Abstract
Background
Diarrheagenic Escherichia coli (DEC) signifies as an important etiological agent of moderate‐to‐severe diarrhea. This study was primarily focused on molecular identification of DEC pathotypes; their association with serogroups and estimates of resistance profiles against different antibiotics regime.
Methods
Five hundred seventy‐two stool specimens from diarrhea patients were investigated for DEC pathotypes. Molecular pathotypes were identified by amplification of virulence genes associated with distinct pathotypes followed by sequencing. Diarrhea is a self‐limiting disease, however, severity and persistence of infection suggest antibiotic use. Therefore, AST and MIC were determined against common antibiotic regimen. Correlations between molecular pathotypes and serogroups were analyzed by somatic “O” antigen serotyping.
Results
The present findings reveal incidence of DEC as an etiological agent up to a level of 21% among all diarrheal age groups. DEC infection rate was higher in children. Enteropathogenic E. coli EPEC, a molecular pathotype of DEC, was found as a predominant pathotype with highest frequency of 13.7%. Two other molecular pathotypes enterotoxigenic E. coli (ETEC) and enteroaggregative E. coli (EAEC) accounted for 5.7% and 1.3%, respectively for all diarrhea incidences. Serological analysis deciphered somatic antigens O26, O2, and O3 as major serogroups identified among EPEC, ETEC, and EAEC pathotypes, respectively. All DEC pathotypes exhibited high levels of antibiotic resistance except for cotrimoxazole and norfloxacin.
Conclusion
Comprehensive molecular characterization of DEC pathotypes, their incidence estimates, and antibiogram patterns will help in ascertaining better diagnostic and therapeutic measures in management of diarrheal diseases.
Keywords: antimicrobial resistance, childhood diarrhea, diarrhea, diarrheagenic E. coli, serogroups
1. INTRODUCTION
Diarrheal diseases are major cause of morbidity and mortality in low‐ to middle‐income countries and estimated to be second leading cause of mortality among children < 5 years of age, resulting in 0.5 million deaths globally.1 Sub‐Saharan and South East Asian regions account for highest burden of the disease (>72%).2 Unfortunately, India bears highest toll of the disease which demands acceleration in interventions for diarrhea prevention and cure.2 Despite the well‐known fact that diarrheal diseases are transmitted by fecal oral route3 inexorable outbreaks continue to be a scourge globally.4 Infectious milieu of diarrhea shed light on its multifactorial nature and vast array of disease etiology.5 Virulence arsenal of etiological agents varies with geographical features and become significantly evident in choice of treatment protocol. Therefore, for a successful treatment regime, identification of etiological agents is of utmost significance.
Escherichia coli is enormously versatile bacterium which elaborates its commensal and pathogenic potential in human host. Diarrheagenic E. coli (DEC) is reported as one of the leading causes of gastrointestinal disorders worldwide and signified as an important issue to address in public health.6, 7, 8, 9 In low‐ to middle‐income countries, >40% of diarrheal episodes among children are caused by diarrheagenic E. coli pathotypes.10 These pathotypes also play a considerable role in diarrhea morbidity in the Indian population.11, 12, 13 Remarkably, distinct DEC pathotypes display specific virulence arsenal which transforms the predominant repertoire available for diagnostic and therapeutic approaches. DEC is further catalogued into various pathotypes based upon occurrence of these unique virulence determinants contributing to specific pathophysiology,14 viz. Enteropathogenic E. coli (eae, bfpA), enterotoxigenic E. coli (eltB, and estA), enteroaggregative E. coli (pCVD), enterohemorrhagic E. coli (vt1 and vt2), and enteroinvasive E. coli (ial).14, 15, 16
Enteropathogenic E. coli (EPEC) is frequently associated with diarrhea incidences from both community and healthcare settings. EPEC has been categorized into atypical and typical EPEC by the presence of eae gene alone and simultaneous expression of bfpA and eae genes, respectively.14, 15
Enterotoxigenic E. coli (ETEC), another E. coli, has been reported as a significant pathogenic form responsible for diarrhea in travelers and population inhabiting endemic regions globally.12, 17, 18 ETEC in the stool specimen may be confirmed by amplification of two marker genes estA and eltB, which encode heat stable and heat labile secretory enterotoxins, respectively.
Since the last decade, several reports have been published for identification of adherent enteroaggregative E. coli (EAEC) as an emerging enteropathogen responsible for adult and childhood diarrhea worldwide.19, 20, 21, 22 The plasmid‐encoded gene probe pCVD which elucidate aggregative phenotype is utilized for identification of EAEC in diagnostic and epidemiological studies.12, 18, 23
Another molecular pathotypes of E. coli, enterohemorrhagic E. coli (EHEC), a subgroup of Shiga toxin‐producing E. coli and enteroinvasive E. coli (EIEC) cause a devastating form of gastrointestinal infections which may lead to severe life‐threatening complications like hemolytic uremic syndrome (HUS). EHEC colonizes large intestine and secretes toxins.14 EIEC invades small bowel enterocytes and is regarded a true intracellular pathogen. However, both EIEC and EHEC display low levels of incidences.6, 24
Escherichia coli serogrouping is used as a conventional method for pathogen characterization and diagnosis.14, 15 Besides, potential use of O antigen characterization, DEC associations with O antigens varies across different geographical regions.25, 26 As DEC pathotypes possess a large number of different “O” somatic antigen; therefore, their continuous monitoring is helpful in subtyping strains and enhancing phylogenetic studies.
As diarrheal disease is generally self‐limiting, antidiarrheal agents are not usually recommended for treatment of diarrhea.27 However, traveler's diarrhea, persistent diarrhea, and acute invasive diarrhea display high severity of infection and extended recovery periods which reinforce the use of antimicrobials such as ampicillin, norfloxacin, nalidixic acid, cefixime, and cotrimoxazole.27, 28, 29, 30
Several investigations have been conducted to study the prevalence of DEC pathotypes in different parts of India.12, 13, 31, 32 However, comprehensive study regarding DEC‐mediated diarrhea is sparse, and inclusive epidemiological studies are not available from Northern hilly regions of the country. This study focuses on investigating the prevalence of diarrheagenic E. coli pathotypes with exhibited serogroups in Himachal Pradesh, a northern hilly state of India. Molecular methods were utilized to better define DEC incidences, their etiology and clinical outcomes. Correlation of DEC pathotypes with different age groups and clinical symptoms was also analyzed. The study also encompasses resistance patterns of identified E. coli pathotypes, which will be useful in treatment regimes for tackling these specific pathogens.
2. MATERIALS AND METHODS
2.1. Culture media and reagents
MacConkey agar, eosin methylene blue agar, Muller Hinton agar, nutrient agar, LB broth, and agar for conventional culture techniques were purchased from Hi‐media Laboratories Pvt. Ltd., Mumbai, India. Agarose, saturated phenol, sodium acetate, antibiotic disks (ampicillin 10 μg, cefixime 5 μg, cotrimoxazole 75 μg, norfloxacin 10 μg, and nalidixic acid 30 μg), and E‐strips were also purchased from Hi‐media Laboratories Pvt. Ltd., Mumbai, India. PCR master mix and 100‐bp DNA ladder were purchased from Promega, Thermo Fisher Scientific, and New England Biolabs (NEB). Chloroform, isoamyl alcohol, and ethanol of analytical grades were purchased from Merck. Primers utilized in this study were purchased from Integrated DNA Technologies, Bangalore, India.
