Abstract
Background
Technical innovation of autoimmune blistering dermatoses (ABDs) diagnosis aimed at multiplex approach. Two multiparametric ELISA tests are commercially available for ABDs serology. The aim was to compare diagnostic accuracy of multiparametric and monospecific ELISAs and to examine the diagnostic value/agreement of multivariant ELISA in compliance with traditional diagnostic setup for ABDs.
Methods
In total, 128 sera from suspected ABDs patients were studied (27 sera in order to compare ELISAs). Multivariant ELISA (detection of IgG against desmoglein 1 and 3 ‐ DSG1/3; BP180, BP230, envoplakin, type VII collagen), monovariant ELISA, and statistical analysis were performed.
Results
With the use of sera from patients with suspected ABDs, the multiparametric ELISA yield an agreement of 84% with traditional stepwise diagnostics. Multivariant ELISA with BP180 and BP230 showed 87.5% and 80% sensitivity, 87.5% and 91% specificity, 87.5% reliability as well as 87.5% and 80% positive predictive value, 87.5% and 91% negative predictive value, respectively, in relation to monospecific ELISA. Multivariant ELISA with DSG1 and DSG3 showed 50% and 80% sensitivity, 100% and 80% specificity, 85% and 80% reliability as well as 100% and 57% positive predictive value, 82% and 92% negative predictive value, respectively, in relation to monospecific ELISA. A better rate of agreement was observed among ELISA systems with BP180 and BP230, than with ELISA systems with DSG1 and DSG3.
Conclusion
Multivariant ELISA test combined with clinical examinations and DIF is recommended as a minimal approach to diagnosing ABDs in ethnic Slavs.
Keywords: autoantibodies, early diagnosis, enzyme‐linked immunosorbent assay, immunologic tests, skin diseases, vesiculobullous
1. INTRODUCTION
Autoimmune blistering dermatoses belong to the complex, heterogeneous organ‐specific autoimmune diseases, which are characterized by autoantibodies against structural components of the skin.1, 2 The main target antigens involve: desmosomal cadherins, desmoglein 1 and 3 (DSG1, DSG3), for pemphigus circle; hemidesmosomal proteins, BP180 and BP230, for bullous pemphigoid (BP); envoplakin for paraneoplastic pemphigus (PNP) and type VII collagen for epidermolysis bullosa acquisita (EBA).
Due to variety of clinical presentations and overlapping clinical symptoms, the precise diagnosis of ABDs based on the clinical picture alone is not possible. In case of clinical suspicion of ABDs, the diagnostic pathway should be performed.3 This pathway consists of various optical/biochemical/molecular techniques (histopathology, indirect immunofluorescence—IIF, direct immunofluorescence—DIF, immunoenzymatic tests—ELISA), what makes the diagnosis of ABDs difficult (hard to accept), time‐consuming, and costly. The detection of autoantibodies produced in ABD patients is essential in the diagnostic workup. For a long time, antigen specificity of autoantibodies may be determined in monospecific (individual) assays.4, 5, 6 However, in cases where identification of multiple antibodies is relevant for a diseases circle (such as ABDs), screening by multiplex test, allowing analysis in a single test run, is considered as an efficient diagnostic first step. Technical innovation of immunoassays aimed at multiplex approach,7 like IIF multiplex biochip8, 9, 10 and multivariant profile ELISA.11, 12, 13, 14, 15 Most recently, two multiparametric ELISA tests are commercially available (Euroimmun, Germany; MBL, Nagoya, Japan) for ABDs serology.11, 12, 13, 14, 15 New tests provide capabilities for efficient IgG circulating autoantibodies screening and characterization in one test. A lately developed multivariant profile ELISAs is a combination of six (BP180‐NC16A‐4X, BP230, DSG1, DSG3, envoplakin, type VII collagen; Euroimmun, Germany)11 or five (DSG1, DSG3, BP180, BP230, and type VII collagen; MBL)12 antigens enabling the simultaneous detection of corresponding IgG autoantibodies. Each antigen is coated in a separate well of the ELISA strip for convenient parallel analysis. The idea of applying a single procedure multiantigen test for diagnosing autoimmune diseases is not new, as a multiantigen blot‐type test for diagnosing autoimmune connective tissue diseases had previously been developed, undergone additions to be even more comprehensive, and is routinely used.16
Diagnostic accuracy of IIF biochip mosaic was demonstrated and discussed in our previous work.10 The aim of this study was to compare the diagnostic accuracy of multiparametric and monospecific (individual) ELISA tests in routine laboratory diagnostics of autoimmune blistering dermatoses and to examine the diagnostic value/agreement of multivariant ELISA in compliance with traditional diagnostic setup for ABDs patients in a Central European university dermatology department.
