Abstract
Background
Iodiated contrast‐induced nephropathy (CIN) is a serious complication of contrast‐enhanced imaging. The aim of this study was to evaluate the diagnostic sensitivities and specificities of serum cystatin C (sCys C) and serum creatinine (sCr) for CIN and to further investigate difference of the incidence, risk factors, and in‐hospital and 3‐month prognosis of CIN according to sCys C criteria and sCr criteria.
Methods
We prospectively evaluated 213 patients who underwent angiography. The sCr and sCys C concentrations were detected before and at 48 hours, 72 hours after the procedure. The incidence, risk factors, and in‐hospital and 3‐month prognosis of CIN were analyzed. Receiver operating characteristic curve (ROC) analysis was performed for sCr and sCys C 48 hours after procedure.
Results
The incidence of CIN was 24.4% (sCys C criteria) and 8% (sCr criteria). Diabetes mellitus, dehydration, and hypoalbuminemia were independent risk factors for CIN. Area under the ROC of sCys C 48 hours after procedure was not superior to sCr (0.715 vs 0.790, P=.178). The mortality of patients with CIN in sCr criteria increased significantly (P<.05).
Conclusion
In this study, the incidence and risk factors of CIN were related to diagnostic criteria. The sCys C was not superior to sCr for predicting CIN in the patients who underwent angiography.
Keywords: acute kidney injury, contrast media, contrast‐induced nephropathy, cystatin C, risk factors
1. Introduction
In recent years, with the rapid development of medical imaging technology and interventional radiology, contrast agents have been used more and more widely. Acute kidney injury (AKI) induced by contrast media which is known as contrast‐induced nephropathy (CIN) has become the third most common cause of hospital‐acquired AKI.1 CIN is related with prolonged hospitalization and higher mortality.2 In that case, only timely diagnosis can help us deal with CIN more effectively in the early stage, and at the same time, avoid overtreatment of non‐CIN patients.
Contrast‐induced nephropathy is diagnosed as the dynamic changes in serum creatinine (sCr) after exposure to iodine contrast media. One of the most common accepted definitions was formulated by European Society of Urogenital Radiology (ESUR).3 However, sCr is not an ideal glomerular filtration rate (GFR) biomarker because it can be affected by a large number of nonrenal factors such as muscle mass, age, race, gender, and nutritional status.
Another serum marker investigated for evaluating renal function is cystatin C. Serum cystatin C (sCys C) is freely filtered by the glomerulus and is then reabsorbed completely.4 Compared with sCr, sCys C concentration is less affected by muscle mass, age, gender, or nutritional status and therefore more reliably predict deterioration of renal function.4, 5, 6 sCys C was used as a reliable biomarker for the early diagnosis and prognosis of CIN.6, 7, 8, 9 But Ribichini et al.10 reported that variations from the sCr baseline offer better diagnostic accuracy for predicting CIN at an earlier stage than similar variations in sCys C. Therefore, whether sCys C is superior to sCr in diagnosis of CIN is still controversial.
The aim of our study was to determine the diagnostic sensitivities and specificities of sCr and sCys C for CIN and to further investigate difference of the incidence, risk factors, and in‐hospital and 3‐month prognosis of CIN according to sCr criteria and sCys C criteria in patients who underwent angiography.
2. Methods
2.1. Patients
Two hundred and thirteen patients who were older than 14 years underwent angiography from April 2011 to October 2011 in Second Xiangya Hospital of Central South University were enrolled in this prospective study. Patients with allergy history, acute kidney injury, cardiogenic shock, administration of nephrotoxic drugs, and in the pregnancy were excluded. The study was approved by the ethics committee of our institution, and all participating patients provided written informed consent and follow‐up treatment was provided to all patients.
2.2. Study protocol
All patients received nonionic contrast agent (Ioversol, 320 mg iodine/mL, Jiangsu Hengrui Pharmaceutical Co., Ltd, Jiangsu, China) and fluid intake of at least 2 L during the 24 hours before angiography. No additional medical prophylaxis was applied.
