Abstract
Background
Previous studies indicated that some routine hematological parameters are associated with the prognosis of ischemic stroke (IS), but none of study has evaluated them simultaneously. The aim of this study was to investigate the prognostic value of routine hematological parameters in IS patients.
Methods
Using medical record database, we retrospectively reviewed the patients with IS admitted in Gansu Province Hospital between June 2014 and July 2015. The prognostic value of routine hematological parameters on admission was analyzed using logistic regression model, receiver operating characteristic (ROC) curve analysis and Cox proportional hazards model.
Results
Patients with hospital mortality had significantly higher white blood cell (WBC), neutrophil, neutrophil to lymphocyte ratio (NLR), red blood cell distribution width (RDW) and National Institutes of Health Stroke Scale (NIHSS), while their lymphocyte, monocyte, and eosinophil were significantly lower. The area under ROC curve (AUC) for eosinophil, neutrophil, WBC, RDW, NLR, monocyte, and lymphocyte were 0.74 (95% CI, 0.67‐0.82), 0.76 (95% CI, 0.67‐0.84), 0.72 (95% CI, 0.64‐0.81), 0.65 (95% CI, 0.56‐0.73), 0.76 (95% CI, 0.68‐0.84), 0.67 (95% CI, 0.59‐0.76), and 0.75 (95% CI, 0.67‐0.83), respectively. In a multivariable logistical regression model, only WBC, NLR, and NIHSS were independently associated with hospital mortality. In a multivariable model, age, NIHSS, RDW, NLR, and eosinophil were independent prognostic factors for all‐cause mortality.
Conclusion
Red blood cell distribution width, NLR and eosinophil are independent prognostic factors for IS.
Keywords: hematological parameters, ischemic stroke, prognosis, red blood cell distribution, retrospective study
1. INTRODUCTION
Ischemic stroke (IS) is one of the leading causes of disability and death around world.1, 2, 3 Prognostic assessment is crucial for treatment selection.4 However, prognostic assessment is really a challenge for clinicians. Although accumulated prognostic factors have been widely validated by previous studies, these factors have some limitations, such as high observer variability and cost.5 Besides, these factors, when used alone or in combination, cannot predict the prognosis of IS patients adequately.5 Therefore, it is of great value to explore more factors with low cost and variability.
During past years, some studies have revealed that routine hematological parameters (eg, red blood cell distribution width [RDW]6, 7 and neutrophil to lymphocyte ratio [NLR]8, 9) are associated with prognosis of IS. However, to the best of our knowledge, none of the studies has investigated the prognostic value of RDW and NLR simultaneously. In another words, it remains unknown whether the prognostic value of RDW is independent of NLR, and vice versa. Besides, whether other hematological parameters are associated with IS prognosis remains unknown. Therefore, we performed this study to simultaneously investigate the prognostic value of routine hematological parameters for IS.
2. MATERIALS AND METHODS
2.1. Subjects
Using medical record database, we retrospectively reviewed the patients with IS admitted in Gansu hospital between June 2014 and July 2015. The inclusion criterion was first onset of IS confirmed by findings of CT or MRI. The exclusion criteria were as follows: age <18 years; complicated with malignant disease or end stage liver or renal disease; received blood transfusion 4 months before admission; trauma induced IS; the period from onset to admission more than 48 hours.
Because this is a retrospective study based on medical records database, inform consent was waived. The Ethic Committee of Gansu Province Hospital approved this study.
2.2. Data extraction
Following clinical data on admission were extracted from the medical record database: age, gender, hypertension, diabetes, hyperlipidemia and coronary artery disease, white blood cell (WBC), neutrophil percentage, lymphocyte percentage, monocyte percentage, eosinophil percentage, hemoglobin concentration, red blood cell, hematocrit, mean corpuscular volume, mean corpuscular volume, mean corpuscular hemoglobin concentration (MCHC), RDW, platelet, National Institutes of Health Stroke Scale (NIHSS) and modified Rankin Scale at discharge. In our hospital, most of the stroke patients were followed up approximately quarterly after discharging. Therefore, the follow‐up data were included in this study. The endpoint of this study is all‐cause mortality.