2. MATERIALS AND METHODS
This work was approved by the local Ethical Committee of the Poznan University of Medical Sciences in Poland.
2.1. Patients and serum samples
In total, 128 patients suspected of having ABDs before initiation of treatments were tested.
Sera from 128 ABD suspected patients were investigated to assess the diagnostic agreement between multivariant profile ELISA and traditional stepwise diagnostic strategy (combination of DIF, IIF as well as monospecific ELISA). Altogether, sera from 27 affected patients and sera from nine non‐affected patients were evaluated to examine the diagnostic accuracy of multiparametric ELISA in relation to monospecific ELISA.
Patients were recruited at the Autoimmune Blistering Dermatoses Section, Department of Dermatology, Poznan University of Medical Sciences, Poland. Patients in the examined groups—pemphigus group and BP group—had to meet following criteria: clinical features—flaccid blisters and erosions on the skin and mucous membranes in pemphigus; tense cutaneous blisters with no or transient involvement of mucosal surfaces in BP, in combination with at least one of the positive diagnostic test, including: (i) typical immunoglobulins deposits detected with direct immunofluorescence (DIF) of perilesional skin (the diagnosis of BP was made in patients having IgG/IgG4 and/or IgG1 non‐U‐pattern deposits along the dermal–epidermal junction; the diagnosis of pemphigus was made in patients having IgG/IgG4/IgG1 fish‐net like pattern, diagnosis will be corroborated with appropriate IgG ELISA in serum samples), (ii) positive pattern in indirect immunofluorescence, and (iii) molecular characterization of antigens (ELISA tests). The serum used in the serological tests was taken at the time of hospital admission/ambulatory care. Five ml of blood serum were obtained from each subject. The samples were centrifuged for 10 minutes at 822 g. Thereafter, they were stored at−20°C until performing ELISAs.
2.2. Immunoenzymatic assays
2.2.1. Monospecific/individual ELISA
Commercially available individual ELISAs (Euroimmun; Luebeck, Germany) were used with recombinant separate/single protein of DSG1, DSG3, BP180, BP230, with the manufacturer's cutoff value of 20 RU/ml. Anti‐BP180‐NC16A‐4X ELISA includes four copies of domain NC16A fused to a polyhistidine tag to enhance protein expression. Anti‐BP230‐CF ELISA contains an amplified fragment of C‐terminal globular domain. Anti‐DSG1 IgG and anti‐DSG3 IgG were measured with an ELISA assay utilizing recombinant proteins DSG1 and DSG3, consisting of the extracellular domain of DSG1 and DSG3, respectively (five subdomains).
2.2.2. Multivariant profile ELISA
The novel mutiparametric ELISA comprising six different antigens (BP180, BP230, DSG1, DSG3, envoplakin, type VII collagen—these are the same domains that are applied in individual ELISA) was performed. Each antigen was coated in a separate well and a semiquantitative evaluation was carried out with the manufacturer's ratio of 1.
All measurements were made in the ELISA plate reader (Asys Expert 96) equipped with Microwin 2000 software by a single operator following the manufacturer's instructions.
