Abstract
Background
Rapid and practical point‐of‐care testing (POCT) devices become more popular, especially in blood donation centers for determining predonation hemoglobin (Hb) concentrations. The purpose of this study was to evaluate accordance between the POCT methods and the venous method as the reference to Hb screening.
Methods
A total of 353 subjects with no known significant health problems were included in the study. Hb screening was performed by two different POCT methods, a noninvasive method (Haemospect, MBR, Germany) and an invasive method (HemoControl, EKF Diagnostic, Germany), and a venous method as the reference (Sysmex XE‐2100, Sysmex Europe, Germany). The obtained results were compared.
Results
The sensitivity and the specificity values of the invasive POCT method (83.3%, 87.9%) were higher than the noninvasive POCT method (66.7%, 77.1%). The Bland‐Altman analysis was evaluated for both sexes and the bias of the noninvasive POCT method of the males (−0.97 g/dL) was higher than the bias of the invasive POCT method of the males (−0.07 g/dL). We found a better correlation between the invasive POCT method (r = .908) compared with the venous method than the noninvasive POCT method (r = .634).
Conclusion
Predonation Hb measurements must be performed with accurate, precise, and practical methods. Although the noninvasive POCT method was practical and painless, it had lower levels of specificity and sensitivity, and more false deferral and pass rates than the invasive POCT method. The POCT methods agreeable to the venous method as the reference might be suitable for Hb screening especially for centers of excessive numbers of blood donation.
Keywords: anemia, blood donation, blood donor questionnaire, hemoglobin screening, point‐of‐care testing
1. INTRODUCTION
Blood donation is a voluntary procedure. Donation of blood and blood components, as well as donor eligibility needs to be determined and evaluated by a physical assessment, a health history determination and hemoglobin (Hb) measurement before blood donation.1, 2, 3 In Turkey, there are about 2.7 million blood donation requirements per year.4 About 2.1 million blood donations are provided by the Turkish Red Crescent and 600 000 blood donations are provided by local stations in the universities. Blood donation requirements in Turkey are a physical determination, conduction of the Turkish Red Crescent Blood Donor Questionnaire,5 and Hb measurement with point‐of‐care testing (POCT) for eligible donors. The Hb levels for donor eligibility are more than 13.5 g/dL for males and more than 12.5 g/dL for females.
The determination of hemoglobin levels prior to donation is usually performed through the invasive capillary POCT method. It is rapid and cheap, but painful and has a risk of infection for donors. The gold standard for hemoglobin measurement is performed with the automated cell analyzer. However, it is time consuming and risky for health care personnel because of the needles. Consequently, noninvasive POCT devices which are user‐friendly and that provide donor safety are developed, which skips a painful step like pricking the finger or venipuncture.
Owing to being a country with high blood donation numbers, evaluation of the suitability and accuracy of the POCT devices is essential. Therefore, we aimed to compare the POCT devices with the reference method of Hb measurement as part of the blood donor screening process of this study.
2. MATERIALS AND METHODS
2.1. Study protocol
Participants of this study consisted of voluntary individuals with no known significant health problems and considered themselves as healthy individuals who were admitted to the Ankara Atatürk Training and Research Hospital for health examinations. Subjects who met the eligibility requirements of blood donation according to the Turkish Red Crescent Blood Donor Questionnaire were included in the present study.5 In our country, prior to donation to the Turkish Red Crescent, the Blood Donor Questionnaire is conducted and the Hb levels of individuals who are in good health, who are between 16 and 65 years old, who weigh at least 50 kg and pass the physical and health history assessments, are measured with a POCT device. To be an eligible blood donor, obtained Hb values must be between 12.5 and 16.5 g/dL for females and between 13.5 and 18 g/dL for males according to the Turkish Red Crescent Blood Donor Criteria.5 This study was approved by the Ethics Committee for Human Studies of Ankara Atatürk Training and Research Hospital. All patients gave their informed and written consent to participate. The study conformed to the Declaration of Helsinki.
