Abstract
Introduction
Vascular cells are regulated by continuous hemodynamic forces in vivo, and mechanical forces such as shear stress are proposed to involve in the progression of cardiovascular diseases such as atherosclerosis. Lamin A/C makes up the nuclear lamina, which structurally supports the nucleus while also functionally participates in chromatin organization and gene transcription. Diseases caused by lamin or other nuclear proteins are called laminopathies. One example, Hutchinson Gilford Progeria Syndrome (HGPS) where young patients show signs of accelerated aging, is caused by de novo mutations on the lamin A/C gene. Vasculature of HGPS patients shares many similarities with people of advanced age, suggesting a role for lamin in vascular aging.
Methods
In this study, we examined how arterial shear stress affects lamin A/C expression in bovine aortic endothelial cells at different population doubling levels (PDL). We also used fluorescence image analysis to examine nuclear shape changes with shear stress and PDL.
Results
Our results suggest that laminar shear stress downregulated lamin A/C expression in low PDL cells, but the effect was reversed in high PDL cells. Nuclear shape changes were more prominent after shear stress in low PDL cells. Moreover, lamin A/C accumulated more at the nuclear periphery after exposure to shear stress.
Conclusions
Overall, our results indicate that both shear stress and cell passage can have an impact on lamin expressions at transcriptional and translational levels, as we continue to understand the effect of shear stress on endothelial lamina as part of the vascular aging process.
Keywords: Nuclear lamin, Lamin A/C, Shear stress
Introduction
Cardiovascular disease is often associated with many risk factors, such as obesity, unbalanced diet, hypertension, and the use of tobacco or alcohol.33 Atherosclerosis, for example, is a chronic inflammation process in arteries, and the plaque buildup over the progression can narrow the arteries and eventually lead to arterial occlusion. Studies suggest that hemodynamic forces such as shear stress may play a role in atherosclerosis formation, where altered or disturbed shear patterns were observed at athero-prone sites, such as curvature and branching points.27 Studies have also shown a potential athero-protective role of uniform high shear stress on vascular cells through a myriad of mechanotransduction pathways.9
Endothelium is the main subject of shear stress generated by blood flow due to the physiological structure of blood vessels, and its ability to transduce and respond to shear stress has been reported.51,52 Research also suggests that endothelial cells can distinguish different patterns of mechanical forces,3 and that shear stress can activate or suppress mechanosensitive pathways in endothelial cells.37 Many candidates in endothelial mechanotransduction pathways have also been proposed and studied, including transcription factors, cytoskeleton components, transmembrane proteins, mechanosensitive ion channels, and focal adhesion.10 Discoveries of new mechano-sensitive candidates are still in progress. In this paper, we mainly focused on lamin A/C, a structural protein of the nuclear lamina, since the mutations in lamin and lamin-associated proteins have caused defects in nuclear mechanical properties and gene regulation.25,48 Based on the above findings, it is suggested that nuclear lamins could also be part of endothelial mechanotransduction pathways.
A-type lamins (lamin A/C) and B-types lamins (lamin B1 and B2) are major components of nuclear lamina in mammals, and they formed separate filamentous networks and followed different assembly mechanisms during nuclear lamina formation after mitosis.31 Moreover, B-type lamins were associated with chromatin, and engaged in nuclear envelope assembly earlier than A-type lamins. Besides acting as supporting structures for nuclear shape,24 lamins were also found to interact with chromatin and be involved in many mechanotransduction pathways. For instance, the activation of p38 mitogen-activated protein kinases (MAPK) was shown to upregulate lamin B1 expression in human primary fibroblasts during oxidative stress.4,15 Abnormal activation profile of extracellular signal-regulated kinase (ERK) pathway together with reduced cell spreading and contraction were observed in human skin fibroblasts with mutant lamin A/C, indicating defective endogenous tensional forces inside the cells through ERK pathway.12,34
Notably, the absence or mutation of lamin A/C can result in many tissue specific disorders known as laminopathies, including muscular dystrophy, adipose tissue abnormalities, axonal neuropathy and dilated cardiomyopathy.8,54 Among these laminopathies is Hutchinson Gilford Progeria Syndrome (HGPS), a rare genetic disorder caused by point mutation at LMNA gene that encodes lamin A/C.13 This mutation will lead to a deletion of 50 amino acids from the sequence, which contains a recognition site for ZMPTE24 (zinc metallopeptidase STE24) that is responsible for the cleavage of the last 15 amino acids at C-terminal polypeptide on prelamin A (lamin A precursor). This results in the production of an uncleaved progerin with permanently farnesylated C-terminal instead of the mature lamin A.54 Patients with HGPS appeared normal at birth, but showed many aging symptoms after few years, such as delayed growth, wrinkled skin and stiffened arteries,36 and they often died in their teens on average.41 Although HGPS patients did not show all aspects of physiological aging such as increased susceptibility to tumor formation,32 this mutation in LMNA can induce many events that are considered to be hallmarks for aging, such as decreased compliance and increased stiffness in arteries at tissue level, and reduced telomere length and modifications in histone methylation at cellular and molecular levels.5,18,36,44,47 Therefore, HGPS can be regarded as a model for prelamin A-dependent aging, which may also contribute to the normal aging.
HGPS patients share something else with people of advanced age: the development of cardiovascular diseases such as atherosclerosis; and HGPS patients often died from stroke or heart attack at an average age of 13.41 Nuclei with defective lamina in aged cells shared similarities with those of HGPS cells, such as distorted nuclear shape and increased mechano-sensitivity.53 However, while progerin was responsible for signs of aging in HGPS,7,30 a much lower level of progerin was observed in healthy cells from either young or old donors, compared to HGPS cells, including cells in vascular system.28 – 30,36 On another hand, the accumulation of prelamin A was reported in vascular smooth muscle cells (VSMCs) from old donors,26,42 and the roles of prelamin A in accelerating cell senescence and DNA damage have also been observed.22 Therefore, prelamin A could be another mechanism in vascular aging, and either prelamin A or progerin accumulation may contribute to signs of aging and the development of atherosclerosis.
Much effort has been devoted to explore how lamin A/C affects nuclear mechanical properties as well as gene regulation. Nuclear defects caused by lamin A/C deficiency have been reported,17,25 and some shear stress-induced pathways involving lamin A/C have also been recognized.19 While research has identified many downstream factors regulated by lamin A/C, here we examined if endothelial lamin A/C expression itself is sensitive to mechanical forces such as shear stress, and if that response is dependent on cell passage. In this paper, we focused on evaluating how lamin A/C expression pattern was influenced by laminar shear stress, and our results indicate that both shear stress and cell passage level could have an impact on lamin A/C expressions at both transcriptional and translational levels.
