Abstract
Background
Hydroelectrolytic disorders are common in clinical situations and may be harmful to the patient, especially those involving plasma sodium and potassium dosages. Among the possible methods for the dosages are flame photometry, ion‐selective electrode (ISE) and colorimetric enzymatic method.
Methods
We analyzed 175 samples in the three different methods cited from patients attending the laboratory of the University Hospital of the Federal University of Juiz de Fora. The values obtained were statistically treated using SPSS 19.0 software. The present study aims to evaluate the impact of the use of these different methods in the determination of plasma sodium and potassium.
Results
The averages obtained for sodium and potassium measurements by flame photometry were similar (P > .05) to the means obtained for the two electrolytes by ISE. The averages obtained by the colorimetric enzymatic method presented statistical difference in relation to ISE, both for sodium and potassium. In the correlation analysis, both flame photometry and colorimetric enzymatic showed a strong correlation with the ISE method for both dosages.
Conclusion
At the first time in the same work sodium and potassium were analyzed by three different methods and the results allowed us to conclude that the methods showed a positive and strong correlation, and can be applied in the clinical routine.
Keywords: clinical analysis, flame photometry, hydroelectrolytic disorders, ion‐selective electrode, laboratory methods
1. INTRODUCTION
Hydroelectrolytic disorders are very common in many clinical and surgical situations, and may be fatal if not corrected. Thus, adequate determinations of these disorders are extremely important such as those involving plasma sodium (Na+) and potassium (K+) assay.1
Sodium is a fundamental ion in organic function and represents most frequent ion in extracellular fluids, with a concentration rate between 135 and 145 mEq/L.2, 3, 4, 5 Among disorders caused by changes in serum sodium concentration, hypernatremia has a low frequency and is well tolerated and usually requires simple therapeutic procedures.6, 7 However, hyponatremia, a major hydroelectrolytic disorder in the hospitalized individuals, is associated with a series of unfavorable outcomes such as intensive care unit hospitalization, prolonged hospitalization, both increasing treatment costs and mortality. In addition, inadequate management of a hyponatremic patient can induce severe neurological damage and even death.4
Potassium is the second most abundant cation in organism. It represents main intracellular ion with 98% found inside cells (140‐150 mEq/L), especially in skeletal muscle8 and its plasma concentration is between 3.5 and 5.5 mEq/L.8, 9, 10 Disorders in the potassium homeostasis are frequent, especially in hospitalized patients under different medicines treatment. As the potassium concentration in human serum is small a physiological tolerance to these changes is small too.9 Although low serum potassium is the disorder most commonly diagnosed by physicians, the hyperkalemia as important as it.8
As exposed, sodium and potassium are essential electrolytes for human homeostasis and its level changes can induce serious complications to the patient. Thus, when most precise, sensitive and/or specific is the method to ions determination, more subsidies the medical clinic will has for correct interventions. Among the possible methods for sodium and potassium determinations we have flame photometry, ion‐selective electrode (ISE), and enzymatic colorimetric method.
Flame photometry is one of the oldest direct potentiometric methods commonly used to measure the concentrations of sodium, potassium, and lithium in serum and urine samples. The flame photometry principle is based on chemical property of alkaline‐earth metals on high temperatures absorbed energy from heat source being conducted to state of excitation in their atomic form. When they return to initial energy state the radiation is release at specific wavelengths being some in region of the visible spectrum. Thus, the emission is proportional to concentration of ions present at the study sample. If an appropriate photodetector is placed behind the filter, an electrical signal is obtained being proportional to concentration of element in solution.11 The flame photometer for use in human samples represented a major advance in laboratory routine and has been presented as a convenient method. This has made possible Na+ and K+ analysis in a shorter time using small blood and urine samples.12
The ISE method consists in the use of specific membranes to ions Na+ and K+. This methodology form a potential by difference in concentrations each side of membrane. In this system two electrodes should be used where the reference electrode having constant potential. The selective electrode for an ion develops a voltage that varies with sample concentration. Thus, from difference between the potentials of the reference electrode and the measuring electrode, the ion concentration in solution is calculated and expressed by Nernst Equation.13, 14
Some advantages of the ISE method can be reliability, robustness, selectivity, sensitivity and use of small sample volume. Moreover, it presents fast results and does not undergo interference due to changes in sample, such as lipemia, hemolysis, and jaundice, as only ionized particles of electrolytes are measured. These characteristics make the ISE an important methodology that can be applied in clinical and industrial trials.15, 16
In most routine diagnostic laboratories, ISE is method of choice for Na+ and K+ assay. However, cost and complexity of integrating this technique into routine analyzers has taken to the development of more easily adaptable spectrophotometric methods.17, 18
The kinetic method used to determine serum Na+ concentration by enzymatic reaction has as principle the activation of reaction between sodium‐dependent β‐D‐galactosidase and its substrate O‐nitrophenyl‐β‐D‐galactopyranose (ONPG) by converting it to O‐nitrophenyl and galactose by Na+ present in the sample. The rate of formation of the product at 405 nm is proportional to amount of sodium present in the sample (Figure 1).19, 20, 21
Figure 1.