2.3. Statistical analysis
The accuracy of multiparametric ELISA was evaluated by calculating diagnostic sensitivity, diagnostic specificity, diagnostic reliability as well as positive and negative predictive values in relation to monospecific ELISA using the dedicated MedCalc Software 2015 (Ostend, Belgium, www.medcalc.org). Estimates of sensitivity and specificity were calculated by tabulating the number of correctly classified samples. For statistical evaluation, we used Fisher's exact test.
Cohen's kappa was used to evaluate the interrate analytical agreements among the two ELISA systems for each of the antibodies tested.
3. RESULTS
3.1. Diagnostic value/agreement of multivariant profile ELISA in compliance with traditional diagnostic setup for ABDs
With the use of sera from patients with suspected ABD, the multiparametric ELISA yield an agreement of 84% with traditional stepwise diagnostics (multiplex ELISA was in line with data from the traditional diagnostic algorithm—described above—in 112 individuals).
The comparison of results agreement of multivariant profile ELISA and DIF demonstrated 7% of incompatibilities—six individuals (4.7%) present negative DIF with positive results of multivariant profile ELISA, whereas three individuals (2.3%) present positive DIF with negative results of multivariant profile ELISA.
In five elderly patients with itchy polymorphic rash but equivocal DIF of perilesional lower limb, armpit or arm skin elevated levels of IgG antibodies to BP230 and/or BP180 were found; in four with multiparametric ELISA, and in one with monovalent BP180 ELISA but not with multiparametric ELISA.
In 5.5% of patients, positive results for envoplakin were obtained, whereas all of those results were near the cutoff value (borderline, slightly above the cutoff ratio). None of the patients with IgG antibodies to envoplakin presented with clinical mucocutaneous features of PNP at the time of serum testing.
In only 3.1% of patients, positive results for type VII collagen were obtained. All such patients had clinical features of EBA at the time of serum testing.
3.2. Comparison of diagnostic accuracy between multiparametric ELISA and monospecific ELISA
The diagnostic sensitivity, specificity, and reliability as well as positive and negative predictive values of multiparametric ELISA in comparison with monospecific ELISA are shown in Table 1.
Table 1.
Calculation of the diagnostic sensitivity, specificity, reliability, and predictive values of multivariant ELISA in relation to monospecific ELISA in diagnosis of autoimmune blistering dermatoses
| Diagnostic accuracy of multivariant ELISA in relation to individual ELISA | |||||
|---|---|---|---|---|---|
| Multivariant vs individual ELISA | Sensitivity (%) | Specificity (%) | Positive predictive value (PPV) (%) | Negative predictive value (NPV) (%) | Reliability (%) |
| DSG1 | 50 | 100 | 100 | 82 | 85 |
| DSG3 | 80 | 80 | 57 | 92 | 80 |
| BP180 | 87.5 | 87.5 | 87.5 | 87.5 | 87.5 |
| BP230 | 80 | 91 | 80 | 91 | 87.5 |
DSG1, desmoglein 1; DSG3, desmoglein 3.
A better rate of agreement was observed among ELISA systems with BP180 and BP230, than with ELISA systems with DSG1 and DSG3. The interrate agreements (kappa values) among methods are presented in Table 2.
Table 2.
Interrate agreements (kappa values) among ELISA systems for the antibodies tested
| Multiparametric ELISA vs monospecific ELISA | |
|---|---|
| DSG1 | 0.583 |
| DSG3 | 0.529 |
| BP180 | 0.75 |
| BP230 | 0.709 |
DSG1, desmoglein 1; DSG3, desmoglein 3.
