Abstract
Background
Hyaluronic acid (HA) has been found to be an important trigger of atherosclerosis. In this study, we investigate the possible association of serum HA with cardiovascular disease risk in a population of low/intermediate risk for cardiovascular events.
Methods
We enrolled 200 subjects with low/intermediate risk for developing cardiovascular disease. High specific C‐reactive protein (hsCRP) was used as an indicator of preclinical atherosclerosis. The Framingham score was used to calculate the cardiovascular risk.
Results
Participants with dyslipidemia had significantly higher levels of serum HA than those without dyslipidemia (t‐test, P = 0.05), higher levels of hsCRP (Kruskal–Wallis test, P = 0.04), and higher cardiovascular risk according to the Framingham score (Kruskal–Wallis test, P = 0.05). Serum HA concentration correlated significantly with the Framingham score for risk for coronary heart disease over the next 10 years (Spearman r = 0.152, P = 0.02). Diabetic volunteers had significantly higher HA than those without diabetes (t‐test, P = 0.02). Participants with metabolic syndrome had higher serum HA levels and higher hsCRP (Kruskal–Wallis test, P = 0.01) compared to volunteers without metabolic syndrome (t‐test, P = 0.03).
Conclusions
Serum HA should be explored as an early marker of atheromatosis and cardiovascular risk.
Keywords: atherosclerosis, cardiovascular risk, diabetes, dyslipidemia, early marker, hyaluronic acid, metabolic syndrome
Introduction
Cardiovascular disease is currently the leading cause of death in developing countries with smoking, obesity, physical inactivity, hyperlipidemia, hypertension, and diabetes mellitus being the most important risk factors. Early intervention not only for disease prevention but also for early diagnosis is important.
Hyaluronic acid (HA) is a nonsulfate glycosaminoglycan composed of repeating disaccharide groups, D‐glucuronic acid, and N‐acetyl‐D‐glucosamine. It is synthesized by cells of the arterial wall (fibroblasts, endothelial cells, and smooth muscle cells) and hepatic stellate cells 1. Its role is to retain tissue water, maintain the osmotic balance, and regulate cellular processes such as migration, adhesion, and proliferation. HA interaction with cell membrane receptors contributes to morphogenesis, tissue remodeling, and inflammation 2. Different length HA molecules induce different functions; high molecular weight HA (106 Da) inhibits angiogenesis, whereas HA molecules of 3–25 disaccharide units induce angiogenesis. Along with other glycosaminoglycans (GAGs), HA has an important role in the interaction of vascular tissue and blood components. GAGs reduce the interaction between endothelial cells and leukocytes 3, contributing to the regulation of redox state; they are crucially involved in the mediation of shear‐induced nitric oxide release as well as physiologic anticoagulation.
Hyaluronic acid is increased in vascular plaques 2 and its high metabolism causes their destabilization 4. Use of HA in angioplasty stents inhibits vessel restenosis 5 and clot formation 6. Studies in rats have shown that HA is increased in serum and in heart muscle on the first and third day after myocardial infarction 7. Hyaluronic acid binds water in the heart muscle and improves both the mechanical and electrophysiological functions of the heart. Thus, it was suggested that application of HA in the heart muscle after infarction could be a possible treatment 8. Moreover, it has been shown that serum HA in patients before coronary artery bypass grafting (CABG) is an indicator of possible occurrence of arterial fibrillation postoperatively 9. Serum HA has been used to assess the severity of myocardial fibrosis in chronic congestive heart failure (CHF) 10 and its increase is associated with heart transplants rejection in rats 11. In patients with chronic renal failure, serum HA correlates with the adhesion molecules soluble vascular cell adhesion molecule‐1 (sVCAM‐1) and sICAM‐1, which play an important role in atherogenesis 12.
We investigated the possible association of serum HA with the risk of cardiovascular disease in a population of low/intermediate risk for cardiovascular events.
Materials and Methods
Study Population
We enrolled 200 subjects of both gender, aged 35–70, and low/intermediate cardiovascular risk. hsCRP (high specific C‐reactive protein) was used as an indicator of preclinical atherosclerosis, whereas the Framingham score was used to calculate the cardiovascular risk 13.
Sample Analysis
Serum samples from all participants were stored at 20°C till analyzed for HA. Serum glucose, urea, creatinine, Alanine Transaminase (ALT/SGPT), Aspartate Aminotransferase (AST/SGOT), Gamma‐glutamyl Transferase (GGT), cholesterol, High‐density lipoprotein (HDL), Low‐density lipoprotein (LDL), triglycerides, and total bilirubin (TBIL) concentrations were measured using the A25 Clinical Chemistry Analyser (Biosystems S.A., Barcelona, Spain). Serum hs‐CRP was measured by turbidimetric/immunoturbidimetric method using the Abbott Architect c8000 Clinical Chemistry and Immunoassay Analyser (Abbott Laboratories, Abbott Park, IL).