The sCr and sCys C concentrations were measured before angiography and at 48 hours, and 72 hours after angiography. Three months after the procedure, urinary sediment and sCr level were detected to evaluate the incidence of chronic kidney disease (CKD). All indices were detected in a single hospital‐based laboratory using consistent methodology. The estimated GFR was calculated according to equation: 175 × sCr−1.234 × age−0.179 × 0.79 (if female).11
2.3. Analytical methods
sCys C was measured by colloidal gold particle‐enhanced colorimetric immunoassay, and centration of sCys C and sCr was determined by different automatic biochemical analyzer (Hitachi 7600‐20 and 7170A, Tokyo, Japan, respectively).
2.4. Outcome and definition
The primary outcome for this study was the occurrence of CIN, which was defined as ESUR definition11 or as a rise in concentration of Cys C by more than 25% from preprocedure value within 3 days (Cys C definition).
Secondary outcomes were composite occurrence of all‐cause death and CKD in 3 months after the procedure.
Chronic kidney disease is diagnosed as abnormalities of kidney function or structure and present for >3 months.12 We used a BUN/Scr ratio of more than 20 as the cutoff point for dehydration.13
2.5. Statistical analysis
Continuous data were expressed as means±SD. Categorical variables are presented as absolute numbers and percentages. Comparison of continuous variables was analyzed using paired t tests and the Student's t test. Categorical variables were analyzed with Pearson's chi‐squared test, and a two‐sided 95% confidence interval (CI) was constructed around the point estimate of the odds ratio (OR). A multivariate logistic regression analysis was used to determine independent risk factors of CIN. The receiver operating characteristic curve (ROC) was depicted, and the area under the curve was calculated to plot the sensitivity and specificity of sCr and Cys C. Statistical significance was defined as P<.05.
3. Results
3.1. Clinical basic characteristics
A total of 213 patients (164 male, 49 female, mean age 52.07±14.52 years, ranging from 14 to 87 years, mean weight 60.76±9.52 kg) between April 2011 and October 2011 who underwent angiography were enrolled in this study. The basic characteristics of the patients were as follows: 24 patients more than 70 years old; 107 patients of smoking; 45 patients with hypertension; 22 patients of hypercholesterolemia; 24 patients of diabetes mellitus; 57 patients with hypoalbuminemia; 13 patients with dehydration; 24 patients with chronic kidney disease, which contained stage 1 CKD (nine cases), stage 2 CKD (seven cases), stage 3a CKD (four cases), stage 3b CKD (two cases), and stage 4 CKD (two cases).
3.2. The occurrence of CIN
According to sCr criteria, CIN occurred in 12 patients (5.6%) at 48 hours and 17 patients (8.0%) at 72 hours after contrast media administration. While according to sCys C criteria, CIN occurred in 31 patients (14.6%) at 48 hours and 52 patients (24.4%) at 72 hours after contrast exposure.
3.3. Risk factors analysis for CIN
The baseline characteristics of patients are shown in Tables 1 and 2. Differences of risk factors were found in the indicators including hemoglobin concentration, hypoalbuminemia (Cys C criteria), diabetes mellitus, and dehydration state (sCr criteria) by single‐factor analysis of variance between the CIN and non‐CIN patients. The multivariate logistic regression analysis revealed that dehydration state was the strongest risk factor for CIN, followed by diabetes mellitus and hypoalbuminemia (Table 3).
Table 1.