4. DISCUSSION
Our findings revealed that new multiplex test format for IgG autoantibodies determination in ABDs enabling efficient serological diagnostics with good agreement (84%) with traditional diagnostic stepwise, was in line with data on distinct population.11, 12, 13 The small discrepancies may be related with the absence of certain target structures (eg laminin 332, p200 antigen, some epitopes of DSG1/DSG3/BP180/BP230 such as BP180 ectodomains) and the lack of another immunoglobulin class/subclass detection (particularly IgA class).
As described previously,11 multivariant profile ELISA should provide quite similar diagnostic value for ABDs serology with the monospecific ELISA systems used commercially. Because ELISA profile was created on the basis of the individual kits, their sensitivities and specificities should be comparable.11 However, our findings give new light on this issue demonstrating the discrepancy between diagnostic accuracy of some target antigen detected with different ELISA systems. Therefore, we should still bear in mind that there may be differences in sensitivity and specificity among commercially available ELISA systems for ABDs, which was also disclosed in this article. Presented findings revealed that the best specificity (100%) showed multiparametric ELISA with anti‐DSG1 in contrast to the lowest sensitivity (50%) of this antigen detection in relation to the monospecific ELISA. The highest reliability (87.5%) showed both multivariant ELISA with BP180 and BP230. Van Beek et al11 showed that sensitivities of multivariant ELISA ranged from 85.7% (PNP) to 100% (pemphigus vulgaris) and specificities from 97.3% (BP) to 100% (EBA), which is slightly contrasted to our data. Probably, the performance of multiparametric ELISA with DSG1 may be improved (increased sensitivity, without affecting the specificity) by setting the ratio level. As previously noted,17 the low sensitivity of some commercial ELISA systems reflects the high cutoff values rather than methodological problems in the assays. Moreover, as we formerly indicated,14 a comparison of multivalent and monovalent ELISAs revealed inconsistent results near cutoff values, which may be associated with cutoff point sharing. Thus, perhaps the unified ratio for all six antigens in multiparametric ELISA may provide some unclear results, which are difficult to understand and likely to prevent proper conclusions (Figure 1) particularly for junior and inexperienced practicing dermatologists who have algorithms and consensuses imprinted on their minds. The readings unmatching with learned clinical picture can be a perplexing parlance for them. Likewise, the interpretation and reporting of plenty results of borderline significance, particularly in case of envoplakin, should be regarded as an up‐to‐date problem. According to the interpretation of Cohen's kappa18, the interrate agreement among tests with BP180 and BP230 was moderate with a kappa value of 0.75 and 0.709, respectively. Conversely, the detection of anti‐DSG1 IgG not only showed a low sensitivity, but even striking difference among tests—weak level of agreement was observed for anti‐DSG1 and anti‐DSG3 IgG autoantibodies (0.583 and 0.529, respectively). This difference is likely due to lack of the receiver operator characteristic (ROC) curve analysis with the individual cutoff point for each immobilized antigen. Hence, the performance of test could be further optimized by ROC curve analysis and establishing an in‐house cutoff for Poland. Therefore, additional study should be required to investigate the performance of the ELISA systems using larger samples from ABD patients. A limitation of our work is the relative small number of ABD samples for tests comparison, however, it was difficult to collect samples from patients with active and untreated ABDs because of epidemiologic reasons. Then, obtained results may facilitate further comparison of autoantibodies level between multiparametric and monospecific ELISAs.
Figure 1.