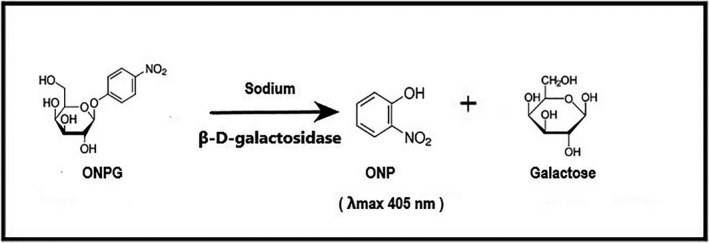
Enzymatic reaction has as principle the activation of reaction between sodium‐dependent β‐D‐galactosidase and its substrate O‐nitrophenyl‐β‐D‐galactopyranose (ONPG) by converting it to O‐nitrophenyl (ONP) and galactose by Na+ present in the sample resulting in the formation of the yellow (λmax 405 nm) compound ONP
The enzymatic reaction for K+ assay is based on conversion of phosphoenolpyruvate into pyruvate by K+ dependent pyruvate kinase. In presence of reduced nicotinamide adenine dinucleotide (NADH), the pyruvate generated is converted to lactate in a reaction catalyzed by lactate dehydrogenase. The K+ concentration present in the sample is proportional to reduction of the optical density at 380 nm as a function of NADH oxidation to nicotinamide adenine oxidized dinucleotide (NAD) (Figure 2).17, 21
Figure 2.
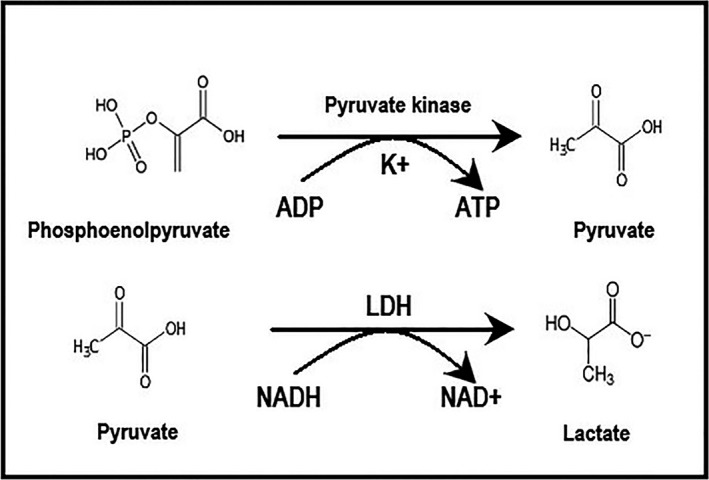
The enzymatic reaction for K+ assay is based on conversion of phosphoenolpyruvate into pyruvate by K+ dependent pyruvate kinase. In presence of reduced nicotinamide adenine dinucleotide (NADH), the pyruvate generated is converted to lactate in a reaction catalyzed by lactate dehydrogenase (LDH). The K+ concentration present in the sample is proportional to reduction of the optical density at 380 nm as a function of NADH oxidation to nicotinamide adenine oxidized dinucleotide (NAD +)
The development of new enzymatic reagents has conferred stability to liquid form, allowing analysis of ions together other biochemical dosages, getting practicality to analytical process.21
No research was done comparing the three different methodologies routinely employed. Thus, the present study aimed to compare use of flame photometry, ISE and colorimetric enzymatic in sodium and potassium determination in human serum.
2. MATERIALS AND METHODS
2.1. Sample
The study was approved by the Research Ethics Committee of the Federal University of Juiz de Fora, under the number 36301514.0000.5133.