Measurement of HA Concentration
Serum HA was measured by a latex agglutination method (Wako Chemicals GmbH, Neuss, Germany) that was applied in Siemens ADVIA 1800 Clinical Chemistry Analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY). This assay contains a hyaluronic acid binding protein (HABP), which binds specifically the HA in serum sample. To make an insoluble aggregate, latex particles coated by anti‐HABP antibody are added and the latex binds to above complex. As a result, the insoluble aggregate increases turbidity in the solution. The degree of turbidity of the solution can be measured optically and is proportional to the concentration of hyaluronic acid in the sample. The limit of detection of this method is 5.8 μg/l 14.
Statistical Analysis
The correlation of serum HA levels with demographic characteristics, biochemical markers, hsCRP, and Framingham cardiovascular risk score was calculated using Pearson's correlation coefficient and Spearman's correlation coefficient. Differences in HA serum value between groups were evaluated using Student's t‐test, Kruskal–Wallis, Mann–Whitney, ANOVA, and chi‐square test. All analyses were conducted using the SPSS statistical package (Statistical Package for Social Sciences v. 17, Chicago, IL) and STAT‐GRAPHICS PLUS version 5.1 (Graphic Software System, Warrenton, VA, USA).
Results
We enrolled 200 subjects (113 women and 87 men), mean age 49.9 years (±0.55), and Body Mass Index (BMI) 28.3 (range = 17.01–49.54). A total of 64 were smokers, 19 had been diagnosed with Type II diabetes, and 118 with hypercholesterolemia receiving lipid‐lowering therapy (statins) (Table 1). All participants had normal hsCRP values. One hundred and sixty‐eight of 200 participants were low risk for developing cardiovascular disease (disease incidence being less or equal to 10% for the next 10 years), whereas 32 subjects had Framingham score between 10% and 20%, which accounts for average cardiovascular risk.
Table 1.
Distribution of Baseline Characteristics in Study Population
| Characteristic | |
| Age (years) | 49.9 (0.55) |
| Male/female | 87 (44)/113 (56) |
| BMI (kg/m2) | 28.3 (4.5) |
| Diabetes mellitus | 19 (10) |
| Smoker | 64 (32) |
| Dyslipidemia | 142 (71) |
| Lipid‐lowering treatment | 118 (59) |
| Biochemical markers | |
| Glucose (mg/dl) | 95.4 (2.2) |
| Creatinine (mg/dl) | 0.82 (0.01) |
| Urea (mg/dl) | 32.2 (1.2) |
| SGOT (U/l) | 28.4 (0.8) |
| SGPT (U/l) | 32.3 (2.3) |
| GGT (U/l) | 22.8 (1.1) |
| Cholesterol (mg/dl) | 187.8 (2.4) |
| HDL – cholesterol (mg/dL) | 41.8 (0.7) |
| LDL – cholesterol (mg/dl) | 121.4 (2.2) |
| Triglycerides (mg/dl) | 107.7 (74) |
| Uric acid (mg/dl) | 5.0 (8.1) |
| Cardiovascular risk factors | |
| Haluronic acid (ng/ml) | 65.5 (2.6) |
| Framingham risk score (%) | 2 (5) |
| Framingham risk (Low/Intermediate) | 168/32 |
| hsCRP (mg/l) | 0.13 (0.2) |
| Hematological markers | |
| Hemoglobin (g/dl) | 13.6 (0.58) |
| White blood cells (109/l) | 7.239 (128) |
| Platelets (109/l) | 238 (4) |
For categorical: n (%); for continuous: mean (SEM), except for BMI, hsCRP, triglycerides, Framingham risk score, which are expressed as median (interquartile range).
Serum HA was positively associated with BMI (r = 0.170, P = 0.02), age (r = 0.225, P = 0.01), and triglycerides (r = 0.142, P = 0.02). Serum HA concentration had a positive correlation with urea and creatinine levels (r = 0.136, P = 0.05 and r = 0.138, P = 0.05). Serum HA also correlated significantly with the Framingham score for risk for coronary heart disease over the next 10 years (r = 0.152, P = 0.02); (Fig. 1). We did not find significant correlation between hsCRP and HA concentration.
Figure 1.
