Abstract
Background
The assessment of the coagulation status using thromboelastography (TEG) in Chinese population has less been reported. This study aimed to establish reliable reference values for kaolin‐activated TEG in Chinese volunteers.
Methods
A total of 1681 Chinese adult individuals were recruited for this study. The reference individuals were stratified by gender and age, and the TEG values were measured on the basis of strict quality control. The 95% reference values were determined using nonparametric statistical methods.
Results
The sex‐related 95% reference values were reaction time (R):4.2‐8.7 minutes; clotting time (K): 1.2‐3.2 minutes; alpha angle (α): 47.0‐72.3 degree; maximum amplitude (MA): 49.1‐70.5 mm for males, and R: 3.7‐9.0 minutes; K: 1.0‐3.2 minutes; α: 48.4‐74.4 degree; MA: 46.8‐72.4 mm for females. Also, the TEG parameters indicated a relatively more hypercoagulable profile in both female and elder groups.
Conclusions
This study established the reference values for kaolin‐activated TEG in the target Chinese population, which might provide a reference for both clinical and laboratory studies.
Keywords: Chinese, coagulation, reference values, thromboelastography
1. Introduction
In 1948, Hartert first described a new method called thromboelastography for detecting blood coagulation.1 The method uses whole blood to carry out qualitative rather than quantitative analysis. It can be used to evaluate several aspects of the coagulation process: initiation of the clot, propagation kinetics, clot firmness, and fibrinolysis. On the basis of this technology, two manufacturers, Haemonetics Corp. (Niles, IL, USA) and TEM International GmbH (Munich, Germany), have produced their own thromboelastographic instruments. The former is thrombelastograph (TEG), and the latter is the rotation thrombelastometry (ROTEM). The technology and methodology of thromboelastography have been discussed in detail.2, 3, 4
Due to the limitations of the TEG's technology, the method has been applied in clinical practice for nearly half a century. Another reason is the dominance of routine coagulation tests (RCTs) including the activated partial thromboplastin time (APTT) and the prothrombin time (PT).5 However, the reproducibility has improved with the continuous standardization of the methodology. Digitalization of the procedure, combined with rapid assessment and relatively simple operation for bedside as a point‐of‐care testing (POCT), has increased the interest in, and application of, TEG.6
After the approval of TEG by the Food and Drug Administration, its application in clinical practice has been widely reported in the world, especially in the diagnosis of traumatic bleeding, and liver and cardiac surgery.6, 7, 8 Meanwhile, studies comparing TEG and conventional coagulation tests have been continuing.9, 10 TEG has also been included in the authoritative guidelines on blood management recently.11, 12
Considering that the current TEG reference range of most domestic laboratories mainly refers to the instrument or reagent specifications from manufacturers, it cannot represent the overall level of the Chinese population, and could not be accurately applied in medical diagnosis and treatment. Hence, it was necessary to establish a reference range of TEG parameters for the target population.
2. Materials and Methods
2.1. Subject selection
The study was approved and conducted at the First Affiliated Hospital of Anhui Medical University from January 2014 through April 2016. A total of 2221 volunteers, aged 18‐65 years, from different regions were recruited, and informed written consents were obtained from all the participants. The questionnaire forms according to the Clinical and Laboratory Standards Institute (CLSI) C28‐A3 document recommendations were collected.13 Clinical history, physical examination, and certain clinical laboratory tests of all the volunteers were recorded. Accordingly, strict exclusion criteria were established:
Diagnosed with acute or chronic diseases in the last 10 months;
Undergone surgery or blood donation in the last 6 months;
Had a habit of smoking or drinking;
Diagnosed with an abnormality of the hematological system (anemia or coagulation dysfunction) on laboratory examination;
Taken any prescription or over‐the‐counter drugs or hormonal contraceptive (female) in the previous month;
Pregnant women.
2.2. Blood sample collection
Before the collection of blood samples between 7 and 9 am, all of the participants were in a state of limosis and calmness; no emotional or stressful situation occurred. A collection of forearm venous blood complied with the Chinese National Guide to Clinical Laboratory Procedures (the fourth version); the tourniquets were carefully used. Blood samples were collected by venipuncture using 22‐gauge needles into 1.8‐mL tubes (Becton Dickinson, Plymouth, Devon, UK) with 3.2% sodium citrate (0.129 mol/L). Extra 2 mL of blood (EDTA‐anticoagulated) was drawn for a complete blood count (Sysmex XE2100, Shiojiri, Japan), and another 2 mL of blood was collected in a separation gel vacutainer for serum biochemical tests (Modular P 800, Roche, Mannheim, Germany). The samples were gently mixed, kept at room temperature, and transported to the laboratory within 30 minutes.
