Abstract
Background
Bilirubin played a great role in antioxidation and anticancer and has been considered as a promising prognostic factor of non‐liver disease‐related death in various cancers. The aim of this study was to assess the prognostic value of pre‐treatment serum bilirubin in stage IV CRC patients.
Methods
Serum bilirubin including TBIL, DBIL, and IBI which were tested at pre‐treatment were investigated in 154 stage IV CRC patients in Zhongda Hospital, Nanjing, China, from July 2005 to July 2011. X‐tile program was used to determine the optimal cut‐off values of these three biomarkers. Kaplan‐Meier analysis, univariate, and multivariate cox regression as well as time‐dependent ROC curve analysis were performed to evaluate the relations between serum bilirubin and survival outcomes.
Results
We got the results that the optimal cut‐off points of serum TBIL, DBIL, and IBI levels were 12.9, 6.1, and 4.8 μmol/L, respectively. Univariate analysis showed that elevated TBIL, DBIL, and CEA were significantly associated with poor 5‐year OS in stage IV CRC patients. Multivariate cox analysis indicated that the high DBIL (HR=1.603, 95%CI=1.053‐2.442, P<.028) and CEA (HR=1.785, 95%CI=1.123‐2.837, P=.014) could be identified as independent factors for poor OS. Furthermore, time‐dependent ROC curves demonstrated that high DBIL had similar prognostic efficacy as elevated CEA for poor OS (AUC=0.63 and 0.61, respectively).
Conclusions
Pre‐treatment elevated TBIL and DBIL levels were associated with poor OS in stage IV CRC patients. Moreover, DBIL could be considered as an independent prognostic biomarker for OS. Furthermore, DBIL had similar prognostic efficacy as CEA for OS.
Keywords: bilirubin, colorectal cancer, overall survival, prognosis
Abbreviations:
- CRC
colorectal cancer
- HR
hazard ratio
- 95%CI
95% confidential interval
- TBIL
total bilirubin
- DBIL
direct bilirubin
- IBI
indirect bilirubin
- OS
overall survival
- LFTs
Liver function tests
- CEA
carcinoembryonic antigen
- CA19‐9
carbohydrate antigen 19‐9
- UGT1A1
uridine diphosphate glucuronosyltransferase 1A1
- ROC
receiver operating characteristic
- NSCLC
non‐small‐cell lung cancer
1. INTRODUCTION
Colorectal cancer (CRC) is one of the most common cancers worldwide and it also has been supposed as the fourth most commonly leading cause of cancer death in male and the third in female.1 With limitation of useful early diagnostic biomarkers and invasiveness of colonoscopy, it was reported approximately 25% CRC patients present with metastases at initial diagnosis,2 which was the key contributor to poor 5‐year overall survival outcome reported for CRC, though obvious improvements of treatment regimens had been implement recently. As we knew, the primary treatment for metastatic CRC was surgery or systemic chemo‐radiotherapy. However, it has become increasingly difficult to identify the best way to integrate systemic chemotherapy with curative surgery for metastatic CRC patients.3 Thus, finding optimal markers to identify optional therapeutic options for certain subgroups of CRC patients would have enormous clinical benefits. Compared with traditional prognostic markers, such as tumor size, tumor TNM stage and differential grade, blood‐based biomarkers became more and more popular and posed greater potential to predict prognosis and guide treatment for CRC patients because it is easily accessible and minimally invasive.
Liver function tests (LFTs), a group of blood tests, were frequently included as baseline tests for inpatients and usually used for reflecting the liver function, such as the integrity and functionality of hepatocytes, and conditions of biliary tract. Bilirubin, the end point product of heme catabolism, was a member of LFTs. It was historically considered to have no physiological function.4 Interestingly, nowadays bilirubin has been demonstrated by many experimental and clinical researches to play important protective roles in antioxidant and anticancer, giving a rise in the poor ability of patients with cancer scavenging plasma free redical.5, 6, 7 Moreover, an inverse relationship between serum bilirubin level and cancer risk also has been reported in CRC.4, 8, 9, 10 At same time, serum bilirubin was also widely investigated as a useful prognostic indicator for non‐liver disease‐related mortality in various cancers, including CRC.11, 12, 13, 14, 15 However, as for Zhang's study, they only reported stage II‐III CRC patients.15 And Cao's study conducted in northern Chinese population, only reported patients with rectal cancer, the cut‐off value was evaluated by ROC curve, and only stage I‐III patients were included.12 In a word, the relationship between bilirubin level and survival of stage IV CRC has not been clearly delineated.