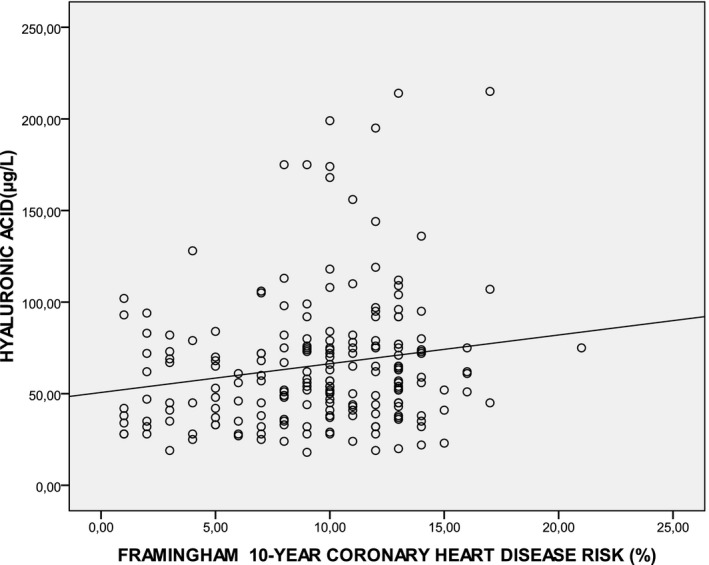
Correlation between serum concentration of HA (μg/l) and Framingham 10‐year coronary heart disease risk (%) (r = 0.152, P = 0.03).
Dyslipidemia
Participants with dyslipidemia had significantly higher levels of serum HA (68.8 ± 3.2 μg/l) than those without dyslipidemia (57.5 ± 3.8 μg/l; t‐test, P = 0.05) (Fig 2a) and significantly higher levels of hsCRP (median = 0.15, range = 0.02–1.27) mg/l) than without (median = 0.10, range = 0.02–1.22 mg/l; Kruskal–Wallis test, P = 0.04); (Fig. 2b), (Table 2). Dyslipidemia group showed significantly higher cardiovascular risk according to the Framingham score (Kruskal–Wallis test, P = 0.05) and higher levels of uric acid (5.2 ± 1.03 ng/ml) than those without (4.8 ± 1.03 ng/ml; t‐test, P = 0.03).
Figure 2.
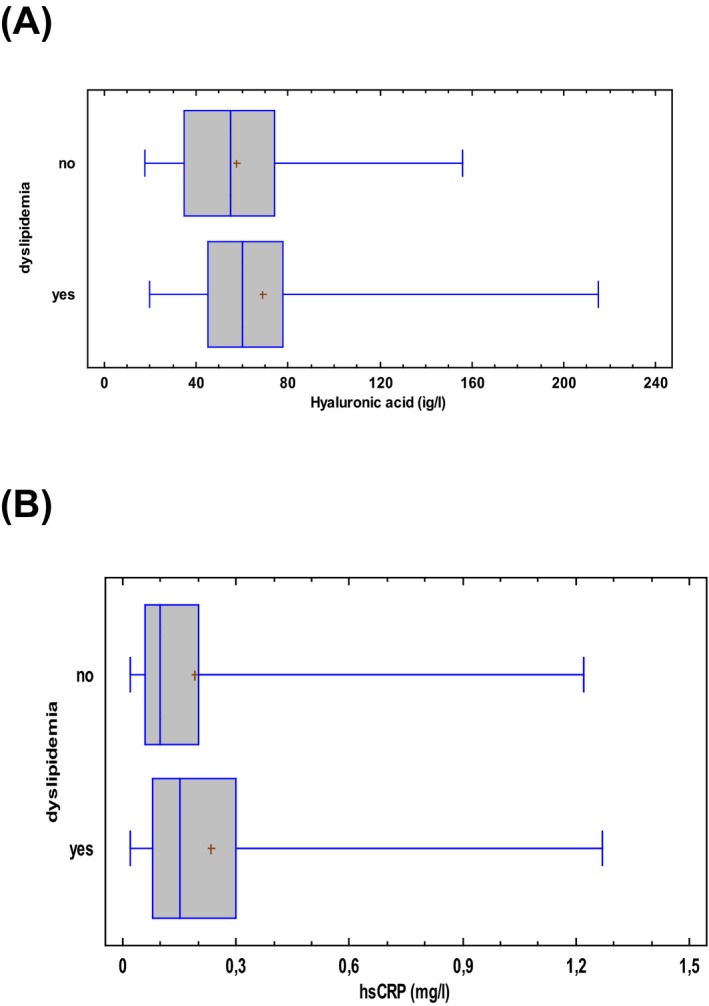
Box plot (5–95th percentiles) of (a) serum hyaluronic acid levels (μg/l) and (b) hsCRP levels (mg/l) in participants without and with dyslipidemia.
Table 2.