In this study, the Questionnaire was conducted on the subjects with no known significant health problems and complete blood count (CBC), biochemistry, and hormone test requests. With the Questionnaire, the voluntary individuals were questioned about the presence of hepatitis B and C, HIV, cardiovascular diseases, rheumatic diseases, diabetes mellitus, genetic diseases, cancer, hepatitis B carriage, smoking, and pregnancy status. Of eligible individuals for blood donation according to the Questionnaire, Hb levels were analyzed with a noninvasive POCT method (Haemospect, MBR Optical Systems GmbH& Co. KG, Wuppertal, Germany), an invasive POCT method (HemoControl, EKF Diagnostic GmbH, Barleben, Germany), and a venous method (Sysmex XE‐2100, Sysmex Europe GmbH, Norderstedt, Germany), respectively. During measurements of POCT devices, venous results of the patients were not known. After the Hb screening, routine biochemistry and hormone test results were examined, individuals with lipid profile changes, increased levels of HbA1C, impaired liver, thyroid, and renal function tests were excluded from the study.
2.2. Noninvasive POCT method
The noninvasive POCT method runs on battery power based on transcutaneous reflection spectroscopy.6 The palm side of the finger of the right hand is attached to button sensors of the device. A sensor head placed on the skin reflects a white light into the underlying tissue (0.5‐0.9 mm) over a waveguide. The tissue components absorb some projected light, but some of it is reflected back on the device. Eventually, the spectrometer breaks the light down into its divided wavelengths and an electronic measurement unit tests it. The quantitative value is represented on the device. The measuring range of the noninvasive POCT method is between 9 and 18 g/dL.7
2.3. Invasive POCT method
The measurement of Hb concentration of the invasive POCT method device is based on the Vanzetti′s azide methemoglobin method. Capillary measurements were implemented with disposable cuvettes from the middle finger of the left hand. The fingertip was cleaned with an alcohol cotton and pricked safely with a lancet. The first three drops were removed with a cotton wool swab and the cuvette was filled with the subsequent drop that was 8‐10 μL, as suggested by the manufacturer. The cuvette was affixed to the invasive POCT method device and photometrically analyzed at 570 nm within 25‐60 seconds. The measuring range of the invasive POCT method is between 0 and 25.6 g/dL.8
2.4. Venous method
A venous sample was taken for CBC evaluation with the automated blood cell counter (Sysmex XE‐2100). The sodium lauryl sulfate (SLS)‐Hb method is used for the measurement of Hb concentration of the venous method. Lipoproteins of the cell membrane of the red blood cells are dissolved with SLS to release Hb and are converted into SLS‐Hb. The measurement of the concentration of SLS‐Hb is performed as light absorbance at 555 nm.9, 10 The measuring range of the venous method is between 0 and 25 g/dL.
2.5. Precision study
All devices (Haemospect, HemoControl, and Sysmex XE‐2100) were well calibrated and controlled; manufacturers’ instructions were followed while performing the Hb measurement. The HemoControl was calibrated with the manufacturers’ calibration cuvette (Hb value was 15.6 g/dL) during the study period.
To investigate the precision of the venous method and the invasive POCT device, two levels of commercial quality control materials for each device were analyzed. For the noninvasive POCT method, two healthy volunteers (their Hb values were stable) who did not participate in the blood donation process were included in the analysis of within‐day and between‐day imprecision. The palm side of the fingers of these subjects was measured by the noninvasive POCT device. The within‐day and between‐day CVs were calculated. The within‐day CV's of the Haemospect, the HemoControl, and the Sysmex XE‐2100 were 7.08, 2.45, and 1.04 at 11.8, 6.3, and 7.9 g/dL concentrations; 3.2, 1.28, and 1.22 at 18.0, 13.0, and 13.9 g/dL concentrations, respectively. The between‐day CV's of the Haemospect, the HemoControl, and the Sysmex XE‐2100 were 4.37, 1.81, and 1.02 at 11.8, 6.3, and 5.8 g/dL concentrations; 4.42, 1.69, and 0.82 at 18.0, 13.0, and 12.4 g/dL concentrations, respectively.