Hence, this retrospective study was conducted in eastern Chinese population to investigate the prognostic impacts of serum bilirubin levels including total bilirubin (TBIL), direct bilirubin (DBIL) and indirect bilirubin (IBI) in 154 stage IV CRC patients. To our knowledge, this is the first study to assess serum bilirubin in stage IV CRC patients.
2. MATERIALS AND METHODS
2.1. Study population
Between July 2005 and July 2011, 154 patients with stage IV CRC who were confirmed by histology or cytology in Zhongda Hospital, Jiangsu, China, were enrolled in this retrospective analysis. The inclusion criteria were as followed: (1) age ≥18 years; (2) TBIL, DBIL and IBI were determined within 2 weeks before initial medical therapy. The exclusion criteria were as followed: (1) previous diagnosis of malignancy; (2) mingled with other cancers; (3) underlying known hepatobiliary and pancreatic diseases; (4) elevated parameters in liver function tests (AST>40 U/L; ALT>50 U/L); (5) with any reasons resulting in jaundice; and (6) with clinical parameters and laboratory results loss. At last, 154 patients were enrolled in this study and informed consents were obtained from all eligible patients. This study was approved by the ethics committee of Southeast University.
2.2. Clinical parameters and laboratory results
The following clinical parameters were evaluated: age, gender, tumor site, treatment type and metastatic organ, which were retrieved from medical records. At the same time, laboratory results including routine tumor markers for CRC, such as carcinoembryonic antigen (CEA) (upper physiological value: 5 ng/mL) and carbohydrate antigen 19‐9 (CA19‐9) (upper physiological value: 37 U/mL) measured using electrochemiluminescence by ELECSYS 2010 (Roche, Basel, Switzerland) and biochemical data (TBIL, DBIL and IBI) detected by Olympus AU5421 automatic analytical system (Olympus Co. Ltd., Tokyo, Japan) were also collected from medical records. All enrolled patients’ blood samples were obtained at 6‐8 clock in the morning before initial medical therapy.
2.3. Follow‐up
Patients were followed up regularly until death or dating up to September 1, 2016 according to 7th edition of the TNM‐UICC/AJCC classification for CRC (every 3‐6 months for first 2 years, every 6 months for the third to fifth years). The overall survival (OS) was defined as the time from the date of diagnosis to last follow‐up or death. Follow‐up data for patients were computed from medical records, physical examinations, laboratory examinations, or telephone follow‐up.
2.4. Statistical analyses
IBM SPSS Statistical 20.0 (SPSS Inc. Chicago, IL, USA) and R 3.3.0 software (Institute for Statistics and Mathematics, Vienna, Austria) were used for statistical analysis. Kolmogorov‐Smirnow test was selected to assess the normality of calculated parameters. Student's t test was used for normal distributed parameters, otherwise Mann‐Whitney U test was performed. Chi‐square test was used to compare categorical variables. For clinical practice, the continuous variables were changed to categorical variables, and the cut‐off values of TBIL, DBIL and IBI were determined by X‐tile 3.6.1 (Yale University, New Haven, CT, USA).16 OS curves were established according to the Kaplan‐Meier method and the differences were analyzed by the log‐rank test. To identify the independent factors, multivariate Cox regression analyses were performed. Age, gender, and other variables with a P value <.10 in the univariate cox regression analysis were entered into the multivariate cox regression model using backward conditional method. Time‐dependent receiver operating characteristic (ROC) curve analysis was used to further compare the performance of significant variables in multivariate analysis in predicting survival outcome. Two‐tailed P values of <.05 were considered to be statistically significant.