Distribution of Characteristics in Participants With and Without Hypercholesterolemia Treatment, Diabetes Mellitus, and Metabolic Syndrome
| Characteristic | HA (μg/l) | P‐value | hs‐CRP (mg/dl) | P‐value | Framingham risk score (Low/Intermediate) | P‐value |
|---|---|---|---|---|---|---|
| Dyslipidemia | ||||||
| Yes (n = 142) | 68.8 (3.2) | 0.05 | 0.15 (0.02–1.27) | 0.04 | 118/24 | 0.377 |
| No (n = 58) | 57.5 (3.8) | 0.10 (0.02–1.22) | 50/8 | |||
| Hypercholesterolemia treatment | ||||||
| Yes (n = 119) | 70.0 (3.8) | 0.04 | 0.15 (0.02–1.27) | 0.05 | 100/18 | 0.438 |
| No (n = 81) | 59.1 (3.1) | 0.11 (0.02–1.22) | 68/14 | |||
| Diabetic mellitus | ||||||
| Yes (n = 19) | 90.9 (10.7) | 0.02 | 0.12 (0.03–1.19) | 0.442 | 12/7 | 0.017 |
| No (n = 181) | 62.8 (2.5) | 0.13 (0.02–1.27) | 156/25 | |||
| Metabolic syndrome | ||||||
| Yes (n = 27) | 85.2 (9.7) | 0.03 | 0.2 (0.04–1.19) | 0.001 | 24/3 | 0.001 |
| No (n = 173) | 62.4 (2.5) | 0.1 (0.02–1.27) | 120/53 | |||
For categorical: n (% of participants); for continuous: mean (SEM), except for BMI, hsCRP, which are expressed as median (range).
P‐values were derived from t‐test for HA, Kruskal–Wallis test for hsCRP, and chi‐square test for Framingham risk score.
Hypercholesterolemia Treatment
About 119 participants, treated with statins for hypercholesterolemia, had significantly higher HA serum levels (70.0 ± 3.8 μg/l) and hsCRP (median = 0.15 mg/l, range = 0.02–1.27 mg/l) compared to the 81 not receiving lipid‐lowering therapy (HA = 59.1 ± 3.1 μg/l; t‐test, P = 0.04; hsCRP median = 0.11, range 0.02–1.22 mg/l, Kruskal–Wallis test, P = 0.05); (Table 2). SGOT and SGPT levels did not differ significantly between the two groups. Fourteen participants who were not treated for hypercholesterolemia, but with triglycerides more than 150 mg/dl, had significantly higher HA serum levels (70.7 ± 6.3 μg/l) compared to 68 participants who were not on any therapy and had triglyceride levels less than 150 mg/dl (56.6 ± 4.9 μg/l) (Kruskal–Wallis test, P = 0.04). A total of 39 subjects under statins and LDL levels higher than 130 mg/dl had significant higher HA (69 ± 5.6 μg/l) than 31 subjects receiving treatment and LDL levels higher than 130 mg/dl (55.7 ± 4.9 μg/l) (Kruskal–Wallis, P = 0.05).
Diabetes and Hyperglycemia
About 19 diabetic volunteers had higher serum HA than nondiabetic controls (90.9 ± 10.7 μg/l vs. 62.8 ± 2.5 μg/l; t‐test, P = 0.02). None of the diabetic patients had complications and only five (three men and two women) had cholesterol higher than 200 mg/dl. No association of hyaluronic acid with HbA1c was found in diabetic patients. In total, 26 volunteers with glucose levels higher than 120 mg/dl, regardless of diagnosis of diabetes, had significantly higher serum HA compared to those who had glucose levels less than 120 mg/dl (76.7 ± 8.8 μg/l vs. 63.4 ± 2.6 μg/l; t‐test, P = 0.03). They also had a higher risk for developing heart disease, according to Framingham score (chi‐square test, P = 0.04); (Table 2).
Metabolic Syndrome
The diagnosis of metabolic syndrome was made according to NCEP/ATP III 2001 criteria and 27 of 200 participants had metabolic syndrome, and they had higher levels of serum HA (85.2 ± 9.7 μg/l) (t‐test, P = 0.03) (Fig. 3a) and higher hsCRP (median = 0.2, range = 0.04–1.19 mg/l) (Fig. 3b) compared to volunteers without metabolic syndrome (HA: 62.4 ± 2.5 μg/l, P = 0.03, t‐test; hsCRP: median = 0.1 range = 0.02–1.27 mg/l, Kruskal–Wallis test, P = 0.01). Subjects with metabolic syndrome were found to have higher risk for cardiovascular disease, as expected (chi‐square test, P = 0.001); (Table 2).
Figure 3.