2.3. Statistical analysis
Normally distribution was tested using the Kolmogorov‐Smirnov test. The continuous variables were compared using the Students' t‐test or Mann‐Whitney U test, if appropriate. Categorical data were compared using the Chi‐square test. The predictive value of routine hematological parameters for hospital mortality was assessed using receiver operating characteristic (ROC) curve analysis and forwards conditional logistic regression model. The long‐term prognostic value of routine hematological parameters was assessed using Cox regression model. All analyses were performed using SPSS 22.0, and P <.05 was defined as statistically significant.
3. RESULTS
3.1. Routine hematological parameters and short‐term prognosis
Table 1 lists the characteristics of subjects. A total of 362 subjects were enrolled and 49 of them died in hospital. The patients with hospital mortality were older (P=.05) and had significantly higher WBC, neutrophil, NLR, RDW, and NIHSS (P<.05 for all), while their lymphocyte, monocyte, and eosinophil were significantly lower (P<.05 for all), indicating that these factors are potential prognostic factors for IS.
Table 1.
Clinical characteristics of subjects
| Characteristics | All subjects | Hospital mortality | ||
|---|---|---|---|---|
| Yes | No | P | ||
| Sample size | 362 | 49 | 313 | — |
| Gender, M/F | 216/146 | 30/19 | 186/127 | .81 |
| Age, years | 63 (52, 76) | 68 (57, 79) | 62 (52, 75) | .05 |
| Hypertension, Y/N | 292/70 | 39/10 | 253/60 | .84 |
| CAD, Y/N | 47/315 | 8/41 | 39/274 | .45 |
| Diabetes, Y/N | 50/312 | 7/42 | 43/270 | .92 |
| Hyperlipidemia, Y/N | 63/299 | 4/45 | 59/254 | .07 |
| WBC, 109/L | 7.3 (6.0, 10.3) | 11.7 (7.5, 14) | 7.1 (5.9, 9.6) | <.01 |
| Neu, % | 72 (62, 82) | 82 (77, 91) | 71 (62, 80) | <.01 |
| Lym, % | 19 (10, 29) | 8.4 (4.3, 14.6) | 20.1 (11.8, 29.0) | <.01 |
| NLR | 4.0 (2.2, 8.0) | 9.5 (5.3, 21.7) | 3.6 (2.1, 6.8) | <.01 |
| Mon, % | 6.4 (5.1, 8.0) | 5.4 (3.4, 6.5) | 6.6 (5.5, 8.1) | <.01 |
| Eos, % | 0.8 (0.1, 1.9) | 0.1 (0.0, 0.5) | 0.9 (0.2, 1.9) | <.01 |
| RBC, 109/L | 4.63 (4.07, 5.06) | 4.52 (3.92, 5.07) | 4.64 (4.13, 5.06) | .30 |
| Hemoglobin, g/L | 143 (127, 157) | 143 (128, 157) | 143 (127, 157) | .97 |
| Hematocrit, % | 0.43 (0.39, 0.47) | 0.43 (0.38, 0.47) | 0.43 (0.39, 0.47) | .66 |
| MCV, fl | 93 (90, 97) | 94 (90, 99) | 93 (90, 97) | .13 |
| MCH, pg | 31 (30, 33) | 31 (30, 33) | 31 (30, 33) | .38 |
| MCHC, g/L | 334 (325, 343) | 334 (324, 343) | 333 (326, 343) | .60 |
| RDW, % | 13.3 (12.5, 14.7) | 14.7 (13.0, 15.6) | 13.2 (12.5, 14.5) | <.01 |
| Platelet count, 109/L | 189 (132, 233) | 179 (125, 251) | 190 (133, 233) | .96 |
| NIHSS, score | 9 (5, 13) | 12 (9, 17) | 8 (4, 13) | <.01 |
Data were presented as median and quartile, and compared with Mann‐Whitney U test. Categorical data were presented as absolute value and compared with Chi‐square test.
