Abstract
Background
New high‐performance liquid chromatography (HPLC) method was developed for the determination of vitamin K1 and two forms of vitamin K2 (MK‐4 and MK‐7) in human serum, and the levels of vitamin K were determined in 350 samples of postmenopausal women.
Methods
Vitamin K was determined by HPLC with fluorescence detection after postcolumn zinc reduction. The detection was performed at 246 nm (excitation) and 430 nm (emission). The internal standard and 2 mL of ethanol were added to 500 μL of serum. The mixture was extracted with 4 mL of hexane, and solid phase extraction was then used.
Results
The HLPC method was fully validated. The intra‐ and interday accuracy and precision were evaluated on two QC samples by multiple analysis, and CV were less than 10%. The limit of quantification for MK‐4 was found at 0.04 ng/mL, for K1 0.03 ng/mL, and for MK‐7 0.03 ng/mL. The mean recoveries of the corresponding compounds were 98%‐110%. Serum levels of MK‐4, K1, and MK‐7 in postmenopausal women with osteoporosis were 0.890 ± 0.291 ng/mL, 0.433 ± 0.394 ng/mL, and 1.002 ± 1.020 ng/mL, respectively (mean ± SD). Serum levels of MK‐4, K1, and MK‐7 in postmenopausal women without osteoporosis were 0.825 ± 0.266 ng/mL, 0.493 ± 0.399 ng/mL, and 1.186 ± 1.076 ng/mL, respectively (mean ± SD).
Conclusion
New HPLC method for the determination of vitamins K1, MK‐4, and MK‐7 in serum was evaluated and validated. This method is highly specific and sensitive with the low limit of quantification.
Keywords: HPLC, menaquinones, MK‐4, MK‐7, phylloquinone, vitamin K
1. INTRODUCTION
Vitamin K belongs to the family of structurally similar fat‐soluble 2‐methyl‐1,4‐naphthoquinones. The members of vitamin K family have various side chains.1, 2 There are three types of vitamin K, phylloquinone (vitamin K1), menaquinone (vitamin K2), and menadione (vitamin K3). In the Western diet, phylloquinone (PK) is a predominant dietary form and is produced by plants and algae (eg, green leafy vegetables such as kale and spinach).3, 4, 5 Menaquinones (MK) are synthesized by gut bacteria and are found in fermented foods such as dairy products, natto (fermented soybeans), and animal products (eg, liver, egg yolk, meat). Menaquinones have a varying number of isoprenoid residues in their side chains. The main important forms of menaquinones are MK with four (MK‐4) and seven (MK‐7) isoprenoid residues. Menaquinones‐4 is the most common form of vitamin K2 in animal products.5, 6, 7 Menadion is produced synthetically 8.
The half‐time of PK and MK‐4 is 1.5‐2 hours. The half‐time of MKs with longer side chains is about 72 hours. Vitamin K is absorbed from a gut. Vitamin K has neither the recommended daily dosage nor the reference level. The adequate intake (AI) of vitamin K is about 120 μg/d for adult men and 90 μg/d for adult women.3, 9
Deficiency of vitamin K1 could cause anemia and bleeding (coagulopathy). Osteoporosis and vascular calcification are associated with the deficiency of vitamin K2. Newborns are at higher risk of vitamin K1 deficiency, and postmenopausal women are at higher risk of vitamin K2 deficiency. There is no known toxicity associated with high doses of vitamin K.3
Vitamin K function is blocked by coumadin (anticoagulants). Patients with anticoagulant treatment often suffer from a lack of vitamin K in their body.4 Vitamin K is usually determined by high‐performance liquid chromatography (HPLC) with fluorescence detection with postcolumn zinc reduction.7
Vitamin K acts as a cofactor in the enzymatic carboxylation by gamma‐glutamyl‐carboxylase of glutamic acid residues forming gamma‐carboxyglutamic acid in proteins. Vitamin K1 is the best‐known member of the vitamin K family. Vitamin K1 is very important for blood coagulation.2 Vitamin K has different functions. It has a procoagulant activity (prothrombin, factor II, VII, IX, X) and also an anticoagulant activity (protein C, S, Z). A deficiency of vitamin K1 increases prothrombin time. Vitamin K reduces risk of vascular calcification and osteoporosis (matrix Gla protein, osteocalcin) and is related to “calcification paradox”.10 Vitamins K2 and K3 exhibit anticancer activity in vitro and in vivo for cell lines of ovaries, liver, colon, leukemia, breast, etc.2, 10
2. MATERIAL AND METHODS
Analytical standards of phylloquinone and MK‐4 were obtained from Sigma‐Aldrich (Czech Republic). MK‐7 was obtained from NattoPharma (Norway). Acetic acid and sodium acetate were purchased from Penta (Czech Republic); zinc chloride was obtained from Merck (Czech Republic). All used solvents acetonitrile, 2‐propanole, methanol, and hexane (HPLC grade) were purchased from Sigma‐Aldrich (Czech Republic).