3. RESULTS
3.1. The optimal cut‐off values for TBIL, DBIL, and IBI
The optimal cut‐off values calculated by X‐tile were 12.9 μmol/L for TBIL, 6.1 μmol/L for DBIL and 4.8 μmol/L for IBI (Figure 1). Patients were subsequently divided into high group and low group according to the optimal cut‐off levels. The cut‐off values for CEA and CA19‐9 were 5 ng/mL and 37 U/mL, respectively, which was according to the reference range reported by Clinical Laboratory Department in Zhongda Hospital.
Figure 1.
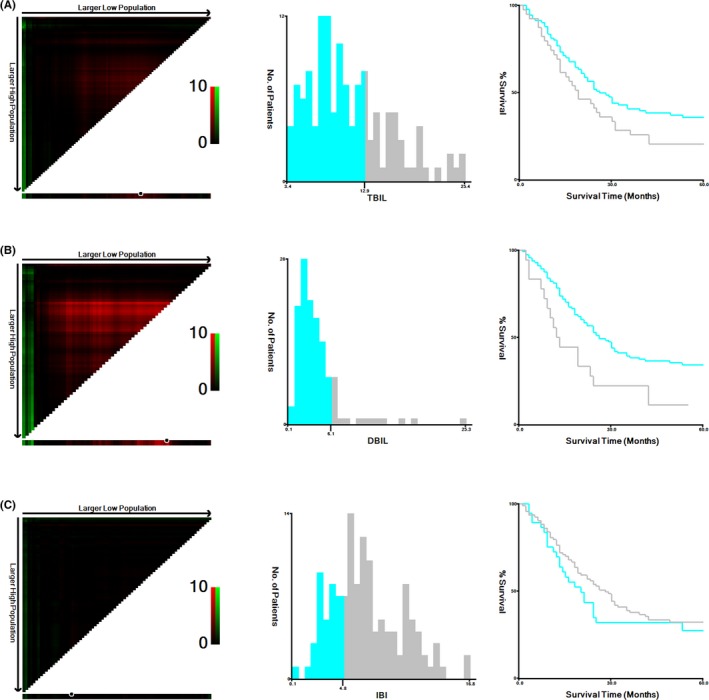
X‐tile analyses for 5‐year overall survival. The sample of CRC patients was equally divided into training and validation sets. X‐tile plots of training sets were shown in the left panels, with plots of matched validation sets shown in the smaller inset. The optimal cut‐off values highlighted by the black circles in left rectangular panels were also shown in histograms of the entire cohort (middle panels), and Kaplan‐Meier plots were displayed in right panels. P values were determined by using the cut‐off values defined in training sets and applying them to validation sets. The optimal cut‐off values of TBIL, DBIL, and IBI for OS were 12.9 μmol/L, 6.1 μmol/L, and 4.8 μmol/L, respectively. (A) TBIL. (B) DBIL. (C) IBI.CRC, colorectal cancer; TBIL, total bilirubin; DBIL, direct bilirubin; IBI, indirect bilirubin; OS, overall survival
3.2. Baseline characteristics of patients based on TBIL, DBIL, and IBI
Clinical baseline characteristics were summarized in Table 1. A total of 154 patients had a median age of 64 years (range 25‐86) and were mostly males (65.6%). Colon was the most common location site of the primary tumor (61.7%). A total of 99 patients (64.3%) underwent operation for CRC and up to 115 (74.7%) CRC patients underwent chemo‐radiotherapy. The majority of CRC patients had high CEA level (70.8%). During the deadline of follow‐up, 44 (28.6%) were still alive and 110 (71.4%) patients were dead. The median OS was 22.5 months (range 1‐60). To study the correlation of TBIL, DBIL, and IBI with baseline characteristics of patients, comparison between the high and low groups for TBIL, DBIL and IBI was carried out. Our results revealed that TBIL and DBIL were significantly associated with CEA (P all<.05). Moreover, DBIL was also closely associated with gender (P=.014).