Materials and Methods
Cell Culture
Bovine aortic endothelial cells (BAEC from Lonza Company, USA) were grown in Dulbecco’s Modification of Eagle’s Medium (DMEM, Corning) containing 10% of FBS (Fetal Bovine Serum, JR Scientific), 1% of l-glutamine (Cellgro) and 2% Penicillin streptomycin (Cellgro). Cells were maintained in a humidified incubator at 37 °C supplemented with 5% CO2, and subculture was conducted at a ratio of 1:6 when cells were reaching 95% confluency.
Population Doubling Level
Population doubling level was utilized to denote cell age, which is an indication of the number of times that cells have divided.38 Assuming that cells were growing at an exponential growth rate, population doubling level was calculated as , where is the cell population at confluency in the flask, is the initial amount of cell population, and is the number of generations that cells have gone through in the flask. We chose to describe cells as “low” to describe PDL of up to 13 after first passage, “middle” as PDL estimated at 45 5, and “high” refers to PDL estimated at 60 5.
Shear Stress Experiment
To apply shear stress on cells, a parallel flow chamber was used to generate laminar flow on cells as indicated.43 Navier–Stokes equation was used to describe the motion of the media based on its viscous property, and after simplification, shear stress () was calculated as , where is the viscosity, is the flow rate, and are geometrical parameters of the flow chamber. Therefore, shear stress () depended on the flow rate and viscosity of flow, with constant area and height of the flow chamber. Shear stress was set to 15 dyne/cm2, which was controlled by volumetric flow rate. Cells were exposed to shear for 6 h.
Western Blot Analysis
After shear experiment, the monolayer of BAEC was washed with PBS, and lysed and collected by RIPA lysis buffer containing 20 mM Tris HCl, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton-X, 1 mM DTT, 0.5 mM PMSF and 150 mM Protease inhibitor. Protein samples were loaded on a polyacrylamide gel and transferred to a PVDF membrane. Anti-lamin A/C antibody (Cell Signaling, #4777), anti-actin antibody (Sigma A2066), and the corresponding secondary antibodies conjugated to HRP (Bio-rad) as well as chemiluminescence HRP substrate reagents were used in subsequent incubation steps.
Quantitative RT-PCR Analysis
Quantitative real-time polymerase chain reaction was performed to examine lamin A/C transcription level. Cells were first collected from glass slides, after which RNA was purified by the RNA isolation kit (Thermo Scientific). Then reverse transcription was conducted by a cDNA reverse transcription kit (Applied Biosystem), and SYBR green dye (Applied Biosystems) was utilized in the following cDNA amplification. Primer pairs for target gene (LMNA) from Bio-rad had the context sequence of 140 base pairs of the lamin gene. Primer pairs for control gene GAPDH (Forward primer: 5′-GCAAGTTCAACGGCACAGTCA-3′; Reverse primer: 5′-ACCAGCATCACCCCACTTGAT-3′, Invitrogen) were also used.
Considering the actual efficiencies of primers: 90.35 and 89.72% for target gene (LMNA) and control gene (GAPDH), respectively, Pfaffl method39 was applied to analyze RT-PCR results, whose formula is shown below:
where or is the efficiency of LMNA or GAPDH primers, or denotes difference in thresholds between control samples and treated samples after amplification step for each gene. Any result greater than 1 indicates upregulated lamin A/C transcription, and any value smaller than 1 denotes downregulated lamin A/C transcription.
Immunohistochemistry
To access cell morphological information and to confirm the Western Blot results, immunostaining was conducted to examine the effects of mechanical forces on cells. Cells were fixed by 4% paraformaldehyde (PFA) to cross link proteins.50 Then fixed cells were blocked by 1% BSA and incubated with primary antibody of lamin A/C (Cell Signaling) at 1:100 dilutions and secondary antibody with FITC (SC-2099, Santa Cruz) at 1:100 ratios. For nucleus staining, after the blocking step, cells were incubated with Hoechst blue stain solutions at 1:1000 dilution for 15 min in the dark before imaging. Fluorescence was detected by a fluorescent microscope (Leica DMI6000B). Images were acquired by a digital camera (Leica DFC350 FX) with fluorescence filter cube (Excitation filter BP 340–380 nm, suppression filter LP 425 nm) and GFP cube (Excitation filter BP 470/40 nm, suppression filter LP 525/50 nm). A 40× objective (Leica HCX PL FLUOTAR L 40× with NA of 0.60) and Leica Application Suite X software were used for image acquisition.
Image Analysis
To characterize cell morphology, nuclei (on average about 45) were picked for each data group, and shape factors (area, elongation and circularity/shape index) were measured and calculated by the following formulas: Elongation = , and Circularity = , using the ImageJ software (NIH). To quantify how lamin A/C localized within the nucleus, analysis was performed to calculate the ratio of lamin A/C at the periphery over the whole nucleus (including the periphery).40 Around 20 nuclei were chosen for each data point. For consistency, the periphery intensity was acquired by averaging eight points at nuclear perimeter and on the evenly-distributed radial lines from the midpoint of Feret’s diameter.
Image analysis and measurements were all done with ImageJ software. Images were firstly converted into greyscale, and nuclei were identified by using the threshold function, after which shape factors as well as intensity were measured in ImageJ.
Statistical Analysis
To maintain consistency, every data point was repeated at least three times independently. To quantitatively show significant changes, unpaired t test was performed between two groups, and a comparison with P value less than 0.05 was regarded as significant. One-way ANOVA was performed for multiple groups, and was followed by Turkey multiple comparison procedure by SAS at . Data were expressed as mean SEM.