HPLC analysis was performed using an Agilent 1260 HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a fluorescence detector. The separation was carried out on a reversed phase column LiChroCART RP 18, Superspher 100 (4.6 × 125 mm, particle size 4 μm, Merck, Czech Republic) with postcolumn zinc reduction (powder zinc 99.995%, Sigma‐Aldrich). The column was operated at 22°C (laboratory temperature). The mobile phase consisted of methanol 85%, 2‐propanol 9%, acetonitrile 5%, and methanol solution 1% (10 mM zinc chloride, 5 mM natrium acetate, 5 mM acetic acid). The flow rate of the mobile phase was 0.8 mL/min. The vitamin K derivate (Immundiagnostik AG, Germany) was used as an internal standard. Fluorescence detection was carried out at an excitation wavelength of 246 nm and an emission wavelength of 430 nm. The injected volume of prepared samples was 50 μL. The chromatograms were processed by OpenLab (Chem 32, Agilent Technologies). The total run time per sample was 20 minutes (Figure 1).
Figure 1.
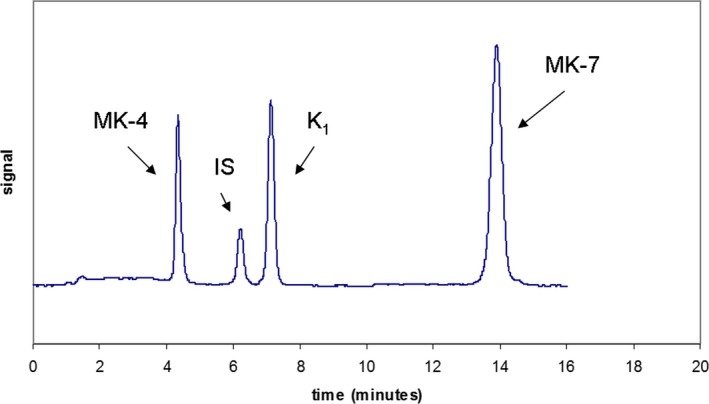
Analysis of vitamin K
2.1. Preparation of standards and control materials
All stock vitamin K solutions were prepared by diluting certified material with ethanol to a final concentration of 200 ng/mL. Standards were prepared using pooled serum from healthy volunteers. Aliquots of serum were spiked with known amounts of K1, MK‐4, and MK‐7. Standard concentration used 0.1, 0.3, 0.6, 1, 1.5, 2, 4, 8, and 15 ng/mL. Working standard solutions were stored at −20°C. Control samples were prepared at concentrations of 0.5 and 1.5 ng/mL. Vitamin K‐depleted serum was prepared by exposing pool serum to UV light over 24 hours.
For determination, we used 0.5 mL of serum sample (control, calibrator); 10 μL of internal standard (Immundiagnotik AG) and 2 mL of ethanol were added. Sample was briefly mixed, and 4 mL of hexane was added. The mixture was shaken for 5 minutes and then centrifuged 10 minutes at 3727 g. The upper layer was quantitatively transferred to a glass tube, and the lower layer was re‐extracted with another 4 mL of hexane and centrifuged. The organic layer was then evaporated under a stream of nitrogen. The lipid extract was dissolved with 2 mL of hexane, and SPE extraction was performed. The sample was loaded onto the silica Sep‐pak extraction cartridges (Waters, USA) connected to a Visiprep Solid Phase Extraction Vacuum Manifold (Sigma‐Aldrich), which was washed before with 3 × 3 mL of hexane. The cartridges were washed again with 3 × 3 mL of hexane, and vitamin K was then eluted with 3 × 3 mL of diethylether:hexane (3:97). The sample was then evaporated under a stream of nitrogen, and the residue was dissolved with 100 μL of isopropanol. 50 μL of sample was injected into the column, which was connected with postcolumn reactor freshly filled with zinc dust (Sigma‐Aldrich).