Baseline characteristics of the patients with CIN vs without CIN (Cys C criteria)
| Characteristic | CIN (N=52) | Non‐CIN (N=161) | P |
|---|---|---|---|
| Age (y) | 50.04±13.16 | 52.73±14.92 | .247 |
| Age >70 y, n (%) | 3 (5.8) | 21 (13.0) | .149 |
| Male, n (%) | 39 (75.0) | 125 (77.6) | .694 |
| Smoke habit, n (%) | 31 (59.6) | 76 (47.2) | .120 |
| Weight (kg) | 60.48±8.82 | 60.85±9.76 | .808 |
| Hemoglobin concentration (g/L) | 119.96±22.97 | 127.36±21.26 | .034 |
| Baseline serum creatinine (μmol/L) | 76.30±35.93 | 71.94±20.41 | .408 |
| Chronic kidney disease, n (%) | 6 (11.5) | 18 (11.2) | .943 |
| Diabetes mellitus, n (%) | 4 (7.7) | 20 (12.4) | .348 |
| Hypertension, n (%) | 8 (15.4) | 37 (23.0) | .243 |
| Hypercholesterolemia, n (%) | 7 (13.5) | 15 (9.3) | .393 |
| Hypoalbuminemia, n (%) | 22 (42.3) | 35 (21.7) | .004 |
| Dehydration state, n (%) | 6 (11.5) | 7 (4.3) | .090 |
| Baseline eGFR (mL/min/1.73 m²) | 98.63±23.76 | 99.26±20.31 | .853 |
| Baseline eGFR <90 (mL/min/1.73 m²) | 14 (26.9) | 48 (29.8) | .690 |
| Baseline serum CysC level (mg/L) | 1.65±0.73 | 1.74±0.63 | .389 |
| Contrast media volume (mL) | 69.00±55.15 | 58.64±46.00 | .181 |
| Multiple CM administrations within 2 wk, n (%) | 28 (53.8) | 92 (57.1) | .677 |
| In‐hospital stay length (d) | 13.71±7.63 | 12.94±5.65 | .503 |
CIN, contrast‐induced nephropathy; eGFR, estimated glomerular filtration rate; CysC, cystatin C; CM, contrast media.
Table 2.
Baseline characteristics of the patients with CIN vs without CIN (sCr criteria)
| Characteristic | CIN (N=17) | Non‐CIN (N=196) | P |
|---|---|---|---|
| Age (y) | 54.65±19.85 | 51.85± 14.01 | .576 |
| Age >70 y, n (%) | 4 (23.5) | 20 (10.2) | .108 |
| Male, n (%) | 14 (82.4) | 150 (76.5) | .768 |
| Smoke habit, n (%) | 7 (41.2) | 100 (51.0) | .436 |
| Weight (kg) | 62.79±9.92 | 60.58±9.49 | .211 |
| Hemoglobin concentration (g/L) | 128.53±22.56 | 125.30±21.85 | .560 |
| Baseline serum creatinine (μmol/L) | 74.96±19.22 | 72.84±25.55 | .738 |
| Chronic kidney disease, n (%) | 4 (23.5) | 20 (10.2) | .108 |
| Diabetes mellitus, n (%) | 7 (41.2) | 17 (8.7) | .001 |
| Hypertension, n (%) | 5 (29.4) | 40 (20.4) | .365 |
| Hypercholesterolemia, n (%) | 5 (29.4) | 17 (8.7) | .020 |
| Hypoalbuminemia, n (%) | 5 (29.4) | 52 (26.5) | .780 |
| Dehydration state, n (%) | 5 (29.4) | 8 (4.1) | .002 |
| Baseline eGFR (mL/min/1.73 m²) | 96.71±20.95 | 99.31±21.20 | .628 |
| Baseline eGFR <90 (mL/min/1.73 m²) | 8 (47.1) | 54 (27.6) | .100 |
| Baseline serum CysC level (mg/L) | 1.83±0.66 | 1.71±0.66 | .459 |
| Contrast media volume (mL) | 50.35±23.81 | 62.11±9.96 | .339 |
| Multiple CM administrations within 2 wk, n (%) | 7 (41.2) | 113 (57.7) | .189 |
| In‐hospital stay length (d) | 16.41±9.61 | 12.84±5.74 | .150 |
CIN, contrast‐induced nephropathy; eGFR, estimated glomerular filtration rate; CysC, cystatin C; CM, contrast media.
Table 3.
Variables related to the risk of CIN based on the multivariate logistic regression analysis
| Variable | OR | 95% CI | P‐value |
|---|---|---|---|
| Serum CysC criteria | |||
| Hypoalbuminemia | 2.640 | 1.357‐5.136 | .004 |
| sCr criteria | |||
| Diabetes mellitus | 5.450 | 1.707‐17.393 | .004 |
| Dehydration state | 6.510 | 1.662‐25.503 | .007 |
CIN, contrast‐induced nephropathy; OR, odds ratio; CI, confidence interval; CysC, cystatin C; sCr, serum creatinine.