We used 175 samples from patients attending at Laboratory of the University Hospital (UH) of the Federal University of Juiz de Fora, Minas Gerais, Brazil, from October to December 2016. Patients with age among 18 and 65 years (adulthood in Brazil) were selected for this study. There was no restriction of race or gender to participate of the study. During the analysis, BD Vacutainer® Gel BD SST® II Advance tubes with capacity to 5 mL of blood were used to obtain blood by venipuncture according standard protocol of UH laboratory.
The samples were sent to laboratory and centrifuged for 10 minutes at 1050 g. The supernatant serum was collected from blood samples for electrolyte assay and biochemical analysis. The samples with a lipemic or hemolysate aspect were not included in the study because they interfere with enzymatic colorimetric and flame photometry methodologies.21 The total time to performing all the procedures was approximately 1 hour.
2.2. Sodium and potassium analysis
The automated analysis by the ISE method is routinely performed on Labmax 560 device from Labtest (Labtest Diagnostica SA, Minas Gerais, Brazil). The ISE was used as the standard method of study since the UH laboratory performs routinely internal quality control of analyzes by ControlLab® and external quality control through proficiency testing. ControlLab® is the company that makes the proficiency test in partnership with Brazilian Society of Clinical Pathology/Laboratory Medicine (SBPC/ML) accredited by National Institute of Metrology Quality and Technology (INMETRO) and recognized by the Brazilian Ministry of Health. Na+ and K+ assay made by the colorimetric enzymatic method were performed using commercial reagent for Labtest Labmax 560 whose determination principle was previously described. Before performing these tests two internal enzyme control levels were analyzed for both Na+ and K+ and reagents were calibrated with their own calibrators supplied together kit. The flame photometry analysis was performed on FC‐280 photometer from CELM company (São Paulo, Brazil), using the standard lithium reference of same company.
2.3. Statistical analysis
To characterize the sample, descriptive statistics were used, such as mean and standard deviation. The paired t test was used to compare Na+ and K+ rates by ISE method (reference in this study) with those obtained by flame photometry and by colorimetric enzymatic method. A 95% confidence interval and a significance level of 5% were adopted. Pearson's correlation was also used to evaluate the existence and level of correlation between variables. Data were analyzed using SPSS 19.0 (SPSS Inc., Chicago, United States).
3. RESULTS
Interference by endogenous and exogenous substances with assays for clinical analytes is a common problem in laboratory medicine. Seventeen serum samples were excluded due to hemolysis and lipemia; hemolysis has a direct effect on several tests. The cellular components of erythrocytes may in fact raise the analyte concentration as a contaminant. Lipemia is the result of circulating chylomicrons which are large lipid particles. They cause interference by turbidity or light scattering and volume displacement.
After Na+ and K+ assay by ISE, flame photometry and enzymatic colorimetric methods, we observed that results obtained from different methodologies showed small mean and standard deviation, showing little difference between the results obtained by different methods (Table 1).
Table 1.
Summary of serum Na+ and K+ assay comparisons with ISE, flame photometry and enzymatic colorimetric
| Samples | Mean mEq/L | Standard deviation | Minimum | Maximum | |
|---|---|---|---|---|---|
| Na+ assay | |||||
| ISE | 175 | 138.52 | 4.74 | 124.10 | 154.40 |
| Flame photometry | 175 | 138.19 | 4.56 | 128.00 | 152.00 |
| Enzymatic colorimetric | 175 | 139.29 | 4.90 | 124.63 | 152.32 |
| K+ assay | |||||
| ISE | 175 | 4.16 | 0.76 | 1.85 | 6.55 |
| Flame photometry | 175 | 4.18 | 0.75 | 1.80 | 6.50 |
| Enzymatic colorimetric | 175 | 4.50 | 0.65 | 2.56 | 7.22 |
ISE, ion‐selective electrode.
As showed in Table 1, we observed that variability of data in relation to mean (coefficient of variation/CV), showed small difference in Na+ rate by ISE and flame photometry (0.012) as well as ISE and enzymatic colorimetric (0.0178). Similarly the K+ rates by ISE and flame photometry (0.004) as well as ISE and enzymatic colorimetric (0.039) were very low.