Table 1.
Baseline characteristics of stage IV patients with colorectal cancer based on TBIL, DBIL, and IBI
| Characteristics | Total patients | TBIL (N=154) (μmol/L) | P * | DBIL (N=154) (μmol/L) | P * | IBI (N=154) (μmol/L) | P * | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (N=154) | ≦12.9 | >12.9 | ≦6.1 | >6.1 | ≦4.8 | >4.8 | ||||
| (N=98) | (N=56) | (N=119) | (N=35) | (N=42) | (N=112) | |||||
| Age (y) | ||||||||||
| ≦64 | 83 (53.9%) | 49 (31.8%) | 34 (22.1%) | .199 | 62 (40.3%) | 21 (13.6%) | .410 | 18 (11.7%) | 65 (42.2%) | .092 |
| >64 | 71 (46.1%) | 49 (31.8%) | 22 (14.3%) | 57 (37.0%) | 14 (9.1%) | 24 (15.6%) | 47 (30.5%) | |||
| Gender | ||||||||||
| Male | 101 (65.6%) | 61 (39.6%) | 29 (26.0%) | .249 | 72 (46.8%) | 29 (18.8%) | .014 | 29 (18.8%) | 72 (46.8%) | .580 |
| Female | 53 (34.4%) | 101 (24.0%) | 8 (10.4%) | 47 (30.5%) | 6 (3.9%) | 13 (8.4%) | 40 (26.0%) | |||
| Tumor site | ||||||||||
| Rectal | 59 (38.3%) | 37 (24.0%) | 22 (14.3%) | .851 | 47 (30.5%) | 12 (7.8%) | .577 | 15 (9.7%) | 44 (28.6%) | .685 |
| Colon | 95 (61.7%) | 61 (39.6%) | 34 (22.1%) | 72 (46.8%) | 23 (14.9%) | 27 (17.5%) | 68 (44.2%) | |||
| Operation | ||||||||||
| No | 55 (35.7%) | 31 (20.1%) | 24 (15.6%) | .162 | 40 (26.0%) | 15 (9.7%) | .316 | 12 (7.8%) | 43 (27.9%) | .257 |
| Yes | 99 (64.3%) | 67 (43.5%) | 2 (20.8%) | 79 (51.3%) | 20 (13.0%) | 30 (19.5%) | 69 (44.8%) | |||
| Chemo‐radiotherapy | ||||||||||
| No | 39 (25.3%) | 24 (15.6%) | 15 (9.7%) | .753 | 31 (20.1%) | 8 (5.2%) | .703 | 11 (7.1%) | 28 (18.2%) | .880 |
| Yes | 115 (74.7%) | 74 (48.1%) | 41 (26.6%) | 88 (57.1%) | 27 (17.5%) | 31 (20.1%) | 84 (54.5%) | |||
| CEA | ||||||||||
| <5 ng/mL | 45 (29.2%) | 36 (23.4%) | 9 (5.8%) | .007 | 42 (27.3%) | 3 (1.9%) | .002 | 15 (9.7%) | 30 (19.5%) | .278 |
| ≥5 ng/mL | 109 (70.8%) | 114 (40.3%) | 12 (30.5%) | 77 (50.0%) | 32 (20.8%) | 27 (17.5%) | 43 (53.2%) | |||
| CA 19‐9 | ||||||||||
| <37 U/mL | 74 (48.1%) | 49 (31.8%) | 25 (16.2%) | .522 | 62 (40.3%) | 12 (7.8%) | .064 | 20 (13.0%) | 54 (35.1%) | .948 |
| ≥37 U/mL | 80 (51.9%) | 49 (31.8%) | 31 (20.1%) | 57 (37.0%) | 23 (14.9%) | 22 (14.3%) | 58 (37.7%) | |||
TBIL, total bilirubin; DBIL, direct bilirubin; IBI, indirect bilirubin; CEA, carcinoembryonic antigen; CA19‐9, carbohydrate antigen 19‐9.