3.4. Changes of renal function parameters after contrast media administration
sCys C and sCr significantly increased; eGFR level decreased 48 and 72 hours after contrast media administration in patients with CIN (P<.05). No significant difference in sCys C, sCr, and eGFR levels was observed 48 and 72 hours after contrast media administration in patients without CIN (P>.05; Table 4).
Table 4.
Time‐course changes in serum creatinine, CysC, and eGFR in the two groups
| Variable | CIN (n=17) | P‐value | Non‐CIN (n=196) | P‐value |
|---|---|---|---|---|
| sCr (μmol/L) | ||||
| Baseline | 74.96±19.22 | 72.84±25.55 | ||
| 48 h after procedure | 110.11±43.09a | .000 | 72.53±28.94 | .691 |
| 72 h after procedure | 113.26±55.97a | .008 | 72.78±33.42 | .954 |
| sCysC (mg/L) | ||||
| Baseline | 1.83±0.66 | 1.71±0.66 | ||
| 48 h after procedure | 2.43±1.26a | .015 | 1.71±0.68 | .899 |
| 72 h after procedure | 2.81±2.04a | .045 | 1.76±0.72 | .155 |
| eGFR (mL/min/1.73 m²) | ||||
| Baseline | 96.71±20.95 | 99.31±21.20 | ||
| 48 h after procedure | 71.74±29.58a | .000 | 100.14±24.03 | .184 |
| 72 h after procedure | 73.33±30.3a | .001 | 100.38±24.79 | .114 |
P<.05 as compared with baseline values.
CIN, contrast‐induced nephropathy; eGFR, estimated glomerular filtration rate; Cys C, cystatin C.
3.5. Characteristics of sCr and Cys C as diagnostic markers
Receiver operating characteristic curve analysis showed that the AUC for sCr and sCys C at 48 hours was 0.790 (P<.05, 95% CI=0.655‐0.924) and 0.715 (P<.05, 95% CI=0.586‐0.843), respectively, which were not significantly different (P=.178). When the cutoff values of sCr and sCys C were set at 90.4 μmol/L and 1.605 mg/L, respectively, sensitivity and specificity of the two markers used for the diagnosis were 70.6%, 88.2%, 92.3%, and 53.1%, respectively (Figure 1).
Figure 1.
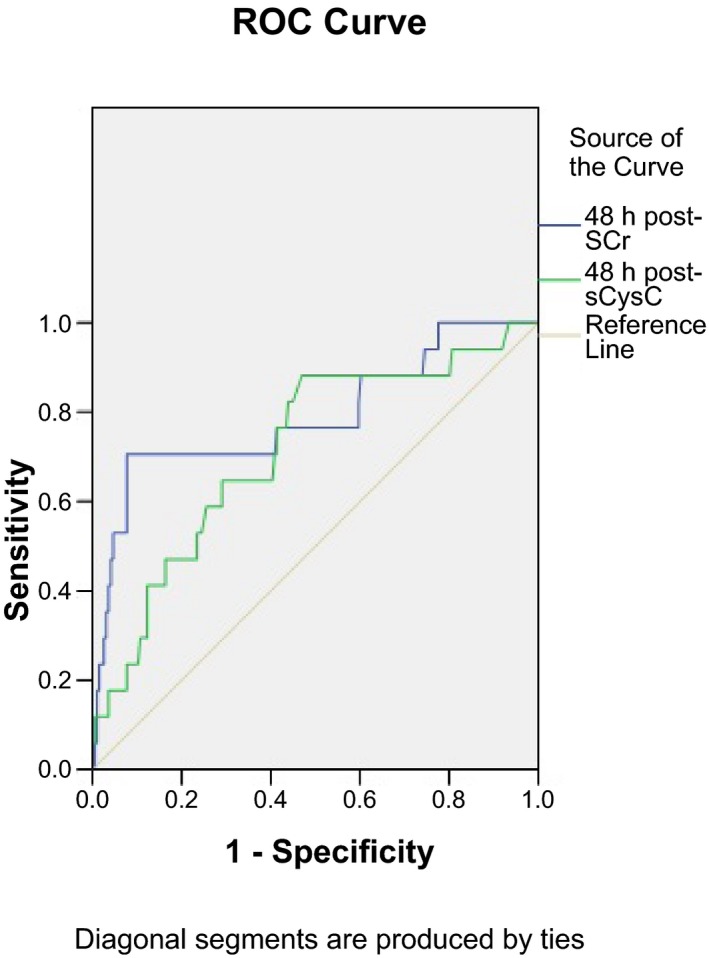
Receiver operating characteristic (ROC) curves showing the different diagnostic sensitivities and specificities for diagnosis of contrast‐induced nephropathy (CIN) of serum creatinine and serum Cys C at 48 h after procedure
3.6. Hospital and 3‐month outcomes
As shown in Table 5, there was no patient required renal replacement therapy nor died after the procedure in hospital. No patient progressed to CKD or end‐stage renal disease in 3 months after the procedure. During the 3‐month follow‐up period, eight patients died. The mortality of CIN group was significantly higher than non‐CIN group according to the sCr criteria (P<.05).