The allowable total analytical error (TEa) was calculated using internal and external quality control results according to Ricos C. et al study11 for the venous method, served as a reference and the obtained 4.09% value was under the TEa limit of Hb (±7%).12 TEa of the POCT devices were not calculated because there were no external quality control materials for the POCT devices.
2.6. Statistical analysis
Descriptive statistics were calculated for the three methods using SPSS version 22 (Statistical Package for the Social Sciences, Chicago, IL, USA). Values are expressed as mean ± SD, with a P < .05 indicating significance. The distribution of the data was evaluated by a Kolmogorov‐Smirnov test. The biochemical/hematological parameters of the groups were compared using independent t test and Mann‐Whitney U test. Correlation analyses were performed using Pearson's correlation coefficient. The coherence with the reference method was obtained by plotting Bland‐Altman graphs using Medcalc software version 16.8 (MedCalc Software bvba, Ostend, Belgium). Sensitivity, specificity, negative and positive predictive values (NPV and PPV), and their confidence intervals were calculated using a MedCalc diagnostic test evaluation calculator.13
3. RESULTS
A total of 353 subjects (148 males and 205 females) were included in this study. The cases included in the study were in the range of 18‐65 years old with a mean age of 34.05 ± 11.09 years. They were tested for two consecutive trials by the noninvasive POCT method and the invasive POCT method. For all trials, the Hb levels were compared to the values obtained from the venous method of venous samples that served as a reference. The Hb values of the 11 subjects using the noninvasive POCT method were excluded because of the “invalid measurement” signals in the device (Hb values of these cases were between 8.7 and 13.3 g/dL using the venous method). The characteristics of subjects for noninvasive, invasive and venous Hb measurements are shown in Table 1. The average Hb measurements for males were, 14.61, 15.51, and 15.58 g/dL; for females, 12.71, 12.96, and 12.94 using the noninvasive POCT method, the invasive POCT method and the venous method, respectively (Table 1).
Table 1.
Characteristics of subjects for the noninvasive POCT method, the invasive POCT method, and the venous method
| Noninvasive POCT method | Invasive POCT method | Venous method | |
|---|---|---|---|
| Subjects (n) | 342 | 353 | 353 |
| Age (years) | 33.96 ± 10.97 | 34.05 ± 11.09 | 34.05 ± 11.09 |
| Total Hb (g/dL) | 13.53 ± 1.33 | 14.03 ± 1.92 | 14.05 ± 1.78 |
| Women (n) | 194 | 205 | 205 |
| Age (years) | 33.32 ± 10.46 | 33.52 ± 10.70 | 33.52 ± 10.70 |
| Total Hb (g/dL) | 12.71 ± 0.91 | 12.96 ± 1.44 | 12.94 ± 1.25 |
| Men (n) | 148 | 148 | 148 |
| Age (years) | 34.79 ± 11.59 | 34.79 ± 11.59 | 34.79 ± 11.59 |
| Total Hb (g/dL) | 14.61 ± 0.99 | 15.51 ± 1.46 | 15.58 ± 1.17 |
Values are mean ± SD.
The subjects were divided into two groups as eligible and ineligible. This was determined according to their venous Hb concentration and eligibility for blood donation. The biochemical/hematological parameters of the groups were compared as a result of statistical analysis and there was no statistically significant difference between the groups according to biochemical/hematological parameters of the groups (P > .05).