We measured 350 patients’ serum samples from postmenopausal women with and without osteoporosis. All serum samples were obtained in tubes with a clot activator (Vacuette, Germany), and the tubes were protected from light, centrifuged 10 minutes at 3727 g and immediately stored at −80°C. The samples were stored maximum 3 months before the analysis.
3. RESULTS
The HPLC method has been successfully validated. The calibration curves were constructed by plotting the peak area ratios of vitamin K standards to the internal standard against the concentration of vitamin K. The assays were linear (r 2 was 0.9992 for MK‐4, r 2 was 0.9993 for K1, r 2 was 0.9995 for MK‐7) across the whole range of concentrations. The intra‐ and interday accuracy and precision were evaluated on two QC samples by multiple analysis (n = 20). The intraday CV for MK‐4 were 8.4% and 8.4%, for K1 were 7.3% and 8.6%, and for MK‐7 were 9.5% and 6.9%. Interday CV for MK‐4 were 9.4% and 9.7%, for K1 were 9.9% and 8.7, and for MK‐7 were 9.9% and 9.6%. The within‐day accuracy was expressed by the calculated bias between observed and theoretical concentrations for MK‐4 were 5.2% and 0.07%, for K1 were 4.5% and 3.9, and for MK‐7 were 2.5 and 2.5%. The mean recoveries of the corresponding compounds were 98%‐110%. No interference has been found between MK‐4, K1, and MK‐7, or the internal standard (Figure 2). The limit of quantification for MK‐4 was found at 0.04 ng/mL, for K1 0.03 ng/mL, and for MK‐7 0.03 ng/mL. Retention times of MK‐4, IS, K1, and MK‐7 were 4.2 minutes, 6.1 minutes, 7.0 minutes, and 14.1 minutes respectively. Quantification was based on the peak area ratio of vitamin K to the internal standard.
Figure 2.
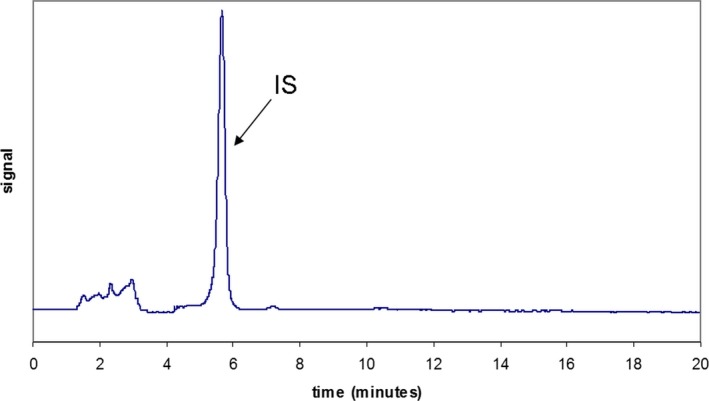
Analysis of blank serum with IS
The stability of vitamins was tested. We tested the stability of vitamins in the fridge (2‐8°C) for 7 days (Table 1), and the stability in the freezer for 3 months (Table 2). Our results showed that the vitamins were not stable in the fridge. Mean decreases of 51.6% for MK‐4, 16.8% for K1, and 23.0% for MK‐7 were noted after 7 days storage of serum in the refrigerator. The vitamins stored in the freezer were stable throughout the storage. Mean decreases of all vitamins were lower than 1%.
Table 1.
Sample stability in the fridge
| Sample stability—in the fridge, sample protected from light (4‐8°C) | |||
|---|---|---|---|
| Period | MK‐4 (ng/mL) | K1( )(ng/mL) | MK‐7 (ng/mL) |
| Original value | 1.004 | 1.051 | 1.320 |
| After 2 d | 0.864 | 1.029 | 1.242 |
| After 5 d | 0.658 | 0.927 | 1.131 |
| After 7 d | 0.486 | 0.874 | 1.016 |
Table 2.
