factor-like growth factor mRNA levels in human vascular endothelial cells. Biochem. Biophys. Res. Commun. 1993;197:256–262. doi: 10.1006/bbrc.1993.2469. [DOI] [PubMed] [Google Scholar]
- 44.Nikolova G, Strilic B, Lammert E. The vascular niche and its basement membrane. Trends Cell Biol. 2007;17:19–25. doi: 10.1016/j.tcb.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Nugent MA, Edelman ER. Kinetics of basic fibroblast growth factor binding to its receptor and heparan sulfate proteoglycan: a mechanism for cooperactivity. Biochemistry. 1992;31:8876–8883. doi: 10.1021/bi00152a026. [DOI] [PubMed] [Google Scholar]
- 46.Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, et al. Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat. Cell Biol. 2014;16:1045–1056. doi: 10.1038/ncb3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ousman SS, David S. MIP-1alpha, MCP-1, GM-CSF, and TNF-alpha control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord. J. Neurosci. 2001;21:4649–4656. doi: 10.1523/JNEUROSCI.21-13-04649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Passerini AG, Milsted A, Rittgers SE. Shear stress magnitude and directionality modulate growth factor gene expression in preconditioned vascular endothelial cells. J. Vasc. Surg. 2003;37:182–190. doi: 10.1067/mva.2003.66. [DOI] [PubMed] [Google Scholar]
- 50.Pastrana E, Cheng LC, Doetsch F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc. Natl. Acad. Sci. USA. 2009;106:6387–6392. doi: 10.1073/pnas.0810407106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puglianiello A, Germani D, Rossi P, Cianfarani S. IGF-I stimulates chemotaxis of human neuroblasts. Involvement of type 1 IGF receptor, IGF binding proteins, phosphatidylinositol-3 kinase pathway and plasmin system. J. Endocrinol. 2000;165:123–131. doi: 10.1677/joe.0.1650123. [DOI] [PubMed] [Google Scholar]
- 52.Reisig K, Clyne AM. Fibroblast growth factor-2 binding to the endothelial basement membrane peaks at a physiologically relevant shear stress. Matrix Biol. 2010;29:586–593. doi: 10.1016/j.matbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 53.Reneman RS, Hoeks AP. Wall shear stress as measured in vivo: consequences for the design of the arterial system. Med. Biol. Eng. Comput. 2008;46:499–507. doi: 10.1007/s11517-008-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saksela O, Moscatelli D, Sommer A, Rifkin DB. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J. Cell Biol. 1988;107:743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Santaguida S, Janigro D, Hossain M, Oby E, Rapp E, Cucullo L. Side by side comparison between dynamic versus static models of blood-brain barrier in vitro: a permeability study. Brain Res. 2006;1109:1–13. doi: 10.1016/j.brainres.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 56.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 57.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi B, Ding J, Liu Y, Zhuang X, Zhuang X, Chen X, et al. ERK1/2 pathway-mediated differentiation of IGF-1-transfected spinal cord-derived neural stem cells into oligodendrocytes. PLoS ONE. 2014;9:e106038. doi: 10.1371/journal.pone.0106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sirko S, von Holst A, Wizenmann A, Gotz M, Faissner A. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development. 2007;134:2727–2738. doi: 10.1242/dev.02871. [DOI] [PubMed] [Google Scholar]
- 60.Stepp DW, Nishikawa Y, Chilian WM. Regulation of shear stress in the canine coronary microcirculation. Circulation. 1999;100:1555–1561. doi: 10.1161/01.CIR.100.14.1555. [DOI] [PubMed] [Google Scholar]
- 61.Sun L, Liu S, Sun Q, Li Z, Xu F, Hou C, et al. Inhibition of TROY promotes OPC differentiation and increases therapeutic efficacy of OPC graft for spinal cord injury. Stem Cells Dev. 2014;23:2104–2118. doi: 10.1089/scd.2013.0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki Y, Yanagisawa M, Yagi H, Nakatani Y, Yu RK. Involvement of beta1-integrin up-regulation in basic fibroblast growth factor- and epidermal growth factor-induced proliferation of mouse neuroepithelial cells. J. Biol. Chem. 2010;285:18443–18451. doi: 10.1074/jbc.M110.114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tham M, Ramasamy S, Gan HT, Ramachandran A, Poonepalli A, Yu YH, et al. CSPG is a secreted factor that stimulates neural stem cell survival possibly by enhanced EGFR signaling. PLoS ONE. 2010;5:e15341. doi: 10.1371/journal.pone.0015341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomas JA, Deaton RA, Hastings NE, Shang Y, Moehle CW, Eriksson U, et al. PDGF-DD, a novel mediator of smooth muscle cell phenotypic modulation, is upregulated in endothelial cells exposed to atherosclerosis-prone flow patterns. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H442–H452. doi: 10.1152/ajpheart.00165.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu SM, Tan KS, Chen H, Beh TT, Yeo HC, Ng SK, et al. Enhanced production of neuroprogenitors, dopaminergic neurons, and identification of target genes by overexpression of sonic hedgehog in human embryonic stem cells. Stem Cells Dev. 2012;21:729–741. doi: 10.1089/scd.2011.0134. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J. Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X, Zhang L, Cheng X, Guo Y, Sun X, Chen G, et al. IGF-1 promotes Brn-4 expression and neuronal differentiation of neural stem cells via the PI3 K/Akt pathway. PLoS ONE. 2014;9:e113801. doi: 10.1371/journal.pone.0113801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.