The paired t test was performed comparing the values obtained for Na+ and K+ by the ISE method (reference of our study) with those obtained by Flame photometry and by the Enzymatic method. As show in Table 2, we can observe that in Na+ assay there was no significant difference between the means obtained by ISE method and flame photometry (P = .126). However, the comparison between the ISE and the enzymatic colorimetric showed a significant difference (P < .001). Similarly, K+ assay showed no significant difference between the ISE and flame photometry (P = .343). However, like to Na+ analysis the comparison between ISE and colorimetric enzymatic showed a significant difference for K+ assay (P < .001).
Table 2.
Analysis of the average between Na+ and K+ assay
| Methods | Mean difference | Confidence Interval of the 95% | t test | Degrees of freedom | P‐Value | |
|---|---|---|---|---|---|---|
| Minor | Major | |||||
| Na+ | ||||||
| ISE ‐ Flame photometry | 0.3247 | −0.0923 | 0.7417 | 1.537 | 174 | .126 |
| ISE ‐Enzymatic colorimetric | −0.7704 | −1.1786 | −0.3622 | −3.725 | 174 | <.001 |
| K+ | ||||||
| ISE ‐ Flame photometry | −0.0174 | −0.0536 | 0.0188 | −0.950 | 174 | .343 |
| ISE‐Enzymatic colorimetric | −0.3409 | −0.3889 | −0. 2928 | −13.989 | 174 | <.001 |
ISE, ion‐selective electrode.
In our study, the results were also analyzed to verify the existence or not of correlation between the techniques, having as standard the ISE method. These findings are agreed with data of Figures 3, 4, 5, 6. Figure 3 shows a positive correlation between the Na+ values obtained by ISE and flame photometry (r = .820), as well as a positive correlation between the ISE values and the colorimetric enzymatic method (r = .840) (Figure 4).
Figure 3.
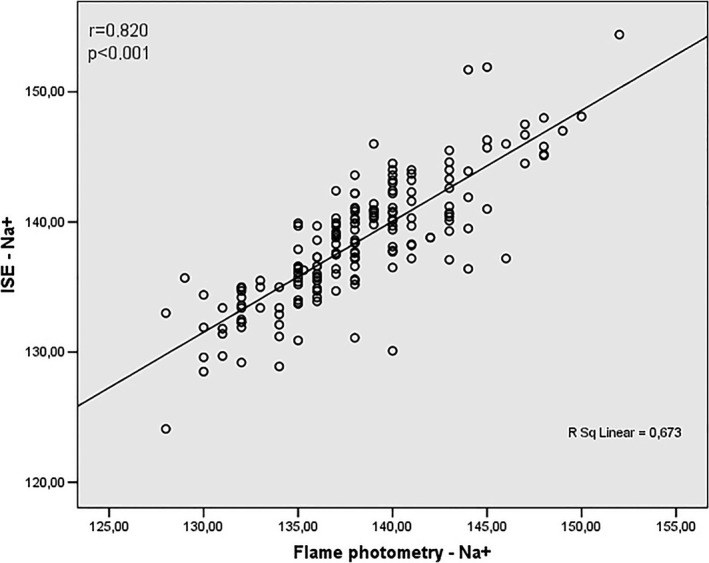
Correlation analysis between values obtained by Na+ assay using ISE and flame photometry methods
Figure 4.
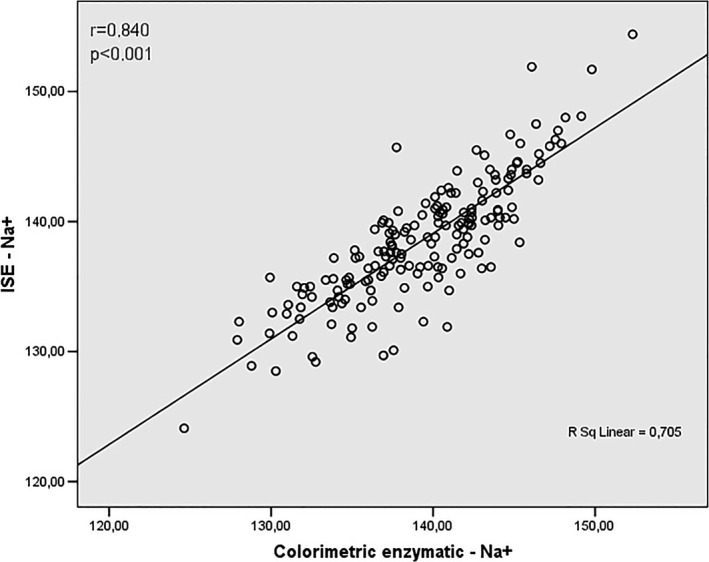
Correlation analysis between values obtained by Na+ assay using ISE and colorimetric enzymatic methods
Figure 5.