2.2. Study sites and clinical specimens
From February 2013 to April 2016, a total of 572 stool specimens of diarrheal patients aged between 13 days and 85 years were collected. Samples were collected from patients with primary complaint of three or more loose stools/day who were admitted to Regional hospital, Solan and tertiary care hospital Indira Gandhi medical college (IGMC), Shimla. Information on gender, age, geographic origin, and clinical symptoms was obtained by means of standard questionnaire. Patients presented with loose stool as chief complication but also reported to have other clinical manifestations such as dehydration, vomit, fever, abdominal pain, and mucus, in common. Written informed consents were taken from patients or patient's parent or legal guardians in case of children.
Stool specimens from diarrheal patients were collected in sterile containers and transported immediately to the laboratory after collection. All experiments included in the study were authorized by the Institutional Ethics Committee (IEC/Project no‐04‐2014).
2.3. Isolation and detection of DEC
The stool specimens were enriched in Luria Broth and streaked onto MacConkey agar, eosin methylene blue agar and incubated for 24 to 48 hours at 37°C. Typical lactose fermenting pink colored colonies from MacConkey agar were selected and subcultured on Luria‐Bertani agar. Following overnight incubation, lactose fermenting colonies were subjected to a series of standard biochemical tests; IMViC (indole, methyl red, Voges‐Proskauer, citrate), triple sugar iron agar, urease agar, and motility tests.18 Bacterial strains with characteristic IMViC pattern ++–– were biochemically characterized as E. coli strains.
2.4. DNA extraction and 16S rRNA gene characterization
Biochemically confirmed strains were initially molecularly characterized using 16S rRNA gene for E. coli.33 DNA extraction of E. coli strains was performed by phenol‐chloroform method. PCR thermo cycling conditions for 16S rRNA gene were standardized at following condition. Initial denaturation was performed at 94°C for 5 minutes, final denaturation 94°C for 30 seconds, primer annealing at 52°C for 30 seconds, and initial extension at 72°C for 30 seconds for 35 cycles, with a final extension of 7 minutes at 72°C. PCR was set up with 25 μl reaction mixture having 12.5 μL 2 × PCR Master Mix, 0.2 μmol/L of each primer, 300 ng/μL of template DNA, and nuclease‐free water. PCR products were evaluated with a 1.5% 1XTAE (Tris‐acetic acid‐ethylene diamine tetracetate buffer) agarose gel at 50 mV for 30 minutes. A 100‐bp molecular marker was run concurrently. PCR products were visualized under ultraviolet light trans‐illumination.
2.5. Molecular characterization of DEC pathotypes
DEC molecular pathotypes were identified by amplification of virulent genes. Primer sequences were selected from two published studies and are given in Table S1.17, 18 Initially, molecular pathotypes were amplified in a multiplex PCR followed by single gene PCR for identification and reproducibility of specific DEC pathotypes. Different molecular pathotypes were identified on the basis of amplification of following amplicons of genes; ETEC encoded heat stable (estA 147bp17, 18) and heat labile toxin (eltB of 322bp17 or 508 bp18) genes, EPEC encoded bundle pilus‐forming gene (bfpA 367bp17, 18) and intimin gene (eae of 83018 or 376 bp17 amplicon), EHEC encoded verocytotoxins (vt1 130bp17 and vt2 298bp17), EIEC encoded invasion gene (ial 320bp17), and EAEC plasmid encoded aggregative phenotype specific (pCVD 630bp17, 18) were targeted in the PCR. During EPEC pathotype identification, eae and bfpA genes amplify at 367bp and 376 bp, respectively,17 which could not be resolved by agarose gel electrophoresis; therefore, eae gene (830 bp) and bfpA gene (367 bp) were stringently amplified by single gene PCR. PCR thermocycling conditions for pathotypes were same as described above for amplification of 16S rRNA gene. Amplified PCR products were further confirmed by commercial Sanger sequencing at various time intervals during study. Sequenced DEC pathotypes were taken as positive control in PCR.
2.6. Serological characterization
Identification of bacterial somatic O antigen was performed by standard agglutination test using 176 “O”‐specific antisera.34 For serogroups characterization, biochemically and molecularly confirmed E. coli isolates were screened at National Salmonella and E. coli center at Central Research Institute, Kasauli (H.P.). Briefly, test strain was inoculated into 5 mL nutrient broth and incubated at 37°C for overnight with agitation. Bacterial growth was boiled at 100°C for one hour, and then formalin was added to a final concentration of 0.3% (Test antigen). For testing with pooled sera, 50 μL of 16 pools of O antisera was added to 96‐well plate. Then 50 μL of test antigen was added to each well. A negative control well was set up with 50 μL each of antigen and saline, respectively. Plates were incubated at 37°C overnight and observed for agglutination reaction. Test strain showing agglutination in all wells including negative control, strain was regarded “rough.” If agglutination was seen with single pool, then next agglutination test was set up with factor sera constituting the pool. But if agglutination was seen with more than one pool, then antigen was titrated against all sera constituting the pools. The test antigen which even did not show agglutination following antigen preparation at 121°C for 2½ hours was regarded as “untypeable.”
2.7. Antimicrobial susceptibility test (AST) and minimum inhibitory concentration determination (MIC)
The antimicrobial susceptibility of the PCR‐positive E. coli pathotypes was determined by standard Kirby Bauer's disk diffusion method35 against ampicillin (10 μg), cefixime (5 μg), cotrimoxazole (25 μg), nalidixic acid (30 μg), and norfloxacin (10 μg) according to CLSI and ICMR guidelines.30, 36 Minimum inhibitory concentrations for ampicillin (0.016‐256 μg), cefixime (0.016‐256 μg), cotrimoxazole (0.016‐256 μg), nalidixic acid (0.016‐256 μg), and norfloxacin (0.016‐256 μg) were determined using the E test. Escherichia coli ATCC 25922 was used as reference strains for quality control in AST and MIC tests. Results were interpreted according to CLSI and ICMR guidelines.30, 36
2.8. Statistical analysis
The age of the patients was classified into five groups, viz., <2 years, 3‐5 years, 6‐17 years, 18‐65 years, and >65 years. In statistical analysis >65 years age represented the most normative group because elderly from developing countries are more prone to diarrheal infection due to immunocompromised status.37 In a similar study by Dutta et al,12 2012, elderly age group comprising subjects >65 years of age were also taken as reference for comparative statistical analysis. Similarly, categorized DEC was compared among each age group with >65 years as reference group. Fisher's exact test was performed to establish mutual relatedness among the three types of DEC pathotypes. P values of ≤.05 were considered as statistically significant and calculated the odds ratio (OR) at the 95% confidence interval (CI). Chi‐square (bivariate analysis) was used to compare clinical symptoms in DEC‐positive and DEC‐negative populations.
3. RESULTS
3.1. Identification and molecular characterization of E. coli
During February 2013 to April 2016, a total of 572 stool specimens were collected from diarrheal patients admitted to regional (Govt. hospital Solan) and tertiary care hospital (Indira Gandhi Medical College, Shimla) in Himachal Pradesh, a hilly state of India. Hospitalized patients presented a very wide age group window ranging from 13 days to 85 years. A total of two hundred forty‐seven (n = 247) patients < 5 years of age and three hundred twenty‐five patients (n = 325) aged >5 years were analyzed in this study. Standard microbiological techniques and biochemical assays showed the presence of diarrheagenic E. coli in stool specimens of diarrhea patients.