2.3. TEG assay
Two TEG analyzers 5000 (Haemoscope Corp., Niles, IL, USA) were operated by three qualified technicians to ensure the consistency of sample pretreatment. All the analyses were performed according to standard operating procedures. Briefly, 1 mL of the sample was transferred to a kaolin vial (Haemoscope Corp.), which was inverted softly to activate the sample appropriately. Then, 340 μL of the sample from the kaolin‐activated blood was added to a 37°C prewarmed disposable cup containing 20 μL of calcium chloride (0.2 mol/L). The assay was continued for at least 30 minutes until recording of the following four main parameters: R (clot reaction time), K (time from clot formation till the amplitude reached 20 mm), α (angle measured from a tangent line drawn to the curve of the TEG tracing starting from the point of clot reaction time), and maximum amplitude (MA, amplitude measured at the widest point of TEG tracing).
The daily maintenance of the two TEG analyzers (with four testing channels) was performed according to the manufacturer's recommendations. Level I Control (Haemoscope Corp.) was tested on every working day as the internal quality control (IQC) standard. For ensuring the precision of the test, Levey‐Jennings charts were drawn to verify which values fitted well with the reference ranges provided by the manufacturer.
2.4. Statistical analyses
The Statistical Package for the Social Sciences (SPSS 19.0 version; SPSS, Chicago, IL, USA) was used to manage and analyze the data. Descriptive statistics was represented by the mean±standard deviation, or with a subsequent coefficient of variation for IQC. Gaussian or “normal” probability distribution was determined using the one‐sample Kolmogorov‐Smirnov test. The presence of outliers was determined using the Dixon's test. Differences among gender groups or age groups were evaluated using the homogeneity test of variance and independent‐sample t test for normally distributed values, and the Mann‐Whitney U test for data with non‐normal distribution. Statistical significance was set at P<.05. The 95% reference range was calculated as (P2.5, P97.5) by the method of percentiles.
3. Results
After screening, the outliers were censored from the original subjects, and a total of 1681 volunteers were enrolled in the present study (859 males and 822 females). The sample size was in accordance with the rigorous CLSI guidelines for determining laboratory reference ranges.13
Table 1 shows the detailed demographic and medical characteristics of the participants. The average age was not different between genders. Twenty consecutive kaolin‐activated TEG IQCs including four channels are summarized in Table 2, and the Levey‐Jennings chart of one parameter (MA) is presented in Figure 1. The precision on TEG was associated with coefficients of variance (CVs) between 1.9% and 18.0% for different parameters, with the lowest CVs seen for α.
Table 1.
Demographics and baseline characteristics
| Total | Male | Female | |
|---|---|---|---|
| n=1681 | n=859 | n=822 | |
| Age (y) | 47.3±10.0 | 46.9±10.0 | 47.6±10.1 |
| Habitual residence | |||
| City | 1240 (73.8%) | 643 (74.9%) | 597 (72.6%) |
| Town | 441 (26.2%) | 216 (25.1%) | 225 (27.4%) |
| Basic examination | |||
| Body mass index (kg/m2) | 21.9±2.3 | 22.5±2.2 | 21.3±2.1 |
| Systolic pressure (mm Hg) | 129.8±9.0 | 130.8±9.4 | 128.7±8.5 |
| Diastolic pressure (mm Hg) | 78.4±3.1 | 78.8±3.5 | 78.0±2.7 |
| Blood examination | |||
| Hemoglobin (g/L) | 138.5±14.2 | 147.6±10.0 | 129.0±11.5 |
| Hematocrit | 0.41±0.04 | 0.41±0.04 | 0.41±0.04 |
| Platelet counts (×109/L) | 208.6±48.0 | 217.1±45.0 | 199.8±49.5 |
| Fasting glucose (mmol/L) | 5.1±0.7 | 5.2±0.6 | 5.1±0.7 |
| Triglyceride (mmol/L) | 1.17±0.35 | 1.18±0.36 | 1.16±0.34 |
Values are presented as the mean±standard deviation or N (%).
Table 2.