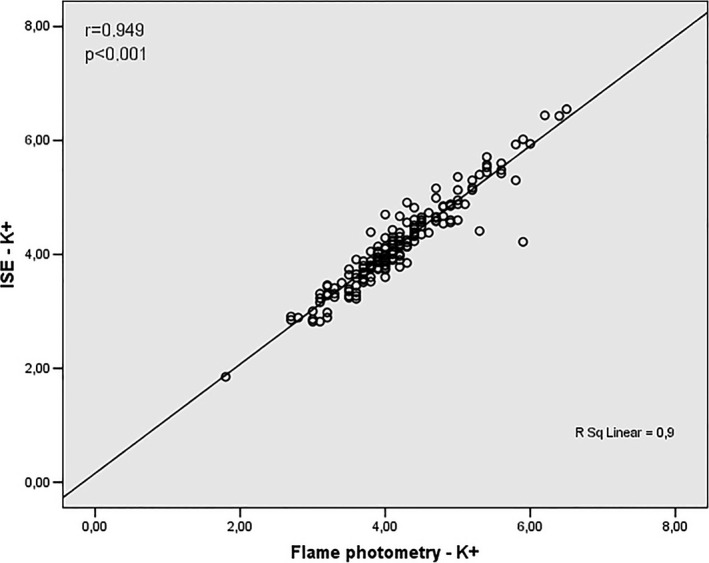
Correlation analysis between values obtained by K+ assay using ISE and flame photometry methods
Figure 6.
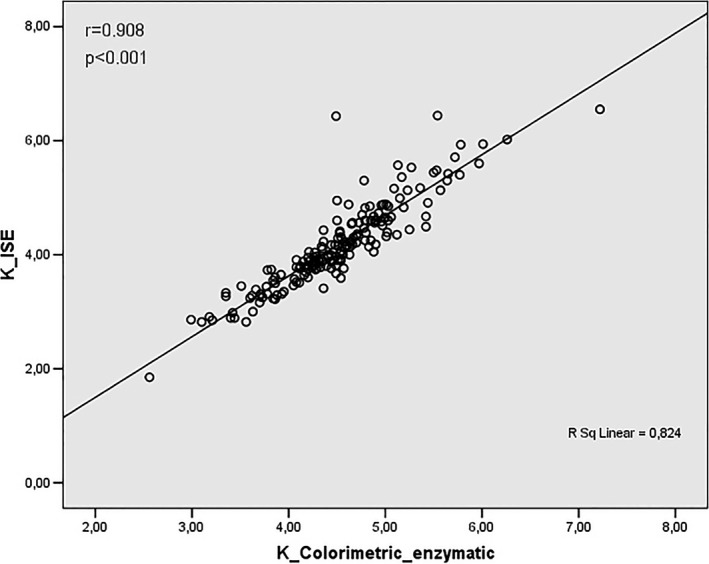
Correlation analysis between values obtained by K+ assay using ISE and colorimetric enzymatic methods
As shown in Figures 5 and 6 K+ assay revealed correlation between ISE and flame photometry (r = .949) and ISE and enzymatic colorimetric (r = .908) respectively.
4. DISCUSSION
Seventeen serum samples were excluded due to hemolysis and/or lipemia, once they have a direct effect on several tests. According Albert,22 the hemolysis increases the concentration of potassium per 3 mmol/L for each 1 g/L of hemoglobin being that lipemia causes interference by turbidity or light scattering and volume displacement. Although the literature date say that ISE does not undergo interference due to changes in the sample, such as lipemia and hemolysis,15, 16 colorimetric enzyme and flame photometry methods can be impaired. Thus, these samples could impair the correlation between them and ISE method.
According Leite,23 different techniques can be used to validate an analytical result. Therefore is relevant to analyze the same sample by another technique, in order to verify the magnitude of result. Serum sodium and potassium disorders are common in both inpatients and out patients, especially in emergency patients, where disnatremias and discalemias are reported as independent predictors of mortality.24 In this article, the results showed in Table 1 allow us to infer that methods are effective for Na+ and K+ assay.