CAD, coronary artery disease; WBC, white blood cell; Neu, neutrophil percentage; Lym, lymphocyte percentage; NLR, neutrophil to lymphocyte ratio; Mon, monocyte percentage; Eos, eosinophil percentage; RBC, red blood cell; MCV, mean corpuscular volume; MCH, mean corpuscular volume; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width.
3.2. Routine hematological parameters and hospital mortality
Figure 1 is a ROC curve depicting the predictive value of routine hematological index for hospital mortality. The areas under curve (AUCs) were: eosinophil 0.74 (95%CI, 0.67‐0.82), neutrophil 0.76 (95%CI, 0.67‐0.84), WBC 0.72 (95%CI, 0.64‐0.81), RDW 0.65 (95%CI, 0.56‐0.73), NLR 0.76 (95%CI, 0.68‐0.84), monocyte 0.67 (95%CI, 0.59‐0.76), lymphocyte 0.75 (95% CI, 0.67‐0.83).
Figure 1.
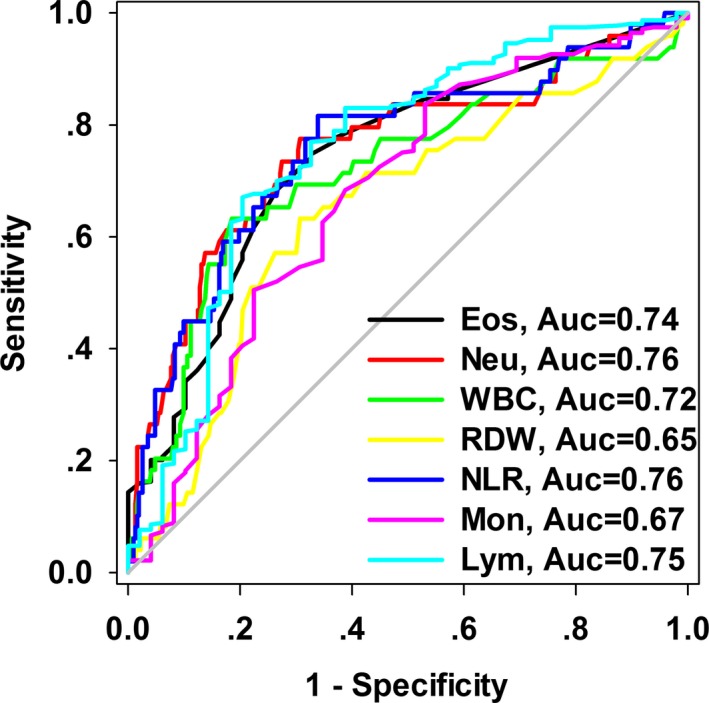
Receiver operating characteristics curve depicting the predictive value of routine hematological parameters for hospital mortality
We further used forward conditional multivariable logistic regression model to analyze the relationship between routine hematological parameter and hospital mortality. As shown in Table 2, age, WBC, eosinophil, monocyte, NIHSS, RDW, and NLR were significantly associated with hospital mortality in a univariable analysis. However, in a multivariable model, only WBC, NLR, and NIHSS were independently associated with hospital mortality, with ORs of 1.09 (95%CI, 1.01‐1.18), 1.07 (95%CI, 1.03‐1.11), and 1.17 (95%CI, 1.09‐1.25), respectively.
Table 2.