Sample stability in the freezer
| Sample stability—in the freezer, sample protected from light (−80°C) | |||
|---|---|---|---|
| Period | MK‐4 (ng/mL) | K1 (ng/mL) | MK‐7 (ng/mL) |
| Original value | 1.004 | 1.051 | 1.320 |
| After 1 mo | 1.002 | 1.046 | 1.320 |
| After 2 mo | 1.000 | 1.043 | 1.318 |
| After 3 mo | 0.996 | 1.045 | 1.311 |
We measured the levels of vitamin K in two groups of patients. The first group were postmenopausal women with osteoporosis aged 67.6 ± 7.7 years (mean ± SD, n = 192), and the second group were postmenopausal women without osteoporosis aged 64.6 ± 8.3 years (mean ± SD, n = 158). In accordance with the WHO consensus, osteoporosis is diagnosed when the densitometry shows −2.5 SD in the T‐score downward. The first group with osteoporosis included women with a T‐score of the lumbar spine and left femoral neck below −2.5. Our data showed a large variability in vitamin K levels. Serum levels of MK‐4, K1, and MK‐7 from postmenopausal women with osteoporosis were 0.890 ± 0.291 ng/mL, 0.433 ± 0.394 ng/mL, and 1.002 ± 1.020 ng/mL, respectively (mean ± SD). Serum levels of MK‐4, K1, and MK‐7 in postmenopausal women without osteoporosis were 0.825 ± 0.266 ng/mL, 0.493 ± 0.399 ng/mL, and 1.186 ± 1.076 ng/mL, respectively (mean ± SD).
4. DISCUSSION
Vitamin K plays a key role in the blood coagulation, but it is also essential for bone and vascular health.2, 10, 11, 12, 13, 14, 15, 16 Especially over the last thirty years, different methods for quantification of vitamin K in serum have been developed.17, 18, 19, 20, 21 The most commonly used methods for determination of vitamin K are HPLC methods with fluorescent or electrochemical detection. Recently, there have been published some methods based on liquid chromatography connected with tandem mass spectrometry (LC‐MS). Determination of vitamin K levels is quite difficult, because of low concentration of circulating form of vitamin K in plasma, and interfering compounds in plasma, especially triglycerides.
We describe a HPLC method for determination of vitamins K1, MK‐4, and MK‐7 with low limit of quantification. Wang et al19 used HPLC method with fluorescence detection after zinc postcolumn reduction for determination of vitamin K1. They tested, similarly as we, a proprietary vitamin K derivate from Immundiagnostik as an internal standard together with MK‐6. However, they had problems with MK‐6 due to the asymmetric peak. Their limit of quantification was 0.01 ng/L. Marinova et al20 developed a HPLC method with fluorescent detection for determination of vitamins K1 and MK‐4. They used vitamin K1 (25) as internal standard. The limit of quantification was 0.0625 ng per injection for both forms of vitamins. The analysis time was about 13 minutes long.
Kamao et al22 published HPLC method also with fluorescent detection for measuring of vitamins K1, MK‐4, and MK‐7. They tested two HPLC systems. The analysis time was quite long approximately 40 minutes. Ahmed et al23 also described the determination of vitamins K1, MK‐4, and MK‐7 by HPLC, but with online photoreactor and peroxyoxalate chemiluminescence detection. Authors had a high limit of detection 1.4, 1.7, and 5.5 ng/mL for vitamins K1, MK‐4, and MK‐7. The disadvantage of this method was a big amount of patient samples (1 mL of plasma), quite complicated equipment, and the analysis time was about 30 minutes long.
Recently, there have been also other methods based on liquid chromatography with tandem mass spectrometry (LC‐MS/MS) published. These methods primarily use atmospheric pressure chemical ionization (APCI). For example, Song et al24 determined vitamin K1 by HPLC‐APCI‐MS without SPE extraction, only with liquid‐liquid extraction step. The analysis time was about 13 minutes long, and the limit of quantification was 0.3 ng/mL, it is still quite high.