The bold represent that P value was statistically significant.
*Difference between groups was tested by Chi‐square test.
3.3. The association between baseline characteristics and clinical prognosis
The association between baseline characteristics and OS in stage IV CRC patients were listed in Table 2. Our results showed that TBIL (>12.9 μmol/L), DBIL (>6.1 μmol/L), tumor site (colon), chemo‐radiotherapy (yes), CEA (≥5 ng/mL), and CA19‐9 (≥37 U/mL) were significantly associated with decreased OS. Clinical baseline characteristics for the prediction of clinical prognosis were further investigated by univariate and multivariate analysis with Cox regression model. The significant characteristics in univariate analysis above were entered into the multivariate cox regression model to further determine the influence on OS (Table 2). Our results indicated that location of colon (HR=1.657, 95%CI=1.102‐2.490, P=.015), elevated CEA (HR=1.785, 95%CI=1.123‐2.837, P=.014), and high level of DBIL (HR=1.603, 95%CI=1.053‐2.442, P=.028) identified by multivariate Cox regression could be considered to be independent markers for poor OS of CRC patients. Moreover, in univariate analysis, the OS was better in patients with high IBI than in those with low IBI (HR=0.794), but there was no statistical significance between them (P=.284). Time‐dependent ROC curves demonstrated that high DBIL had similar prognostic efficacy as elevated CEA for reduced 5‐year OS (AUC=0.63 and 0.61, respectively).
Table 2.
Univariate/multivariate analysis for overall survival
| Characteristics | 5‐year OS | OS | |||||
|---|---|---|---|---|---|---|---|
| Total patients (N=154) | Number of death (N=110) | Log‐rank P value | Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | ||||
| Age (y) | |||||||
| ≤ 64 | 83 | 55 (66.27%) | .152 | 1 | .195 | ||
| >64 | 71 | 55 (77.46%) | 1.309 (0.900‐1.903) | ||||
| Gender | |||||||
| Male | 101 | 73 (72.28%) | .778 | 1 | .781 | ||
| Female | 53 | 37 (69.81%) | 0.945 (0.636‐1.404) | ||||
| Location | |||||||
| Rectal | 59 | 35 (59.32%) | .005 | 1 | .006 | 1 | .015 |
| Colon | 95 | 75 (78.95%) | 1.759 (1.176‐2.630) | 1.657 (1.102‐2.490) | |||
| Operation | |||||||
| No | 55 | 42 (76.36%) | .193 | 1 | .200 | ||
| Yes | 99 | 68 (68.69%) | 0.778 (0.529‐1.143) | ||||
| Chemo‐radiotherapy | |||||||
| No | 39 | 19 (48.72%) | .012 | 1 | .014 | 1 | .082 |
| Yes | 115 | 91 (79.13%) | 1.864 (1.132‐3.067) | 1.566 (0.945‐2.597) | |||
| CEA | |||||||
| <5 ng/mL | 45 | 24 (53.33%) | .002 | 1 | .003 | 1 | .014 |
| ≥5 ng/mL | 109 | 86 (78.90%) | 1.995 (1.267‐3.142) | 1.785 (1.123‐2.837) | |||
| CA19‐9 | |||||||
| <37 U/mL | 74 | 45 (60.81%) | .015 | 1 | .017 | 1 | .555 |
| ≥37 U/mL | 80 | 65 (81.25%) | 1.590 (1.086‐2.328) | 1.139 (0.740‐1.754) | |||
| TBIL | |||||||
| ≤12.9 μmol/L | 98 | 63 (64.29%) | .022 | 1 | .025 | 1 | .859 |
| >12.9 μmol/L | 56 | 47 (83.93%) | 1.540 (1.054‐2.248) | 1.053 (0.593‐1.870) | |||
| DBIL | |||||||
| ≤6.1 μmol/L | 119 | 78 (65.55%) | .002 | 1 | .003 | 1 | .028 |
| >6.1 μmol/L | 35 | 32 (91.43%) | 1.875 (1.241‐2.834) | 1.603 (1.053‐2.442) | |||
| IBI | |||||||
| ≤4.8 μmol/L | 42 | 30 (71.43%) | .276 | 1 | .284 | ||
| >4.8 μmol/L | 112 | 80 (71.43%) | 0.794 (0.521‐1.210) | ||||
TBIL, total bilirubin; DBIL, direct bilirubin; IBI, indirect bilirubin; CEA, carcinoembryonic antigen; CA19‐9, carbohydrate antigen 19‐9; HR, hazard ratio; 95%CI, 95% confidential interval.