Summary of 20 consecutive daily internal quality controls
| R (min) | K (min) | α (degree) | MA (mm) | |
|---|---|---|---|---|
| Channel 1 | 0.74±0.13 (17.0%) | 0.81±0.04 (5.1%) | 83.39±1.56 (1.9%) | 52.36±2.50 (4.8%) |
| Channel 2 | 0.72±0.10 (13.7%) | 0.83±0.04 (5.0%) | 84.29±2.94 (3.5%) | 53.89±3.48 (6.5%) |
| Channel 3 | 0.76±0.11 (14.1%) | 0.82±0.04 (4.9%) | 82.52±2.79 (3.4%) | 51.34±3.13 (6.1%) |
| Channel 4 | 0.73±0.13 (18.0%) | 0.80±0.04 (4.6%) | 84.06±2.38 (2.8%) | 52.43±3.47 (6.6%) |
| Manufacturer's RRs | 0‐2 | 0‐2 | 79‐88 | 43‐63 |
| Manufacturer's CVs | 13.4% | 4.4% | 2.8% | 6.3% |
α, alpha angle; CVs, coefficients of variance; K, clotting time; MA, maximum amplitude; R, reaction time; RRs, reference ranges; SD, standard deviation.
Values are presented as the mean±SD (CV) unless otherwise indicated.
Figure 1.
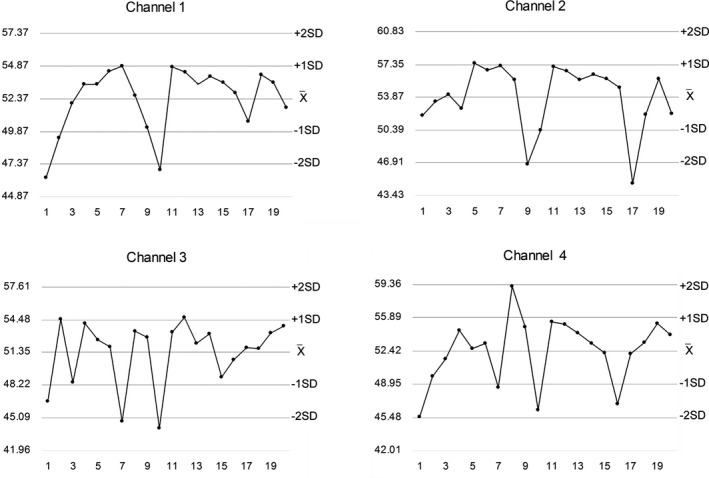
Internal quality control chart of thromboelastography's maximum amplitude values (mm) from four channels. The X‐axis indicates the measuring time (weekday). The reference range in the manufacturer's specification was 43‐63 mm
The gender groups were divided into two age subgroups to explore the factors that might affect the TEG parameters (Table 3). The K time and α demonstrated statistically significant differences (P<.05) between the younger group (18‐45 years old) and elder group (45‐65 years old) for both genders, suggesting that the latter was more hypercoagulable than the former in combination with other parameters. Meanwhile, the four main parameters were all significantly different (P<.05) between genders, with women possessing a more hypercoagulable profile compared with men.
Table 3.
Comparison of thromboelastography results after grouping by sex and age
| Male | P a | Female | P b | P c | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total | 18‐45 y | 46‐65 y | Total | 18‐45 y | 46‐65 y | ||||
| n | 859 | 379 | 480 | — | 822 | 358 | 464 | — | .814* |
| Age (y) | 46.9±10.0 | 37.6±5.8 | 54.3±5.4 | — | 47.6±10.1 | 38.3±5.9 | 54.8±5.8 | — | .231 |
| R (min) | 6.0±1.2 | 6.2±1.2 | 5.9±1.2 | .001 | 5.9±1.3 | 6.0±1.2 | 5.9±1.4 | .120 | .020 |
| K (min) | 2.0±0.5 | 2.0±0.5 | 1.9±0.5 | <.001 | 1.8±0.6 | 1.8±0.6 | 1.7±0.5 | .003 | <.001 |
| α (degree) | 62.3±6.0 | 61.6±5.7 | 62.8±6.1 | <.001 | 65.0±6.4 | 64.6±6.4 | 65.3±6.4 | .051 | <.001 |
| MA (mm) | 59.7±5.4 | 59.1±5.1 | 60.2±5.6 | .002 | 62.0±6.2 | 60.8±6.3 | 62.9±6.0 | <.001 | <.001 |
α, alpha angle; K, clotting time; MA, maximum amplitude; R, reaction time; SD, standard deviation.