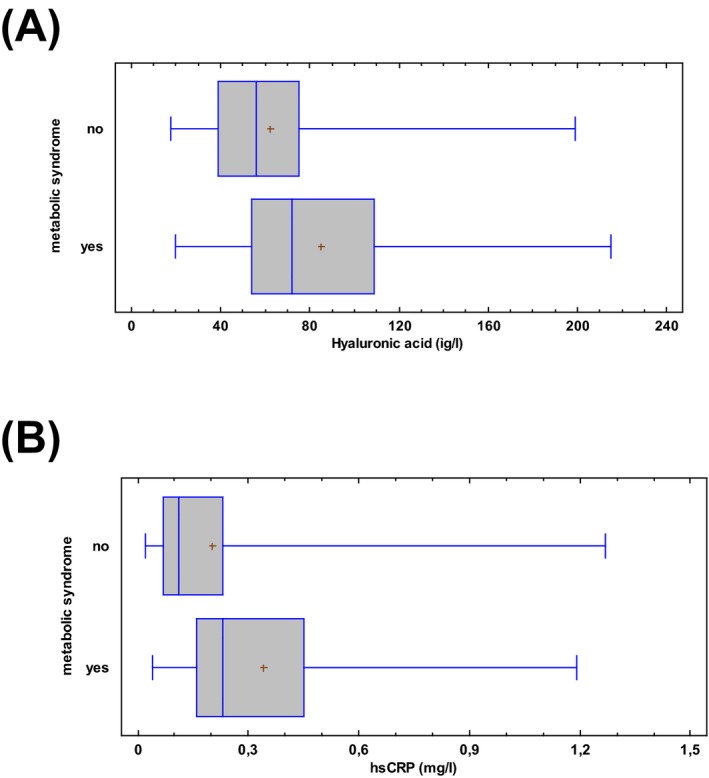
Box plot (5–95th percentiles) of (a) serum hyaluronic acid levels (μg/l) and (b) hsCRP levels (mg/l) in participants without and with metabolic syndrome.
Discussion
Vascular wall proteoglycans mediate shear‐induced release of nitric oxide and contribute to the endothelial permeability barrier, regulation of redox state, inhibition of coagulation, as well as leukocyte and platelet adhesion 15. Damage of proteoglycans can occur under exposure to atherogenic agents, such as oxidized LDL 15, 16, hypercholesterolemia 17, 18, and hyperglycemia 19. Glycosaminoglycans also form complexes with lipoproteins in the aortic wall and activate atherosclerotic mechanisms leading to cardiovascular disease 20. On the other hand, HDL inhibits proteoglycan synthesis and formation of complexes with lipoproteins 21, inhibiting atherogenesis.
Serum HA increases in several pathologies such as rheumatoid arthritis 22 and liver cirrhosis 23. The role of HA in atherogenesis has been investigated extensively and it is well demonstrated that HA contributes in the early development of atherosclerosis. Initial studies showed that its concentration at the wall of the human aorta decreases with advanced atherosclerosis 24. Examination of arteries from adults and neonates 2 showed that during the early stages of atherosclerotic lesions, there is diffuse intimal thickening, associated with a strong expression of HA around the foam cells of the fibrous plaque. Hyaluronic acid is produced by the majority cells, especially smooth muscle cells (VSMCs), endothelial, and fibroblast cells after CD44 stimulation. CD44 and RHAMM stimulation induces chemotaxis of VSMCs. VSMCs are normally found in the vascular tunica media, and they migrate to the intima in the early stages of atherosclerosis and generate large quantities of extracellular matrix components, including HA. Consequently, HA binds and transfers cytokines which contribute at a later stage and change the composition of the extracellular matrix. Precisely, macrophages and T lymphocytes migrate to areas that plaque is about to develop and accumulate in the interstitial space of the vessel wall, creating the original core. The low molecular weight HA molecules, which are increased in inflammatory conditions, such as diabetes and vascular remodeling, regulate the production of chemokines and cytokines by leukocytes, such as IL‐6 and IL‐8. IL‐6 induces the proliferation of VSMCs and the expression of VCAM‐1 (vascular cell adhesion protein 1) 12, whereas IL‐8 promotes the adhesion of monocytes 25.
It has been found that oxidized LDL (OxLDL) and high levels of HA are important triggers of atherosclerosis. The load of aortic OxLDL causes overexpression of HAS2 in VSMCs through LOX‐1 receptor (Lectin‐like oxidized low‐density lipoprotein (LDL) receptor‐1) and HA deposition in the pericellular space 26. LDL–HA complexes have been found in atherosclerotic lesions 27. The retention of LDL in the intimal space with macrophages and trapped cholesterol contributes to the formation of foam cells. Sparks et al. 17 showed that plasma cholesterol correlates positively with the lipids and the levels of HA in the wall of the aorta.