Analyzing routine hematological parameters and hospital mortality using multivariable logistic regression model
| Univariable | Multivariable | |||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Age, per 1 year | 1.03 (1.00‐1.05) | .05 | — | — |
| WBC, per 109/L | 1.18 (1.11‐1.26) | <.01 | 1.09 (1.01‐1.18) | .02 |
| Eos, per 1% | 0.40 (0.25‐0.64) | <.01 | — | — |
| Mon, per 1% | 0.79 (0.69‐0.90) | <.01 | — | — |
| NLR, per 1 | 1.10 (1.06‐1.13) | <.01 | 1.07 (1.03‐1.11) | <.01 |
| RDW, per 1% | 1.32 (1.12‐1.55) | <.01 | — | — |
| NIHSS, per 1 | 1.18 (1.10‐1.26) | <.01 | 1.17 (1.09‐1.25) | <.01 |
OR, odds ratio; WBC, white blood cell; NLR, neutrophil to lymphocyte ratio; Eos, eosinophil; Mon, monocyte; RDW, red blood cell distribution width.
Forward conditional logistic regression model was used and the variables included in model including age, gender, WBC, eosinophil, monocyte, RDW, NIHSS, hypertension, diabetes, hyperlipidemia, coronary artery disease. Age, gender, WBC, eosinophil, monocyte, RDW and NIHSS were expressed as continuous variable, and hypertension, diabetes, hyperlipidemia and coronary artery disease were expressed as categorical variable.
3.3. Routine hematological parameters and long‐term prognosis
Next, we used Cox model to analyze the relationship between routine hematological parameters and long‐term mortality. The median follow‐up time was 12 months and the endpoint is all‐cause mortality. As shown in Table 3, age, NIHSS, RDW, eosinophil, monocyte, NLR, and WBC were prognostic factors in a univaribale model. However, in a multivariable model, only age, NIHSS, RDW, NLR, and eosinophil were independent prognostic factors for all‐cause mortality.
Table 3.
Analyzing prognostic value of routine hematological parameters and long‐term prognosis
| Univariable | Multivariable | |||
|---|---|---|---|---|
| HR (95%CI) | P | HR (95%CI) | P | |
| Age, per 1 year | 1.03 (1.01‐1.05) | <.01 | 1.02 (1.00‐1.04) | .03 |
| NIHSS, per 1 score | 1.10 (1.05‐1.15) | <.01 | 1.06 (1.01‐1.11) | .01 |
| RDW, per 1% | 1.31 (1.20‐1.43) | <.01 | 1.17 (1.07‐1.28) | <.01 |
| NLR, per 1 | 1.05 (1.03‐1.07) | <.01 | 1.03 (1.01‐1.05) | <.01 |
| Eos, per 1% | 0.62 (0.48‐0.79) | <.01 | 0.75 (0.59‐0.95) | .02 |
| WBC, per 109/L | 1.11 (1.07‐1.15) | <.01 | — | — |
| Mon, per 1% | 0.91 (0.83‐0.99) | .04 | — | — |
HR, hazard ratio; NLR, neutrophil to lymphocyte ratio; RDW, red blood cell distribution width; Eos, eosinophil percentage; WBC, white blood cell.
Forward conditional Cox model was used and the variables included in model including age, gender, WBC, eosinophil, monocyte, RDW, NIHSS, hypertension, diabetes, hyperlipidemia, coronary artery disease. Age, gender, WBC, eosinophil, monocyte, RDW and NIHSS were expressed as continuous variable, and hypertension, diabetes, hyperlipidemia and coronary artery disease were expressed as categorical variable.
4. DISCUSSION
Although some studies have investigated the prognostic value of routine hematological parameters, especially RDW and NLR, for IS, this study has strengthen. First, to the best of knowledge, this is the first study simultaneously investigating the prognostic value of all hematological parameters for IS, and we found that the prognostic value of NLR was independent of RDW. Second, we found that eosinophil was also an independent prognostic factor for IS, and the prognostic value of eosinophil for IS has not been reported by previous studies.