There is a close physiological variation of electrolytic values, and the most varied morbid conditions can lead alterations of these values, disturbing the homeostasis of the electrolytes.16 It is important to report that accuracy in determination of serum sodium concentration is essential for diagnosis and safe treatment of dysnatremias.25 Assay of potassium should follow the same criteria, since its serum concentration range is small, as well as the organism's tolerance to these modifications.10
Langhoff and Steiness,26 with support of a potentiometric analyzer specific for sodium and potassium, obtained similar results like flame photometry and both instruments showed linearity within a physiological range for sodium and potassium concentrations. In addition, the instruments showed similar precisions, agreeing our study, since comparison of these methods there was no statistically difference between the Na+ and K+ assay.
In recent study conducted by Albert et al,22 were evaluated 630 serum samples with aim to compare two different methods (ISE and flame photometer) of same laboratory for Na+ and K+ analysis. As shown the value of Na+ and K+ obtained by ISE and flame photometer are in accordance with reference values, demonstrating 95% of confidence. Thus, we demonstrate that results between the two methods are to agree, since they do not differ enough to cause clinical complications.
The results for Na+ and K+ obtained by ISE compared to colorimetric enzyme were statistically significant (P < .001). These data were analyzed in accordance with values provided by ControlLab® and the manufacturer of colorimetric enzymatic reagent, since standards were properly analyzed and accepted before assay. We emphasize that limit values for calculation of Na+ and K+ are ± 8 mmol/L and ± 10% for K+, respectively, while variation of enzyme reagent is ± 15% for both electrolytes.
Concerning to Na+ assay none of 175 samples evaluated by colorimetric method presented results outside of rate proposed by ControlLab® in comparison to results obtained by ISE though established significant statistical difference. According K+ assay to 175 samples we showed that 93 (53%) has a change rate of ± 10% as proposed by ControlLab® when compared to value obtained by ISE method while others 82 samples (47%) had a change rate of ± 15% as proposed by colorimetric enzymatic assay kit.
According Yu et al,27 enzymes play an important role in laboratory reactions, however, they generally suffer from lower stability and less tolerance of temperature, pH condition compared with general chemical product. This, perhaps, may explain the statistical difference found between the ISE and colorimetric enzyme methods. However, it is possible to observe that differences were small within the clinical tolerance without changes that could lead others interpretations.
In addition, degree of difference in samples assay would not change the classifications of the natremias and calemias, not being able to cause immediate risk to patients.
Although few studies have been comparing Na+ and K+ assay by ISE and colorimetric enzymatic methods, Quiles et al20 developed a study proposing an automated method based on principles of injection flow analysis for enzymatic determination of serum sodium. Like our study (r = .840, Figure 4), the correlation presented by Quiles20 was excellent (r = .9716) when compared to direct potentiometric method, ie ISE. Thus, the proposed method proved to be a valid alternative to ISE on determination of serum sodium concentration.
These analyses are supported by Fernandez‐Homero.28 Their Analysis of 15 sample serum to K+ by ISE and enzymatic colorimetric showed a positive correlation of the r = .9931. Koch, Parrish and Ladenson,29 using a Du Pont analyzer evaluated Na+ and K+ assay by direct potentiometry (ISE). They compared these results with results in three different equipments to ISE and flame photometer as principle. The analysis between Du Pont aca ISE and flame photometer using 103 serum samples showed a solid correlation of r = .962 and r = .995 to Na+ and K+ respectively.
Thus, these results corroborate our study, since we showed a solid correlation between ISE and flame photometry for Na+ (r = .820) and K+ (r = .949).
Colorimetric enzymatic methodology has been undergoes constant evolution. As shown in this article, statistical differences between sodium and potassium by colorimetric enzymatic technology were observed in relation to the ISE method (P < .001), however, with a strong correlation (0.840 Na+ and 0.908 K+). Advances in enzymatic determination are a promise for this tool. Electrocatalysis of molecules is a hot topic in biological and energy‐related chemistry. In the work of Zhao et al,30 was developed a new system to study the electrocatalytic efficiency of a single catechol molecule for NADH oxidation by single functionalized nanoparticle collision at ultramicroelectrodes. NADH molecules could be catalyzed per second by a single catechol molecule, suggesting the successful establishment of this novel catalytic system. Studies like this show that enzymatic methodologies continue to evolve and could be used as a promising platform for research on other molecular and diagnostic electrocatalytic systems.