Tammi et al. 21 isolated and measured glycosaminoglycans in the inner and tunica media of the aorta of rats, which were on diet high in lipids. The diet caused a rapid increase in glycosaminoglycan concentration, mainly sulfate glycosaminoglycans. However, hyaluronic acid, total cholesterol, and collagen were not significantly increased in the aorta. There was no evidence of macroscopic atherosclerotic lesions, even after long feeding periods. It was considered that early deposition of glycosaminoglycans due to diet reflects an homeostatic regulation rather than a pathological accumulation. This is supported by the rapid reversibility of the increase in GAGs, when the standard laboratory diet was restored.
The relationship between hyperglycemia and HA in the atherogenesis is also important. High glucose concentration stimulates the synthesis of HA through activation of HA synthases (HAS) 1, 2, and 3 28.
Hyaluronic acid has an active role in the intimal hyperplasia of blood vessels, as well as in the development and contraction of atherosclerotic plaque both at initial stages and later in restenosis of the vessel after its surgical opening 29. Cleavage of HA to low molecular weight fragments (LMW‐HA) is associated with inflammation and matrix metalloproteinase activity (MMP‐9). Bot et al. 4 analyzed the atherosclerotic and adjacent fibrous carotid plaques and found that the activity of the hyaluronidase of LMW‐HA and CD44 levels was elevated only in atherosclerotic plaques.
In our study, participants who had dyslipidemia had higher serum HA than those who had not. Dyslipidemic participants had a higher risk of developing cardiovascular disease according to Framingham score, higher hsCRP values, and higher levels of uric acid, which is another risk factor for developing atherosclerosis 30. Although the participants in our study were at low and intermediate risk for cardiovascular disease and had normal values of hsCRP, serum HA changes related to what had been shown in the vessel wall of atherosclerotic lesions 18. The two groups were similar in age and BMI (known to correlate with the levels of HA). Also, both groups were similar in transaminases levels. We know that HA originates from hepatic stellate cells, and hepatocytes and Kupffer cells are the main sites of HA uptake and degradation 31, 32. Our participants had normal transaminases levels.
Similar findings were showed in participants treated with lipid‐lowering treatment. We found that they had higher serum HA and hsCRP, compared with those who were not on treatment. Similarly, subjects with metabolic syndrome had higher serum HA and hsCRP than those without. These findings further support our hypothesis that HA could serve as a sensitive indicator of cardiovascular risk. SGOT and SGPT of all subjects were within the normal range and showed no differences between groups. Hence, the change in HA levels should not be attributed to liver dysfunction, as HA clearance is performed via the receptors of liver endothelial cells.
Our findings parallel those of the study by Heickendorff et al. 33, who found that diabetic patients had increased HA in tunica media of the vessel wall within atherosclerotic lesions; HA correlated positively with years of diagnosis of diabetes. This has been also observed in the study by Mine et al. 34, where patients with diabetes had higher serum HA and hsCRP compared to nondiabetic participants; serum HA was higher, particularly in patients with diabetic vasculopathy, contrary to other studies 34. We found no association of serum HA with hsCRP. This may be explained by the fact that we had enrolled volunteers with current low risk for cardiovascular disease and normal levels of hsCRP.
People with nondiabetic hyperglycemia (>120 mg/dl) have a higher risk of cardiovascular disease 35. Our findings are in agreement. In addition, we found that alongside the difference in cardiovascular risk between the two groups, there is a statistically significant difference in the levels of serum HA. This further supports the claim that serum HA varies according to the risk for heart disease.
Serum HA has been associated with endothelial dysfunction markers, such as sVCAM‐1, von Willebrand Factor (vWF), and angiopoeitin‐2 in patients with chronic renal failure. HA levels correlated with hemodialysis time period and have been proven to be a predictor for death during hemodialysis and for the development of heart disease 12, 36, 37. These variations in the levels of HA have been demonstrated both in the vessel wall and serum of these patients.
One of the limitations of our study is that we do not provide information on the size distribution of the HA molecular size in serum. As the role of HA oligosaccharides in inflammation, as well as the biological activity of the polymer fragments, is well recognized 25. However, the methodology of our study and our analysis cannot provide this information. Further future studies addressing this issue would be of great interest.
Conclusively, serum HA could serve as an early indicator of atheromatosis and cardiovascular risk. Although we have not revealed a correlation of HA with cholesterol, the existence of a significant relationship with the presence of dyslipidemia provides strong evidence toward this direction. Supportive to our findings is the fact that HA of the wall of blood vessels is not known to correlate with plasma cholesterol, but only with LDL and triglycerides (parameters defining dyslipidemia). Our findings showed that HA is not associated with the values of biochemical markers of lipid profile alone, but in general, with the risk a healthy individual has to develop cardiovascular disease. We had chosen subjects with low or intermediate risk to demonstrate early changes in serum HA and, as a next step, it would be useful in future to add subjects with high cardiovascular risk to evaluate the value of HA as predictor of developing heart disease.