3.2. Incidences of DEC molecular pathotypes in study population
Identification of diarrheagenic E. coli pathotypes was performed on the basis of biochemical, molecular, and serological assays. All biochemically characterized E. coli were also confirmed by 16S rRNA gene amplification (Figure S1A). Further, DEC molecular pathotypes were identified by amplification of virulence gene‐specific primers selected according to pathotype classification system devised by Nataro and Kaper14 (Figures S1B‐D). Overall, diarrheagenic E. coli accounted for a proportion of approximately 21% (n = 120/572) in hospitalized patients. Among distinct DEC pathotypes, EPEC (n = 79/572, 13.8%) was found to be predominant pathotype followed by ETEC (n = 33/572, 5.8%) and EAEC (n = 8/572, 1.4%). Pathotypes belonging to classes EHEC and EIEC of DEC were not found in analyzed specimens. The amplified products of PCR were further confirmed by sequencing, and partial coding sequences obtained were found to be 100% similar to targeted reference genes. The gene sequences were submitted to NCBI database (NCBI accessions: KX911251, KX911252, KX911253, and KX911255) and were utilized as positive control in subsequent PCR analysis (Data [Link], [Link], [Link], [Link], [Link], [Link]).
3.3. Distribution of virulent genomic elements among DEC molecular pathotypes
Characterization of DEC molecular pathotype was ascertained on amplification of either distinct gene or combination of genes (Table S1). In this study, eae gene of atypical EPEC (62.5% eae gene, n = 75/120) was most prevalent as compared to typical EPEC (3.3% eae & bfpA, n = 4/120). In case of ETEC‐infected patients, strains harboring estA (18.3%, n = 22/120) were more prevalent than strains possessing both estA and eltB genes (10%, n = 12). All EAEC strains (n = 8) possessed pCVD (6.6%, 8 of 120) gene.
3.4. Clinical symptoms Vs DEC molecular pathotypes
Clinical symptoms of DEC pathotype‐mediated infection vary from acute to persistent diarrhea, febrile, or afebrile, with or without symptoms of dehydration. Besides, loose stools as a common illness among study population, symptoms of fever, vomit, dehydration, mucus, and abdominal pain were also observed. To ascertain DEC molecular pathotype‐specific clinical symptoms, chi‐square analysis was performed, and P values and odds ratio (OR) at 95% confidence interval (CI) were calculated (Table 1). For comparison, DEC‐positive population (n = 120) was taken as positive control, and population without DEC infection (n = 452) was utilized as negative control. Symptoms of watery stools, visible mucus were found statistically associated with DEC pathotype infection. Other pathophysiological features such as vomiting, severe dehydration, and fever were also observed with higher frequency in EPEC and ETEC pathotypes, but similar cases were also observed in DEC‐negative population therefore statistically insignificant. While, EAEC infection was found primarily associated with the frequent bowl movements (>6 episodes of watery stool), fever, vomiting, and dehydration.
Table 1.
Comparative analysis of clinical features associated with DEC‐positive and DEC‐negative patients by chi‐square test
| Clinical symptoms observed | DEC positive (n = 120,%) | DEC negative (n = 452,%) | P value (at 95% CI) |
|---|---|---|---|
| Vomiting | 30 (25) | 109 (24.1) | .840 |
| Fever | 25 (20.8) | 62 (13.7) | .0536 |
| Dehydration | 25 (20.8) | 107 (23.6) | .5117 |
| Watery diarrhea | 20 (16.6) | 11 (2.4) | .0001a |
| Mucus | 6 (5) | 4 (0.8) | .0022a |
| Abdominal pain | 3 (2.5) | 6 (1.3) | .3589 |
Statistically significant values.
3.5. Age group distribution of DEC molecular pathotypes
For determination of high‐risk age groups, study population was stratified into five various age groups, viz. children 0‐2 years (n = 202) and 3‐5 years (n = 45), adolescent 6‐17 years (n = 41), adult 18‐65 years (n = 257), and elderly >65 years (n = 27). Our study revealed uniform abundance of EPEC and ETEC infections in all age segments, however, children < 5 years (<2 years & 3‐5 years) of age showed higher incidence rates as compared to any other age group (Figure 1). Although EAEC pathotype was detected with low frequency, but enteropathogen was predominantly found in children population (5.2%, 7/572) as compared to adult diarrheal patients (0.3%, 1/572) (Figure 1).
Figure 1.
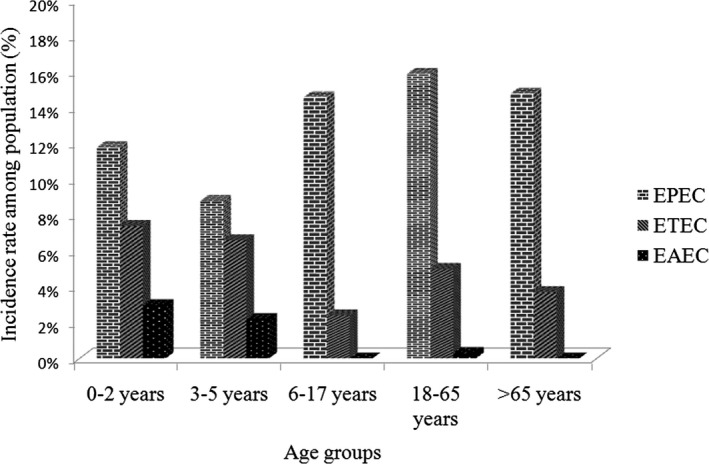
Prevalence of diarrheagenic Escherichia coli (DEC) pathotypes among different age groups. X‐axis represents different age groups under study, and Y‐axis represents proportions of different pathotypes. EPEC= Enteropathogenic E. coli, ETEC= Enterotoxigenic E. coli, EAEC= Enteroaggregative E. coli
To recognize specificity of any DEC pathotype to particular age groups, bivariate Fisher analysis was performed (Table 2). Statistically significant correlations were observed for EPEC, ETEC, and EAEC pathotypes with those of children <2 years of age. However in adult age group (17‐65 years), only EPEC and ETEC prevalence were correlated significantly.
Table 2.
Bivariate analysis of age wise distribution of diarrheagenic Escherichia coli pathotypes using Fisher's exact test
| Age group | DEC pathotype | Odd Ratio (at 95% CI) | P‐ value |
|---|---|---|---|
| 0‐2 yrs | EPEC (24/79) | 8.182 (2.684‐24.94) | .0001a |
| ETEC (15/33) | 26.67 (3.248‐219) | .0001a | |
| EAEC (6/8) | 44.20 (1.794‐1089) | .0070a | |
| 3‐5 yrs | EPEC (4/79) | 1.00 (0.2411‐4.418) | 1.0000 |
| ETEC (3/33) | 3.1 (0.3050‐31.50) | .6312 | |
| EAEC (1/8) | 3.4 (0.1194‐96.78) | 1.0000 | |
| 6‐17 yrs | EPEC (6/79) | 1.541 (0.4175‐5.688) | .7480 |
| ETEC (1/33) | 1.000 (0.05988‐16.70) | 1.0000 | |
| 18‐65 yrs | EPEC (41/79) | 20.23 (6.743‐60.69) | <.0001a |
| ETEC (13/33) | 20.80 (2.522‐171.5) | .0005a | |
| EAEC (1/8) | 3.400 (0.1194‐96.78) | 1.0000 | |
| >65 yrs as reference category | |||
Statistically significant values.