Values are presented as the mean±SD unless otherwise indicated.
P a or P b, comparison of two age groups in males or females; P c, comparison of all volunteers in genders.
*Pearson chi‐square test P value.
Since the study population did not follow a normal distribution for most of the parameters, the reference values for measured TEG variables were established in accordance with gender and expressed as (P2.5‐P97.5) by the percentile method (Table 4). Finally, the diagnostic inconsistency between the manufacturer's and the authors’ reference values was evaluated (Table 4). It suggested that using different R or α reference values might lead to a completely different diagnostic advice.
Table 4.
Comparison of the manufacturer's and the present study's reference values
| Manufacturer's RRs | Present study's RRs | Inconsistency in diagnosis | |||
|---|---|---|---|---|---|
| Male | Female | A(−) and B(+) | A(+) and B(−) | ||
| R (min) | 5‐10 | 4.2‐8.7 | 3.7‐9.0 | 27 (1.61%) | 273 (16.24%) |
| K (min) | 1‐3 | 1.2‐3.2 | 1.0‐3.2 | 12 (0.71%) | 23 (1.37%) |
| α (degree) | 53‐72 | 47.0‐72.3 | 48.4‐74.4 | 0 | 114 (6.78%) |
| MA (mm) | 50‐70 | 49.1‐70.5 | 46.5‐72.4 | 0 | 57 (3.39%) |
α, alpha angle; MA, maximum amplitude; K, clotting time; R, reaction time; RRs, reference ranges.
Values are presented as the P2.5th to P97.5th by the percentile method.
‘+’ indicates abnormal coagulation; ‘−’ indicates normal coagulation.
A, Manufacturer's RRs; B, present study's RRs.
4. Discussion
Routine coagulation tests using platelet count, PTs, and APTTs play a significant role in screening for coagulation abnormalities and selective application of hemostatic interventions in the transfusion algorithms,14 and monitoring the existing anticoagulants (i.e., heparin and vitamin K antagonists). However, these time‐consuming tests have been questioned, and must be interpreted with caution as they do not reflect the in vivo hemostatic response: the interaction between the vessel wall, platelets, fibrinogen, and circulating coagulation factors. Besides, these tests have never been validated for predicting hemorrhagic tendency.2, 10
As an emerging viscoelastic POCT, TEG provides a more thorough evaluation of an individual's hemostatic state than RCTs do through measuring clot strength beyond initial fibrin formation: the end point of tests such as PT‐INR and APTT. Besides guiding blood component and pharmacological intervention in the identification of hypocoagulable and fibrinolytic states, TEG allows the recognition and subsequent thromboprophylaxis of hypercoagulable states. Normal parameters, typical pathophysiologic tracings, and more detailed explanation and clinical recommendations of TEG have been reviewed by Jr et al.15 and Bolliger et al.16
In a comparative study of three POCT devices to detect hemostatic changes, Espinosa et al.10 found that even if the TEG seemed to be more sensitive, its use was still associated with potential problems. The manufacturer's proposed normal values for TEG were not validated,17 and they did not provide any information about factors that might influence the results, such as gender, age, or underlying medical conditions. In other studies including the present study, the fact that TEG might be oversensitive in the identification of coagulation abnormalities was also observed.18, 19 Specifically, the value of R time might have had a higher false‐positive rate (>15%) if the reference range pre‐defined by the manufacturer was used in the present study. It meant that patients might have received an opposite diagnosis of coagulation function or even inappropriate treatment of blood transfusion. Therefore, each institution should determine its own reference values20 and establish standardized procedures to minimize variability before adopting TEG.
According to the recommendations in the C28‐A3 guideline, the preanalytical and analytical aspects are essential when establishing reference intervals.13, 20 The former is known to have the highest errors in the total test process,21 and quality control plays an important role in the latter. QC is extremely important in ensuring precise and accurate results, which can be achieved using both IQC and external quality assessment (EQA). However, a few published data are available on the precision of TEG tests or the variability in results between centers.22 Chitlur and Lusher23 reported precision on TEG with CVs between 6% and 60% for the different parameters, with the lowest CVs seen for R and MA. Published data in the manufacturer's specification exhibited the kaolin‐activated TEG with CVs between 2.8% and 13.4% for the four main parameters, which were lower than the values in the present study. Moreover, it was found that the present IQC results were in the manufacturer's range; still, some data remained out of the mean±2 standard deviation (SD) or even mean±3SD. Therefore, it is believed that the IQC values of TEG parameters provided by the manufacturer may be somewhat overbroad. Unfortunately, a high‐budget national EQA has not been carried out, as TEG has not been popular in China yet. Kitchen et al.22 have introduced their advanced experience from the UK National External Quality Assessment Scheme for blood coagulation.