Results
Lamin A/C Expression Pattern Changes with Increasing PDL
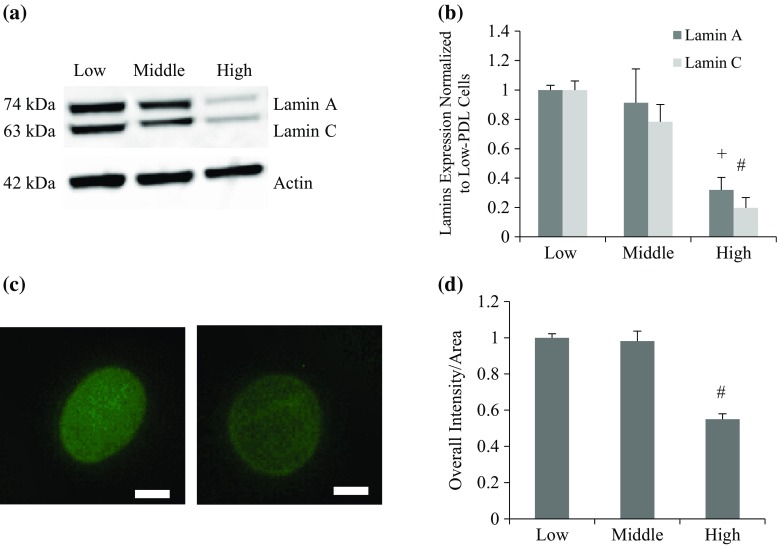
Reduced lamin A/C protein expression was observed in high PDL endothelial cells (Figs. 1a and 1b), and immunostaining of lamin A/C antibody followed by quantification of the fluorescence was performed to verify the decreasing trend (Figs. 1c and 1d). Lamin A and C expression decreased significantly in higher PDL cells compared to lower PDL cells. This expression change coincides with similar findings in different types of cells (osteoblasts, dermal fibroblasts and cardiomyocytes) in mice.1,11 Considering lamin A/C as a supporting structure at the nuclear envelope, its reduction over cell division may contribute to nuclear fragility by the mechanical loadings during cardiac cycles. Besides quantitative amount changes of A-type lamins in cells with different PDL, we also analyzed their distribution within the nucleus based on immunostaining images. We saw from Fig. 1c, that lamin distribution within the nucleus changed from a more even distribution throughout the nucleus in early stage, to a skewed distribution with more accumulations at the nuclear periphery at higher PDL. To rule out the area effect on intensity, we quantified mean intensity, which was calculated as overall intensity per area of each nucleus. Nuclei were visualized by staining lamin A/C using immunohistochemistry (Fig. 1c), and more than 30 nuclei were included for each data point in Fig. 1d. The result showed that the overall intensity decreased (significantly in high PDL cells), regardless of nuclei area, a trend that is consistent with Western results (Figs. 1a and 1b).
Figure 1.
Expression patterns of lamin A/C in BAEC with varied PDL. (a) Representative Western Blot images for cells at low, middle, or high PDL. (b) Quantitative result of repeated Western blots. One-way ANOVA was conducted followed by Turkey–Kramer method. Actin was used to normalize lamin A/C expression in each group, and all data points were then normalized to low PDL cells for lamin A and lamin C. Both lamin A and lamin C showed significant decreases with higher PDL (+ p < 0.01 and # p < 0.001 for lamin A and lamin C, respectively. (c) Representative images of nuclei stained with lamin A/C antibody. Left and right panels are BAEC with low and high PDL, respectively. (d) Quantitative image analysis result based on fluorescent images of BAEC stained with lamin A/C antibody. The result showed that mean intensity (overall intensity per nuclear area) decreased with PDL number, normalized with respect to low PDL values (# p < 0.001 of middle and old-PDL cells compared to low PDL group). Significance was observed at level between low and high as well as middle and high PDL groups. Scale bar: 5 µm.
Nuclear Shape is Modulated After 6-h Shear Regardless of Cell Age
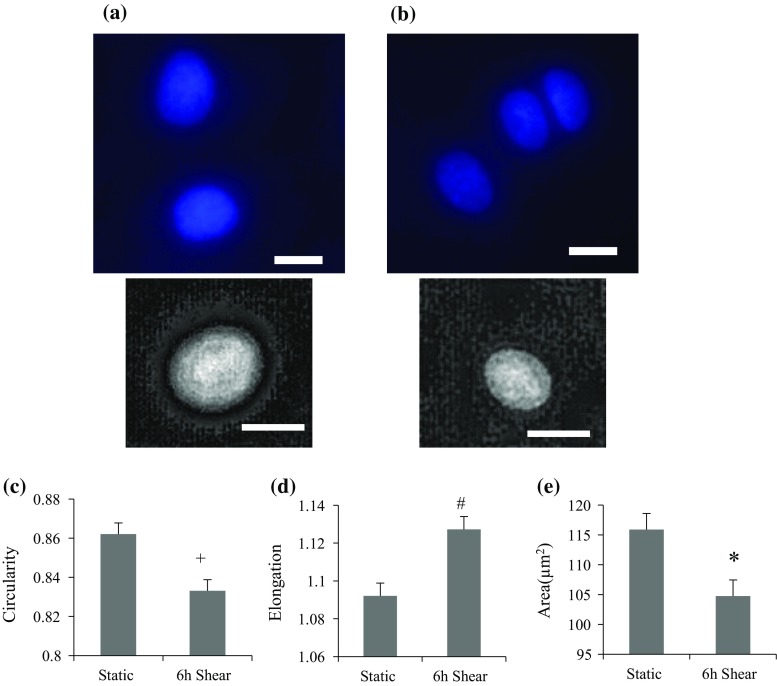
We next examined the effect of shear stress on nuclear shape in endothelial cells (PDL up to 50). Cells were seeded on glass slides and were exposed to 15 dyne/cm2 of shear stress for 6 h. Shear stress was applied in the horizontal direction. Another slide that has been plated at the same time was placed in an incubator as a static control. To visualize the nucleus, we stained both control and sheared cells by Hoechst fluorescent stain (Figs. 2a and 2b, respectively). A minimum of 50 nuclei in both static and shear cases were analyzed, and shape factors (circularity, elongation and area) were measured and displayed in Figs. 2c, 2d and 2e, respectively. Our result shows that the nucleus was elongated and less circular toward shear stress, and nuclear area was decreased after shear. These data suggest that cells were adjusting their nuclear shape in response to shear stress, which could be an effort to minimize exerted force on nuclei.20
Figure 2.
Shape analysis of endothelial nuclei after shear. At least 50 nuclei were assessed for each group. Nuclei were stained by Hoechst solution in static state (a) or after shear experiment (b). Top panels show Hoechst stained nuclei, and bottom images are deconvoluted images. Shear stress was applied horizontally in the image. Circularity (c), elongation (d) and nuclear area (e) changed significantly in BAEC after 6-h shear. Significant decreases in circularity (+ p < 0.01) and area (*p < 0.05), and increase in elongation (# p < 0.001) were observed after shear experiment. PDL effect was not considered here. Scale bar: 10 µm.