The electrolyte assay is a fundamental clinical practice where the need for rapid results has allowed the emergence of automated methods such as ISE and enzymatic colorimetric.27 Was described that electrode selective is the reference method to analyze Na+ and K+. However, researches for new methods are necessary to continue improve laboratorial analysis and the quality of life of the patients. Ying et al,31 compared to traditional wire electrodes with wireless asymmetric nanopore electrode and this exhibited a high signal‐to‐noise ratio by increasing the current resolution from nanoamperes to picoamperes. This author showed that asymmetric nanopore electrode is highly sensitive and promising for the future imaging of electron transfer dynamics in live cells and research in new techniques.
Against the above, the results have been showing that methods are safe and can be used in Na+ and K+ assay, since there is a solid correlation between obtained results. However, in order to establish the best methodology for Na+/K+ assay, further analysis are necessary such as cost/benefit, reagents stability, frequency of apparatus calibrations among others since these were not aim of this study. For this goal, further studies are required.
Thus, we can infer that analysis by ISE, flame photometer and colorimetric enzymatic method can be used in Na+ and K+ assay on clinical practice.
Garcia RA, Vanelli CP, Pereira Junior OdS, Corrêa JOdA. Comparative analysis for strength serum sodium and potassium in three different methods: Flame photometry, ion‐selective electrode (ISE) and colorimetric enzymatic. J Clin Lab Anal. 2018;32:e22594 10.1002/jcla.22594
REFERENCES
- 1. Peres LAB, Tanaka TM, Sato CM, et al. Injúria renal aguda e distúrbios hidroeletrolíticos em pacientes submetidos à cirurgia geral em hospital universitário. Rev Med. 2010;12:79‐84. [Google Scholar]
- 2. Neto OMV, Neto MM. Distúrbios do equilíbrio hidroeletrolítico. Medicina, Ribeirão Preto. 2003;36:325‐337. [Google Scholar]
- 3. Mata LS, Gusmão D, Almeida ARP. Encefalopatia hemorrágica hipernatrêmica: relato de caso e revisão da literatura. Rev Bras Ter Intensiva. 2010;22:305‐309. [PubMed] [Google Scholar]
- 4. Rocha PN. Hiponatremia: conceitos básicos e abordagem prática. J Bras Nefrol. 2011;33:248‐260. [DOI] [PubMed] [Google Scholar]
- 5. Arampatzis S, Funk GC, Leichtle AB, et al. Impact of diuretic therapy‐associated electrolyte disorders present on admission to the emergency department: a cross‐sectional analysis. BMC Med. 2013;11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gusmão F, Abdulkader R. Hipernatremia. Medicinanet [home page on the Internet]. 2008. http://www.medicinanet.com.br/conteudos/revisoes/1344/hipernatremia.htm. Accessed November 5, 2017.
- 7. Dutra VF, Tallo FS, Rodrigues FT, et al. Desequilíbrios hidroeletrolíticos na sala de emergência. Rev Bras Clin Med. São Paulo 2012;10:410‐419. [Google Scholar]
- 8. Daly K, Farrington E. Hypokalemia and hyperkalemia in infants and children: pathophysiology and treatment. J Pediatr Health Care. 2013;27:486‐496. [DOI] [PubMed] [Google Scholar]
- 9. Palmer BF, Dubose TD. Disorders of potassium metabolism In: Schrier RW, ed. Renal and electrolyte disorders, 7th edn Philadelphia: Wolters Kluwer; 2010:137‐165. [Google Scholar]
- 10. Hinkle C. Electrolyte disorders in the cardiac patient. Crit Care Nurs Clin North Am. 2011;23:635‐643. [DOI] [PubMed] [Google Scholar]
- 11. Lyra WS. Espectrometria de Emissão em Chama Baseada em Imagens Digitais. 2008. Dissertação de mestrado. Programa de pós‐graduação em química, Universidade Federal da Paraíba.