3.6. Serogroup analysis of DEC pathotypes
Molecular pathotypes of DEC were characterized for E. coli somatic O antigen and were found associated with at least twenty‐three different O serogroups (Figure 2). Serologic analysis revealed 60.8% (73/120) of E. coli isolates as diarrhea‐associated serotypes. Serogroups O2, O26, O35, and O41 were the most commonly characterized with a prevalence of 41% (30/73).
Figure 2.
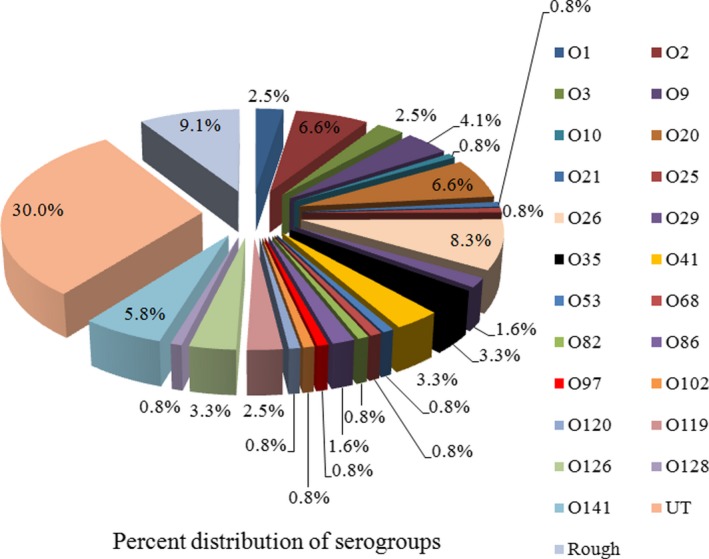
Distribution of different O serogroup among diarrheagenic Escherichia coli (DEC) isolates. UT (untypeable), Rough (Nonagglutinable with antisera)
Figure 3 shows O26 and O2 as most commonly isolated among EPEC and ETEC pathotypes, respectively. O26 is often associated with classical attaching and effacing group (EPEC) and non‐O157‐EHEC strains.14 We observed serogroups O2, O25, and O128 in ETEC strains only. Apart from classical serogroups, EAEC predominantly belonged to serogroup O3 (2.5%). However, higher proportions of strains belonging to EPEC, ETEC, and EAEC pathotypes remained untypeable (30%) and did not agglutinate with O antiserum (9%).
Figure 3.
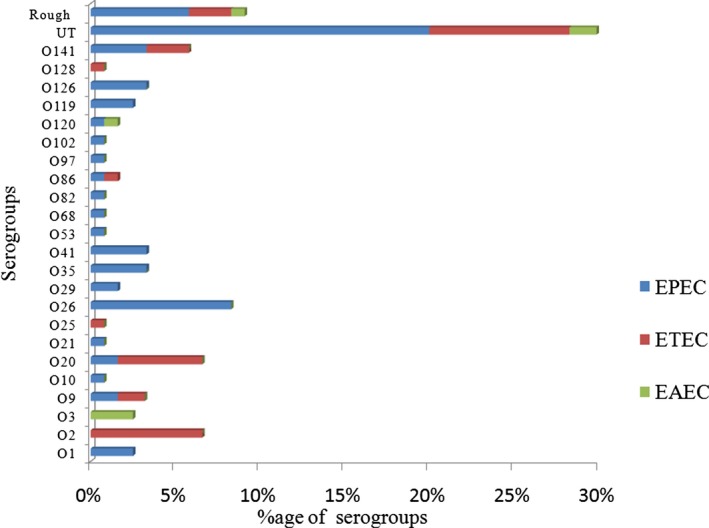
Relationship of virulent genotype with serogroups of diarrheagenic Escherichia coli strains characterized from diarrhea patients
Correlation between DEC virulent genes and O serogroups revealed eae gene of EPEC was most commonly associated with more number of O serogroups (Figure 4). estA and eltB genes of ETEC toxins were observed with O2, O20, O25, O102, and O141 serogroups while serogroup O9 was observed with estA only. The pCVD gene of EAEC was found to be associated majorly with one serogroup O3.
Figure 4.
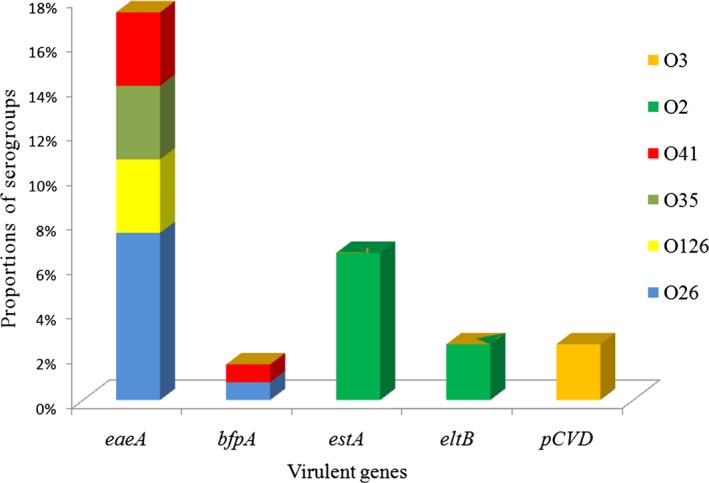
Representing association of diarrheagenic Escherichia coli (DEC) virulent genes with top six O serogroups
3.7. Antimicrobial resistance in DEC molecular pathotypes
Several studies have been performed for analyzing antibiotic resistance patterns among diarrheagenic E. coli isolates.27, 28, 29, 30 Therefore, molecular pathotypes of DEC were also screened for antibiogram patterns by antimicrobial susceptibility test (AST) and minimum inhibitory concentration (MIC) according to CLSI guidelines.36 Antibiotics utilized in screening were chosen on the basis of ICMR recommendations30 (Figure S2A & Figure S2B). AST and MIC revealed that a majority of diarrheagenic E. coli strains were sensitive for cotrimoxazole (36%), while <20% were sensitive for cefixime, norfloxacin, ampicillin, and nalidixic acid (Table 3). Proportions of intermediate strains against all five antibiotic were <5%. DEC pathotypes exhibited alarming rates of resistance against widely used antimicrobials; ampicillin, cefixime, nalidixic acid, and norfloxacin (approximately 80%).
Table 3.
Antibiotic resistance among the different diarrheagenic Escherichia coli groups in patients with diarrhea
| Characteristics (Resistant) | P value | OR (95% CI) | |
|---|---|---|---|
| AMP | EPEC 82.2% (65) | .0026** | 2.792 (1.456‐5.353) |
| ETEC 78.7% (25) | < .0001** | 3.473 (1.866‐6.462) | |
| EAEC 75% (6) | < .0001** | 4.895 (2.668‐8.979) | |
| CPM | EPEC 77.2% (61) | .0311* | 2.052 (1.108‐3.801) |
| ETEC 81.8% (26) | .0004** | 3.329 (1.721‐6.440) | |
| EAEC 75% (6) | < .0001** | 4.895 (2.668‐8.979) | |
| NAL | EPEC 79.7% (67) | .0078** | 2.452 (1.299‐4.627) |
| ETEC 81.8% (27) | < .0001** | 4.205 (2.209‐8.005) | |
| EAEC 62.5% (5) | < .0001** | 3.807 (2.078‐6.974) | |
| NOR | EPEC 79.7% (67) | .0078** | 2.452 (1.299‐4.627) |
| ETEC 69.6% (22) | .0135* | 2.154 (1.205‐3.849) | |
| EAEC 62.5% (5) | < .0001** | 3.807 (2.078‐6.974) | |
| COT | EPEC 62% (49) | Reference category | |
| ETEC 51.5% (16) | |||
| EAEC 37.5% (3) | |||
AMP, ampicillin; COT, cotrimoxazole; CPM, cefixime; NOR, norfloxacin, and NAL, nalidixic acid. Statistically significant values (*P < .05, ** P < .01 (chi‐square test), for the comparison of the resistance percentage among the different E. coli).