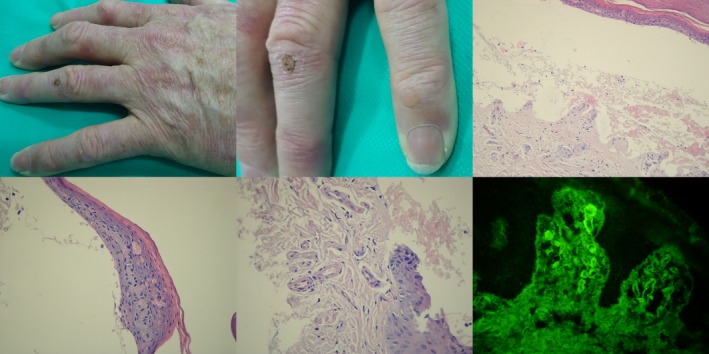
A middle‐aged male with clinical (milia and tense blister on skin over joins of the hands), H+E (subepidermal blister showing no inflammation with festooned papillae appearance, caterpillar bodies, and thickening of superficial dermal vessels), and DIF (IgG1 cuff‐like deposits in upper dermis vessels) features of porphyria cutanea tarda having grossly elevated level of uroporhyrins in urea, nevertheless slightly elevated level of serum IgG antibodies to both BP80 (1.3) and BP230 (1.6) (both cutoffs below 1.0) with multiparametric ELISA
Interestingly, the low percentage of only slightly positive (“borderline”) results for envoplakin in a setting of our patients (5.5%) who had no clinical mucocutaneous features of PNP may suggest the necessity of replacing envoplakin (target antigen of PNP) with laminin 332, which is one of the target antigens in mucous membrane pemphigoid (MMP). According to our observations/experience supported with the literature reports19 about the epidemiological data on ABDs, MMP seems to be more important epidemiological problem than PNP. Bertram et al19 showed in the prospective analysis on European population (period of 18 months) that incidence calculated for MMP was 2.0 per 1 million subjects per year, whereas PNP is so rare that no epidemiological data were gathered during this time period. Thus, it seems that laminin 332 represents a significant, but unfortunately still missing, parameter for clinicians in order to ABDs serological screening. A comprehensive European/American business survey did not reveal laminin 332 ELISA kit for commercial use, however, the IIF including transfected cells with laminin 332 has just been developed.20 The very concept of MMP affecting conjunctivae is still controversial to some researchers who call to change diagnostic criteria of this potentially devastating disease as a prerequisite to improve its management.21 Expanding this concept, it may be that chronic erosive subepithelial oral lesions form a continuum of pathologic processes involving both antibody‐mediated and cell‐mediated immunity in various combinations with “pure” MMP at one end of the continuum and “pure” oral erosive lichen planus at the other. Thus, multiparametric ELISA should be, visionary and ethnicity oriented—PNP poses a medical problem in a multiethnic societies like the USA, the UK, or Germany; or in homogenous East Asian countries like Japan, contrary to Central European Poland which still has relatively homogeneous population. The clinical unreliability of readings for envoplakin should be considered as a caveat of the test evaluated here, whereas clinical reliability of readings for type VII collagen should be stressed.
It is universally agreed that a definitive detection of autoimmune phenomena in ABDs requires DIF. Thus, a fascinating issue remains the agreement between the multiparametric ELISA and DIF for ABDs recognition. Van Beek at al11 demonstrated a very high agreement for pemphigus (93.6%) and a substantial agreement for BP (71.4%). Our findings indicated 7% of incompatibilities between these methods, whereas most cases (4.7%) present negative DIF with positive results of multivariant profile ELISA. Thus, several factors may have impact on it: reactivity to target antigens not included in the ELISA profile,11 other than IgG class of autoantibodies, or perhaps the proper determination of the optimum site for skin biopsy for DIF. Elderly individuals with elevated levels of serum IgG antibodies to BP180 and/or BP230 but equivocal DIF readings, which are influenced by spatial–temporal evolution of lesions and plausibly by different density of BP antigens in various body areas, might have prodromal stage of BP which further indicates usefulness of performing multiparametric ELISA. First, this multiparametric ELISA, having type VII collagen, facilitates the reliable diagnosis of EBA, a disease which, especially in its mechanobullous form, can run relentlessly progressive, refractory to traditional treatments course, and can have serious comorbidities, including inflammatory bowel disease. Nevertheless, it should be noted that examined multivariant profile ELISA (Euroimmun, Germany) gives only a semiquantitative results, thus it may be insufficient to monitor the course and therapy of ABDs. The second commercially available multiparametric ELISA (MBL) enables qualitative assessment of IgG autoantibodies, however, it possesses less antigens for ABDs screening.