Nuclear Morphology Changes More After 6-h Shear in Low PDL Cells
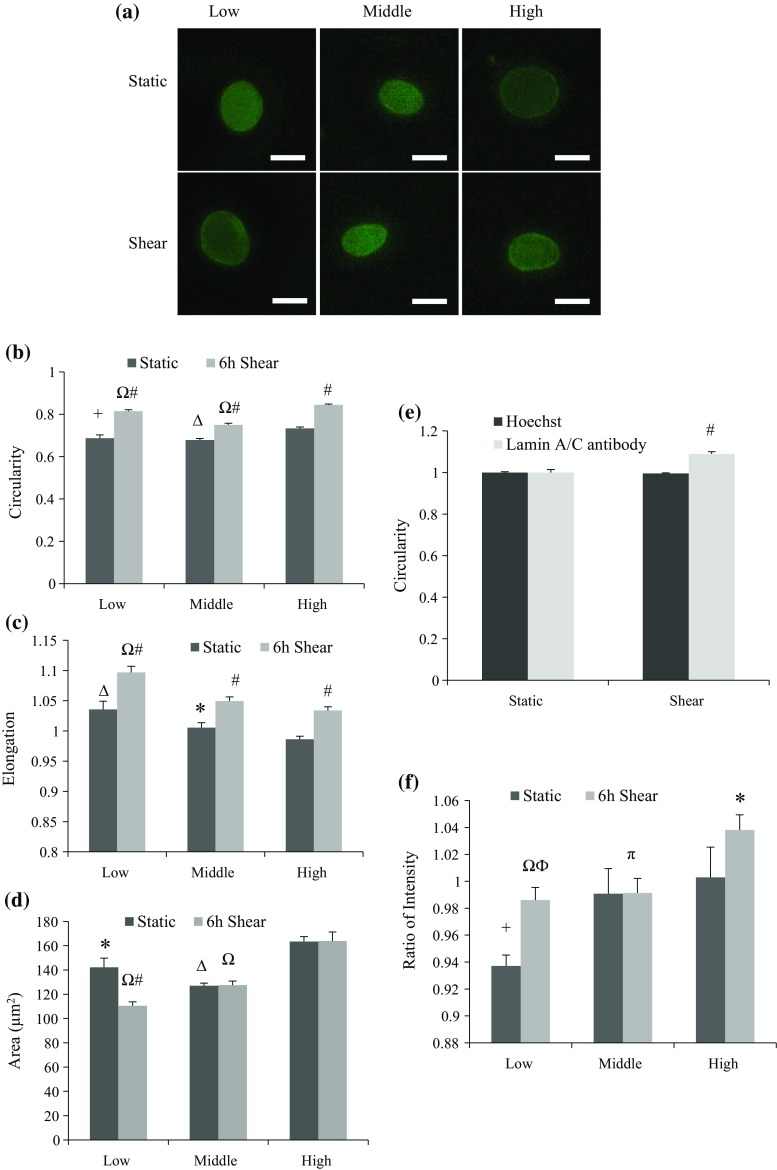
To further explore whether the cell doubling process influenced nuclear shape modulations toward shear stress, cells were divided into low, middle, and high PDL groups. Nuclear area, elongation and circularity were measured again by staining lamin A/C protein rather than using Hoechst DNA stain. Representative images in Fig. 3a show the stained nuclei before and after exposure to 6-h horizontal laminar shear stress. As shown in Figs. 3b and 3c, both circularity and elongation increased in cell nuclei that were exposed to shear stress at all PDL stages, while the overall area only decreased in low PDL cells after shearing (Fig. 3d). Parallel comparisons between groups with different PDL indicate that as the PDL went up in either static or sheared cells, increased area, elevated circularity and reduced elongation were observed in cell nuclei. Those differences suggest changes in nuclear mechanical properties in higher PDL cells that may result from lamin A/C reduction and redistribution within the nucleus. The data also suggest that cell PDL may affect nuclear lamin expression, as well as its ability to provide structural support to the nucleus.
Figure 3.
Shape analysis of endothelial nuclei for groups with different PDL. Cells were labeled with lamin A/C antibody either in static condition or after 6-h shear. At least 28 nuclei were picked for each analysis. (a) Fluorescent images of nuclei stained by anti-lamin A/C antibody before and after shear. Upper panel shows cells in static state, and lower panel shows cells after 6-h shear. Low, middle, and high PDL cells were presented in separate columns, from left to right. Scale bar: 10 µm. (b) Circularity of nuclei with varied PDL before and after shear. All groups of cells had increased circularity by shear stress (# p < 0.001 compared to static), and when compared with high PDL group, low and middle PDL groups all showed significant decrease in either static or shear case (+ p < 0.01, Δ p < 0.001 compared with high PDL group in static state, and Ω p < 0.001 compared with high PDL group after 6-h shear). (c.) Elongation of nuclei with varied PDL before and after shear. All groups of cells had elongated nuclei by shear stress (# p < 0.001 compared to static), and when compared with high PDL group, low PDL group showed significant increase in either static or shear case (Δ p < 0.001 and Ω p < 0.001 compared with high PDL cells in static and shear case, respectively), while middle PDL group only showed significance in control cells compared with high PDL control cells (*p < 0.05). (d) Nuclear area (µm2) of cells with varied PDL before and after shear. Cells with low PDL had decreased area after shear (# p < 0.001), while no significance was observed within either middle or high PDL groups. When compared with the high PDL group, low and middle PDL groups all showed significant area decrease in either static or shear case (*p < 0.05, Δ p < 0.001 compared with high PDL in static state, and Ω p < 0.001 compared with high PDL after 6-h shear). (e) Circularity of nuclei with middle PDL before and after shear stained either by Hoechst solution or anti-lamin A/C antibody. Only nuclei labeled with anti-lamin A/C antibody showed increased circularity after exposed to shear stress (# p < 0.001 compared to static). (f) Ratio of lamin A/C intensity at the nuclear periphery over whole nucleus. Both low and high PDL group showed significant increase in intensity ratio after shear (ϕ p < 0.01 and *p < 0.05, respectively). Significant increase in ratio was also observed in high PDL in static state (+ p < 0.05 compared to low PDL static cells). Moreover, sheared high PDL group showed significant increase compared to the other two (Ω p < 0.001 and π p < 0.05, respectively).
Interestingly, when we compared Figs. 2 and 3, we saw fairly consistent results. We observed a decrease in nuclear area and an increase in elongation after applied shear stress regardless of PDL. Only the circularity of the nuclei stained by lamin A/C antibody (Fig. 3b) was inconsistent with Fig. 2c, where Hoechst stain was used. Further validation was performed using cells at the same PDL level (middle PDL). Cells were sheared and stained by those two reagents. Following quantitative analysis, the result confirmed slightly decreased circularity in cells stained by Hochest solution but increased circularity in cells stained by anti-lamin A/C antibody (Fig. 3e).