On correlating resistance pattern with distinct DEC pathotype, EPEC strains were observed to be most resistant against all tested drugs. ETEC strains were found 18‐49% sensitive against all tested antibiotics. EAEC pathotypes showed highest sensitivity for cotrimoxazole (62.5%), norfloxacin (37.5%), nalidixic acid, and cefixime (25% in both). Table 3 showed statistical analysis using chi‐square test performed to evaluate pathotype‐specific antibiotic resistance level. All molecular DEC pathotypes were found to possess significant levels of antibiotic resistance against all antibiotics.
4. DISCUSSION
Successful interventions in management of infectious diseases need identification of the etiological agent and treatment of clinical symptoms manifested during disease. Diarrhea is a multifactorial illness associated with wide spectrum of pathogens including viruses, bacteria, and parasites.38, 39 Conventional microbiological techniques combined with molecular identification system incredibly increases reproducibility and scalability of etiological agent characterization.40 However, in context of diarrhea, the viral agents have been explored to a greater extent as compared to others.41, 42, 43 By virtue of phenotypic and genotypic attributes, E. coli elaborates capacities from important gut commensal to pathogen of intestinal as well as extraintestinal infections.15, 44 E. coli possesses a repertoire of virulent elements which lead to segregation of this bacterium into diverse kinds of pathotypes and genotypes. Lack of uniform surveillance system for bacterial pathogens underestimates their role in diarrhea incidences. In our previous study, we have deciphered the role of viral agents of diarrhea in Himachal Pradesh, a Northern hilly state of India.45 The current study was aimed at elucidating the frequency of DEC pathotypes using virulence gene markers in moderate‐to‐severe diarrhea population of Himachal Pradesh. Prior to this study, there have been no reports from present region addressing DEC incidences. Therefore, it is of utmost importance to address DEC‐associated diarrheal incidences to provide a comprehensive view of diarrhea etiology within the region which will facilitate further epidemiological and therapeutic prospects.
The diarrhea study cases involved in comprehensive investigation belonged to a broad window of age, from 13 days to 85 years. Therefore, the study population was stratified into five different age groups (0‐2 years, 3‐5 years, 6‐17 years, 18‐65 years, and >65 years), and age group >65 years was taken as reference group for statistical analysis. The incidence rates of diarrheagenic E. coli were observed up to 21% as the sole pathogen and approximately 6% as mixed infection with Rotavirus. Our study shows moderate DEC infection rates, similar to the reports from developing world.46, 47, 48, 49 However, reports from other parts of India and neighboring countries showed 10‐35% variation in DEC incidence rates.11, 12, 13, 50, 51 Sporadic outbreaks with 42% to 65% of incidences are also reported from different regions of India.52, 53, 54 Globally, prevalence of E. coli as an etiological agent of diarrhea is well reported between 30% and 40% cases.55, 56 DEC coinfection with other enteric pathogens is greatly known to aggravate symptoms and duration of diarrhea.50, 57
Our observations indicate higher proportions of DEC pathotypes associated with childhood diarrhea than any other age set. In a recent study conducted in Mexico, Canizalez‐Roman and coworkers49 also reported higher DEC incidences in children population. In addition, higher frequencies of DEC pathotypes in moderate‐to‐severe cases of childhood diarrhea are reported all over the globe.5, 12, 29, 56, 58 Previous studies established that DEC preponderance among children may be due to their compromised immune level and intimate attachment of pathogens to the tender epithelial mucosa.14, 59 DEC infection‐induced alterations in intestinal physiology and microbiota composition remain restricted to the postnatal period also.59 Therefore, DEC infection might predispose children < 5 years to sequelae of diarrheal episodes.
By molecular identification approach, DEC molecular pathotype EPEC is observed with highest frequency among all diarrheal patients. Recurrent isolation of EPEC from severe diarrhea cases is implicated especially in pediatric populations.60 Persistent diarrhea is the most common clinical presentation in EPEC infection, and this enteropathogen possesses an innate propensity to persist longer in intestine than other pathotypes.14 EPEC is typically categorized into two classes, atypical EPEC having eae gene and typical EPEC possess combination of bfpA and eae genes.61 High frequencies of the eae gene in current study underpin the importance of atypical EPEC as predominant diarrheal pathogen in the region. Low frequency of bfpA observed in the present study suggests its fewer incidence rates in the population similar to the previous reports.62, 63 Both eae and bfpA genes are responsible for intimate attachment to the surfaces via intimin and bundle‐forming pilus. In addition, EPEC also possesses different combination of fimbriae and type III secretion system protein for producing attaching and effacing phenotypes. On global level, EPEC alone contributes for 5%‐10% cases of pediatric diarrhea.58, 61, 64 Our observations coincide with various epidemiological studies from different parts of world which reported EPEC as the main DEC pathotype affecting children and adults with similar frequency.65, 66, 67, 68
Another DEC molecular pathotype ETEC‐specific clinical outcomes rely upon the secretion of two enterotoxins, viz heat labile (estA gene) and heat stable (eltB gene) toxins. These toxins result in secretory diarrhea via Cl− secretion through the cystic fibrosis transport receptor (CFTR) and cyclic guanosine monophosphate (cGMP).14 Among the ETEC‐positive patients, estA gene was more frequently isolated than eltB alone, or estA and eltB in combination are similar to other studies.12, 69 For many years, ETEC has been implicated as the major cause of traveler's diarrhea70. In the present study, ETEC showed varying prevalence among all ages, and similar observations were reported from the northern part of the country.69, 71
EAEC pathotype is known to cause disease via multiple mechanisms; adherence to mucosa, secretion of toxins, and mucosal inflammation.22 The EAEC enteropathogen was identified by using pCVD gene probe. We observed EAEC predominantly in children (n = 7/8) followed by elderly age group (1/8). Other studies have also shown prevalence of pCVD‐positive E. coli in the stool specimens of adults and childhood diarrhea, and this can be as high as 11%.72 Current findings strengthen evidences that EAEC is an emerging diarrheal agent in the South East Asian children population.73
As different DEC molecular pathotypes exhibit surface to invasive pathophysiology resulting in different clinical outcomes. We found that clinical symptoms of watery stools and mucus were significantly associated with DEC pathotype infection. Present observations reinforce the conviction that DEC pathotype is considerably responsible for severe gastrointestinal infections associated with childhood and adult diarrhea.39
Characterization of E. coli somatic “O” antigen still appears to be useful technique for conventional identification of certain DEC pathotypes.14, 74, 75, 76 The serogroup O26 was most commonly observed followed by O2, O41, O35, O126, and O1. Similar to our study, serogroups O26, O2 were found to be associated severe diarrhea cases.61 Interestingly, few isolates belong to untypeable or rough classes in various categories of DEC pathotypes. From the literature, E. coli serogroups are much related to identification of clonal variant of DEC pathotypes rather than precise identification.14, 15, 75, 76
The global spread of antimicrobial resistant strains threatens the effective prevention and treatment of enteric infections caused by Gram‐negative bacteria. E. coli has become increasingly resistant to conventional and commonly used antibiotics in hospital and community settings,26, 77 and certainly poses serious threat to the management of infectious diseases.