According to their Hb values, donors were divided into two groups as eligible for blood donation (ie ≥12.5 g/dL for female and ≥13.5 g/dL for male) and ineligible for blood donation (ie <12.5 g/dL for female and <13.5 g/dL for male). Based on these groups (3 ineligible donors with high Hb values were not included); sensitivity, specificity, and negative and positive predictive values (NPV and PPV) were calculated for each analyzer. Sensitivity shows that the percentage of donors classified as ineligible by the venous method were classified as ineligible by the test method too. Specificity shows that the percentage of donors classified as eligible by the venous method were determined as acceptable by the test method too. Comparing sensitivity, specificity, and negative and positive predictive values (NPV and PPV), the invasive POCT method was superior to the noninvasive POCT method for all results. But the noninvasive POCT method and the invasive POCT method had low PPV values: 51.28% and 68.63%, respectively. The percentage of donors that were determined falsely with the noninvasive and invasive POCT methods was 25.66% and 13.14%, respectively. Fifty‐seven of the volunteers were determined falsely as ineligible using the noninvasive POCT method due to its poor sensitivity. Thirty of the anemic volunteers were determined as eligible donors using the noninvasive POCT method. On the other hand, this number was only 14 using the invasive POCT method (Table 2).
Table 2.
Performance of the noninvasive and the invasive capillary Hb screening in comparison to the venous Hb measurement as a reference
| Eligible donors | Ineligible donors | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|---|---|
| Noninvasive POCT method | 249 | 90 | 66.67 (55.95‐76.26) | 77.11% (71.38‐82.18) | 51.28% (44.54‐57.98) | 86.49% (82.58‐89.62) |
| Pass | 192 | 30 | ||||
| Fail | 57 | 60 | ||||
| Invasive POCT method | 266 | 84 | 83.33% (73.62‐90.58) | 87.97% (83.44‐91.62) | 68.63% (60.92‐75.43) | 94.35% (91.18‐96.43) |
| Pass | 234 | 14 | ||||
| Fail | 32 | 70 |
Predonation screening using POCT methods and the venous method was shown in Table 3. “Rejected” shows the number of donors rejected by each POCT device. “Rejected correctly” shows the number of donors rejected by both the venous method and each POCT device. “Accepted correctly” shows the number of donors accepted by both the venous method and each POCT device. “Accordance” shows the percentage of donors classified correctly, eligible, and ineligible by the test method in comparison to the venous method. The accordance of eligible and ineligible donors between the POCT methods and the venous method was 66.37% for the noninvasive method and 81.71% for the invasive method. The noninvasive POCT method showed poor accordance with the venous method in total and female donors. Fifty‐two of the donors were determined correctly as the ineligible donors using the invasive POCT method, but the noninvasive POCT method determined only 33 donors. Ten of the volunteers were defined falsely as ineligible for both of the POCT devices that were defined as eligible donors with the venous method.
Table 3.
Predonation screening using the noninvasive and invasive POCT methods according to the venous reference value
| Donors | Noninvasive POCT method | Invasive POCT method |
|---|---|---|
| Total | ||
| Screened donors | 339 | 350 |
| Rejected by venous method | 61 | 66 |
| Rejected | 90 | 84 |
| Rejected correctly | 33 | 52 |
| Rejected falsely too low | 57 | 32 |
| Accepted correctly | 192 | 234 |
| Accepted falsely too high | 30 | 14 |
| Accordance (%) | 66.37 | 81.71 |
| Women | ||
| Screened donors | 193 | 204 |
| Rejected by venous method | 55 | 60 |
| Rejected | 74 | 66 |
| Rejected correctly | 27 | 48 |
| Rejected falsely too low | 46 | 18 |
| Accepted correctly | 92 | 126 |
| Accepted falsely too high | 28 | 12 |
| Accordance (%) | 61.65 | 85.29 |
| Men | ||
| Screened donors | 146 | 146 |
| Rejected by venous method | 6 | 6 |
| Rejected | 16 | 18 |
| Rejected correctly | 6 | 4 |
| Rejected falsely too low | 11 | 14 |
| Accepted correctly | 100 | 108 |
| Accepted falsely too high | 2 | 2 |
| Accordance (%) | 72.60 | 76.71 |
The agreement with results obtained by different methods was demonstrated with two different plots, according to Bland‐Altman. The invasive POCT method showed perfect agreement with the venous method owing to the bias (−0.02 g/dL, 95% CI = −1.59‐1.56) (Figure 1). But the noninvasive POCT method showed poor agreement with the venous method owing to the bias of −0.58 g/dL (95% CI = −3.28‐2.1). There was a negative relation between the average and the mean difference of the measurements of the noninvasive POCT method and the venous method of total donors (r = −.340, P < .001). The noninvasive POCT method showed higher bias (−0.97 g/dL, 95% CI = −3.59‐1.65) than the invasive POCT method (−0.07 g/dL, 95% CI = −1.92–1.77) for males (Figure 1). A scatter plot of the invasive POCT method vs the venous method showed a linear distribution, while the scatter plot of the noninvasive POCT method vs the venous method showed a wider distribution (Figure 2). The correlation between each POCT method and the venous method was compared. There was a higher correlation between the invasive POCT method (r = .908, P < .001) and the venous method than the noninvasive POCT method and the venous method (r = .634, P < .001).