The bold represent that P value was statistically significant.
4. DISCUSSION
In this retrospective study of 154 stage IV CRC patients, we indicated that pre‐treatment elevated TBIL and DBIL levels were associated with poor OS, and DBIL was an independent prognostic factor for OS. To the best of our knowledge, our study was the first report on the prognostic role of DBIL in stage IV CRC patients.
X‐tile program, a robust graphical tool verified by Yale University was used to determine the optimum cut‐off values for TBIL, DBIL, and IBI, which were 12.9, 6.1, and 4.8 μmol/L, respectively.16 However, these cut‐off values varied from previous studies (date were listed in Table 3).11, 12, 13, 14, 15 With the aspect to CRC, Gao used the threshold 2.6 μmol/L as cut‐off value to classified the DBIL for stage I‐IV rectal cancer,12 and Zhang chose 3.6 μmol/L for stage II‐III CRC,15 which were slightly lower than our cut‐off value of DBIL. Number of patients from different tumor stages and different geographic regions may be could explain this phenomenon. Furthermore, different reference ranges of routine biochemistry tests in different medical centers could also contribute to this difference.
Table 3.
Published studies on the association between serum bilirubin level and survival of cancer patients
| Study | Year | Cancer | Number | Method | TNM stage | Country | Ethnicity | Survival | Factors | Cut‐off value | Significant in univariate analysis | Significant in multivariate analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deng et al.11 | 2015 | NPC | 1327 | ROC | I‐IV | Guangzhou | Asian | OS | IBI | 9.7 μmol/L | Yes | No |
| Deng et al.11 | 2015 | NPC | 1327 | ROC | I‐IV | Guangzhou | Asian | DMFS | IBI | 9.7 μmol/L | Yes | Yes |
| Li et al.13 | 2015 | NSCLC | 1617 | ROC | I‐III | Guangdong | Asian | OS,DFS, LRFS, DMFS | TBIL | 9.50 μmol/L | Yes | No |
| Li et al.13 | 2015 | NSCLC | 1617 | ROC | I‐III | Guangdong | Asian | OS, DFS, LRFS, DMFS | DBIL | 3.45 μmol/L | Yes | No |
| Li et al.13 | 2015 | NSCLC | 1617 | ROC | I‐III | Guangdong | Asian | OS, DFS, LRFS, DMFS | IBI | 6.95 μmol/L | Yes | Yes |
| Liu et al.14 | 2015 | Breast cancer | 2425 | Spline curves | I‐III | Houston | Caucasian | OS | TBIL | 0.2 mg/dl | Yes | Yes |
| Cao et al.12 | 2016 | Rectal cancer | 469 | ROC | I‐IV | Beijing | Asian | OS | DBIL | 2.6 μmol/L | Yes | Yes |
| Zhang et al.15 | 2016 | CRC | 986 | X‐tile | II‐III | Shanghai | Asian | OS, DFS | DBIL | 3.6 μmol/L | Yes | Yes |
| This study | 2016 | CRC | 154 | X‐tile | IV | Nanjing | Asian | OS | TBIL | 12.9 μmol/L | Yes | No |
| This study | 2016 | CRC | 154 | X‐tile | IV | Nanjing | Asian | OS | DBIL | 6.1 μmol/L | Yes | Yes |
| This study | 2016 | CRC | 154 | X‐tile | IV | Nanjing | Asian | OS | IBI | 4.8 μmol/L | No | No |
ROC, receiver operating characteristic; NSCLC, non‐small‐cell lung cancer; NPC, nasopharyngeal carcinoma; CRC, colorectal cancer; TBIL, total bilirubin; DBIL, direct bilirubin; IBI, indirect bilirubin; OS, overall survival; LRFS, locoregional relapse‐free survival; DMFS, distant metastasis‐free survival; DFS, disease‐free survival; RFS, relapse‐free survival.