The underlying mechanism of the prognostic value of RDW, NLR, and eosinophil for IS remains largely unknown. We hypothesized that the prognostic value of these routine hematological parameters can be explained at least partially by inflammation and stress. Previous studies indicated that both RDW and NLR are inflammatory markers, because they are associated with some well‐known inflammatory markers. For example, a previous study revealed that RDW is positively correlated with inflammatory markers,10 such as C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR). In a recent study, a positive correlation between NLR, CRP and ESR was also observed in general populations visited hospital for healthy checking.11 Besides, some prospective cohort studies also indicated that increased RDW and NLR are associated with high risk or poor prognosis of some inflammatory related diseases, such as stroke,12, 13 myocardial infarction14, 15, 16 and sepsis.17, 18 On the other hand, the prognosis of IS is largely determined by the strength of inflammatory response, as accumulated studies indicated that increased inflammatory markers, such as CRP19, 20 and interleukin‐6 (IL‐6)21, 22 are associated with unfavorable outcomes of IS. Therefore, the prognostic value of NLR and RDW for IS may be mediated by inflammation response.
Besides RDW and NLR, we found that decreased eosinophil percentage is associated with high all‐cause mortality in IS. This finding is biologically plausible. Previews studies indicated that peripheral eosinophil percentage is decreased under stress response.23 Therefore, decreased eosinophil in IS patients may reflex the strong stress response. On the other hand, some studies revealed that strong stress response is associated with poor outcomes in IS patients. For example, hypercortisolemia, a marker of the strong stress response, is associated with unfavorable outcomes of IS.24 Therefore, the prognostic value of eosinophil for IS may be mediated by stress response.
Prognostic markers for IS have attracted much attention during past decades, because prognosis can greatly affect the therapy approaches selection for IS. To date, many novel prognostic markers for IS have been identified.5 However, compared with these biomarkers, routine hematological parameters have strengths, including low cost, objective test with low observer variation, easily obtained. Therefore, it may be more preferred in clinical practice.
The major limitation of this study is the retrospective design and single center study. Therefore, the participant selection bias cannot be avoided. Besides, some of clinical data were absence in medical records; therefore, we cannot adjust more clinical data in the multivariable regression analysis. Further studies with prospective design and larger sample size and more clinical data are needed to investigate the prognostic value of hematological parameters for IS.
Taken together, this study indicated that routine hematological parameters, including NLR, eosinophil, and RDW are useful and independent prognostic factors for IS.
Fan L, Gui L, Chai E‐Q, Wei C‐J. Routine hematological parameters are associated with short‐ and long‐term prognosis of patients with ischemic stroke. J Clin Lab Anal. 2018;32:e22244 10.1002/jcla.22244
REFERENCES
- 1. Jiang G, Li W, Wang D, Shen C, Ji Y, Zheng W. Epidemiological transition and distribution of stroke incidence in Tianjin, China, 1988‐2010. Public Health. 2016;131:11‐19. [DOI] [PubMed] [Google Scholar]
- 2. Li L, Yiin GS, Geraghty OC, et al. Incidence, outcome, risk factors, and long‐term prognosis of cryptogenic transient ischaemic attack and ischaemic stroke: a population‐based study. Lancet Neurol. 2015;14:903‐913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maredza M, Bertram MY, Tollman SM. Disease burden of stroke in rural South Africa: an estimate of incidence, mortality and disability adjusted life years. BMC Neurol. 2015;15:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maldonado NJ, Kazmi SO, Suarez JI. Update in the management of acute ischemic stroke. Crit Care Clin. 2014;30:673‐697. [DOI] [PubMed] [Google Scholar]
- 5. Whiteley W, Chong WL, Sengupta A, Sandercock P. Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke. 2009;40:e380‐e389. [DOI] [PubMed] [Google Scholar]