Figure 1.
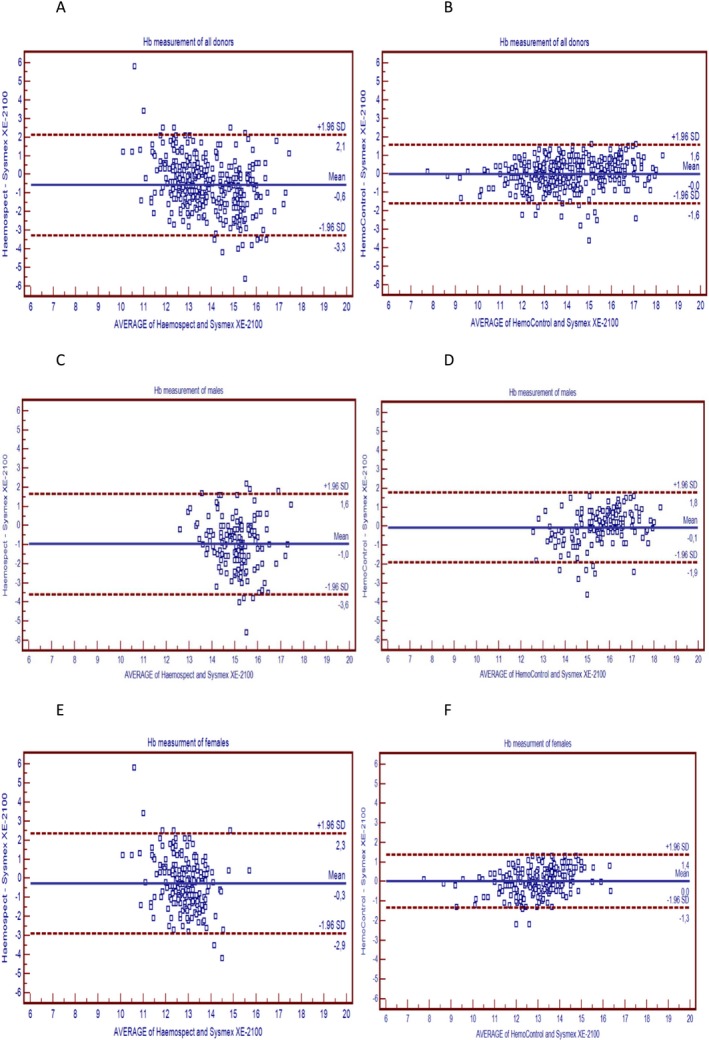
(A) Bland‐Altman plots of the difference between the Sysmex XE‐2100, the Haemospect for all donors. (B) Bland‐Altman plots of the difference between the Sysmex XE‐ 2100, the HemoControl for all donors. (C) Bland‐Altman plots of the difference between the Sysmex XE‐2100, the Haemospect for males. (D) Bland‐Altman plots of the difference between the Sysmex XE‐2100, the HemoControl for males. (E) Bland‐Altman plots of the difference between the Sysmex XE‐2100, the Haemospect for females. (F) Bland‐Altman plots of the difference between the Sysmex XE‐2100, the HemoControl for females.