The differences in the parameters of TEG were observed among genders in the present study. Compared with males, females had lower R and K values, whereas α and MA values were higher, which meant that women might have a more hypercoagulable profile compared with men. This was consistent with several other studies.19, 24, 25 Also, Polak et al.26 studied the changes in TEG parameters of women during pregnancy. They found that the main parameters of TEG indicated the blood coagulation of pregnant women was higher than that of the control group. Although no satisfactory, detailed explanation exists for coagulation changes during pregnancy so far,26 and why females have higher levels of clotting factors,27 it is worthwhile to discuss the necessity of determining different normal range values for males and females in clinical practice.
Besides gender, this study also investigated the possible effect of age on TEG results.28 Some statistically significant differences were found between age groups benefiting from a large sample size. The blood was in a state of relatively high coagulation in the elder group, especially in men. Age‐related differences in kaolin‐activated TEG variables have not been widely explored to date; only a few studies have focused on the parameters of TEG in healthy newborns or children.29, 30 A possible explanation for this is that TEG, being a global assessment of hemostasis, is inherently insensitive to identify changes between different ages in the hemostatic system.29 However, the present findings indicated the necessity to investigate the standardization of age grouping in future studies.
The limitation of the present study was the lack of validation of the reference intervals, which required the participation of multicenter laboratories in the local areas. In addition, as China has not yet performed the EQA for TEG, the accuracy of the present results may be affected.
In conclusion, this study showed the sex‐related reference values for kaolin‐activated TEG in volunteers of Anhui province, China. The manufacturer's reference values may not be appropriate for different populations. It is difficult but necessary to establish a reference interval that involves several important links, together with TEG's varieties of specimen and activator. Therefore, the present results may be useful in clinical practice.
Acknowledgments
This work was supported by the Department of Blood Transfusion and Department of Laboratory Medicine at the First Affiliated Hospital of Anhui Medical University (Hefei, China). The authors thank all the subjects who participated in this study.
Sun J‐B, Bian M‐H, Zhong T, et al. Reference values for kaolin‐activated thromboelastography in volunteers of Anhui Province in China. J Clin Lab Anal. 2017;31:e22128 10.1002/jcla.22128
References
- 1. Hartert H. Blutgerinnungsstudien mit der thromboelastographie, einem neuen Untersuchungsverfahren. Klin Wochenschr. 1948;26:557–583. [DOI] [PubMed] [Google Scholar]
- 2. Ganter MT, Hofer CK. Coagulation monitoring: current techniques and clinical use of viscoelastic point‐of‐care coagulation devices. Anesth Analg. 2008;106:1366–1375. [DOI] [PubMed] [Google Scholar]
- 3. Luddington RJ. Thromboelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. [DOI] [PubMed] [Google Scholar]
- 4. Tomori T, Hupalo D, Teranishi K, et al. Evaluation of coagulation stages of hemorrhaged swine: comparison of thromboelastography and rotational elastometry. Blood Coagul Fibrinolysis. 2010;21:20–27. [DOI] [PubMed] [Google Scholar]
- 5. Favaloro EJ, Lippi G. Coagulation update: what's new in hemostasis testing? Thromb Res. 2011;127(Suppl 2):S13–S16. [DOI] [PubMed] [Google Scholar]
- 6. Reikvam H, Steien E, Hauge B, et al. Thrombelastography. Transfus Apher Sci. 2009;40:119–123. [DOI] [PubMed] [Google Scholar]
- 7. Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–896. [PMC free article] [PubMed] [Google Scholar]
- 8. Bolliger D, Tanaka KA. Roles of thrombelastography and thromboelastometry for patient blood management in cardiac surgery. Transfus Med Rev. 2013;27:213–220. [DOI] [PubMed] [Google Scholar]