Riphagen et al25 published a LC‐MS method with APCI for determination of vitamins K1, MK‐4, and MK‐7 with a simplified pretreatment sample procedure. But the limit of quantification for MK‐7 was only 2.2 ng/mL. Therefore, this method is definitely not suitable for monitoring the levels in normal population, because the levels of vitamin MK‐7 are much lower. These authors used the pretreatment of the sample without SPE extraction but with a very bad limit of quantification of MK‐7. Especially based on this study, it is evident that SPE extraction is necessary for the removal of interfering substances, which affect mainly MK‐7.
In the literature, it is possible to find the published reference range for vitamin K1 (0.11.‐1.01 ng/mL,26 0.1‐3.2 ng/mL,27 and 0.22‐2.28 ng/mL,28 but it is impossible to find the reference range for menaquinones, so there is currently no established threshold of menaquinones that would indicate insufficiency or deficiency. However, it is not currently known if the recommended intake is sufficient to meet all physiological needs. Therefore, it is very difficult to derive clinical outcomes in patients as well as to compare the levels of vitamin K. The different plasma levels of phylloquinone and menaquinones have been reported, and the published data differ significantly across countries.21 Kamao et al22 measured the levels of vitamin K in 20 healthy subjects and in 10 osteoporotic patients treated with MK‐4. The mean concentration ± SD in healthy subject for MK‐7 was 16.27 ± 20.58 ng/mL. Kaneki et al17 published mean ± SD for MK‐7 3.82 ± 3.11 ng/mL, and Suhara et al29 published mean ± SD for MK‐7 6.37 ± 7.45 ng/mL in healthy adults. But these data were obtained from the Japanese population, where the levels of MK‐7 differ greatly from European or American ones, because Japanese diet is rich in natto (fermented soybean food), containing the biggest amount of MK‐7. Fusaro et al30 found median concentration of K1, MK‐4, and MK‐7 0.63 ng/mL, 0.51 ng/mL, and 1.09 ng/mL, respectively, in hemodialysis patients.
Kaneki et al17 also published data from France and the UK, and those do not correlate with our data. In Table 3, we summarized the published levels in different populations. It is obvious that the data vary across populations. It is a question whether the data are so significantly different due to various analytical methods, and because it is necessary to standardize them, or whether the concentrations vary with the type of meal pattern. Additional research in this area is needed. It is also important to mention that some published data were measured in very small groups of patients.22, 23, 29, 31, 32, 37 It is quite interesting that our results in postmenopausal women with osteoporosis and without osteoporosis are very similar; however, the difference was statistically significant. Especially, the differences in the Japanese population are more evident. Authors Kaneki et al17 found out the difference between the levels of MK‐7 about 2.97 ng/mL in Japanese women with and without osteoporosis living in eastern Japan, but only 0.35 ng/mL in Japanese women living in western Japan. The levels of MK‐7 were significantly higher in both groups of postmenopausal women from eastern Japan, because in this part of Japan natto is frequently eaten. Shea et al38 published the difference of MK‐7 between Japanese women with and without osteoporosis 0.499 ng/mL. Comparable to our results are the differences of MK‐7 in the UK about 0.18 ng/mL and in France about 0.12 ng/mL,17 but these data were measured in small groups of patients. Vitamin K (especially K2) plays an essential role in mineral utilization and activates proteins responsible for the deposition of calcium and phosphorus salts in bones. Low vitamin K concentrations are associated with increased risks of osteoporotic fractures in the elderly and vascular calcification.21, 39 It was published that serum vitamin K concentrations are significantly associated with dietary intake of these vitamins.25 It is not entirely clear why there are no significant differences between populations but also between postmenopausal women in individual populations, and if only nutritional and metabolic factors play role. Therefore, further investigations are necessary to be performed.
Table 3.