Our conclusion is that the use of multivariant profile ELISA test combined with clinical examinations and DIF can be recommended, as a minimal workup, for reliable diagnosis of ABDs. This multiplex test format is especially suitable for identifying overlapping diseases serving as a rapid, precise, and cost‐effective ABDs IgG class autoantibodies screening measure. Furthermore, it can be an excellent tool to indicate the occurrence of epitope spreading phenomenon during follow‐up (disease shift).
ACKNOWLEDGMENTS
We are grateful to Barbara Jastrzębska for technical assistance. A part of this study was presented at 76th Annual SID Meeting, April 26‐29, 2017, Portland, Oregon, USA (abstract no 151: Ref. 14). This study was partly funded from Poznan University of Medical Sciences grant 502‐14‐02220351‐10256 and from grant of the Polish Ministry of Science and Higher Education “Iuventus Plus” 0127/IPI/2015/73.
Gornowicz‐Porowska J, Seraszek‐Jaros A, Bowszyc‐Dmochowska M, Bartkiewicz P, Kaczmarek E, Dmochowski M. Clinical evaluation of a multiparametric ELISA as a rapid tool for routinely diagnosing IgG‐mediated autoimmune blistering dermatoses in ethnic Slavs. J Clin Lab Anal. 2018;32:e22336 10.1002/jcla.22336
REFERENCES
- 1. Tampoia M, Zucano A, Villalta D, Antico A, Bizzaro N. Anti‐skin specific autoantibodies detected by a new immunofluorescence multiplex biochip method in patients with autoimmune bullous diseases. Dermatology. 2012;225:37‐44. [DOI] [PubMed] [Google Scholar]
- 2. Gornowicz‐Porowska J, Bowszyc‐Dmochowska M, Dmochowski M. Autoimmunity‐driven enzymatic remodeling of the dermal‐epidermal junction in bullous pemphigoid and dermatitis herpetiformis. Autoimmunity. 2012;45:71‐80. [DOI] [PubMed] [Google Scholar]
- 3. Jindal A, Rao R, Bhogal BS. Advanced diagnostic techniques in autoimmune bullous diseases. Indian J Dermatol. 2017;62:268‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmidt E, Dähnrich C, Rosemann A, et al. Novel ELISA systems for antibodies to desmoglein 1 and 3: correlation of disease activity with serum autoantibody levels in individual pemphigus patients. Exp Dermatol. 2010;19:458‐463. [DOI] [PubMed] [Google Scholar]
- 5. Tampoia M, Giavarina D, Di Giorgio C, Bizzaro N. Diagnostic accuracy of enzyme‐linked immunosorbent assays (ELISA) to detect anti‐skin autoantibodies in autoimmune blistering skin diseases: a systematic review and meta‐analysis. Autoimmun Rev. 2012;12:121‐126. [DOI] [PubMed] [Google Scholar]
- 6. Otten JV, Hashimoto T, Hertl M, Payne AS, Sitaru C. Molecular diagnosis in autoimmune skin blistering conditions. Curr Mol Med. 2014;14:69‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Damoiseaux J. Multiparametric autoimmune diagnostics: recent advances. Pathol Lab Med Int. 2016;8:15‐25. [Google Scholar]
- 8. Damoiseaux J, van Rijsingen M, Warnemünde N, Dähnrich C, Fechner K, Tervaert JW. Autoantibody detection in bullous pemphigoid: clinical evaluation of the EUROPLUS™ Dermatology Mosaic. J Immunol Methods. 2012;382:76‐80. [DOI] [PubMed] [Google Scholar]