- 6. Kara H, Degirmenci S, Bayir A, et al. Red cell distribution width and neurological scoring systems in acute stroke patients. Neuropsychiatr Dis Treat. 2015;11:733‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turcato G, Cervellin G, Cappellari M, et al. Early function decline after ischemic stroke can be predicted by a nomogram based on age, use of thrombolysis, RDW and NIHSS score at admission. J Thromb Thrombolysis. 2017;43:394‐400. [DOI] [PubMed] [Google Scholar]
- 8. Zhao L, Dai Q, Chen X, et al. Neutrophil‐to‐lymphocyte ratio predicts length of stay and acute hospital cost in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25:739‐744. [DOI] [PubMed] [Google Scholar]
- 9. Celikbilek A, Ismailogullari S, Zararsiz G. Neutrophil to lymphocyte ratio predicts poor prognosis in ischemic cerebrovascular disease. J Clin Lab Anal. 2014;28:27‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628‐632. [DOI] [PubMed] [Google Scholar]
- 11. Kweon OJ, Lee MK, Kim HJ, Chung JW, Choi SH, Kim HR. Neutropenia and neutrophil‐to‐lymphocyte ratio in a healthy Korean population: race and sex should be considered. Int J Lab Hematol. 2016;38:308‐318. [DOI] [PubMed] [Google Scholar]
- 12. Lappegard J, Ellingsen TS, Skjelbakken T, et al. Red cell distribution width is associated with future risk of incident stroke. The Tromso Study. Thromb Haemost. 2016;115:126‐134. [DOI] [PubMed] [Google Scholar]
- 13. Wang F, Hu S, Ding Y, et al. Neutrophil‐to‐lymphocyte ratio and 30‐day mortality in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2016;25:182‐187. [DOI] [PubMed] [Google Scholar]
- 14. Skjelbakken T, Lappegard J, Ellingsen TS, et al. Red cell distribution width is associated with incident myocardial infarction in a general population: the Tromso Study. J Am Heart Assoc. 2014;3:e001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verdoia M, Barbieri L, Di Giovine G, et al. Neutrophil to lymphocyte ratio and the extent of coronary artery disease: results from a large cohort study. Angiology. 2016;67:75‐82. [DOI] [PubMed] [Google Scholar]
- 16. Arbel Y, Shacham Y, Ziv‐Baran T, et al. Higher neutrophil/lymphocyte ratio is related to lower ejection fraction and higher long‐term all‐cause mortality in ST‐elevation myocardial infarction patients. Can J Cardiol. 2014;30:1177‐1182. [DOI] [PubMed] [Google Scholar]
- 17. Chen CK, Lin SC, Wu CC, Chen LM, Tzeng IS, Chen KF. STARD‐compliant article: the utility of red cell distribution width to predict mortality for septic patients visiting the emergency department. Medicine (Baltimore). 2016;95:e3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu X, Shen Y, Wang H, Ge Q, Fei A, Pan S. Prognostic significance of neutrophil‐to‐lymphocyte ratio in patients with sepsis: a prospective observational study. Mediators Inflamm. 2016;2016:8191254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsuo R, Ago T, Hata J, et al. Plasma C‐reactive protein and clinical outcomes after acute ischemic stroke: a prospective observational study. PLoS One. 2016;11:e0156790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li YM, Liu XY. Serum levels of procalcitonin and high sensitivity C‐reactive protein are associated with long‐term mortality in acute ischemic stroke. J Neurol Sci. 2015;352:68‐73. [DOI] [PubMed] [Google Scholar]
- 21. Kwan J, Horsfield G, Bryant T, et al. IL‐6 is a predictive biomarker for stroke associated infection and future mortality in the elderly after an ischemic stroke. Exp Gerontol. 2013;48:960‐965. [DOI] [PubMed] [Google Scholar]
- 22. Park SY, Kim J, Kim OJ, et al. Predictive value of circulating interleukin‐6 and heart‐type fatty acid binding protein for three months clinical outcome in acute cerebral infarction: multiple blood markers profiling study. Crit Care. 2013;17:R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mathur RB, Sachdev JC. Mental stress and eosinophil count. Indian J Psychol. 1958;2:381‐386. [PubMed] [Google Scholar]
- 24. Slowik A, Turaj W, Pankiewicz J, Dziedzic T, Szermer P, Szczudlik A. Hypercortisolemia in acute stroke is related to the inflammatory response. J Neurol Sci. 2002;196:27‐32. [DOI] [PubMed] [Google Scholar]