Published levels of vitamin K in different populations
| K1 (ng/mL) | MK‐4 (ng/mL) | MK‐7 (ng/mL) | Location | Population | n | Reference |
|---|---|---|---|---|---|---|
| 0.51 ± 0.37 | not measured | 0.29 ± 0.18 | UK | Young normal subjects | 11 | Suttie31 |
| 0.60 ± 0.28 | not measured | 0.33 ± 0.17 | UK | Elderly normal subjects | 17 | Suttie31 |
| 0.10 ± 0.14 | 0.01 ± 0.0004 | 0.35 ± 0.65 | Japan | Healthy postmenopausal women | 23 | Kawana et al32 |
| 0.85 ± 0.99 | not measured | not measured | China | Older men | 86 | Yan et al33 |
| 1.12 ± 1.45 | not measured | not measured | China | Older women | 92 | Yan et al33 |
| 0.69 ± 0.90 | not measured | not measured | USA | Older men | 741 | Booth et al34 |
| 1.81 ± 1.11 | 0.15 ± 0.17 | 16.27 ± 20.58 | Japan | Healthy subjects | 20 | Kamao et al22 |
| 0.62 ± 0.25 | 46.83 ± 46.41 | 4.18 ± 6.28 | Japan | Osteoporotic patients | 10 | Kamao et al22 |
| 1.22 ± 0.57 | 0.39 ± 0.46 | 6.37 ± 7.45 | Japan | Healthy subjects | 20 | Suhara et al29 |
| 0.32 ± 0.24 | 0.02 ± 0.04 | 1.97 ± 2.80 | Japan | Healthy postmenopausal women | 344 | Tsugawa et al35 |
| 1.21 ± 0.15 | 0.65 ± 0.19 | 1.51 ± 0.34 | Japan | Healthy subjects | 6 | Ahmed et al23 |
| 0.68 ± 0.05 | not measured | not measured | USA | African American adults | 180 | Shea et al36 |
| not measured | 2.20 ± 0.38 | < LOD (0.04 ng/mL) | Japan | Healthy female subjects | 10 | Sato et al37 |
| 1.36 ± 1.08 | 0.91 ± 0.85 | 1.95 ± 1.37 | Italy | Healthy blood donors | 62 | Fusaro et al30 |
| 0.61 ± 0.21 | 0.09 ± 0.01 | <LOD (2.86 ng/mL) | Netherlands | Renal transplant recipients | 60 | Riphagen et al25 |
5. CONCLUSION
We described a HPLC method for determination of vitamin K with fluorescence detection after postcolumn reduction. This method is selective and highly sensitive and is suitable for the routine measurement of the levels of phylloquinone and menaquinones in serum. It was published that it is much easier to find out the status of vitamin K by the measurement of undercarboxylated vitamin K‐dependent proteins, where the analytical methods are not so complicated. However, lots of these proteins are affected, for example, by vitamin D, therefore the levels of these proteins may not reflect the actual concentration of vitamin K. In order to improve the outcomes of some medical treatments, especially related to bone metabolism and inhibition of vascular calcification, it is recommended to measure directly the levels of phylloquinone and menaquinones in serum or plasma.
ACKNOWLEDGMENTS
Supported by Ministry of Health, Czech Republic—conceptual development of research organization, Motol University Hospital, Prague, Czech Republic 00064203. Special thanks go to Dr. James Partridge for the language revision of the English study.
Klapkova E, Cepova J, Dunovska K, Prusa R. Determination of vitamins K1, MK‐4, and MK‐7 in human serum of postmenopausal women by HPLC with fluorescence detection. J Clin Lab Anal. 2018;32:e22381 10.1002/jcla.22381
REFERENCES
- 1. Lamson DW, Plaza SM. The Anticancer Effects of Vitamin K. Altern Med Rev. 2003;8:303‐318. [PubMed] [Google Scholar]
- 2. Vitamin K2. Monograph. Altern Med Rev. 2009;14:284‐293. [PubMed] [Google Scholar]
- 3. Food and Agriculture Organization of the United Nations/World Health Organization . 2001. Human vitamin and mineral requirements. Report of a joint FAO/WHO expert consultation, Bangkok, Thailand.