Figure 2.
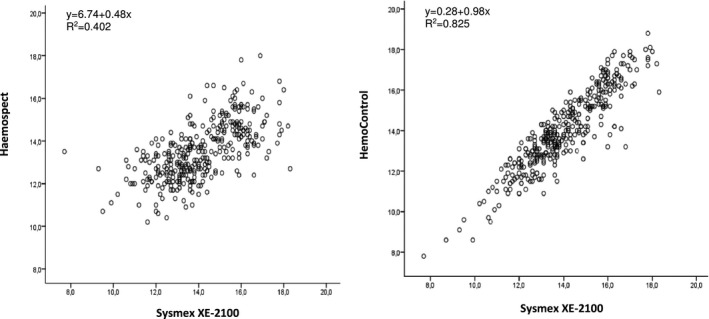
Scatter plots of the Haemospect and the HemoControl
4. DISCUSSION
In this study, measurements obtained from different devices for Hb screening were compared. To our knowledge, this is the first study in Turkey, where there are about 2.7 million blood donations per year and blood donations are provided mainly by the Turkish Red Crescent. The popularity of POCT methods is increasing because of it being easy‐to‐use and most blood donation centers prefer to use POCT devices instead of using the venous method as well as the Turkish Red Crescent. For this reason, it is important to determine the accuracy of POCT devices and the accordance between the POCT devices and the venous method. As distinct from the other studies, biochemical/hematological parameters of eligible and ineligible donor groups were also evaluated. According to our results, there was no difference between these two groups of these parameters (P > .05).
The ideal Hb screening method should be practical, portable, inexpensive, noninvasive, painless, user‐friendly; should offer high sensitivity, specificity, accuracy and precision, low false deferral rates, and false pass rates. Sensitivity (83.33%), specificity (87.97%), and NPV (94.35%) values of the invasive POCT method were high. Owing to its false deferral rates, the PPV (68.63%) value of the invasive POCT method was moderate. The noninvasive POCT method was inefficient for detection of ineligible volunteers owing to its high false deferral (n = 57 donors) and false pass rates (n = 30 donors) (25.66%). Also, donating individuals were negatively affected because of these false pass rates. On the other hand, the real donors were missed because of falsely rejected donors. In the literature, there are studies in which the noninvasive POCT method was observed more sensitive and specific and had a lower bias than our study. However, these studies had lower blood donor numbers than our study.6, 14 The accuracy of the noninvasive POCT method was found similar with the invasive POCT method in other study.15 This may be due to the performance of their POCT devices which may be different from ours.
Both POCT devices are more practical and quicker than the venous method. The measurement durations of the POCT devices are 15 and 40 seconds for the noninvasive POCT method and the invasive POCT method, respectively. But the venous method is time consuming owing to the phlebotomy. Compared with the POCT methods, the invasive method has some disadvantages like pain, causing stress, and an infection risk. So, donors are afraid of blood donation by using invasive methods. In addition, the invasive method may be affected by preanalytical factors like an order of drop used, moisture and not properly filled cuvettes, poor peripheral circulation, and skills of the person using the device. The noninvasive method is advantageous because it causes no risk and pain. But the noninvasive method may also be affected by preanalytical factors such as being affected by pigmented, hennaed, callous and sweaty fingers, position of donors, and finger temperature. Therefore, in this study, Hb measurements were not able to be performed in 11 volunteers using the noninvasive method owing to these factors.