References
- 1. Neuman MG, Cohen LB, Nanau RM. Hyaluronic acid as a non‐invasive biomarker of liver fibrosis. Clinical Biochem 2016;49:302–315. [DOI] [PubMed] [Google Scholar]
- 2. Levesque H, Girard N, Maingonnat C, et al. Localization and solubilization of hyaluronan and of the hyaluronan‐binding protein hyaluronectin in human normal and arteriosclerotic arterial walls. Atherosclerosis 1994;105:51–62. [DOI] [PubMed] [Google Scholar]
- 3. Deed R, Rooney P, Kumar P, et al. Early‐response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non‐angiogenic, high‐molecular‐weight hyaluronan. Int J Cancer 1997;71:251–256. [DOI] [PubMed] [Google Scholar]
- 4. Bot PT, Pasterkamp G, Goumans MJ, et al. Hyaluronic acid metabolism is increased in unstable plaques. Eur J Clin Invest 2010;40:818–827. [DOI] [PubMed] [Google Scholar]
- 5. Travis JA, Hughes MG, Wong JM, Wagner WD, Geary RL. Hyaluronan enhances contraction of collagen by smooth muscle cells and adventitial fibroblasts: role of CD44 and implications for constrictive remodeling. Circ Res 2001;88:77–83. [DOI] [PubMed] [Google Scholar]
- 6. Verheye S, Markou CP, Salame MY, et al. Reduced thrombus formation by hyaluronic acid coating of endovascular devices. Arterioscler Thromb Vasc Biol 2000;20:1168–1172. [DOI] [PubMed] [Google Scholar]
- 7. Ohkawa SI, Sugiura M, Hata R, Nagai Y. Acidic glycosaminoglycans in urine, serum and myocardium of aged patients with myocardial infarction. J Mol Cell Cardiol 1977;9:541–550. [DOI] [PubMed] [Google Scholar]
- 8. Yoon SJ, Fang YH, Lim CH, et al. Regeneration of ischemic heart using hyaluronic acid‐based injectable hydrogel. J Biomed Mater Res B Appl Biomater 2009;91:163–171. [DOI] [PubMed] [Google Scholar]
- 9. Sezai A, Hata M, Niino T, et al. Study of the factors related to atrial fibrillation after coronary artery bypass grafting: a search for a marker to predict the occurrence of atrial fibrillation before surgical intervention. J Thorac Cardiovasc Surg 2009;137:895–900. [DOI] [PubMed] [Google Scholar]
- 10. Li G, Yan QB, Wei LM. Serum concentrations of hyaluronic acid, procollagen type III NH2‐terminal peptide, and laminin in patients with chronic congestive heart failure. Chin Med Sci J 2006;21:175–178. [PubMed] [Google Scholar]
- 11. Johnsson C, Tufveson G. Serum hyaluronan–a potential marker of cardiac allograft rejection? J Heart Lung Transplant 2006;25:544–549. [DOI] [PubMed] [Google Scholar]
- 12. Stenvinkel P, Lindholm B, Heimburger M, Heimburger O. Elevated serum levels of soluble adhesion molecules predict death in pre‐dialysis patients: association with malnutrition, inflammation, and cardiovascular disease. Nephrol Dial Transplant 2000;15:1624–1630. [DOI] [PubMed] [Google Scholar]
- 13. Kannel WB, Dawber TR, Kagan A, Revotskie N, Stokes J 3rd. Factors of risk in the development of coronary heart disease–six year follow‐up experience. The Framingham Study. Ann Intern Med 1961;55:33–50. [DOI] [PubMed] [Google Scholar]
- 14. Papastamataki M, Delaporta P, Premetis E, Kattamis A, Ladis V, Papassotiriou I. Evaluation of liver fibrosis in patients with thalassemia: the important role of hyaluronic acid. Blood Cells Mol Dis 2010;45:215–218. [DOI] [PubMed] [Google Scholar]
- 15. Cardoso LE, Mourao PA. Glycosaminoglycan fractions from human arteries presenting diverse susceptibilities to atherosclerosis have different binding affinities to plasma LDL. Arterioscler Thromb 1994;14:115–124. [DOI] [PubMed] [Google Scholar]
- 16. Iverius PH. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem 1972;247:2607–2613. [PubMed] [Google Scholar]
- 17. Sparks JD, Sparks CE, Kritchevsky D. Hypercholesterolemia and aortic glycosaminoglycans of rabbits fed semi‐purified diets containing sucrose and lactose. Atherosclerosis 1986;60:183–196. [DOI] [PubMed] [Google Scholar]
- 18. Tammi M, Seppala PO, Lehtonen A, Mottonen M. Connective tissue components in normal and atherosclerotic human coronary arteries. Atherosclerosis 1978;29:191–194. [DOI] [PubMed] [Google Scholar]