Lamin A/C Accumulates at the Nuclear Periphery After Shear, Especially in Low and High PDL Cells
Furthermore, our data suggest that shear stress brought more lamin A/C to the nuclear periphery (Fig. 3f). To quantify how lamin A/C localized within the nucleus, quantitative analysis was carried out to measure lamin A/C intensity at the periphery as well as overall nuclear area, based on fluorescent images of cells labeled with anti-lamin A/C antibody. The ratio of intensity was calculated as the ratio of lamin A/C at the periphery over the whole nucleus (including the periphery). Figure 3f shows that shear stress significantly increased peripheral lamin A/C in both and low and high PDL groups after shear experiment. This accumulation was noticeable in the high PDL group, where lamin A/C was more evenly distributed at the nuclear periphery in sheared cells compared to static cells, where lamin A/C was more speckled at the nuclear rim (Fig. 3a). We saw a similar trend earlier in Fig. 1c, where lamin distribution changed from the nuclear interior to the nuclear periphery in higher PDL cells. Quantitative results also showed elevated ratio of lamin A/C intensity at the nuclear periphery over whole nucleus with increasing PDL (Fig. 3f), suggesting that lamin A/C tended to accumulate at the nuclear periphery as PDL increased. This phenomenon in higher PDL cells might be a way to compensate the nuclear mechanical sensitivity due to decreased overall lamin A/C. Overall, our result indicates that shear stress may affect lamin A/C redistribution within the nucleus, in a PDL dependent manner.
Protein Expression of Lamin A/C Responds to Shear Stress Differently Depending on PDL
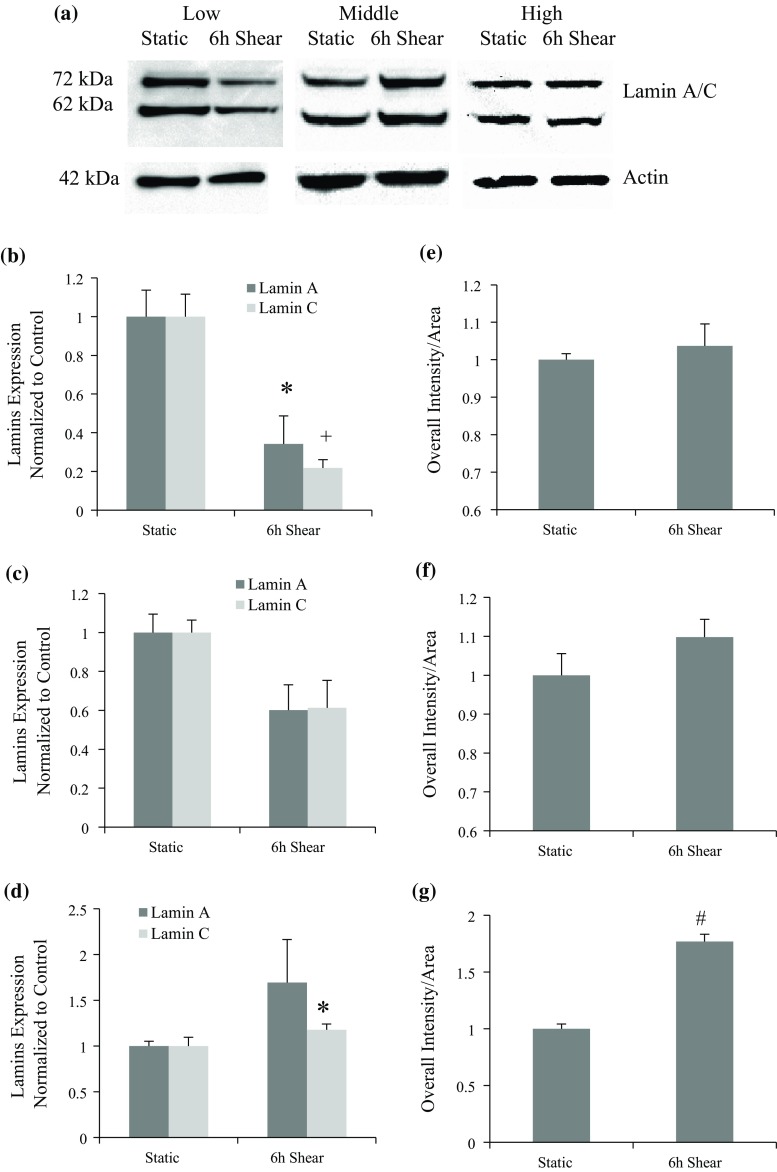
Western Blot was conducted to examine the possible effect that PDL could bring on lamin A/C expression after applying shear stress. Figure 4a shows representative Western blots at different PDL levels. Results show that lamin A/C expression was reduced in response to shear stress in low PDL cells (Fig. 4b). However, this trend dissipated as PDL increased (Fig. 4c), and was reversed in high PDL cells (Fig. 4d). To verify Western Blot results, mean intensity (overall intensity/area) based on lamin immunostaining images was quantified and normalized (Figs. 4e, 4f and 4g). There is a good agreement between Western and fluorescence intensity results for middle and high PDL cells. Interestingly, no significant lamin A/C change was observed in low PDL group after shear based on the result of fluorescence intensity. A similar trend was observed for overall intensity that is independent of changes in area observed in Fig. 3d. The discrepancy between Western blot and fluorescence intensity results for low PDL cells (Figs. 4b and 4e) could be contributed instead to the significant change in lamin distribution, from the nuclear interior to periphery after shear stress, in low PDL cells (Fig. 3f).
Figure 4.
Lamin A/C protein expression after 6-h shear in cells at different PDL levels. (a) Representative blot images of lamin A/C and actin before and after applying laminar shear in groups with varied PDL. (b) Lamin A/C protein expression after 6-h shear in low PDL group, which shows significant decreases compared to control sample (*p < 0.05 for lamin A and + p < 0.01 for lamin C). (c) Lamin A/C protein expression after 6-h shear in middle PDL cells, and no significant difference was observed. (d) Lamin A/C protein expression after 6-h shear in high PDL cells, which shows significant increase for lamin C compared to control sample (*p < 0.05). (e, f, g) Mean intensity of lamin A/C within the nucleus before and after shear for three groups: low, middle, and high PDL cells, respectively. Cells were stained with lamin A/C antibody either in static condition or after 6-h shear, and results show changes with respect to static control. Significant increase was observed in high PDL cells after shear (# p < 0.001 compared with static group).
These results suggest that shear stress can better modulate lamin A/C in low PDL cells, which may facilitate nuclear remodeling, since the high level of lamin A could hinder nuclear shape changes.49 Similar to Western results, the mean intensity was significantly increased in high PDL cells (Fig. 4g). These data further support a regulatory role for shear stress on lamin A/C expression in BAEC that is highly sensitive to cell passage number or PDL. While other studies have shown changes in lamin expression in primary endothelial cells in response to different shear levels,19 this is the first study demonstrating that shear stress affects lamin expression in a PDL dependent manner.