We examined the DEC pathotypes resistance against five antibiotics: ampicillin, cefixime, norfloxacin, nalidixic acid, and cotrimoxazole belonging to class quinolones and β‐lactams. These antibiotics are in accordance with Centre for Disease Control and Prevention and also advised by ICMR.27, 30 In the present study, EPEC was found as most the resistant pathotype, and highest levels of antibiotic resistance were observed against ampicillin. These observations are concordance with previous studies analyzing DEC resistance.78, 79 We observed lowest resistance rates against cotrimoxazole among all DEC pathotypes. However, Sadeghabadi and coworkers reported approximately 80% resistance against cotrimoxazole in diarrheagenic E. coli. Similar reports across the globe also elucidated high levels of resistance against DEC pathotypes.71, 79, 80, 81, 82 Although in our study, proportions of DEC as diarrheal pathogen are limited to a moderate level; however, current study revealed high levels of resistance among DEC pathotypes in hospitalized patients. The observed high resistance rates to antibiotics may be a result of extreme disease severity and persistence of infections among hospitalized patients.
The present study is to our knowledge, the first comprehensive research in the region addressing associations of molecular DEC pathotypes with clinical outcomes and antibiogram patterns. Our findings highlight the importance of continuous DEC pathotype surveillance programs for therapeutic approaches and not the least the benefit of employing comprehensive inspection of antimicrobial resistance in the region. In relation to treatment, a very few studies have evaluated comprehensive importance of drugs for the management of DEC pathotype infection. After introduction of the rotavirus vaccines into national immunization program of India, the next priority must be to identify diarrheal pathogens owing to high morbidity and mortality rates. The study would help in prioritizing diagnostic and therapeutic measures against predominant DEC pathotypes. Exploring the resistant phenotypes would aid in management and spread of multidrug‐resistant strains.
AUTHOR'S CONTRIBUTIONS
Jitendraa Vashistt and Harish Changotra designed the study and fieldwork. Nutan Thakur and Swapnil Jain performed fieldwork with assistance from Neelam Grover in stool specimens collection and transport. Nutan Thakur carried out laboratory research experiments and manuscript writing. Yashwant Kumar performed serogrouping of E. coli strains. Rahul Shrivastava was involved in protocol designing and data presentation.
Supporting information
ACKNOWLEDGMENTS
The authors would like to acknowledge Indian Council of Medical Research, New Delhi, India, for providing financial grants (5/9‐1(26) 2011‐12 ECD‐II) to carry out research work and would like to thank the staff of the Department of pediatrics IGMC, Shimla & regional govt. hospital, Solan, for their diligent support during sample collection.
Thakur N, Jain S, Changotra H, et al. Molecular characterization of diarrheagenic Escherichia coli pathotypes: Association of virulent genes, serogroups, and antibiotic resistance among moderate‐to‐severe diarrhea patients. J Clin Lab Anal. 2018;32:e22388 10.1002/jcla.22388
REFERENCES
- 1. Diarrhoeal disease. [Cited 6 May 17]. Available from: http://www.who.int/mediacentre/factsheets/fs330/en/
- 2. Diarrhoea remains a leading killer of young children, despite the availability of a simple treatment solution. [Cited 6 May 17]. Available from: https://data.unicef.org/topic/child-health/diarrhoeal-disease/#
- 3. Amézquita‐Montes Z, Tamborski M, Kopsombut UG, et al. Genetic relatedness among Escherichia coli pathotypes isolated from food products for human consumption in Cartagena, Colombia. Food Patho & Dis. 2015;12:454‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee WC, Kwon YH. Comparative study on the epidemiological aspects of enterohemorrhagic Escherichia coli infections between Korea and Japan, 2006 to 2010. Korean J Intern Med. 2016;31:579‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kotloff KL, Blackwelder WC, Nasrin D, et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. CID. 2012;55:S232‐S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frank C, Werber D, Cramer JP, et al. Epidemic profile of Shiga‐toxin–producing Escherichia coli O104: H4 outbreak in Germany. N Engl J Med. 2011;365:1771‐1780. [DOI] [PubMed] [Google Scholar]
- 7. Radosavljevic V, Finke EJ, Belojevic G. Analysis of Escherichia coli O104: h4 Outbreak in Germany in 2011 Using Differentiation Method for Unusual Epidemiological Events. Cent Eur J Public Health. 2016;24:9‐15. [DOI] [PubMed] [Google Scholar]
- 8. Guzman‐Hernandez R, Contreras‐Rodriguez A, Hernandez‐Velez R, et al. Mexican unpasteurised fresh cheeses are contaminated with Salmonella spp., non‐O157 Shiga toxin producing Escherichia coli and potential uropathogenic E. coli strains: a public health risk. Int J Food Microbiol. 2016;237:10‐16. [DOI] [PubMed] [Google Scholar]
- 9. Ochi S, Shah M, Odoyo E, et al. An Outbreak of Diarrhea in Mandera, Kenya, Due to Escherichia coli Serogroup O‐Nontypable Strain That Had a Coding Gene for Enteroaggregative E. coli Heat‐Stable Enterotoxin 1. ASTMH. 2016;96:457‐464. 16‐0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miliwebsky E, Schelotto F, Varela G, et al. Human diarrheal infections: diagnosis of diarrheagenic Escherichia coli pathotypes In: Torres AG, ed. Escherichia Coli in the Americas. Cham, Switzerland: Springer International Publishing; 2016:343‐369 [Google Scholar]
- 11. Shetty VA, Kumar SH, Shetty AK, et al. Prevalence and characterization of diarrheagenic Escherichia coli isolated from adults and children in Mangalore, India. J Lab Phy. 2012;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dutta S, Guin S, Ghosh S, et al. Trends in the prevalence of diarrheagenic Escherichia coli among hospitalized diarrheal patients in Kolkata, India. PloS One. 2013;8:e56068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suganya D, Kanimozhi K, Panneerselvam A, et al. Molecular Characterization of Diarrheagenic Escherichia coli from Tiruchirappalli District, Tamil Nadu, India. Int J Curr Microbiol App Sci. 2016;5:478‐484. [Google Scholar]
- 14. Nataro JP, Kaper JB. Diarrheagenic Escherichia coli . Clin Microbiol Rev. 1998;11:142‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli . Nature Rev Microbiol. 2004;2:123‐140. [DOI] [PubMed] [Google Scholar]
- 16. Feng P, Weagant SD, Jinneman K. BAM: diarrheagenic Escherichia coli. 2014. Available from: https://www.fda.gov/food/foodscienceresearch/laboratorymethods/ucm070080.htm
- 17. Nguyen TV, Le Van P, Le Huy C, et al. Detection and characterization of diarrheagenic Escherichia coli from young children in Hanoi, Vietnam. J Clin Microbiol. 2005;43:755‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Panchalingam S, Antonio M, Hossain A, et al. Diagnostic microbiologic methods in the GEMS‐1 case/control study. CID. 2012;55:S294‐S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Weintraub A. Enteroaggregative Escherichia coli: epidemiology, virulence and detection. J Med Microbiol. 2007;56:4‐8. [DOI] [PubMed] [Google Scholar]
- 20. Okhuysen PC, DuPont HL. Enteroaggregative Escherichia coli (EAEC): a cause of acute and persistent diarrhea of worldwide importance. JID. 2010;202:503‐505. [DOI] [PubMed] [Google Scholar]
- 21. Jensen BH, Olsen KE, Struve C, et al. Epidemiology and clinical manifestations of enteroaggregative Escherichia coli . Clin Microbiol Rev. 2014;27:614‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elias WP, Navarro‐Garcia F. Enteroaggregative Escherichia coli (EAEC) In Torres AG, ed. Escherichia Coli in the Americas. Cham, Switzerland: Springer; 2016:27‐57. [Google Scholar]
- 23. Debroy C, Brigh B, Wilson R, et al. Plasmid‐coded DNA fragment developed as a specific gene probe for the identification of enteroaggregative Escherichia coli . J Med Microbiol. 1994;41:393‐398. [DOI] [PubMed] [Google Scholar]
- 24. Rasko DA, Webster DR, Sahl JW, et al. Origins of the Escherichia coli strain causing an outbreak of hemolytic–uremic syndrome in Germany. New Eng J Med. 2011;365:709‐717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campos LC, Franzolin MR, Trabulsi LR. Diarrheagenic Escherichia coli categories among the traditional enteropathogenic E. coli O serogroups: a review. Mem Inst Oswaldo Cruz. 2004;99:545‐552. [DOI] [PubMed] [Google Scholar]
- 26. Tahden M, Manitz J, Baumgardt K, et al. Epidemiological and Ecological Characterization of the EHEC O104: H4 Outbreak in Hamburg, Germany, 2011. PLoS ONE. 2016;11:e0164508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen LH, Hochberg NS, Magill AJ. The Pretravel Consultation; Centre for Disease Control and Prevention. https://wwwnc.cdc.gov/travel/yellowbook/2018/the-pre-travel-consultation/the-pre-travel-consultation.