Table 5.
Major adverse events within 3 months in the two groups
| Clinical outcomes | Cys C criteria | P | sCr criteria | P | ||
|---|---|---|---|---|---|---|
| CIN (N=52) | Non‐CIN (N=161) | CIN (N=17) | Non‐CIN (N=196) | |||
| Renal replacement therapy, n (%) | 0 | 0 | 0 | 0 | ||
| In‐hospital death, n (%) | 0 | 0 | 0 | 0 | ||
| 3‐mo death, n (%) | 4 (7.7) | 4 (2.5) | .102 | 6 (35.3) | 2 (1.0) | .000 |
| Progressed to CKD/ESRD, n (%) | 0 | 0 | 0 | 0 | ||
CKD, chronic kidney disease; ESRD, end‐stage renal disease.
4. Discussion
In the present study, we found that the sCys C was not superior to sCr for predicting CIN in patients who underwent angiography. The incidence and risk factors of CIN were associated with the chosen definitions. The sCr criteria seemed more suitable for diagnosis of CIN in patients who underwent angiography.
Contrast‐induced nephropathy is a serious complication of contrast‐enhanced imaging. The incidence of CIN was reported more than 50% after angiography in patients with renal insufficiency and additional risk factors.3, 14 This study found that the CIN incidence was 24.4% (sCys C criteria) and 8.0% (sCr criteria). It suggested that the incidence of CIN varied depending on the different chosen definitions. It correlates with the literatures.6, 15, 16
The occurrence of CIN is associated with risk factors. The updated ESUR guidelines indicated that risk factors of CIN included CKD, diabetes mellitus, dehydration, age >70 years, use of nephrotoxic drugs, hyperosmotic contrast medium, and high dose of contrast agent, etc.3 Because of the differences in the selection of study population, the definition of CIN, etc., risk factors of CIN reported by the literature were different.13, 17, 18, 19 Our study showed that dehydration state, diabetes mellitus (sCr criteria), and hypoalbuminemia (Cys C criteria) were the strongest predictors at risk of CIN. It further suggested that there was a certain relationship between risk factors of CIN and definition, or standard as well as the selected objects.
Serum creatinine was routinely used to provide useful information for renal injury in clinical practice. However, it is not an ideal indicator to evaluate renal function because of following limitations.6, 20 For example, creatinine excreted in the urine is not solely a result of GFR. Serum creatinine concentration may not change until a significant amount of kidney function is lost. sCys C was highlighted to be a superior biomarker for early‐stage renal injury in comparison with creatinine.4, 5, 20, 21, 22 Furthermore, Briguori et al.6 and Rickli et al.7 studies implied that Cys C might be a reliable marker for the early diagnosis of CIN. Wacker‐Gußmann et al.8 reported that CIN could be independently predicted by sCys C, whereas sCr was not predictive. In another research performed by Ebru et al.9 also indicated that sCysC was a sensitive indicator of CIN. However, diagnostic sensitivity and specificity for sCys C in detecting CIN were not analyzed in these studies. Recently, Ribichini et al.10 revealed that variations from the sCr baseline offer better diagnostic accuracy for predicting CIN at 12 hours than similar variations in sCys C in 166 patients with risk characteristics for developing CIN undergoing coronary and interventions. So whether sCys C is an ideal predictor for CIN than sCr is still disputable. Our study found that sCys C and sCr significantly increased, and eGFR level decreased 48 and 72 hours after contrast media administration in patients with CIN. However, there was no significant difference between the AUC for sCr and sCys C at 48 hours. It further supported that the sCys C is not superior to sCr at 48 hours for predicting CIN in patients who underwent angiography. The reasons for the different diagnostic value of sCys C and sCr in CIN patients may be related to case selection, sample sizes, definitions of AKI used, end point of study design, experimental methods, skill level of inspectors, laboratory equipment, and other factors.