TEa of the reference method was 4.09% and within the recommended allowable error limits for Hb (±7%).12 The within‐day and between‐day CVs of the reference method meet the manufacturer's specifications (<2). To determine the accordance between POCT methods and the venous method, the Bland‐Altman plot and the scatter plot were utilized. Our results showed that Hb levels of all donors were slightly underestimated using POCT methods in comparison to the venous method. The bias of the invasive POCT method and the venous method was almost zero. Similar results were obtained by other studies.14, 16 As distinct from other studies, the Bland‐Altman plots of both sexes were shown in our study, respectively (Figure 1). When the average of measurements of the noninvasive POCT method and the venous method increased, the mean difference of two methods increased negatively (r = −.34, P < .001) so that there was a proportional error. Therefore, in male donors with high Hb levels, the noninvasive POCT method showed high bias due to this proportional error. There is evidence of high correlation (r = .91, P < .001) and low bias (−0.02) between the invasive POCT method and the venous method. The invasive POCT method was observed to be more coherent with the venous method than the noninvasive POCT method.
Comparison of Hb values of capillary blood and venous blood is a controversial issue. Some reports show that Hb values are higher in capillary blood than venous blood,14, 17, 18 whereas the others show the direct opposite.16, 19, 20 Capillary measurements of Hb using POCT methods were lower than the venous method of our study (Table 1). On the other hand, the 10 volunteers were defined falsely as deferral donors with both capillary methods and the reason for this result is unknown.
The accordance of eligible and ineligible donors between the POCT methods and the venous method must be high. Because, about 2.7 million blood donations were performed per year. If the noninvasive POCT method and the invasive POCT method were used, 93 177 and 48 978 donors would define falsely and there would be redundant Hb screening, loss of money, and time. In our study, the numbers of falsely determined female donors were 28 and 12 using the noninvasive POCT method and the invasive POCT method, respectively. In females, anemia occurs where Hb levels are under 12 g/dL and in males under 13 g/dL, according to the WHO definition21 and Hb levels of donors decrease approximately 5 g/L (0.5 g/dL) after the donation.22, 23 Therefore, these false pass rates caused donation‐induced anemia especially for female donors whose Hb levels are at a cut‐off limit. As a result, they might take iron replacement therapy.
In conclusion, to protect the donors’ health prior to blood donation, testing is crucial. The Hb measurements need to be accurate and precise to determine eligible and ineligible donors properly for donation and to prevent loss of money and time. In this study, we compared the Haemospect and the HemoControl devices, but the performance and accuracy of each POCT device may differ. If the disadvantages of mentioned POCT devices are minimized, they can replace the devices using the venous method of Hb measurements. Otherwise, other POCT devices on the market can be provided. Nevertheless, it should be taken into consideration that the results of the POCT devices might be false. Prior to blood donation, the answers given by the volunteers to the questionnaire should be considered important.
Thus, noninvasive POCT devices may encourage an increase in the number of donors for blood donation. Our study might be a reference to blood donation centers using mentioned POCT devices.
DISCLOSURE STATEMENT
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Avcioglu G, Nural C, Yilmaz FM, Baran P, Erel Ö, Yilmaz G. Comparison for noninvasive and invasive point‐of‐care testing methods with reference method of hemoglobin measurement. J Clin Lab Anal. 2018;32:e22309 10.1002/jcla.22309
REFERENCES
- 1. Medicines EDftQo . Guide to the preparation, use and quality assurance of blood components. Recommendation No R (95) 152013.
- 2. Food U. Drug Administration . Title 21, Code of Federal Regulation. www.gpo.gov/fdsys/pkg/CFR-2010-title21-vol7/pdf/CFR-2010-title21-vol7-sec640-3.pdf. Accessed December 12, 2016.
- 3. European Directorate for the Quality of Medicines and Health Care (EDQM) 2016. http://www.edqm.eu/en/Good-practiceguidelines-for-blood-establishments-1587.html?mbID=155. Accessed December 15, 2016.
- 4. Türk Kızılayı Kan Hizmetleri Genel Müdürlüğü. 2015.
- 5. Bakanlığı TS . Ulusal kan ve kan ürünleri rehberi. Türkiye Kan Merkezleri ve Transfüzyon Derneği, İstanbul. 2011.