- 12. Seldin DW. Scientific achievements of John P Peters. Am J Nephrol. 2002;22:192‐196. [DOI] [PubMed] [Google Scholar]
- 13. Kavanagh K, Mills JN. Measuring electrolytes: an evaluation of the IDEXX Vetlyte ion selective electrode method. Aust Vet Pract. 1997;27:97‐102. [Google Scholar]
- 14. Burnett RW, Covington AK, Foghandersen N, et al. Use of ion‐selective electrodes for blood‐electrolyte analysis. Recommendations for nomenclature, definitions and conventions. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Scientific Division Working Group on Selective Electrodes. Clin Chem Lab Med. 2000;38:363‐370. [DOI] [PubMed] [Google Scholar]
- 15. Fernandes JCB, Kubota LT. Eletrodos íon‐seletivos: histórico, mecanismo de resposta, seletividade e revisão dos conceitos. Quim Nova. 2001;24:120‐130. [Google Scholar]
- 16. Giovaninni LH, Kogika MM, Lustoza MD, et al. Comparação sérica e sanguínea do cálcio ionizado, sódio, potássio e cloreto em felinos pelo método eletrodo íon seletivo. Arq Bras Med Vet Zootec. 2007;59:820‐823. [Google Scholar]
- 17. Berry MN, Mazzachi RD, Pejakovlc M, et al. Enzymatic determination of potassium in serum. Clin Chem. 1989;35:817‐820. [PubMed] [Google Scholar]
- 18. Hubl W, Wejbora R, Shafti‐Keramat I, et al. Enzymatic determination of sodium, potassium, and chloride in abnormal (Hemolyzed, Icteric, Lipemic, Paraproteinemic, or Uremic) serum samples compared with indirect determination with ion‐selective electrodes. Clin Chem. 1994;40:1528‐1531. [PubMed] [Google Scholar]
- 19. Berry MN, Mazzachi RD, Pejakovlc M, et al. Enzymatic Determination of Sodium in Serum. Clin Chem. 1988;34:2295‐2298. [PubMed] [Google Scholar]
- 20. Quiles R, Fernández‐Romero JM, Fernández E, et al. Automated enzymatic determination of sodium in serum. Clin Chem. 1993;39(3):500‐503. [PubMed] [Google Scholar]
- 21. Labtest Diagnóstica SA . Manual do Usuário Labmax 560. 2011. 405p.
- 22. Albert V, Subramanian A, Rangarajan K, et al. Agreement of two different laboratory methods used to measure electrolytes. J Lab Physicians. 2011;3:104‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leite F (ed.). Validação em analise química. 4th edn. Campinas, Brazil: Ed. Átomo; 2002. [Google Scholar]
- 24. Pfortmueller CA, Funk G‐C, Leichtle AB, et al. Electrolyte disorders and in‐hospital mortality during prolonged heat periods: a cross‐sectional analysis. Seguro AC, PLoS ONE. 2014;9:e92150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldwasser P, Ayoub I, Barth RH. Pseudohypernatremia and pseudohyponatremia: a linear correction. Nephrol Dial Transplant. 2014;30:252‐257. [DOI] [PubMed] [Google Scholar]
- 26. Langhoff E, Stelness LB. Potentiometric Analysis for Sodium and Potassium in Biological Fluids. Clin Chem. 1982;28:170‐172. [PubMed] [Google Scholar]
- 27. Yu RJ, Ma W, Liu XY, et al. Metal‐linked immunosorbent assay (MeLISA): the enzyme‐free alternative to ELISA for biomarker detection in serum. Theranostics. 2016;6:1732‐1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fernández‐Romero JM, Luque de Castro MD, Quiles RZ. Spectrofluorimetric flow‐injection determination of potassium in serum based on enzyme activation. Anal Chem A. 1995;308:178‐186. [Google Scholar]
- 29. Koch DD, Parrish D, Ladenson JH. Evaluation of a direct potentiometric method for sodium and potassium used in the Du Pont aca. Clin Chem. 1983;29:1090‐1092. [PubMed] [Google Scholar]
- 30. Zhao LJ, Qian RC, Ma W, et al. Electrocatalytic efficiency analysis of catechol molecules for NADH oxidation during nanoparticle collision. Anal Chem. 2016;88:8375‐8379. [DOI] [PubMed] [Google Scholar]
- 31. Ying YL, Hu YX, Gao R, et al. Asymmetric nanopore electrode‐based amplification for electron transfer imaging in live cells. J Am Chem Soc. 2018;140:5385‐5392. [DOI] [PubMed] [Google Scholar]