Our study found that the OS was better in patients with high IBI than in those with low IBI, but there was no statistical significance between them, which indicated that elevated IBI level might be a protective factor for OS. At the same time, a previous study on nasopharyngeal carcinoma (NPC) showed that IBI could inhibit NPC metastasis by inhibiting reactive oxygen species (ROS) production, and acted as a favorable prognostic factor.11 What's more, IBI was also reported to be an independent prognostic factor in NSCLC.13 On the other hand, it was reported that IBI could induce cell apoptosis in colon cancer cell lines by triggering mitochondrial depolarization,17 suggesting that elevated IBI level could predict good outcome. As for the prognostic role of IBI in stage IV CRC, it should be confirmed in further study.
The study revealed some interesting associations between the variates and tumor prognosis. First, we found that the location of tumor was significantly more likely to be colon in patients with poor outcome. It might be due to that colon was the main place of converting unconjugated bilirubin to form urobilinogens by the function of bacterium in enterohepatic recirculation. Second, our study demonstrated that higher DBIL correlated with poor survival in stage IV CRC patients, and could be treated as an independent prognostic biomarker, which was also found in stage I‐IV rectal cancer, stage II‐III CRC, and other malignancies such as NSCLC previously. In addition, this study also showed elevated TBIL level was associated with poor OS, which was consistent with Li's study in NSCLC.13 As we knew, bilirubin was mainly cleared by liver. In hepatocytes, unconjugated bilirubin (or IBI) could be converted into conjugated bilirubin (or DBIL) through conjugation with two molecules of glucuronic acid by the action of UGT1A1 enzyme, which indicated that UGT1A1 enzyme could play essential roles in the level of bilirubin.7 It was reported congenital under‐expression of UGT1A1 resulted into mild, chronic, fluctuating unconjugated hyperbilirubinemia (Gilbert's syndrome), which was due to an extra TA in the (TA)6 locus of the promoter region of UGT1A1, leading to low expression of the enzyme.7 Additionally, recent studies also reported that gene polymorphism of UGT1A1 enzyme was commonly seen in CRC,8 and UGT1A1 gene polymorphism was related to prognosis of irinotecan‐based chemotherapy in patients with advanced CRC,18 thus in advanced CRC patients, the process of IBI converting to DBIL would be influenced, and then poor outcome occurred. Hence, taking the inconvenience and expensiveness of UGT1A1 into consideration, our results proved our hypothesis and indicated that serum bilirubin which was tested routinely could be an available prognostic biomarker for CRC.
On the other hand, our study had some limitations. First, only 154 stage IV patients could not be representative of all stage IV CRC patients in general. Second, only OS which were not able to exclude the influence of cancer unrelated to death was accessed in this study. The last but not least, the lack of an external validation cohort requires further studies.
In conclusion, pre‐treatment elevated TBIL and DBIL levels were associated with poor OS in stage IV CRC patients. Moreover, DBIL could be considered as an independent prognostic biomarker for OS. Furthermore, high DBIL had similar prognostic efficacy as elevated CEA for reduced 5‐year OS.
ACKNOWLEDGMENTS
This work was supported by the Fundamental Research Funds for the Central Universities, University Graduate Student Scientific Innovation Project of Jiangsu (No. SJZZ16_0047).