Contrast‐induced nephropathy not only is a common iatrogenic renal damage after the use of intravascular contrast agent, but also is regarded as a concern existing among the departments of nephrology, cardiology, and radiology. CIN often is transient, but it once happens, resulting in the time of hospitalization prolonged, the costs increased, in‐hospital adverse events and a risk of death increased, as well as 1‐ and 5‐year mortality significantly increased.23, 24 Marenzi et al.25 study showed that the short‐term and long‐term mortality for patients with CIN significantly increased in patients undergoing percutaneous coronary intervention (PCI); CIN is an independent predictor for risk of death after PCI. Wysowski et al.26 reported that CIN is main reason why the contrast agent related to death. In this study, after 3‐month follow‐up, the mortality of patients with CIN increased significantly, which suggested that CIN was one of reasons of death for the patients who underwent angiography within 3 months.
Our study still has some limitations. Firstly, it was single‐center study included a small number of patients and the lack of a control group. Secondly, we did not compare the diagnostic value of Cys C and sCr criteria at an earlier 12 or 24 hours time‐point. Thirdly, we did not further study whether the change of sCr or sCys C had a correlation with the long‐term prognosis, such as 1‐year prognosis or not. Therefore, multicenter trials are still necessary to evaluate the long‐term prognosis of CIN and the role of sCr, sCys C, and other biomarkers in early diagnosis of CIN. Meanwhile, in order to provide a powerful tool for clinicians to monitor acute kidney injury in high‐risk patients, we will select more homogeneous patients and further evaluate the sensitivity and specificity of sCys C and sCr 12, 24, or 48 hours after contrast media administration in the future.
In conclusion, our study suggested that the incidence and risk factors of CIN was related to diagnostic criteria. The sCys C was not superior to sCr for predicting CIN in patients who underwent angiography.
Acknowledgments
The work was supported by the National Science Foundation of China. (81570618), the Scientific Foundation of Hunan Province, China (2010FJ6008).
References
- 1. Au TH, Bruckner A, Mohiuddin SM, et al. The prevention of contrast‐induced nephropathy. Ann Pharmacother. 2014;10:1332–1342. [DOI] [PubMed] [Google Scholar]
- 2. Banda J, Duarte R, Dickens C, et al. Risk factors and outcomes of contrast‐induced nephropathy in hospitalised South Africans. S Afr Med J. 2016;106:699–703. [DOI] [PubMed] [Google Scholar]
- 3. Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;12:2527–2541. [DOI] [PubMed] [Google Scholar]
- 4. Onopiuk A, Tokarzewicz A, Gorodkiewicz E. Cystatin C: a kidney function biomarker. Adv Clin Chem. 2015;68:57–69. [DOI] [PubMed] [Google Scholar]
- 5. Ahlström A, Tallgren M, Peltonen S, Pettilä V. Evolution and predictive power of serum cystatin C in acute renal failure. Clin Nephrol. 2004;5:344–350. [DOI] [PubMed] [Google Scholar]
- 6. Briguori C, Visconti G, Rivera NV, et al. Cystatin C and contrast‐induced acute kidney injury. Circulation. 2010;19:2117–2122. [DOI] [PubMed] [Google Scholar]
- 7. Rickli H, Benou K, Ammann P, et al. Time course of serial cystatin C levels in comparison with serum creatinine after application of radiocontrast media. Clin Nephrol. 2004;2:98–102. [DOI] [PubMed] [Google Scholar]
- 8. Wacker‐Gußmann A, Bühren K, Schultheiss C, et al. Prediction of contrast‐induced nephropathy in patients with serum creatinine levels in the upper normal range by cystatin C: a prospective study in 374 Patients. Am J Roentgenol. 2014;2:452–458. [DOI] [PubMed] [Google Scholar]