- 6. Crowley C, Montenegro‐Bethancourt G, Solomons NW, Schümann K. Validity and correspondence of non‐invasively determined hemoglobin concentrations by two trans‐cutaneous digital measuring devices. Asia Pac J Clin Nutr. 2012;21:191‐200. [PubMed] [Google Scholar]
- 7. MBR Optical Systems Haemospect. https://www.mbr-optical-systems.com/download/flyer-haemospect-en.pdf. Accessed December 10, 2016.
- 8. EKF Diagnostics HemoControl. http://www.ekfdiagnostics.com/res/Hemo%20Control%20Presentation.pdf. Accessed December 10, 2016.
- 9. Sysmex XE‐2100 Brochure. https://www.cmmc.org/cmmclab/IFU/XE-2100.PDF. Accessed December 11, 2016.
- 10. Mieczkowska E, Koncki R, Tymecki Ł. Hemoglobin determination with paired emitter detector diode. Anal Bioanal Chem. 2011;399):3293‐3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ricos C, Ramon F, Salas A, et al. Minimum analytical quality specifications of inter‐laboratory comparisons: agreement among Spanish EQAP organizers. Clin Chem Lab Med. 2012;50:455‐461. [DOI] [PubMed] [Google Scholar]
- 12. Datainnovations Allowable‐Total‐Error‐Table. http://www.datainnovations.com/allowable-total-error-table. Accessed November 10, 2016.
- 13. MedCalc Diagnostic test evaluation calculator. https://www.medcalc.org/calc/diagnostic_test.php. Accessed October 07, 2016.
- 14. Ardin S, Störmer M, Radojska S, Oustianskaia L, Hahn M, Gathof BS. Comparison of three noninvasive methods for hemoglobin screening of blood donors. Transfusion. 2015;55:379‐387. [DOI] [PubMed] [Google Scholar]
- 15. Shah N, Osea E, Martinez G. Accuracy of noninvasive hemoglobin and invasive point‐of‐care hemoglobin testing compared with a laboratory analyzer. Int J Lab Hematol. 2014;36:56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh A, Dubey A, Sonker A, Chaudhary R. Evaluation of various methods of point‐of‐care testing of haemoglobin concentration in blood donors. Blood Transfus. 2015;13:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim MJ, Park Q, Kim MH, Shin JW, Kim HO. Comparison of the accuracy of noninvasive hemoglobin sensor (NBM‐200) and portable hemoglobinometer (HemoCue) with an automated hematology analyzer (LH500) in blood donor screening. Ann Lab Med. 2013;33:261‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tong E, Murphy W, Kinsella A, et al. Capillary and venous haemoglobin levels in blood donors: a 42‐month study of 36 258 paired samples. Vox Sang. 2010;98:547‐553. [DOI] [PubMed] [Google Scholar]
- 19. Gordeuk VR, Brittenham G, Bravo J, Hughes M, Keating L. Prevention of iron deficiency with carbonyl iron in female blood donors. Transfusion. 1990;30:239‐245. [DOI] [PubMed] [Google Scholar]
- 20. Neufeld L, García‐Guerra A, Sánchez‐Francia D, Newton‐Sánchez O, Ramírez‐Villalobos MD, Rivera‐Dommarco J. Hemoglobin measured by Hemocue and a reference method in venous and capillary blood: a validation study. Salud Publica Mex. 2002;44:219‐227. [DOI] [PubMed] [Google Scholar]
- 21. BD Selection WHO Guide Assessing Donor Suitability for Blood Donation. http://www.who.int/bloodsafety/publications/guide_selection_assessing_suitability.pdf. Accessed October 20, 2016.
- 22. Boulton F. Managing donors and iron deficiency. Vox Sang. 2004;87(Suppl 2):22‐24. [DOI] [PubMed] [Google Scholar]
- 23. Gómez‐Simón A, Navarro‐Núñez L, Pérez‐Ceballos E, et al. Evaluation of four rapid methods for hemoglobin screening of whole blood donors in mobile collection settings. Transfus Apheres Sci. 2007;36:235‐242. [DOI] [PubMed] [Google Scholar]