Yang L, Ge L‐Y, Yu T, Liang Y, Yin Y, Chen H. The prognostic impact of serum bilirubin in stage IV colorectal cancer patients. J Clin Lab Anal. 2018;32:e22272 10.1002/jcla.22272
Lin Yang and Lu‐Yao Ge are contributed equally to this work.
REFERENCES
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zuckerman DS, Clark JW. Systemic therapy for metastatic colorectal cancer: current questions. Cancer. 2008;112:1879‐1891. [DOI] [PubMed] [Google Scholar]
- 4. Jiraskova A, Novotny J, Novotny L, et al. Association of serum bilirubin and promoter variations in HMOX1 and UGT1A1 genes with sporadic colorectal cancer. Int J Cancer. 2012;131:1549‐1555. [DOI] [PubMed] [Google Scholar]
- 5. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043‐1046. [DOI] [PubMed] [Google Scholar]
- 6. Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361‐370. [DOI] [PubMed] [Google Scholar]
- 7. Vitek L, Ostrow J. Bilirubin chemistry and metabolism; harmful and protective aspects. Curr Pharm Des. 2009;15:2869‐2883. [DOI] [PubMed] [Google Scholar]
- 8. Liu X, Cheng D, Kuang Q, Liu G, Xu W. Association between UGT1A1*28 polymorphisms and clinical outcomes of irinotecan‐based chemotherapies in colorectal cancer: a meta‐analysis in Caucasians. PLoS ONE. 2013;8:e58489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zucker SD, Horn PS, Sherman KE. Serum bilirubin levels in the U.S. population: gender effect and inverse correlation with colorectal cancer. Hepatology. 2004;40:827‐835. [DOI] [PubMed] [Google Scholar]
- 10. Zucker SD, Benedict M, Sherman KE. Serum bilirubin and risk of colorectal cancer. Aliment Pharmacol Ther. 2006;24:1257‐1259; author reply 9‐61. [DOI] [PubMed] [Google Scholar]
- 11. Deng CC, Xu M, Li J, et al. Unconjugated bilirubin is a novel prognostic biomarker for nasopharyngeal carcinoma and inhibits its metastasis via antioxidation activity. Cancer Prev Res. 2016;9:180‐188. [DOI] [PubMed] [Google Scholar]
- 12. Gao C, Fang L, Li JT, Zhao HC. Significance and prognostic value of increased serum direct bilirubin level for lymph node metastasis in Chinese rectal cancer patients. World J Gastroenterol. 2016;22:2576‐2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li N, Xu M, Cai MY, et al. Elevated serum bilirubin levels are associated with improved survival in patients with curatively resected non‐small‐cell lung cancer. Cancer Epidemiol. 2015;39:763‐768. [DOI] [PubMed] [Google Scholar]
- 14. Liu X, Meng QH, Ye Y, Hildebrandt MA, Gu J, Wu X. Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non‐metastatic breast cancer. Carcinogenesis. 2015;36:243‐248. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Q, Ma X, Xu Q, et al. Nomograms incorporated serum direct bilirubin level for predicting prognosis in stages II and III colorectal cancer after radical resection. Oncotarget. 2016;8:71138‐71146. 10.18632/oncotarget.11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Camp RL, Dolled‐Filhart M, Rimm DL. X‐tile: a new bio‐informatics tool for biomarker assessment and outcome‐based cut‐point optimization. Clin Cancer Res. 2004;10:7252‐7259. [DOI] [PubMed] [Google Scholar]
- 17. Keshavan P, Schwemberger SJ, Smith DL, Babcock GF, Zucker SD. Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int J Cancer. 2004;112:433‐445. [DOI] [PubMed] [Google Scholar]
- 18. Xu C, Tang X, Qu Y, Keyoumu S, Zhou N, Tang Y. UGT1A1 gene polymorphism is associated with toxicity and clinical efficacy of irinotecan‐based chemotherapy in patients with advanced colorectal cancer. Cancer Chemother Pharmacol. 2016;78:119‐130. [DOI] [PubMed] [Google Scholar]