- 9. Ebru AE, Kilic A, Korkmaz FS, et al. Is cystatin‐C superior to creatinine in the early diagnosis of contrast‐induced nephropathy?: a potential new biomarker for an old complication. J Postgrad Med. 2014;2:135–140. [DOI] [PubMed] [Google Scholar]
- 10. Ribichini F, Gambaro G, Graziani MS, et al. Comparison of serum creatinine and cystatin C for early diagnosis of contrast‐induced nephropathy after coronary patients undergoing angiography and interventions. Clin Chem. 2012;2:458–464. [DOI] [PubMed] [Google Scholar]
- 11. Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;10:2937–2944. [DOI] [PubMed] [Google Scholar]
- 12. Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;11:825–830. [DOI] [PubMed] [Google Scholar]
- 13. Pakfetrat M, Nikoo MH, Malekmakan L, et al. Comparison of risk factors for contrast induced acute kidney injury between patients with and without diabetes. Hemodial Int. 2010;4:387–389. [DOI] [PubMed] [Google Scholar]
- 14. Duan SB, Liu FY, Peng YM, et al. A prospective study of contrast nephrotoxicity in patients with chronic renal failure. J Nephrol Dial Transplant. 2001;2:130–132. [Google Scholar]
- 15. Lakhal K, Ehrmann S, Chaari A, et al. Acute kidney injury network definition of contrast‐induced nephropathy in the critically ill: incidence and outcome. J Crit Care. 2011;6:593–599. [DOI] [PubMed] [Google Scholar]
- 16. Centola M, Lucreziotti S, Salerno‐Uriarte D, et al. A comparison between two different definitions of contrast‐induced acute kidney injury in patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol. 2016;210:4–9. [DOI] [PubMed] [Google Scholar]
- 17. Rear R, Bell RM, Hausenloy DJ. Contrast‐induced nephropathy following angiography and cardiac interventions. Heart. 2016;102:638–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Banda J, Duarte R, Dickens C, et al. Risk factors and outcomes of contrast‐induced nephropathy in hospitalised South Africans. S Afr Med J. 2016;7:699–703. [DOI] [PubMed] [Google Scholar]
- 19. Caruso M, Balasus F, Incalcaterra E, et al. Contrast‐induced nephropathy after percutaneous coronary intervention in simple lesions: risk factors and incidence are affected by the definition utilized. Intern Med. 2011;9:983–989. [DOI] [PubMed] [Google Scholar]
- 20. Duan SB, Liu GL, Yu ZQ, Pan P. Urinary KIM‐1, IL‐18 and Cys‐c as early predictive biomarkers in gadolinium‐based contrast‐induced nephropathy in the elderly patients. Clin Nephrol. 2013;11:349–354. [DOI] [PubMed] [Google Scholar]
- 21. Wang M, Zhang L, Yue R, You G, Zeng R. Significance of cystatin C for early diagnosis of contrast‐induced nephropathy in patients undergoing coronary angiography. Med Sci Monit. 2016;22:2956–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cho SY, Hahn WH, Lee HJ, et al. The clinical significance of serum cystatin C in critically ill newborns with normal serum creatinine. J Clin Lab Anal. 2012;26:267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;5:368–375. [DOI] [PubMed] [Google Scholar]
- 24. Weisbord SD, Chen H, Stone RA, et al. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary patients undergoing angiography. J Am Soc Nephrol. 2006;10:2871–2877. [DOI] [PubMed] [Google Scholar]
- 25. Marenzi G, Lauri G, Assanelli E, et al. Contrast‐induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;9:1780–1785. [DOI] [PubMed] [Google Scholar]
- 26. Wysowski DK, Nourjah P. Deaths attributed to X‐ray contrast media on U.S. death certificates. Am J Roentgenol. 2006;3:613–615. [DOI] [PubMed] [Google Scholar]