High PDL Cells Have Elevated mRNA Level of LMNA Gene After 6-h Shear
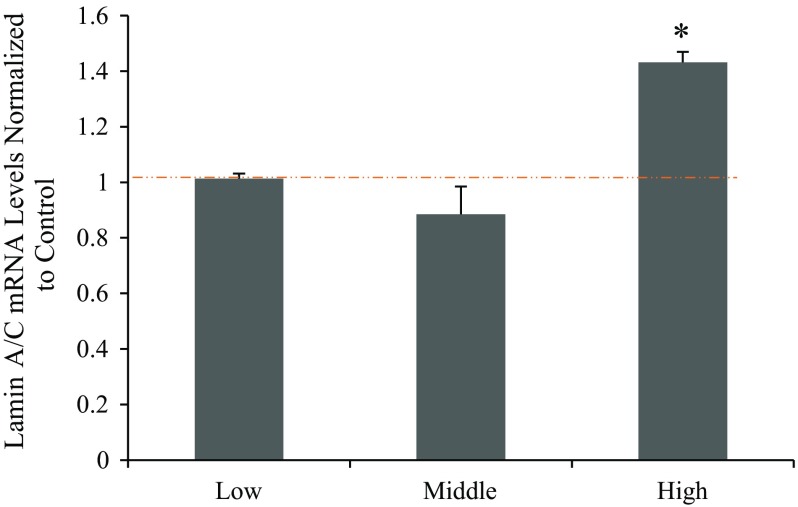
To examine if transcription level of lamin A/C was affected by shear stress, real time qPCR was utilized to investigate transcription levels of lamin A/C in cells (Fig. 5). GAPDH was used as the normalization gene for each plate. The fold increases of LMNA gene for low, middle, and high PDL cells after shear stress were compared to each respective static control group first. Results for these three groups were 1.01 ± 0.02, 0.89 ± 0.02 and 1.43 ± 0.04, respectively. The mRNA synthesis level did not change significantly after shear in low and middle PDL groups, while it was significantly elevated in high PDL group after exposure to shear stress. The mRNA expression level followed a similar trend as the protein expression level, particularly for high PDL cells (Figs. 4d and 4g). This suggests that the expression changes of lamin A/C in low PDL cells due to shear stress could be post transcriptional, while in high PDL cells shear stress affected transcriptional regulation of lamin A/C gene expression. The result further supports our previous findings that mechanical stress such as fluid shear stress plays a role in lamin A/C expression changes, and that the effect of shear stress is dependent on cell division level.
Figure 5.
Fold increase of LMNA (Lamin A/C) mRNA after 6-h shear with varied PDL compared to that of GAPDH. Values greater than 1 indicate increase in mRNA synthesis after shear experiments, and values less than 1 indicate decrease after shear. High PDL cells show significantly higher transcription level of LMNA after exposed to shear stress (*p < 0.05 compared to control cells).
Discussions
Atherosclerosis is believed to be related to the mechanical forces exerted on vascular cells. Hemodynamic forces (shear stress and circumferential stretch force) in vivo are modulating vascular cell behaviors either by remodeling cellular supporting network or by regulating biological pathways. One of the most important risk factors for atherosclerosis is aging. However, the role of vascular mechanical stresses on normal vascular aging, and the molecular pathways that might be involved, are not fully understood. In premature aging syndromes such as HGPS, the nuclear lamina is significantly damaged due to the production of a mutant lamin called progerin. These patients develop atherosclerosis despite young age, and almost always die from cardiovascular events. Since HGPS cell nuclei share many characteristics with cells from healthy elderly people, nuclear lamin could potentially play a significant role in vascular aging. Hemodynamic forces due to blood flow also contribute to maintaining normal physiological profiles of vascular cells. We began to investigate the role that nuclear lamin plays in vascular aging, particularly in endothelial cells that are continuously exposed to shear stress.
In this paper, we mainly focused on how lamin A/C responds to shear stress in endothelial cells in vitro. Furthermore, we looked at the effect of population doubling on cells’ ability to respond to mechanical stress. PD levels were used to indicate low, middle, and high cell passage groups. Although cells in serial subculture documented by PDL are not a direct model for in vivo aging, they still share many common characteristics. For instance, cells with high PDL and cells derived from old donors both showed decreased proliferation capacity and shortened telomere length.45 Elevated senescence in aged endothelium and at atherosclerosis-prone site has also been reported,2,35 which may indicate a changed response toward shear stress in older cells. Further, it is not known how senescent endothelial cells are replaced throughout aging process in vivo.14
Our results demonstrated the regulation of lamin A/C expression by shear stress in a PDL-dependent manner. The overall nuclear morphological changes were first observed in endothelial cells after 6-h shear regardless of PDL. Figure 2 showed that the nuclei were elongated and the average nuclear area was also decreased after shear, which might be a result of changes in nuclear lamina under shear. However, when we looked at cells with low, middle, and high PDL to consider cell doubling effect on lamin A/C, different nuclear shape changes by shear stress were observed (Fig. 3). Hoechst stain was used in Fig. 2, while nuclear perimeter was stained by lamin A/C antibody in Fig. 3. In both cases, nuclear area decreased (particularly for low PDL cells) and elongation increased consistently. However, circularity based on chromatin stain decreased by shear stress, while circularity calculated by lamin staining increased consistently at all PDL levels (Fig. 3e). One explanation would be the difference in the staining reagents. Hoechst stain bound to DNA which mostly dispersed at nucleoplasm, while staining for lamin A/C protein delineated the nuclear periphery and provided information about lamin A/C protein distribution. Cells stained by anti-lamin A/C antibody appeared to have smoother nuclear outline that may contribute to increased circularity. B-type lamins, which interconnect with lamin A/C meshwork and have more physical associations with chromatin, are thought to play a bigger role in anchoring nucleus to the cytoskeleton.16,23,46 Lamin B1 may also allow for different changes in chromatin and nuclear lamina shapes by providing the link between the nucleus and the cytoskeleton. Changes in lamina circularity and lamin A/C distribution can be correlated with changes in B-type lamins to yield a more complete picture of how shear stress affects the nuclear lamina.