- 28. O'Ryan M, Prado V, Pickering LK. A millennium update on pediatric diarrheal illness in the developing world. Semin Pediatr Infect Dis. 2005;16:125‐136. [DOI] [PubMed] [Google Scholar]
- 29. Dupont A, Sommer F, Zhang K, et al. Age‐dependent susceptibility to enteropathogenic Escherichia coli (EPEC) infection in mice. PLoS Pathog. 2016;12:e1005616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Standard operative procedures bacteriology. 2015. Available from: www.icmr.nic.in/Publications/SOP/SOP_Bacteriology.pdf
- 31. Dutta S, Pazhani GP, Nataro JP, et al. Heterogenic virulence in a diarrheagenic Escherichia coli: evidence for an EPEC expressing heat‐labile toxin of ETEC. Int J Med Microbiol. 2015;305:47‐54. [DOI] [PubMed] [Google Scholar]
- 32. Singh T, Das S, Ramachandran V, et al. Antimicrobial resistance, phylogenetic distribution and molecular docking of integrons in multidrug resistant diarrheagenic Escherichia coli isolates from children under five in Delhi, India. Int J Infect Dis. 2016;45:68‐69. [Google Scholar]
- 33. Sabat G, Rose P, Hickey WJ, Harkin JM. Selective and sensitive method for PCR amplification of Escherichia coli 16S rRNA genes in soil. Appl Environ Microbiol. 2000;66:844‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ørskov F, Ørskov I. “Serotyping of Escherichia coli,” In: Bergan T, ed. Methods in Microbiology. Vol. 14 ed. London, UK: Academic Press; 1984:43‐112. [Google Scholar]
- 35. Bauer AW, Perry DM, Kirby WM. Single‐disk antibiotic‐sensitivity testing of staphylococci: an analysis of technique and results. AMA Arch Intern Med. 1959;104:208‐216. [DOI] [PubMed] [Google Scholar]
- 36. Patel JB, Cockerill FR, Bradford PA, et al. Performance standards for antimicrobial susceptibility testing; Twenty‐Fifth Informational Supplement. CLSI. 2015;3:1‐240. [Google Scholar]
- 37. Zhang SX, Zhou YM, Xu W, et al. Impact of co‐infections with enteric pathogens on children suffering from acute diarrhea in southwest China. Infect Dis Pov. 2016;5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151‐2161. [DOI] [PubMed] [Google Scholar]
- 39. Saeed A, Abd H, Sandstrom G. Microbial aetiology of acute diarrhoea in children under five years of age in Khartoum, Sudan. J Med Microbiol. 2015;64:432‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case‐control study. Lancet. 2013;382:209‐222. [DOI] [PubMed] [Google Scholar]
- 41. Badur M, Latha NM, Kumar PR, et al. Prevalence of Rotavirus diarrhea among under‐5 hospitalized children in a Government tertiary hospital, Tirupati. J Dr NTR Uni Health Sci. 2015;4:112. [Google Scholar]
- 42. Kang G. Rotavirus in India: forty years of research. Indian Pediatr. 2016;53:569‐573. [DOI] [PubMed] [Google Scholar]
- 43. Sarkar R, Gladstone B, Warier J, et al. Rotavirus and other diarrheal disease in a birth cohort from Southern Indian community. Indian Pediatr. 2016;53:583‐588. [DOI] [PubMed] [Google Scholar]
- 44. Leimbach A, Hacker J, Dobrindt U. Escherichia coli as an all‐rounder: The thin line between commensalism and pathogenicity. Curr Top Microbiol Immunol. 2013;358:3‐32. [DOI] [PubMed] [Google Scholar]
- 45. Jain S, Thakur N, Grover N, et al. Prevalence of rotavirus, norovirus and enterovirus in diarrheal diseases in Himachal Pradesh, India. Virus Dis. 2016;27:77‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alikhani MY, Hashemi SH, Aslani MM, et al. Prevalence and antibiotic resistance patterns of diarrheagenic Escherichia coli isolated from adolescents and adults in Hamedan, Western Iran. Iran J Microbiol. 2013;5:42‐47. [PMC free article] [PubMed] [Google Scholar]
- 47. Heidary M, Momtaz H, Madani M. Characterization of diarrheagenic antimicrobial resistant Escherichia coli isolated from pediatric patients in Tehran, Iran. Iran Red Crescent Med J. 2014;16:e12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Paredes‐Paredes M, Okhuysen PC, Flores J, et al. Seasonality of diarrheagenic Escherichia coli pathotypes in the US students acquiring diarrhea in Mexico. J Travel Med. 2011;18:121‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Canizalez‐Roman A, Flores‐Villaseñor HM, Gonzalez‐Nuñez E, et al. Surveillance of Diarrheagenic Escherichia coli strains isolated from diarrhea cases from children, adults and elderly at northwest of Mexico. Front Microbiol. 2016;7:1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhu XH, Tian L, Cheng ZJ, et al. Viral and bacterial etiology of acute diarrhea among children under 5 Years of Age in Wuhan, China. Chinese Med J. 2016;129:1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Younas M, Siddiqui F, Noreen Z, et al. Characterization of enteropathogenic Escherichia coli of clinical origin from the pediatric population in Pakistan. Trans R Soc Trop Med Hyg. 2016;110:414‐420. [DOI] [PubMed] [Google Scholar]
- 52. Rajendran P, Ajjampur SSR, Chidambaram D, et al. Pathotypes of diarrheagenic Escherichia coli in children attending a tertiary care hospital in South India. Diagn Microbiol Infect Dis. 2010;68:117‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sudershan R, Kumar RN, Kulkarni B, et al. Escherichia coli pathotypes and their antibiotic resistance in young children with diarrhea in Hyderabad, India. Int J Curr Microbiol App Sci. 2014;3:647‐654. [Google Scholar]
- 54. Purwar S, Bhattacharya D, Metgud S, et al. A cross‐sectional study on aetiology of diarrhoeal disease, India. Indian J Med Microbiol. 2016;34:375. [DOI] [PubMed] [Google Scholar]