- 9. van Beek N, Rentzsch K, Probst C, et al. Serological diagnosis of autoimmune bullous skin diseases: prospective comparison of the BIOCHIP mosaic‐based indirect immunofluorescence technique with the conventional multi‐step single test strategy. Orphanet J Rare Dis. 2012;7:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gornowicz‐Porowska J, Seraszek‐Jaros A, Bowszyc‐Dmochowska M, et al. Accuracy of molecular diagnostics in pemphigus and bullous pemphigoid: comparison of commercial and modified mosaic indirect immunofluorescence tests as well as enzyme‐linked immunosorbent assays. Postepy Dermatol Alergol. 2017;34:21‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Beek N, Dähnrich C, Johannsen N, et al. Prospective studies on the routine use of a novel multivariant enzyme‐linked immunosorbent assay for the diagnosis of autoimmune bullous diseases. J Am Acad Dermatol. 2017;76:889‐894. e5. [DOI] [PubMed] [Google Scholar]
- 12. Horváth ON, Varga R, Kaneda M, Schmidt E, Ruzicka T, Sárdy M. Diagnostic performance of the “MESACUP anti‐Skin profile TEST”. Eur J Dermatol. 2016;26:56‐63. [DOI] [PubMed] [Google Scholar]
- 13. Mende M, Daehnrich C, van Beek N, et al. A multivariant profile ELISA for one‐step diagnostics of autoimmune bullous dermatoses. Satellite Symposium to the 46th Annual ESDR meeting, 5‐7 September 2016, Munich, Germany
- 14. Dmochowski M, Jastrzebska B, Gornowicz‐Porowska J, Bartkiewicz P, Bowszyc‐Dmochowska M. A comparison of multivalent and monovalent ELISA systems for diagnosing IgG‐mediated autoimmune bullous diseases uncommonly reveals inconsistent results near cut‐off values. I Invest Dermatol 2017;137(suppl 1):S26 . 76th Annual SID Meeting, April 26‐29, 2017, Portland, Oregon, USA. [Google Scholar]
- 15. Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157‐169. [DOI] [PubMed] [Google Scholar]
- 16. Lutkowska A, Pietkiewicz P, Gornowicz J, Raptis‐Bolwach M, Dmochowski M, Bowszyc‐Dmochowska M. Typy świecenia immunofluorescencji pośredniej na komórkach HEp‐2 a antygeny wykrywane testem typu blot u chorych diagnozowanych w kierunku choroby tkanki łącznej (Fluorescence patterns in indirect immunofluorescence on HEp‐2 cells and antigens recognized in blot test in patients supposed to have a connective tissue diseases). Derm Klin 2009;11:91‐96. [Google Scholar]
- 17. Ito‐Ihara T, Muso E, Kobayashi S, et al. A comparative study of the diagnostic accuracy of ELISA systems for the detection of anti‐neutrophil cytoplasm antibodies available in Japan and Europe. Clin Exp Rheumatol. 2008;26:1027‐1033. [PubMed] [Google Scholar]
- 18. McHugh ML. Interrater reliability: the kappa statistic. Biochemia Medica. 2012;22:276‐282. [PMC free article] [PubMed] [Google Scholar]
- 19. Bertram F, Bröcker EB, Zillikens D, Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in Lower Franconia, Germany. J Dtsch Dermatol Ges. 2009;7:434‐440. [DOI] [PubMed] [Google Scholar]
- 20. Goletz S, Probst C, Komorowski L, et al. Cell‐based immunofluorescence test applying recombinant laminin 332 for the serological differential diagnosis of pemphigoid. Scientific Conference of the International Pemphigus & Pemphigoid Foundation, June 22‐23, 2017, Luebeck, Germany, Abstract no 37 [Google Scholar]
- 21. Dart JKG. Mucous membrane pemphigoid: should autoantibody detection remain a diagnostic criterion. Scientific Conference of the International Pemphigus & Pemphigoid Foundation, June 22‐23, 2017, Luebeck, Germany. [Google Scholar]