We showed decreased lamin A/C expression with increasing PDL (Fig. 1), which is in good agreement with other observations.1,11 Different regulation patterns of lamin A/C by shear stress were observed in cells at different PDL levels. Our results showed that the lamin A/C were downregulated in low PDL cells, however, the decreasing trend was not significant in cells with middle PDL, and was even reversed in cells with high PDL (Figs. 4b–4d). Quantitative image analysis and statistical results also showed that, as PDL increased, A-type lamins became more localized to the nuclear periphery (Fig. 3f). Therefore, with increased cell doubling level, the overall expression of lamin A/C was suppressed, while its aggregation along the nuclear periphery was more enhanced. On the other hand, the distribution of lamin A/C within the nucleus also changed after 6-h shear, where the lamin A/C accumulated more at the periphery after shear (Fig. 3f), which might contribute to the nuclear shape adjustment toward shear. In addition, the anti-lamin A/C antibody we used has the antigen spanning the sequence around lamin A/C K470 at C terminal. It is also possible that the epitope recognized by the antigen was exposed more at the nuclear periphery after applying laminar shear stress on cells, which can also contribute to the observed redistribution effect.21
Moreover, the stable transcription levels in cells with low PDL indicate that the transcription was not affected by shear stress (Fig. 5), therefore, the downregulation effect in lamin A/C protein expression must happen after transcription, where either the mRNA or the protein was degraded by some regulators when being exposed to laminar shear stress. Meanwhile, the transcription levels in high PDL cells after shear increased, indicating potential regulation by transcription factors of the LMNA gene. Lamin A/C phosphorylation at Ser22 is also associated with myosin-II under environmental tensions, which led to lamin turnover and softening of the nucleus.6 Actomyosin activity and cell contractility under shear stress, plus other factors such as substrate stiffness that may affect lamin phosphorylation, could also contribute to post-transcriptional changes in lamin.
Overall, we provided evidence that both cell passage and fluid shear stress can affect lamin A/C expression and nuclear localization patterns, as well as the support for the hypothesis that PDL level has an impact on the regulatory pathway for lamin A/C induced by laminar shear stress. Similar decrease in lamin A/C expression was observed either by increasing cell passage or by applying shear stress in low PDL cells. In high PDL cells, shear stress actually induced increase in lamin A/C expression. The effect on nuclear shape also varied between low and middle PDL cells: As PDL increased, the nuclear shape was enlarged, while shearing on low PDL cells promoted nuclear elongation as well as reduced nuclear area. Our results showed that lamin A/C is a novel mechano-sensitive nuclear structural protein, whose expression and functions are also affected by cell passage.
In our study, we compared static cells with cells exposed to laminar shear stress at 15 dyne/cm2, which is at a more athero-protective shear level. Shear stress level could also be an important factor, since low level of shear stress would be more inflammatory. A previous report showed reduced lamin A expression in primary rat aortic endothelial cells (within passage 2–4) under low shear stress (5 dynes/cm2) compared with normal shear stress (15 dynes/cm2).19 However, our results showed that only high PDL cells had elevated lamin A/C expression in response to shear stress of 15 dynes/cm2. Although lamin A/C regulation under shear has been confirmed, it is still not known what regulators suppressed lamin A/C protein expression in low PDL cells, and what transcription factors promoted lamin A/C mRNA synthesis in sheared high PDL cells. High passage cells are likely to begin experiencing cell senescence, and changes in lamin expression due to mechanical stress could also contribute to cell senescence. Although elevated laminar shear stress in the vasculature is protective against developing atherosclerosis, its effect could be balanced by cell age.
In HGPS patients, the overexpression of progerin could accelerate cell senescence, especially in those vascular cells that sustain high level of mechanical force and have high proliferative rate,30 which may contribute to atherosclerosis formation. Therefore, the altered lamin A/C expression toward shear stress in senescent cells can also provide a cellular pathway for atherosclerotic development during cell senescence. Future studies could also include using progerin or pre-lamin A accumulation as models for cell aging. Such studies would not only help understand molecular mechanisms of HGPS, but also help clarify the mechano-transduction link between vascular aging and nuclear lamin. Exploring the upstream factors that influence lamin expression would also shed light on understanding how early atherosclerosis develops under hemodynamic forces and its relationship with vascular aging.
Acknowledgments
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
This research does not involve human participants and/or animals.
Informed consent
Informed consent was not needed.
References
- 1.Afilalo J, Sebag IA, Chalifour LE, Rivas D, Akter R, et al. Age-related changes in lamin A/C expression in cardiomyocytes. Am. J. Physiol. 2007;293:H1451–H1456. doi: 10.1152/ajpheart.01194.2006. [DOI] [PubMed] [Google Scholar]
- 2.Aviv H, Khan MY, Skurnick J, Okuda K, Kimura M, et al. Age dependent aneuploidy and telomere length of the human vascular endothelium. Atherosclerosis. 2001;159:281–287. doi: 10.1016/S0021-9150(01)00506-8. [DOI] [PubMed] [Google Scholar]
- 3.Barakat A, Lieu D. Differential responsiveness of vascular endothelial cells to different types of fluid mechanical shear stress. Cell Biochem. Biophys. 2003;38:323–343. doi: 10.1385/CBB:38:3:323. [DOI] [PubMed] [Google Scholar]
- 4.Barascu A, Le Chalony C, Pennarun G, Genet D, Imam N, et al. Oxidative stress induces an ATM-independent senescence pathway through p38 MAPK-mediated lamin B1 accumulation. EMBO J. 2012;31:1080–1094. doi: 10.1038/emboj.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benson EK, Lee SW, Aaronson SA. Role of progerin-induced telomere dysfunction in HGPS premature cellular senescence. J. Cell Sci. 2010;123:2605–2612. doi: 10.1242/jcs.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buxboim A, Swift J, Irianto J, Spinler KR, Dingal PC, et al. Matrix elasticity regulates lamin-A, C phosphorylation and turnover with feedback to actomyosin. Curr. Biol. 2014;24:1909–1917. doi: 10.1016/j.cub.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, et al. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J. Clin. Investig. 2011;121:2833–2844. doi: 10.1172/JCI43578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carboni N, Politano L, Floris M, Mateddu A, Solla E, et al. Overlapping syndromes in laminopathies: a meta-analysis of the reported literature. Acta Myol. 2013;32:7–17. [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Investig. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 10.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duque G, Rivas D. Age-related changes in lamin A/C expression in the osteoarticular system: laminopathies as a potential new aging mechanism. Mech. Ageing Dev. 2006;127:378–383. doi: 10.1016/j.mad.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Emerson LJ, Holt MR, Wheeler MA, Wehnert M, Parsons M, Ellis JA. Defects in cell spreading and ERK1/2 activation in fibroblasts with lamin A/C mutations. BBA-Mol. Basis Dis. 2009;1792:810–821. doi: 10.1016/j.bbadis.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J. Appl. Physiol. 2009;106:326–332. doi: 10.1152/japplphysiol.91353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortuno A, Jose GS, Moreno MU, Diez J, Zalba G. Oxidative stress and vascular remodelling. Exp. Physiol. 2005;90:457–462. doi: 10.1113/expphysiol.2005.030098. [DOI] [PubMed] [Google Scholar]