- 55. Behiry IK, Abada EA, Ahmed EA, et al. Enteropathogenic Escherichia coli associated with diarrhea in children in Cairo, Egypt. Sci World J. 2012;11:2613‐2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Acosta GJ, Vigo NI, Durand D, et al. Diarrheagenic Escherichia coli: prevalence and pathotype distribution in children from Peruvian rural communities. Am J Trop Med Hyg. 2016;95:574‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tobias J, Kassem E, Rubinstein U, et al. Involvement of main diarrheagenic Escherichia coli, with emphasis on enteroaggregative E. coli, in severe non‐epidemic pediatric diarrhea in a high‐income country. BMC Infect Dis. 2015;15:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patzi Vargas S, Zaidi MB, Perez Martinez I, et al. Diarrheagenic Escherichia coli carrying supplementary virulence genes are an important cause of moderate to severe diarrhoeal disease in Mexico. PLoS Negl Trop Dis. 2015;9:e0003510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ochoa TJ, Contreras CA. Enteropathogenic Escherichia coli (EPEC) infection in children. Curr Opinion. Infect Dis. 2011;24:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kallonen T, Boinett CJ. EPEC: a cocktail of virulence. Nature Rev Microbiol. 2016;14:196‐196. [DOI] [PubMed] [Google Scholar]
- 61. Wang L, Wakushima M, Aota T, et al. Specific properties of enteropathogenic Escherichia coli isolates from diarrheal patients and comparison to strains from foods and fecal specimens from cattle, swine, and healthy carriers in Osaka City, Japan. Appl & Env Microbiol. 2013;79:1232‐1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cabal A, García‐Castillo M, Cantón R, et al. Prevalence of Escherichia coli virulence genes in patients with diarrhea and a subpopulation of healthy volunteers in Madrid, Spain. Front Microbiol. 2016;7:641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dias RC, Santos BC, Santos LF, et al. Diarrheagenic Escherichia coli pathotypes investigation revealed atypical enteropathogenic E. coli as putative emerging diarrheal agents in children living in Botucatu, São Paulo State, Brazil. APMIS. 2016;124:299‐308. [DOI] [PubMed] [Google Scholar]
- 64. Sunaifa SM, Roy S, Dhanashree B. Prevalence of Enteropathogenic Escherichia coli (EPEC) in Adult Diarrhea Cases and their Antibiotic Susceptibility Pattern. British Microbiol Res J. 2015;8:560‐566. [Google Scholar]
- 65. Lanata CF, Fischer‐Walker CL, Olascoaga AC. Global causes of diarrheal disease mortality in children< 5 years of age: a systematic review. PLoS ONE. 2013;8:e72788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Taniuchi M, Sobuz SU, Begum S, et al. Etiology of diarrhea in Bangladeshi infants in the first year of life analyzed using molecular methods. JID. 2013;208:1794‐1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Becker‐Dreps S, Bucardo F, Vilchez S, et al. Etiology of childhood diarrhea following rotavirus vaccine introduction: a prospective, population‐based study in Nicaragua. Pediatr Infect Dis J. 2014;2014:1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Platts‐Mills JA, Babji S, Bodhidatta L, et al. Pathogen‐specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL‐ED). Lancet Glob Health. 2015;3:e564‐e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chandra BK, Singh G, Taneja N, et al. Diarrhoeagenic Escherichia coli as a predominant cause of paediatric nosocomial diarrhoea in India. J Med Microbiol. 2012;61:830‐836. [DOI] [PubMed] [Google Scholar]
- 70. Vilchez S, Becker‐Dreps S, Amaya E, et al. Characterization of enterotoxigenic Escherichia coli strains isolated from Nicaraguan children in hospital, primary care and community settings. J Med Microbiol. 2014;2012:729‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rajeshwari K, Uppal B, Singh R, et al. Multidrug resistant enteropathogenic Escherichia coli diarrhea in children. Am J Res Commun. 2015;3:27‐48. [Google Scholar]
- 72. Aslani MM, Alikhani MY, Zavari A, et al. Characterization of enteroaggregative Escherichia coli (EAEC) clinical isolates and their antibiotic resistance pattern. Int J Infect Dis. 2011;2011:e136‐e139. [DOI] [PubMed] [Google Scholar]
- 73. Pabalan N, Singian E, Jarjanazi H, et al. Enteroaggregative Escherichia coli and acute diarrhea in children: a meta‐analysis of South Asian populations. Eur J Clin Microbiol Infect Dis. 2013;32:597‐607. [DOI] [PubMed] [Google Scholar]
- 74. Blanco M, Blanco JE, Blanco J, et al. Distribution and characterization of faecal verotoxin‐producing Escherichia coli (VTEC) isolated from healthy cattle. Veter Microbiol. 1997;54:309‐319. [DOI] [PubMed] [Google Scholar]
- 75. Cho SH, Shin HH, Choi YH, et al. Enteric bacteria isolated from acute diarrheal patients in the Republic of Korea between the year 2004 and 2006. J Microbiol. 2008;46:325. [DOI] [PubMed] [Google Scholar]
- 76. Tilak GP. Role of enteropathogenic Escherichia coli in paediatric diarrhoeas in South India. Materia Socio‐Medica. 2012;24:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gurnee EA, Ndao IM, Johnson JR, et al. Gut colonization of healthy children and their mothers with pathogenic ciprofloxacin‐resistant Escherichia coli . JID. 2015;212:1862‐1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Darehabi HK, Naseri MH, Menbari S, et al. Antibiotic resistance pattern of Escherichia coli groups A, B1, B2 and D isolated from frozen foods and children with diarrhea in Sanandaj, Iran. Int J Entric Pathog. 2013;1:2. [Google Scholar]
- 79. Amaya E, Reyes D, Vilchez S, Paniagua M, et al. Antibiotic resistance patterns of intestinal Escherichia coli isolates from Nicaraguan children. J Med Microbiol. 2014;60:216‐222. [DOI] [PubMed] [Google Scholar]
- 80. Sadeghabadi AF, Ajami A, Fadaei R, et al. Widespread antibiotic resistance of diarrheagenic Escherichia coli and Shigella species. J Res Med Sci. 2014;19:S51. [PMC free article] [PubMed] [Google Scholar]
- 81. Haghi F, Zeighami H, Hajiahmadi F, et al. Frequency and antimicrobial resistance of diarrhoeagenic Escherichia coli from young children in Iran. J Med Microbiol. 2014;63:427‐432. [DOI] [PubMed] [Google Scholar]
- 82. Randrianirina F, Ratsima EH, Ramparany L, et al. Antimicrobial resistance of bacterial enteropathogens isolated from stools in Madagascar. BMC Infect Dis. 2014;14:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