- 4. Stafford DW. The vitamin K cycle. J Thromb Haemost. 2005;3:1873‐1878. [DOI] [PubMed] [Google Scholar]
- 5. Walther B, Karl PK, Booth SL, Boyaval P. Menaquinones, Bacteria, and the Food Supply: The Revalence of Dairy and Fermented Food Products to Vitamin K Requirements. Adv Nutr. 2013;4:463‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Homma K, Wakana N, Suzuki Y, et al. Treatment of Natto, a Fermented Soybean Preparation, to Present Excessive Plasma Vitamin K Concentrations in Patiens Taking Warfarin. J Nutr Sci Vitaminol. 2006;5:297‐301. [DOI] [PubMed] [Google Scholar]
- 7. Iwamoto J. Vitamin K2 Therapy for Postmenopausal Osteoporosis. Nutrients. 2014;6:1971‐1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Erdman JW, MacDonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. Oxford: Wiley‐Blackwell; 2012. [Google Scholar]
- 9. Kamao M, Suhara Y, Tsugawa N, et al. Vitamin K Content of Foods and Dietary Vitamin K Intake in Japanese Young Women. J Nutr Sci Vitaminol. 2007;6:464‐470. [DOI] [PubMed] [Google Scholar]
- 10. Flore R, Ponziani FR, Rienzo TA, et al. Something more to say about calcium homeostasis: the role of vitamin K2 in vascular calcification and osteoporosis. Eur Rev Med Pharmacol Sci. 2013;17:2433‐2440. [PubMed] [Google Scholar]
- 11. Okano T, Shimomura Y, Yamane M, et al. Conversion of phylloquinone (Vitamin K1) into menaquinone‐4 (Vitamin K2) in mice: two possible routes for menaquinone‐4 accumulation in cerebra of mice. J Biol Chem. 2008;283:11270‐11279. [DOI] [PubMed] [Google Scholar]
- 12. Tsugawa N. Cardiovascular Diseases and Fat Soluble Vitamins: Vitamin D and Vitamin K. J Nutr Sci Vitaminol. 2015;61(Suppl):S170‐S172. [DOI] [PubMed] [Google Scholar]
- 13. Knapen MH, Braam LA, Teunissen KJ, Zwijsen RM, Theuwissen E, Vermeer C. Yogurt drink fortified with menaquinone‐7 improves vitamin K status in a healthy population. J Nutr Sci. 2015;4:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maresz K. Proper calcium use: vitamin K2 as a promoter of bone and cardiovascular health. Integr Med (Encinitas). 2015;14:34‐39. [PMC free article] [PubMed] [Google Scholar]
- 15. Park JN, Lee JS, Noh MY, Sung MK. Association between usual Vitamin K intake and anticoagulation in patients under warfarin therapy. Clin Nutr Res. 2015;4:235‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferland G. The discovery of vitamin K and its clinical applications. Ann Nutr Metab. 2012;61:213‐218. [DOI] [PubMed] [Google Scholar]
- 17. Kaneki M, Hedges SJ, Hosoi T, et al. Japanese Fermented Soybean Food as the Major Determinant of the Large Geographic Difference in Circulating Levels of Vitamin K2: possible Implications for Hip‐Fracture Risk. Nutrition. 2001;17:315‐321. [DOI] [PubMed] [Google Scholar]
- 18. Paroni R, Faioni EM, Razzari C, Fontana G, Cattaneo M. Determination of vitamin K1 in plasma by solid phase extraction and HPLC with fluorescence detection. J Chromatogr B. 2009;877:351‐354. [DOI] [PubMed] [Google Scholar]
- 19. Wang LY, Bates CJ, Yan L, Harrington DJ, Shearer MJ, Prentice A. Determination of phylloquinone (vitamin K1) in plasma and serum by HPLC with fluorescence detection. Clin Chim Acta. 2004;347:199‐207. [DOI] [PubMed] [Google Scholar]
- 20. Marinova M, Lütjohann D, Westhofen P, Watzka M, Breuer O, Oldenburg J. A validated HPLC method for the determination of vitamin K in human serum – first application in pharmacological study. Open Clin Chem J. 2011;4:17‐27. [Google Scholar]
- 21. Fusaro M, Gallieni M, Rizzo MA, et al. Vitamin K plasma levels determination in human health. Clin Chem Lab Med. 2017;55:789‐799. [DOI] [PubMed] [Google Scholar]
- 22. Kamao M, Suhara Y, Tsugawa N, Okano T. Determination of plasma vitamin K by high‐performance liquid chromatography with fluorescence detection using vitamin K analogs as internal standards. J Chromatogr B. 2005;816:41‐48. [DOI] [PubMed] [Google Scholar]