- 9. Agren A, Wikman AT, Holmstrom M, et al. Thromboelastography (TEG(R)) compared to conventional coagulation tests in surgical patients‐a laboratory evaluation. Scand J Clin Lab Invest. 2013;73:214–220. [DOI] [PubMed] [Google Scholar]
- 10. Espinosa A, Stenseth R, Videm V, et al. Comparison of three point‐of care testing devices to detect hemostatic changes in adult elective cardiac surgery: a prospective observational study. BMC Anesthesiol. 2014;14:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. STS Task Force and SCA Task Force . Perioperative blood transfusion and blood conservation in cardiac surgery: the STS and SCA clinical practice guideline. Ann Thorac Surg. 2007;83:27–86. [DOI] [PubMed] [Google Scholar]
- 12. Sibylle A, Arash KL, Pierre A, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30:270–382. [DOI] [PubMed] [Google Scholar]
- 13. CLSI Approved Guideline‐Third Edition (C28‐A3) : Defining establishing and verifying reference intervals in the clinical laboratory. Clinical Laboratory and Standards Institute (CLSI), USA, 2010. [Google Scholar]
- 14. Change to American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies . Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. [DOI] [PubMed] [Google Scholar]
- 15. Jr TY, Kavarana MN. Cardiopulmonary bypass and the coagulation system. Prog Pediatr Cardiol. 2005;21:87–115. [Google Scholar]
- 16. Bolliger D, Manfred DS, Kenichi AT. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus Med Rev. 2012;26:1–13. [DOI] [PubMed] [Google Scholar]
- 17. Chitlur M, Sorensen B, Rivard GE, et al. Standardization of thromboelastography: a report from the TEG‐ROTEM working group. Haemophilia. 2011;17:532–537. [DOI] [PubMed] [Google Scholar]
- 18. Cotton BA, Faz G, Hatch Q, et al. Rapid Thromboelastography delivers real‐time results that predict transfusion within 1 hour of admission. J Trauma. 2011;71:407–417. [DOI] [PubMed] [Google Scholar]
- 19. Subramanian A, Albert V, Saxena R, et al. Establishing a normal reference range for thromboelastography in North Indian healthy volunteers. Indian J Pathol Microbiol. 2014;57:43–50. [DOI] [PubMed] [Google Scholar]
- 20. Ozarda Y. Reference intervals: current status, recent developments and future considerations. Biochem Med. 2016;26:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plebani M, Sciacovelli L, Aita A, Chiozza ML. Harmonization of pre‐analytical quality indicators. Biochem Med. 2014;24:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kitchen DP, Kitchen S, Jennings I, et al. Quality assurance and quality control of thrombelastography and rotational Thromboelastometry: the UK NEQAS for blood coagulation experience. Semin Thromb Hemost. 2010;36:757–763. [DOI] [PubMed] [Google Scholar]
- 23. Chitlur M, Lusher J. Standardization of thromboelastography:values and challenges. Semin Thromb Hemost. 2010;36:707–711. [DOI] [PubMed] [Google Scholar]
- 24. Scarpelini S, Rhind SG, Nascimento B, et al. Normal range values for thromboelastography in healthy adult volunteers. Braz J Med Biol Res. 2009;42:1210–1217. [DOI] [PubMed] [Google Scholar]
- 25. Roeloffzen WW, Kluin‐Nelemans HC, Mulder AB, et al. In normal controls, both age and gender affect coagulability as measured by thrombelastography. Anesth Analg. 2010;110:987–994. [DOI] [PubMed] [Google Scholar]
- 26. Polak F, Kolnikova I, Lips M, et al. New recommendations for thromboelastography reference ranges for pregnant women. Thromb Res. 2011;128:e14–e17. [DOI] [PubMed] [Google Scholar]
- 27. Lowe GD, Rumley A, Woodward M, et al. Epidemiology of coagulation factors, inhibitors and activation markers: the Third Glasgow MONICA Survey. I. Illustrative reference ranges by age, sex and hormone use. Br J Haematol. 1997;97:775–784. [DOI] [PubMed] [Google Scholar]
- 28. Ng KF. Changes in thrombelastograph variables associated with aging. Anesth Analg. 2004;99:449–454. [DOI] [PubMed] [Google Scholar]
- 29. Chan KL, Summerhayes RG, Ignjatovic V, et al. Reference values for kaolin‐activated thromboelastography in healthy children. Anesth Analg. 2007;105:1610–1613. [DOI] [PubMed] [Google Scholar]
- 30. Edwards RM, Naik‐Mathuria BJ, Gay AN, et al. Parameters of thromboelastography in healthy newborns. Am J Clin Pathol. 2008;130:99–102. [DOI] [PubMed] [Google Scholar]