- 16.Gerace L, Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980;19:277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- 17.Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 2014;16:376. doi: 10.1038/ncb2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han S, Brunet A. Histone methylation makes its mark on longevity. Trends Cell Biol. 2012;22:42–49. doi: 10.1016/j.tcb.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han Y, Wang L, Yao QP, Zhang P, Liu B, et al. Nuclear envelope proteins Nesprin2 and LaminA regulate proliferation and apoptosis of vascular endothelial cells in response to shear stress. Biochimica et Biophysica Acta. 1853;1165–73:2015. doi: 10.1016/j.bbamcr.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazel AL, Pedley TJ. Vascular endothelial cells minimize the total force on their nuclei. Biophys. J. 2000;78:47–54. doi: 10.1016/S0006-3495(00)76571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ihalainen TO, Aires L, Herzog FA, Schwartlander R, Moeller J, Vogel V. Differential basal-to-apical accessibility of lamin A/C epitopes in the nuclear lamina regulated by changes in cytoskeletal tension. Nat. Mater. 2015;14:1252–1261. doi: 10.1038/nmat4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Infante A, Gago A, de Eguino GR, Calvo-Fernandez T, Gomez-Vallejo V, et al. Prelamin A accumulation and stress conditions induce impaired Oct-1 activity and autophagy in prematurely aged human mesenchymal stem cell. Aging. 2014;6:264–280. doi: 10.18632/aging.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji JY, Lee RT, Vergnes L, Fong LG, Stewart CL, et al. Cell nuclei spin in the absence of lamin b1. J. Biol. Chem. 2007;282:20015–20026. doi: 10.1074/jbc.M611094200. [DOI] [PubMed] [Google Scholar]
- 24.Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J. Biol. Chem. 2006;281:25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 25.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Investig. 2004;113:370–378. doi: 10.1172/JCI200419670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefevre C, Auclair M, Boccara F, Bastard JP, Capeau J, et al. Premature senescence of vascular cells is induced by HIV protease inhibitors implication of prelamin A and reversion by statin. Arterioscler. Thromb. Vasc. Biol. 2010;30:2611-U527. doi: 10.1161/ATVBAHA.110.213603. [DOI] [PubMed] [Google Scholar]
- 27.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 28.McClintock D, Gordon LB, Djabali K. Hutchinson-Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody. Proc. Natl. Acad. Sci. USA. 2006;103:2154–2159. doi: 10.1073/pnas.0511133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClintock, D., D. Ratner, M. Lokuge, D. M. Owens, and L. B. Gordon, et al. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS ONE 2, 2007. [DOI] [PMC free article] [PubMed]
- 30.Miller JD, Ganat YM, Kishinevsky S, Bowman RL, Liu B, et al. Human iPSC-based modeling of late-onset disease via progerin-induced aging. Cell Stem Cell. 2013;13:691–705. doi: 10.1016/j.stem.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moir RD, Yoon M, Khuon S, Goldman RD. Nuclear lamins A and B1: different pathways of assembly during nuclear envelope formation in living cells. J. Cell Biol. 2000;151:1155–1168. doi: 10.1083/jcb.151.6.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mounkes LC, Stewart CL. Aging and nuclear organization: lamins and progeria. Curr. Opin. Cell Biol. 2004;16:322–327. doi: 10.1016/j.ceb.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 34.Muchir A, Shan J, Bonne G, Lehnart SE, Worman HJ. Inhibition of extracellular signal-regulated kinase signaling to prevent cardiomyopathy caused by mutation in the gene encoding A-type lamins. Hum. Mol. Genet. 2009;18:241–247. doi: 10.1093/hmg/ddn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okuda K, Khan MY, Skurnick J, Kimura M, Aviv H, Aviv A. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis. 2000;152:391–398. doi: 10.1016/S0021-9150(99)00482-7. [DOI] [PubMed] [Google Scholar]
- 36.Olive M, Harten I, Mitchell R, Beers JK, Djabali K, et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 2010;30:2301–2309. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Partridge J, Carlsen H, Enesa K, Chaudhury H, Zakkar M, et al. Laminar shear stress acts as a switch to regulate divergent functions of NF-kappa B in endothelial cells. Faseb J. 2007;21:3553–3561. doi: 10.1096/fj.06-8059com. [DOI] [PubMed] [Google Scholar]
- 38.Paul, J. 1975. Cell and Tissue Culture. Edinburgh, New York: Churchill Livingstone; distributed in the U.S.A. by Longman. xii, 484 p., [14] leaves of plates pp.
- 39.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philip JT, Dahl KN. Nuclear mechanotransduction: response of the lamina to extracellular stress with implications in aging. J. Biomech. 2008;41:3164–3170. doi: 10.1016/j.jbiomech.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 41.Pollex RL, Hegele RA. Hutchinson-Gilford progeria syndrome. Clin. Genet. 2004;66:375–381. doi: 10.1111/j.1399-0004.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 42.Ragnauth CD, Warren DT, Liu YW, McNair R, Tajsic T, et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121:2200-U96. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
- 43.Rennier, K., and IUPUI Scholar Works. The role of DAP-kinase in modulating vascular endothelial cell function under fluid shear stress. pp. [2], ix, 72 leaves, 2010.
- 44.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider EL, Mitsui Y. The relationship between in vitro cellular aging and in vivo human age. Proc. Natl. Acad. Sci. USA. 1976;73:3584–3588. doi: 10.1073/pnas.73.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimi T, Pfleghaar K, Kojima SI, Pack CG, Solovei I, et al. The A- and B-type nuclear lamin networks: microdomains involved in chromatin organization and transcription. Gene Dev. 2008;22:3409–3421. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shumaker DK, Dechat T, Kohlmaier A, Adam SA, Bozovsky MR, et al. Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc. Natl. Acad. Sci. USA. 2006;103:8703–8708. doi: 10.1073/pnas.0602569103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solovei I, Wang AS, Thanisch K, Schmidt CS, Krebs S, et al. LBR and lamin A/C sequentially tether peripheral heterochromatin and inversely regulate differentiation. Cell. 2013;152:584–598. doi: 10.1016/j.cell.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thavarajah R, Mudimbaimannar VK, Elizabeth J, Rao UK, Ranganathan K. Chemical and physical basics of routine formaldehyde fixation. J. Oral Maxillofac. Pathol. 2012;16:400–405. doi: 10.4103/0973-029X.102496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Traub O, Berk BC. Laminar shear stress—mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscl. Thromb. Vasc. Biol. 1998;18:677–685. doi: 10.1161/01.ATV.18.5.677. [DOI] [PubMed] [Google Scholar]
- 52.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 53.Vlcek S, Foisner R. Lamins and lamin-associated proteins in aging and disease. Curr. Opin. Cell Biol. 2007;19:298–304. doi: 10.1016/j.ceb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Worman HJ. Nuclear lamins and laminopathies. J. Pathol. 2012;226:316–325. doi: 10.1002/path.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]