- 19. Nieuwdorp M, van Haeften TW, Gouverneur MC, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 2006;55:480–486. [DOI] [PubMed] [Google Scholar]
- 20. Mawhinney TP, Augustyn JM, Fritz KE. Glycosaminoglycan‐lipoprotein complexes from aortas of hypercholesterolemic rabbits. Part 1. Isolation and characterization. Atherosclerosis 1978;31:155–167. [DOI] [PubMed] [Google Scholar]
- 21. Tammi M, Ronnemaa T, Viikari J. Rapid increase of glycosaminoglycans in the aorta of hypercholesterolemic rats; a negative correlation with plasma HDL concentration. Acta Physiol Scand 1979;105:188–194. [DOI] [PubMed] [Google Scholar]
- 22. Nakamura RM. Progress in the use of biochemical and biological markers for evaluation of rheumatoid arthritis. J Clin Lab Anal 2000;14:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen J, Liu C, Chen H, Liu Q, Yang B, Ou Q. Study on noninvasive laboratory tests for fibrosis in chronic HBV infection and their evaluation. J Clin Lab Anal 2013;27:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klynstra FB, Bottcher CJ, van Melsen JA, van der Laan EJ. Distribution and composition of acid mucopolysaccharides in normal and atherosclerotic human aortas. J Atheroscler Res 1967;7:301–309. [DOI] [PubMed] [Google Scholar]
- 25. Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem 2004;279:17079–17084. [DOI] [PubMed] [Google Scholar]
- 26. Viola M, Bartolini B, Vigetti D, et al. Oxidized low density lipoprotein (LDL) affects hyaluronan synthesis in human aortic smooth muscle cells. J Biol Chem 2013;288:29595–29603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Srinivasan SR, Yost K, Radhakrishnamurthy B, Dalferes ER Jr, Berenson GS. Lipoprotein‐hyaluronate associations in human aorta fibrous plaque lesions. Atherosclerosis 1980;36:25–37. [DOI] [PubMed] [Google Scholar]
- 28. Sainio A, Jokela T, Tammi MI, Jarvelainen H. Hyperglycemic conditions modulate connective tissue reorganization by human vascular smooth muscle cells through stimulation of hyaluronan synthesis. Glycobiology 2010;20:1117–1126. [DOI] [PubMed] [Google Scholar]
- 29. Sadowitz B, Seymour K, Gahtan V, Maier KG. The role of hyaluronic acid in atherosclerosis and intimal hyperplasia. J Surg Res 2012;173:e63–e72. [DOI] [PubMed] [Google Scholar]
- 30. Ishizaka N, Ishizaka Y, Toda E, Nagai R, Yamakado M. Association between serum uric acid, metabolic syndrome, and carotid atherosclerosis in Japanese individuals. Arterioscler Thromb Vasc Biol 2005;25:1038–1044. [DOI] [PubMed] [Google Scholar]
- 31. Sharif M, George E, Shepstone L, et al. Serum hyaluronic acid level as a predictor of disease progression in osteoarthritis of the knee. Arthritis Rheum 1995;38:760–767. [DOI] [PubMed] [Google Scholar]
- 32. Kaplan D, Meyer K. Mucopolysaccharides of aorta at various ages. Proc Soc Exp Biol Med 1960;105:78–81. [DOI] [PubMed] [Google Scholar]
- 33. Heickendorff L, Ledet T, Rasmussen LM. Glycosaminoglycans in the human aorta in diabetes mellitus: a study of tunica media from areas with and without atherosclerotic plaque. Diabetologia 1994;37:286–292. [DOI] [PubMed] [Google Scholar]
- 34. Mine S, Okada Y, Kawahara C, Tabata T, Tanaka Y. Serum hyaluronan concentration as a marker of angiopathy in patients with diabetes mellitus. Endocr J 2006;53:761–766. [DOI] [PubMed] [Google Scholar]
- 35. Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta‐analysis of prospective studies. Arch Intern Med 2004;164:2147–2155. [DOI] [PubMed] [Google Scholar]
- 36. Padberg JS, Wiesinger A, di Marco GS, et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 2014;234:335–343. [DOI] [PubMed] [Google Scholar]
- 37. Stenvinkel P, Heimburger O, Wang T, Lindholm B, Bergstrom J, Elinder CG. High serum hyaluronan indicates poor survival in renal replacement therapy. Am J Kidney Dis 1999;34:1083–1088. [DOI] [PubMed] [Google Scholar]