- 23. Ahmed S, Kishikawa N, Nakashima K, Kuroda N. Determination of vitamin K homologues by high‐performance liquid chromatography with on‐line photoreactor and peroxyoxalate chemiluminescence detection. Anal Chim Acta. 2007;591:148‐154. [DOI] [PubMed] [Google Scholar]
- 24. Song Q, Wen A, Ding L, Dai L, Yang L, Qi X. HPLC‐APCI‐MS for the determination of vitamin K(1) in human plasma: method and clinical application. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875:541‐545. [DOI] [PubMed] [Google Scholar]
- 25. Riphagen IJ, van der Molen JC, van Faassen M, et al. Measurement of plasma vitamin K1 (phylloquinone) and K2 (menaquinones‐4 and ‐7) using HPLC‐tandem mass spectrometry. Clin Chem Lab Med. 2016;54:1201‐1210. [DOI] [PubMed] [Google Scholar]
- 26. Sadowski JA, Hood SJ, Dallal GE, Garry PJ. Phylloquinone in plasma from elderly and young adults: factors influencing its concentration. Am J Clin Nutr. 1989;50:100‐108. [DOI] [PubMed] [Google Scholar]
- 27. Kraemer CM. Vitamin K. Medscape Reference. 2015. [homepage on the internet] Medscape Reference. Available from: http://emedicine.medscape.com/article/2088738-overview Accessed June 9, 2017.
- 28. Immundiagnostik AG. Vitamin K1 HPLC Kit: For the determination of vitamin K1 in plasma and serum. 2008, 26 pages. (Vitamin K1 HPLC kit‐Manual).
- 29. Suhara Y, Kamao M, Tsugawa N, Okano T. Method for the determination of vitamin K homologues in human plasma using high‐performance liquid chromatography – tandem mass spectrometry. Anal Chem. 2005;77:757‐763. [DOI] [PubMed] [Google Scholar]
- 30. Fusaro M, Noale M, Viola V, et al. Vitamin K, vertebral fractures, vascular calcifications, and mortality: VItamin K Italian (VIKI) dialysis study. J Bone Miner Res. 2012;27:2271‐2278. [DOI] [PubMed] [Google Scholar]
- 31. Suttie JW. Vitamin K and human nutrition. J Am Diet Assoc. 1992;92:85‐90. [PubMed] [Google Scholar]
- 32. Kawana K, Takahashi M, Hostino H, Kushida K. Circulating levels of vitamin K1, menaquinone‐4, and menaquinone‐7 in healthy elderly Japanese women and patients with vertebral fractures and patients with hip fractures. Endocr Res. 2001;27:337‐343. [DOI] [PubMed] [Google Scholar]
- 33. Yan L, Zhou B, Greenberg D, et al. Vitamin K status of older individuals in northern China is superior to that of older individuals in the UK. Br J Nutr. 2004;92:939‐945. [DOI] [PubMed] [Google Scholar]
- 34. Booth SL, Broe KE, Peterson JW, et al. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab. 2004;89:4904‐4909. [DOI] [PubMed] [Google Scholar]
- 35. Tsugawa N, Shiraki M, Suhara Y, Kamao M, Tahala K, Okano T. Vitamin K status of healthy Japanese women: age‐related vitamin K requirement for gamma‐carboxylation of osteocalcin. Am J Clin Nutr. 2006;83:380‐386. [DOI] [PubMed] [Google Scholar]
- 36. Shea MK, Booth SL, Nettleton JA, Burke GL, Ghen H, Kritchevsky SB. Circulating phylloquinone concentrations of adults in the United States digger according to race and ethnicity. J Nutr. 2012;142:1060‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sato T, Schurgers LJ, Uenishi K. Comparison of menaquinone‐4 and menaquinone‐7 bioavailability in healthy women. Nutr J. 2012;11:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shea MK, Booth SL. Concepts and Controversies in Evaluating Vitamin K Status in Population‐Based Studies. Nutrients. 2016;8:pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Masterjohn CH. On the trail of the elusive X‐factor: A sixty‐two‐year‐old mystery finally solved. 2008, Available from: http://www.westonaprice.org/health-topics/abcs-of-nutrition/on-the-trail-of-the-elusive-x-factor-a-sixty-two-year-old-mystery-finally-solved/ Accessed September 5, 2017.


